Paroxysmal Atrial Fibrillation with Rapid Ventricular Response Following COVID-19 Nasopharyngeal Swab: A Case Report
Abstract
1. Introduction
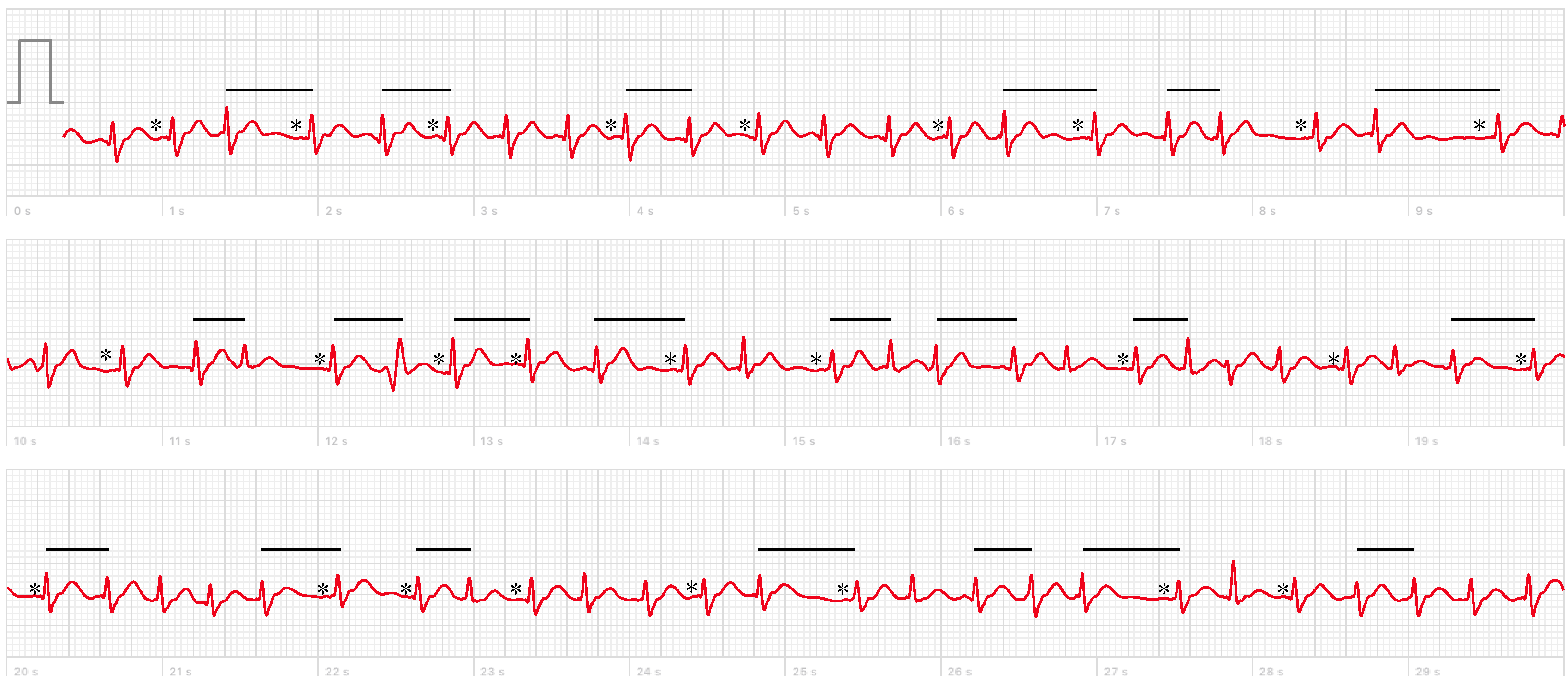
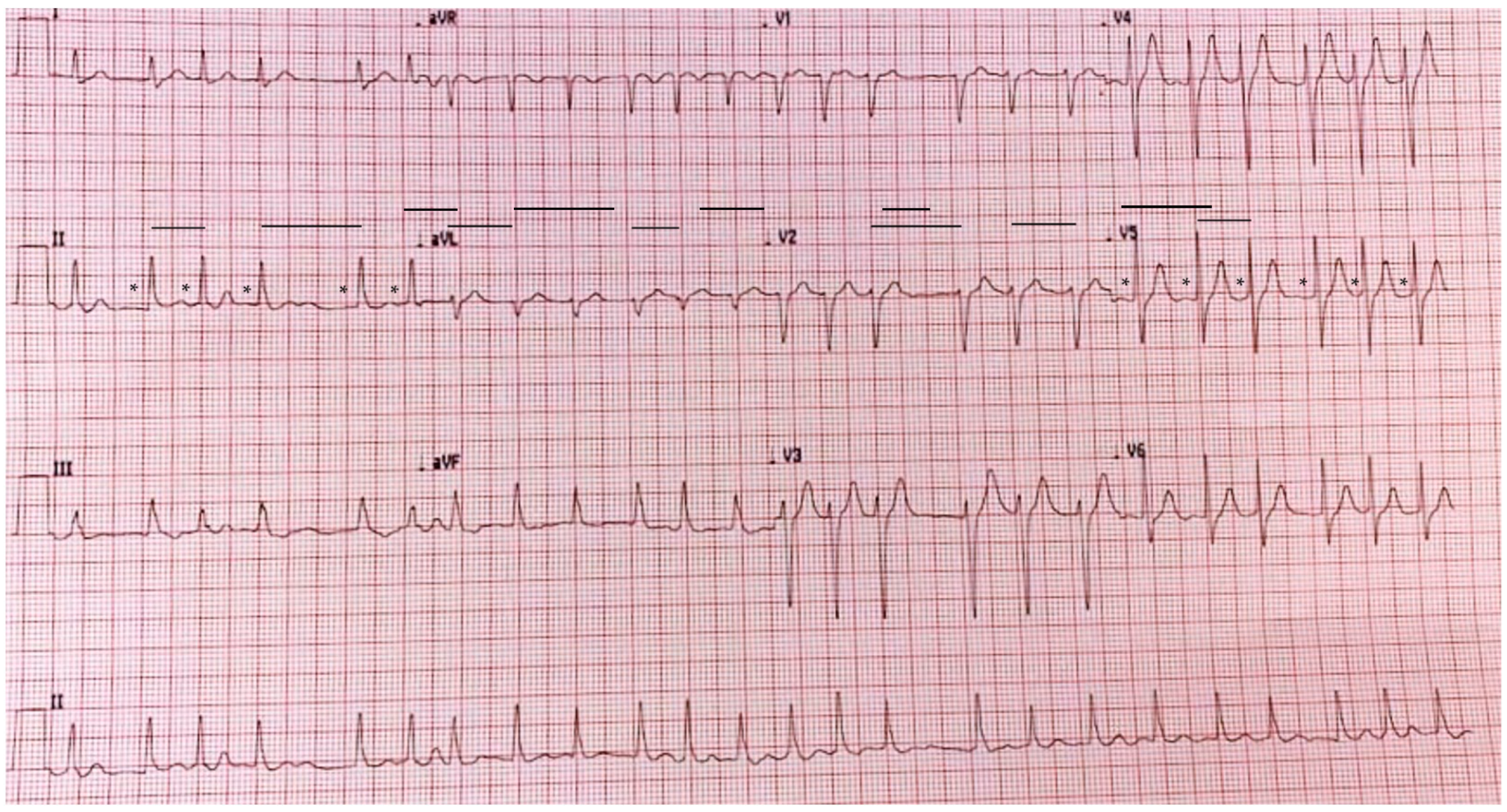
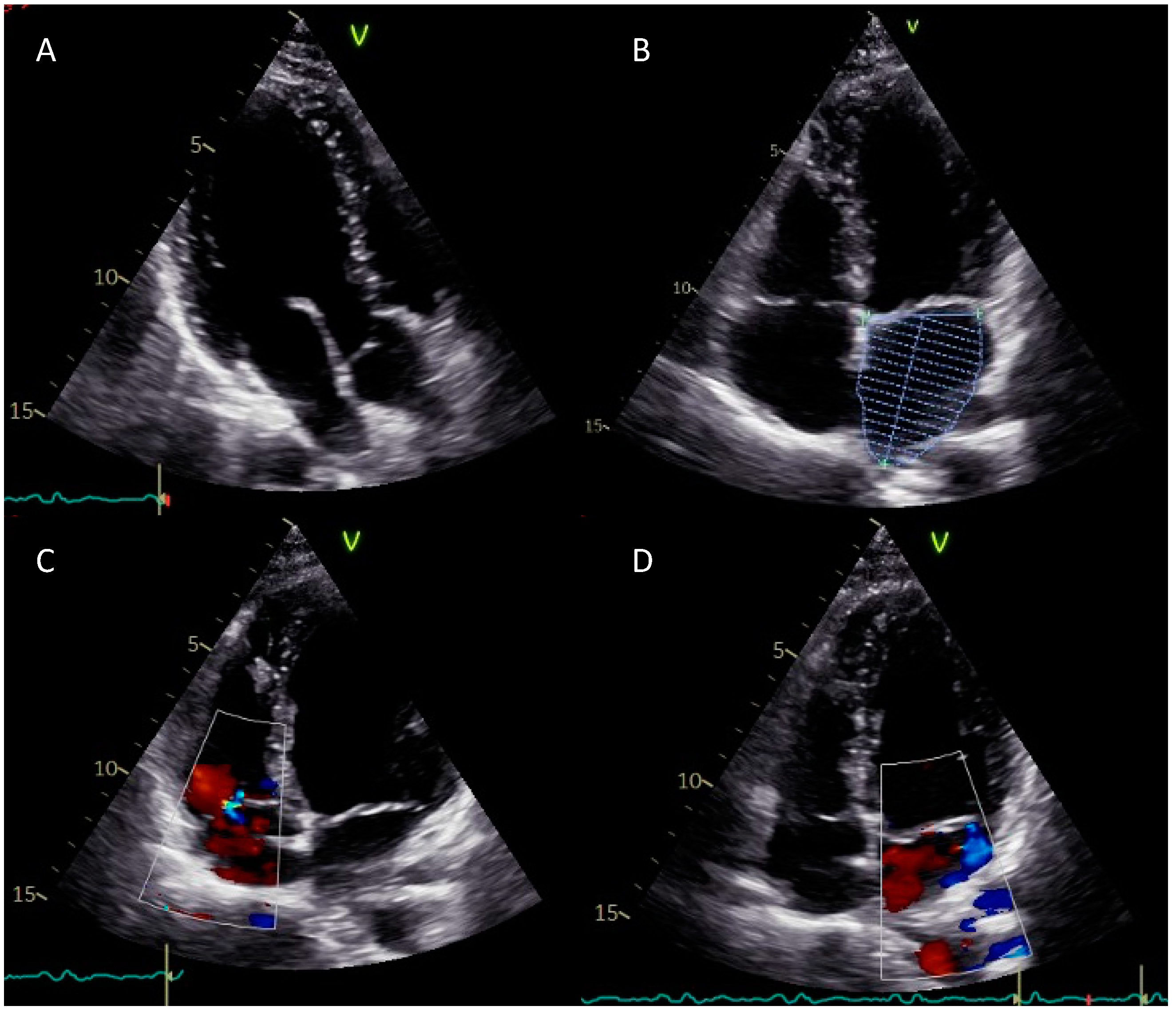
2. Case Report
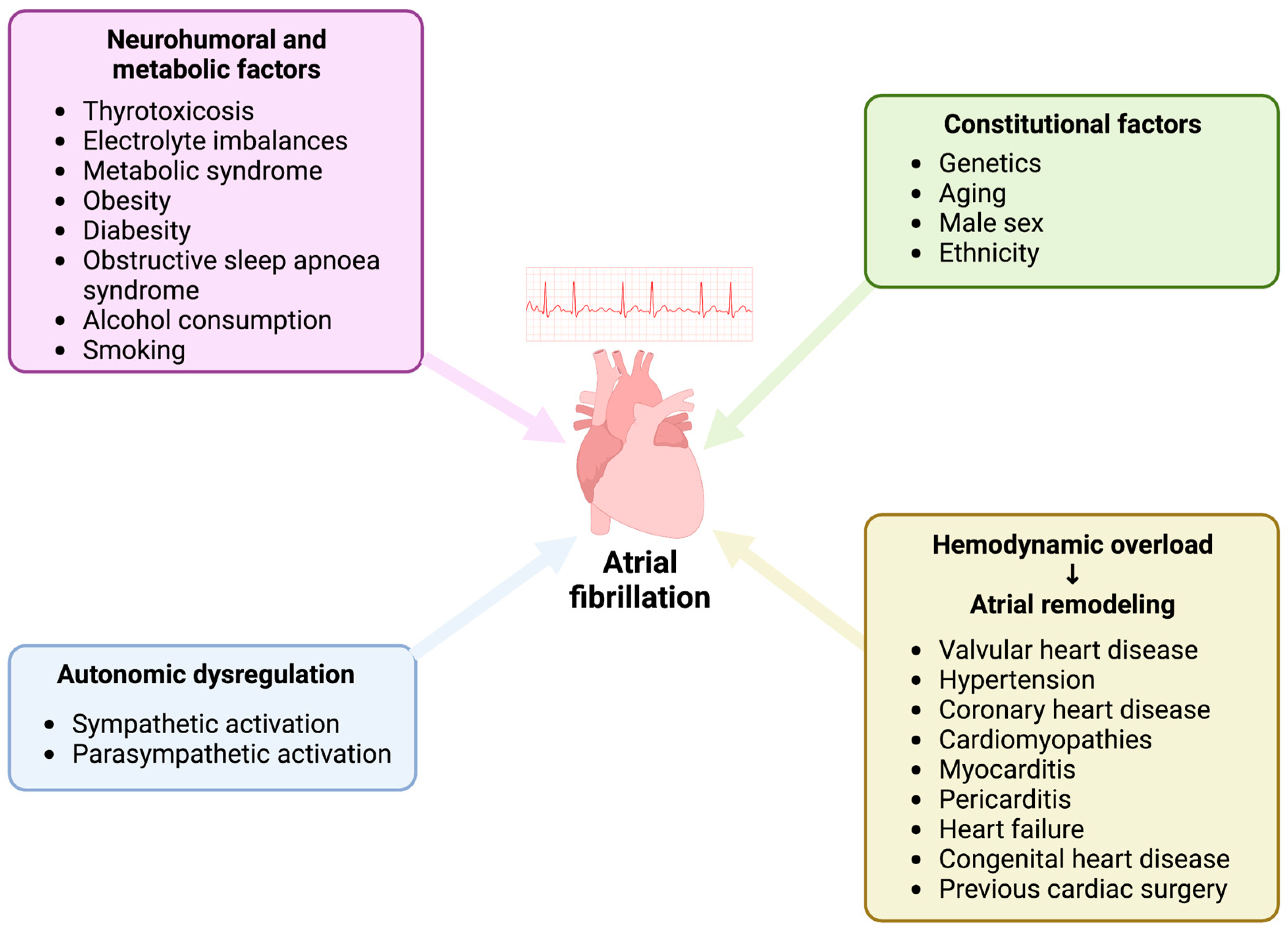
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schnabel, R.B.; Yin, X.; Gona, P.; Larson, M.G.; Beiser, A.S.; McManus, D.D.; Newton-Cheh, C.; Lubitz, S.A.; Magnani, J.W.; Ellinor, P.T.; et al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: A cohort study. Lancet 2015, 386, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial fibrillation. Nat. Rev. Dis. Prim. 2022, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.; Frontera, A.; Bond, R.; Duncan, E.; Thomas, G. Vagal atrial fibrillation: What is it and should we treat it? Int. J. Cardiol. 2015, 201, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Zeng, C.; Liu, X. The cardiac autonomic nervous system: A target for modulation of atrial fibrillation. Clin. Cardiol. 2019, 42, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ilsar, I.; Sabbah, H.N.; Ben David, T.; Mazgalev, T.N. Relationship between right cervical vagus nerve stimulation and atrial fibrillation inducibility: Therapeutic intensities do not increase arrhythmogenesis. Heart Rhythm. 2009, 6, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Gritzfeld, J.F.; Roberts, P.; Roche, L.; El Batrawy, S.; Gordon, S.B. Comparison between nasopharyngeal swab and nasal wash, using culture and PCR, in the detection of potential respiratory pathogens. BMC Res. Notes 2011, 4, 122. [Google Scholar] [CrossRef] [PubMed]
- Hasell, J.; Mathieu, E.; Beltekian, D.; Macdonald, B.; Giattino, C.; Ortiz-Ospina, E.; Roser, M.; Ritchie, H. A cross-country database of COVID-19 testing. Sci. Data 2020, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Rizzo, D.; Longoni, E.; Turra, N.; Urru, S.; Saba, P.P.; Musumano, L.; Bussu, F. Nasopharyngeal swab collection in the suspicion of Covid-19. Am. J. Otolaryngol. 2020, 41, 102551. [Google Scholar] [CrossRef] [PubMed]
- Fabbris, C.; Cestaro, W.; Menegaldo, A.; Spinato, G.; Frezza, D.; Vijendren, A.; Borsetto, D.; Boscolo-Rizzo, P. Is oro/nasopharyngeal swab for SARS-CoV-2 detection a safe procedure? Complications observed among a case series of 4876 consecutive swabs. Am. J. Otolaryngol. 2021, 42, 102758. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Pang, S.; Naclerio, R.M.; Kashima, M. Complications of nasal SARS-CoV-2 testing: A review. J. Investig. Med. 2021, 69, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Bloom, W.R.; Bloom, T.D. Neurally Mediated Syncope Triggered by COVID-19 Nasopharyngeal Swab Specimen Collection: A Case Report. Allergy Rhinol. 2022, 13, 21526567211073794. [Google Scholar] [CrossRef] [PubMed]
- Madanat, L.; Khalife, A.; Sims, M. Asystole During Nasopharyngeal Swab: Is COVID-19 to Blame? Cureus 2021, 13, e15448. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, T.; Mendelowith, D.; Golanov, E.; Spiriev, T.; Arasho, B.; Sandu, N.; Sadr-Eshkevari, P.; Meuwly, C.; Schaller, B.; Trigemino-Cardiac Reflex Examination Group. Trigeminocardiac reflex: The current clinical and physiological knowledge. J. Neurosurg. Anesthesiol. 2015, 27, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Perez, M.V.; Mahaffey, K.W.; Hedlin, H.; Rumsfeld, J.S.; Garcia, A.; Ferris, T.; Balasubramanian, V.; Russo, A.M.; Rajmane, A.; Cheung, L.; et al. Large-Scale Assessment of a Smartwatch to Identify Atrial Fibrillation. N. Engl. J. Med. 2019, 381, 1909–1917. [Google Scholar] [CrossRef]
- Khan, A.A.; Lip, G.Y.H.; Shantsila, A. Heart rate variability in atrial fibrillation: The balance between sympathetic and parasympathetic nervous system. Eur. J. Clin. Investig. 2019, 49, e13174. [Google Scholar] [CrossRef] [PubMed]
- Meuwly, C.; Chowdhury, T.; Sandu, N.; Golanov, E.; Erne, P.; Rosemann, T.; Schaller, B. Definition and Diagnosis of the Trigeminocardiac Reflex: A Grounded Theory Approach for an Update. Front. Neurol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]

| Laboratory Parameter (Unit of Measure) | Value | Normal Range |
|---|---|---|
| Hb (g/dL) | 15.50 | 13.50–17.50 |
| RBC (×106/μL) | 5.09 | 4.30–5.50 |
| WBC (×103/μL) | 6.70 | 4.00–10.00 |
| HCT (%) | 46.00 | 40.00–50.00 |
| MCV (fl) | 90.40 | 83.00–101.00 |
| RDW (%) | 12.40 | 11.00–16.00 |
| PLT (×103/μL) | 196.00 | 150.00–400.00 |
| AST (U/L) | 38.00 | 5.00–34.00 |
| ALT (U/L) | 51.00 | 0.00–55.00 |
| GGT (U/L) | 14.00 | 11.00–59.00 |
| CK (UI/L) | 161.00 | 30.00–200.00 |
| Plasma sodium (mmol/L) | 140.00 | 136.00–145.00 |
| Plasma potassium (mmol/L) | 4.10 | 3.50–5.10 |
| Creatinine (mg/dL) | 0.84 | 0.73–1.18 |
| Cholesterol (mg/dL) | 211.00 | <200.00 (optimal value) |
| HDL cholesterol (mg/dL) | 52.00 | >40 (optimal value) |
| LDL cholesterol (mg/dL) | 138.00 | <100.00 (optimal value) |
| Triglycerides (mg/dL) | 151.00 | 0.00–149.00 |
| Plasma glucose (mg/dL) | 96.00 | 74.00–106.00 |
| Blood urea (mg/dL) | 34.00 | 19.00–43.00 |
| Uric acid (mg/dL) | 5.30 | 3.50–7.20 |
| APTT (seconds) | 31.40 | 23.00–32.00 |
| INR | 1.02 | 0.80–1.20 |
| CRP (mg/dL) | 0.05 | <0.50 |
| Troponin I (pg/mL) | <10.0 | 0.00–34.20 |
| CKMB mass (ng/mL) | <0.3 | 0.00–5.20 |
| Myoglobin (ng/mL) | 38.00 | 0.00–154.90 |
| TSH (μUI/mL) | 1.96 | 0.35–4.94 |
| fT4 (pmol/L) | 11.10 | 9.01–19.50 |
| fT3 (pmol/L) | 5.76 | 2.89–4.88 |
| COVID-19 E gene | Negative | - |
| COVID-19 N gene | Negative | - |
| COVID-19 RdRp/s gene | Negative | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viscusi, M.M.; Ambrosio, L.; Ricciardi, D.; Mangiacapra, F.; Nusca, A.; Paolucci, L.; Ussia, G.P.; Grigioni, F. Paroxysmal Atrial Fibrillation with Rapid Ventricular Response Following COVID-19 Nasopharyngeal Swab: A Case Report. Reports 2023, 6, 15. https://doi.org/10.3390/reports6010015
Viscusi MM, Ambrosio L, Ricciardi D, Mangiacapra F, Nusca A, Paolucci L, Ussia GP, Grigioni F. Paroxysmal Atrial Fibrillation with Rapid Ventricular Response Following COVID-19 Nasopharyngeal Swab: A Case Report. Reports. 2023; 6(1):15. https://doi.org/10.3390/reports6010015
Chicago/Turabian StyleViscusi, Michele Mattia, Luca Ambrosio, Danilo Ricciardi, Fabio Mangiacapra, Annunziata Nusca, Luca Paolucci, Gian Paolo Ussia, and Francesco Grigioni. 2023. "Paroxysmal Atrial Fibrillation with Rapid Ventricular Response Following COVID-19 Nasopharyngeal Swab: A Case Report" Reports 6, no. 1: 15. https://doi.org/10.3390/reports6010015
APA StyleViscusi, M. M., Ambrosio, L., Ricciardi, D., Mangiacapra, F., Nusca, A., Paolucci, L., Ussia, G. P., & Grigioni, F. (2023). Paroxysmal Atrial Fibrillation with Rapid Ventricular Response Following COVID-19 Nasopharyngeal Swab: A Case Report. Reports, 6(1), 15. https://doi.org/10.3390/reports6010015









