Liver Transplantation from a Human Leukocyte Antigen-Matched Sibling Donor: Effectiveness of Direct-Acting Antiviral Therapy against Hepatitis C Virus Infection
Abstract
1. Introduction
2. Successful Re-Treatment with Direct-Acting Antiviral (DAA) Therapy against Hepatitis C Virus (HCV) Infection in a Patient Who Underwent a Living-Donor Liver Transplantation (LDLT) from a Human Leukocyte Antigen (HLA)-Matched Sibling Donor
2.1. Case
2.2. Donor and Living-Donor Liver Transplantation (LDLT)
2.3. Anti-HCV Therapies
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugawara, Y.; Ohtsuka, H.; Kaneko, J.; Ohkubo, T.; Imamura, H.; Makuuchi, M. Successful treatment of hepatitis C virus after liver transplantation from an identical twin. Transplantation 2002, 73, 1850–1851. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.U.; Schiano, T.D.; Min, A.D.; Kim-Schluger, L.; Schwartz, M.E.; Emre, S.; Fishbein, T.M.; Bodenheimer, H.C., Jr.; Miller, C.M. Syngeneic living-donor liver transplantation without the use of immunosuppression. Gastroenterology 2002, 123, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Takada, Y.; Fujimoto, Y.; Koshiba, T.; Haga, H.; Nabeshima, S.; Uemoto, S. Liver transplantation from an identical twin without immunosuppression, with early recurrence of hepatitis C. Am. J. Transplant. 2006, 6, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Toshida, K.; Toshima, T.; Yoshizumi, T.; Harada, N.; Itoh, S.; Nagao, Y.; Wang, H.; Shimagaki, T.; Kurihara, T.; Mori, M. Immunosuppression Free Protocol for Liver Transplant from an Identical Twin Mimicking Positive Donor-Specific Antibodies: A Case Report. Transplant. Proc. 2021, 53, 2576–2579. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Yokosuka, O.; Ehata, T.; Maru, Y.; Imazeki, F.; Saisho, H.; Shiratori, Y.; Omata, M. Detection of GBV-C RNA in patients with non-A-E fulminant hepatitis by reverse-transcription polymerase chain reaction. Hepatology 1997, 25, 1261–1265. [Google Scholar] [CrossRef]
- Enomoto, H.; Ueno, Y.; Hiasa, Y.; Nishikawa, H.; Hige, S.; Takikawa, Y.; Taniai, M.; Ishikawa, T.; Yasui, K.; Takaki, A.; et al. Transition in the etiology of liver cirrhosis in Japan: A nationwide survey. J. Gastroenterol. 2020, 55, 353–362. [Google Scholar] [CrossRef]
- Enomoto, H.; Ueno, Y.; Hiasa, Y.; Nishikawa, H.; Hige, S.; Takikawa, Y.; Taniai, M.; Ishikawa, T.; Yasui, K.; Takaki, A.; et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J. Gastroenterol. 2021, 56, 158–167. [Google Scholar] [CrossRef]
- O’Grady, J.G.; Smith, H.M.; Davies, S.E.; Daniels, H.M.; Donaldson, P.T.; Tan, K.C.; Portmann, B.; Alexander, G.J.; Williams, R. Hepatitis B virus reinfection after orthotopic liver transplantation. Serological and clinical implications. J. Hepatol. 1992, 14, 104–111. [Google Scholar] [CrossRef]
- Taga, S.A.; Washington, M.K.; Terrault, N.; Wright, T.L.; Somberg, K.A.; Ferrell, L.D. Cholestatic hepatitis C in liver allografts. Liver Transpl. Surg. 1998, 4, 304–310. [Google Scholar] [CrossRef][Green Version]
- Shin, E.; Kim, J.H.; Yu, E. Histopathological causes of late liver allograft dysfunction: Analysis at a single institution. Korean J. Pathol. 2013, 47, 21–27. [Google Scholar] [CrossRef]
- Sasaki, R.; Kanda, T.; Ohtsuka, M.; Yasui, S.; Haga, Y.; Nakamura, M.; Yokoyama, M.; Wu, S.; Nakamoto, S.; Arai, M.; et al. Successful Management of Graft Reinfection of HCV Genotype 2 in Living Donor Liver Transplantation from a Hepatitis B Core Antibody-Positive Donor with Sofosbuvir and Ribavirin. Case Rep. Gastroenterol. 2016, 10, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Kobayashi, T.; Ikegami, T.; Miuma, S.; Mizuno, S.; Akamatsu, N.; Takaki, A.; Ishigami, M.; Takatsuki, M.; Sugawara, Y.; et al. Efficacy and safety of glecaprevir and pibrentasvir treatment for 8 or 12 weeks in patients with recurrent hepatitis C after liver transplantation: A Japanese multicenter experience. J. Gastroenterol. 2019, 54, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.S.; Shaikh, A.; Goli, K.; Rich, N.E.; Benhammou, J.N.; Ahmed, A.; Kim, D.; Rana, A.; Goss, J.A.; Naggie, S.; et al. Improved Survival after Liver Transplantation for Patients with HIV and HIV/HCV Coinfection in the INSTI and DAA eras. J. Clin. Infect. Dis. 2022, ciac821. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Ronca, V.; Ebadi, M.; Hansen, B.E.; Hirschfield, G.; Elwir, S.; Alsaed, M.; Milkiewicz, P.; Janik, M.K.; Marschall, H.U.; et al. Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J. Hepatol. 2022, 77, 84–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, Z.L.; Kang, Z.Y.; Xiao, Y.L.; Liu, C.; Li, D.H. Liver graft injury caused by de novo donor-specific HLA antibodies in pediatric liver transplant recipients with low, moderate, and high immunologic risk. Am. J. Surg. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Del Bello, A.; Danjoux, M.; Congy-Jolivet, N.; Lavayssière, L.; Esposito, L.; Muscari, F.; Kamar, N. Histological long-term outcomes from acute antibody-mediated rejection following ABO-compatible liver transplantation. J. Gastroenterol. Hepatol. 2017, 32, 887–893. [Google Scholar] [CrossRef]
- Uebayashi, E.Y.; Okajima, H.; Yamamoto, M.; Ogawa, E.; Okamoto, T.; Haga, H.; Hatano, E. The New Challenge in Pediatric Liver Transplantation: Chronic Antibody-Mediated Rejection. J. Clin. Med. 2022, 11, 4834. [Google Scholar] [CrossRef]
- Kanda, T.; Yokosuka, O.; Fujiwara, K.; Saisho, H.; Shiga, H.; Oda, S.; Okuda, K.; Sugawara, Y.; Makuuchi, M.; Hirasawa, H. Fulminant hepatic failure associated with triazolam. Dig. Dis. Sci. 2002, 47, 1111–1114. [Google Scholar] [CrossRef]
- Sugawara, Y.; Makuuchi, M. Living donor liver transplantation: Present status and recent advances. Br. Med. Bull. 2006, 75–76, 15–28. [Google Scholar] [CrossRef]
- Kanda, T.; Nakamoto, S.; Sasaki, R.; Nakamura, M.; Yasui, S.; Haga, Y.; Ogasawara, S.; Tawada, A.; Arai, M.; Mikami, S.; et al. Sustained Virologic Response at 24 Weeks after the End of Treatment Is a Better Predictor for Treatment Outcome in Real-World HCV-Infected Patients Treated by HCV NS3/4A Protease Inhibitors with Peginterferon plus Ribavirin. Int. J. Med. Sci. 2016, 13, 310–315. [Google Scholar] [CrossRef]
- Korenaga, M.; Murata, K.; Izumi, N.; Tamaki, N.; Yokosuka, O.; Takehara, T.; Sakamoto, N.; Suda, G.; Nishiguchi, S.; Enomoto, H.; et al. No increased risk of hepatocellular carcinoma after eradication of hepatitis C virus by direct-acting antivirals, compared with interferon-based therapy. Glob. Health Med. 2022, 4, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Kubal, C.A.; Mangus, R.S.; Saxena, R.; Lobashevsky, A.; Higgins, N.; Agarwal, A.; Fridell, J.A.; Tector, A.J. Crossmatch-positive liver transplantation in patients receiving thymoglobulin-rituximab induction. Transplantation 2014, 97, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; West, K.A.; Ward, W.W.; Kanakry, J.A.; Flegel, W.A. Rebound and overshoot of donor-specific antibodies to human leukocyte antigens (HLA) during desensitization with plasma exchanges in hematopoietic progenitor cell transplantation: A case report. Transfusion 2021, 61, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Trentadue, G.; Dijkstra, G. Current understanding of alloimmunity of the intestinal graft. Curr. Opin. Organ Transplant. 2015, 20, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, S.O.; Cao, K.; Fernandez-Vina, M.; Kongtim, P.; Malki, M.A.; Fuchs, E.; Luznik, L.; Huang, X.J.; Ciceri, F.; Locatelli, F.; et al. The European Society for Blood and Marrow Transplantation (EBMT) Consensus Guidelines for the Detection and Treatment of Donor-specific Anti-HLA Antibodies (DSA) in Haploidentical Hematopoietic Cell Transplantation. Bone Marrow Transplant. 2018, 53, 521–534. [Google Scholar] [CrossRef]
- Tamura, K.; Tohyama, T.; Watanabe, J.; Nakamura, T.; Ueno, Y.; Inoue, H.; Honjo, M.; Sakamoto, K.; Takai, A.; Ogawa, K.; et al. Preformed donor-specific antibodies are associated with 90-day mortality in living-donor liver transplantation. Hepatol. Res. 2019, 49, 929–941. [Google Scholar] [CrossRef]
- Muro, M.; Moya-Quiles, M.R.; Mrowiec, A. Humoral Response in Liver Allograft Transplantation: A Review of the Role of Anti-Human Leukocyte Antigen (HLA) Antibodies. Curr. Protein Pept. Sci. 2016, 17, 776–784. [Google Scholar] [CrossRef]
- Musat, A.I.; Agni, R.M.; Wai, P.Y.; Pirsch, J.D.; Lorentzen, D.F.; Powell, A.; Leverson, G.E.; Bellingham, J.M.; Fernandez, L.A.; Foley, D.P.; et al. The significance of donor-specific HLA antibodies in rejection and ductopenia development in ABO compatible liver transplantation. Am. J. Transplant. 2011, 11, 500–510. [Google Scholar] [CrossRef]
- Kelly, D.; Verkade, H.J.; Rajanayagam, J.; McKiernan, P.; Mazariegos, G.; Hübscher, S. Late graft hepatitis and fibrosis in pediatric liver allograft recipients: Current concepts and future developments. Liver Transpl. 2016, 22, 1593–1602. [Google Scholar] [CrossRef]
- Oo, Y.H.; Sakaguchi, S. Regulatory T-cell directed therapies in liver diseases. J. Hepatol. 2013, 59, 1127–1134. [Google Scholar] [CrossRef]
- Sagoo, P.; Ali, N.; Garg, G.; Nestle, F.O.; Lechler, R.I.; Lombardi, G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci. Transl. Med. 2011, 3, 83ra42. [Google Scholar] [CrossRef] [PubMed]
- Samonakis, D.N.; Germani, G.; Burroughs, A.K. Immunosuppression and HCV recurrence after liver transplantation. J. Hepatol. 2012, 56, 973–983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Joshi, D.; Pinzani, M.; Carey, I.; Agarwal, K. Recurrent HCV after liver transplantation-mechanisms, assessment and therapy. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Aldridge, R.W.; Mattes, F.M.; Rolando, N.; Rolles, K.; Smith, C.; Shirling, G.; Atkinson, C.; Burroughs, A.K.; Milne, R.S.; Emery, V.C.; et al. Effects of donor/recipient human leukocyte antigen mismatch on human cytomegalovirus replication following liver transplantation. Transpl. Infect. Dis. 2015, 17, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Plotnicky, H.; Touraine, J.L. Cytotoxic T cells from a human chimera induce regression of Epstein-Barr virus-infected allogeneic host cells. Int. Immunol. 1993, 5, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Höhler, T.; Gerken, G.; Notghi, A.; Knolle, P.; Lubjuhn, R.; Taheri, H.; Schneider, P.M.; Meyer zum Büschenfelde, K.H.; Rittner, C. MHC class II genes influence the susceptibility to chronic active hepatitis C. J. Hepatol. 1997, 27, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Minton, E.J.; Smillie, D.; Neal, K.R.; Irving, W.L.; Underwood, J.C.; James, V. Association between MHC class II alleles and clearance of circulating hepatitis C virus. Members of the Trent Hepatitis C Virus Study Group. J. Infect. Dis. 1998, 178, 39–44. [Google Scholar] [CrossRef] [PubMed]
- McKiernan, S.M.; Hagan, R.; Curry, M.; McDonald, G.S.; Nolan, N.; Crowley, J.; Hegarty, J.; Lawlor, E.; Kelleher, D. The MHC is a major determinant of viral status, but not fibrotic stage, in individuals infected with hepatitis C. Gastroenterology 2000, 118, 1124–1130. [Google Scholar] [CrossRef]
- Saito, K.; Ait-Goughoulte, M.; Truscott, S.M.; Meyer, K.; Blazevic, A.; Abate, G.; Ray, R.B.; Hoft, D.F.; Ray, R. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J. Virol. 2008, 82, 3320–3328. [Google Scholar] [CrossRef]
- Kim, H.; Mazumdar, B.; Bose, S.K.; Meyer, K.; Di Bisceglie, A.M.; Hoft, D.F.; Ray, R. Hepatitis C virus-mediated inhibition of cathepsin S increases invariant-chain expression on hepatocyte surface. J. Virol. 2012, 86, 9919–9928. [Google Scholar] [CrossRef]
- Kanda, T.; Lau, G.K.K.; Wei, L.; Moriyama, M.; Yu, M.L.; Chuang, W.L.; Ibrahim, A.; Lesmana, C.R.A.; Sollano, J.; Kumar, M.; et al. APASL HCV guidelines of virus-eradicated patients by DAA on how to monitor HCC occurrence and HBV reactivation. Hepatol. Int. 2019, 13, 649–661. [Google Scholar] [CrossRef]
- Asian Pacific Association for the Study of the Liver (APASL) Hepatitis C Working Party; McCaughan, G.W.; Omata, M.; Amarapurkar, D.; Bowden, S.; Chow, W.C.; Chutaputti, A.; Dore, G.; Gane, E.; Guan, R.; et al. Asian Pacific Association for the Study of the Liver consensus statements on the diagnosis, management and treatment of hepatitis C virus infection. J. Gastroenterol. Hepatol. 2007, 22, 615–633. [Google Scholar] [CrossRef]
- Li, J.; Wu, V.; Pan, C.Q. Direct antiviral therapy for hepatitis C cirrhotic patients in liver transplantation settings: A systematic review. Hepatol. Int. 2022, 16, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Warnaar, N.; Molenaar, I.Q.; Colquhoun, S.D.; Slooff, M.J.; Sherwani, S.; de Wolf, A.M.; Porte, R.J. Intraoperative pulmonary embolism and intracardiac thrombosis complicating liver transplantation: A systematic review. J. Thromb. Haemost. 2008, 6, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.S. Hepatitis C and liver transplantation. Nature 2005, 436, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, M.; Aguilera, V.; Prieto, M.; San Juan, F.; Rayón, J.M.; Benlloch, S.; Berenguer, J. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J. Hepatol. 2006, 44, 717–722. [Google Scholar] [CrossRef]
- Takatsuki, M.; Uemoto, S.; Inomata, Y.; Egawa, H.; Kiuchi, T.; Fujita, S.; Hayashi, M.; Kanematsu, T.; Tanaka, K. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001, 72, 449–454. [Google Scholar] [CrossRef]
- Angelico, R.; Parente, A.; Manzia, T.M. Using a weaning immunosuppression protocol in liver transplantation recipients with hepatocellular carcinoma: A compromise between the risk of recurrence and the risk of rejection? Transl. Gastroenterol. Hepatol. 2017, 2, 74. [Google Scholar] [CrossRef]
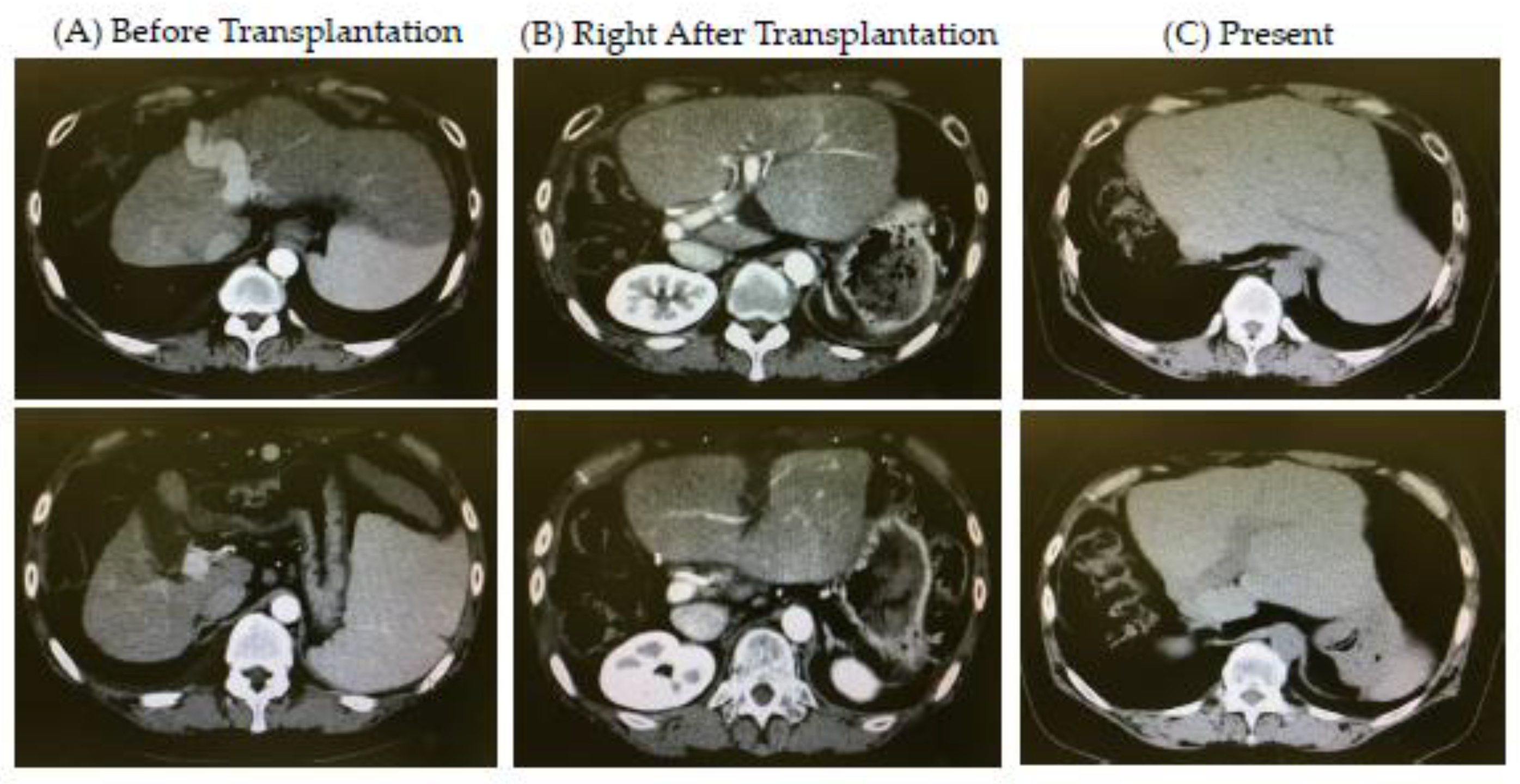
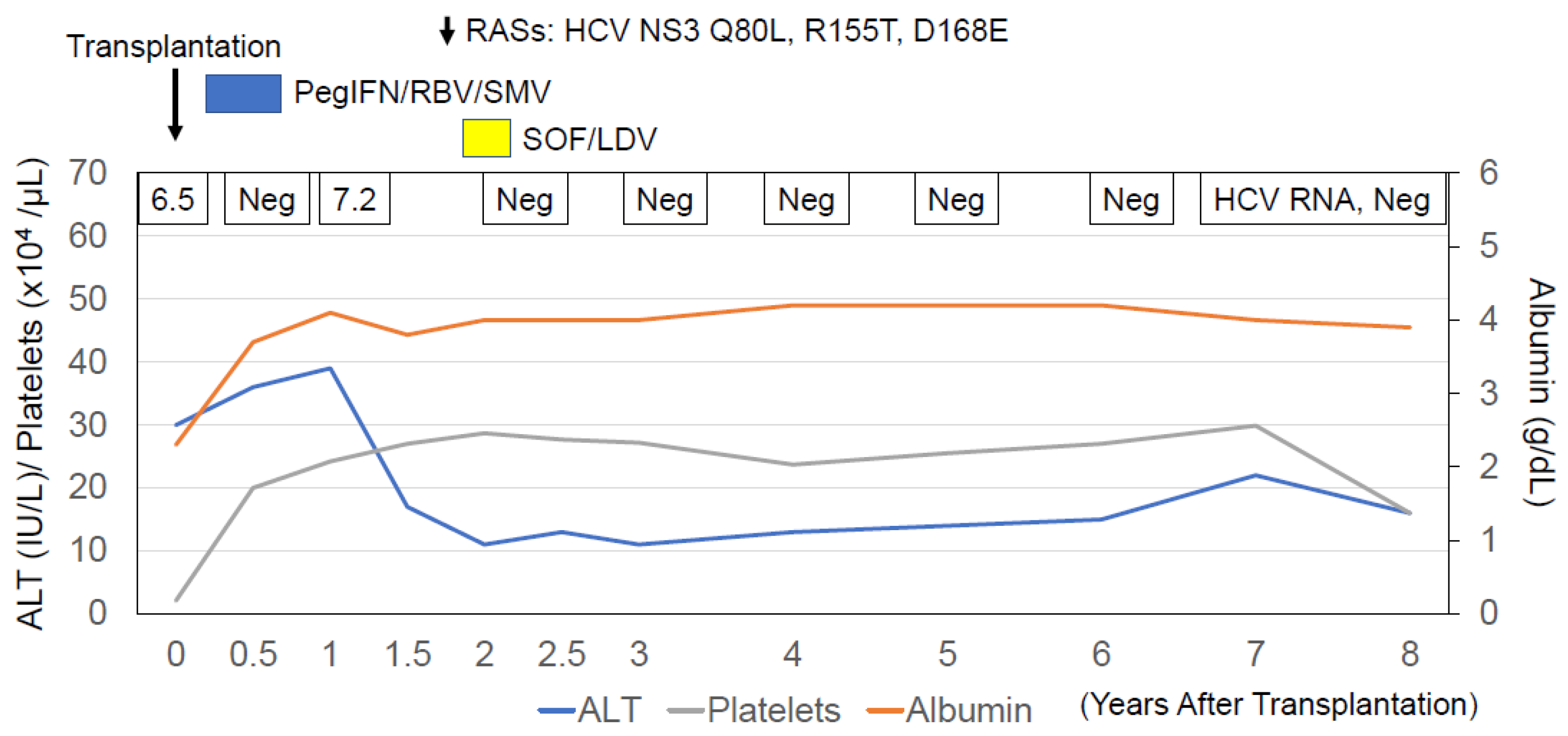
| Item | Values | Item | Values | Item | Values |
|---|---|---|---|---|---|
| WBC | 2000/μL | TP | 5.1 g/dL | HBsAg | Negative |
| RBC | 2,500,000/μL | Albumin | 2.3 g/dL | Anti-HBs | Negative |
| Hemoglobin | 8.5 g/dL | T.CHO | 117 mg/dL | Anti-HBc | Positive |
| Hematocrit | 26.1% | TG | 34 mg/dL | HBV DNA | Undetectable |
| Platelets | 21,000/μL | BUN | 8.6 mg/dL | Anti-HCV | Positive |
| PT | 59% | Creatinine | 0.95 mg/dL | HCV RNA | 6.5 LC/mL |
| PT-INR | 1.38 | CK | 49 IU/L | HCV GT | GT1b |
| AST | 63 IU/L | Amylase | 39 IU/L | Anti-HIV | Negative |
| ALT | 30 IU/L | BS | 87 mg/dL | IgG | 2250 mg/dL |
| LDH | 187 IU/L | HbA1c | 3.9% | IgA | 191 mg/dL |
| γ-GTP | 20 IU/L | NH3 | 54 μg/dL | IgM | 98 mg/dL |
| ALP | 231 IU/L | CRP | <0.10 mg/dL | ANA | Negative |
| T. Bil | 1.8 mg/dL | AFP | 17.7 ng/mL | AMAM2 | Negative |
| D. Bil | 1.0 mg/dL | PIVKA-II | 33 mAU/mL | Anti-LKM1 | Negative |
| Item | Values | Item | Values | Item | Values |
|---|---|---|---|---|---|
| WBC | 7000 /μL | T. Bil | 0.5 mg/dL | AFP | 17.7 ng/mL |
| RBC | 4,150,000 /μL | D. Bil | 0.1 mg/dL | PIVKA-II | 33 mAU/mL |
| Hemoglobin | 12.6 g/dL | TP | 7.0 g/dL | HBsAg | Negative |
| Hematocrit | 40.3% | Albumin | 3.9 g/dL | Anti-HBs | Positive |
| Platelets | 276,000/μL | T.CHO | 235 mg/dL | Anti-HBc | Positive |
| PT | 100% | TG | 85 mg/dL | HBV DNA | Undetectable |
| PT-INR | 0.95 | BUN | 11.3 mg/dL | Anti-HCV | Positive |
| AST | 19 IU/L | Creatinine | 0.76 mg/dL | HCV RNA | Undetectable |
| ALT | 16 IU/L | CK | 85 IU/L | Anti-HIV | Negative |
| LDH | 181 IU/L | BS | 92 mg/dL | ||
| γ-GTP | 43 IU/L | HbA1c | 5.4% | ||
| ALP | 244 IU/L |
| Case | Age (Years)/Gender | Disease(s) of Recipients | Graft | Immunosuppression (Duration) |
|---|---|---|---|---|
| 1 | 49/Male | HCV cirrhosis | Right lateral sector from brother | mPSL (POD 0–30) |
| 2 | 43/Female | HBV cirrhosis and HCC | Right lobe from brother | None |
| 3 | 56/Female | Neuroendocrine tumor metastasis to the liver | Right lobe from sister | None |
| 4 | 51/Male | HCV cirrhosis and HCC | Right lobe | None |
| 5 | 38/Male | HCV cirrhosis | Right lobe | None |
| 6 | In his 40′s/Male | HCV cirrhosis | Left lobe from older brother | mPSL (POD 0–5) |
| 7 | 57/Male | Acute liver failure | Right lobe from younger brother | FK/PSL (post operative 0–7 months) |
| Case | Rejection | Follow-Up Period (years) | Others: HCV GTs and Post-Transplantation Therapeusis | Reference |
| 1 | No | About 1 | HCV GT1b; Mixed lymphocyte cultures, negative; Interferon α 2a plus ribavirin, SVR | [1] |
| 2 | No | About 1 | Lamivudine along with monthly hepatitis B immune globulin infusions | [2] |
| 3 | No | About 0.5 | Sandostatin (Novartis, Switzerland) and interferon | [2] |
| 4 | No | About 1.5 | HCV GT1b; Interferon α plus ribavirin, SVR | [3] |
| 5 | No | About 0.5 | HCV GT1b; Interferon α plus ribavirin | [3] |
| 6 | No | About 8.5 | Mixed lymphocyte cultures, negative; simeprevir/peginterferon α/ribavirin; sofosbuvir /ledipasvir, SVR | Our case |
| 7 | No | About 0.5 | DSA, positive (Class II ≥ 2000 MFI). | [4] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanda, T.; Matsumoto, N.; Ishii, T.; Arima, S.; Shibuya, S.; Honda, M.; Sasaki-Tanaka, R.; Masuzaki, R.; Kanezawa, S.; Ogawa, M.; et al. Liver Transplantation from a Human Leukocyte Antigen-Matched Sibling Donor: Effectiveness of Direct-Acting Antiviral Therapy against Hepatitis C Virus Infection. Reports 2022, 5, 49. https://doi.org/10.3390/reports5040049
Kanda T, Matsumoto N, Ishii T, Arima S, Shibuya S, Honda M, Sasaki-Tanaka R, Masuzaki R, Kanezawa S, Ogawa M, et al. Liver Transplantation from a Human Leukocyte Antigen-Matched Sibling Donor: Effectiveness of Direct-Acting Antiviral Therapy against Hepatitis C Virus Infection. Reports. 2022; 5(4):49. https://doi.org/10.3390/reports5040049
Chicago/Turabian StyleKanda, Tatsuo, Naoki Matsumoto, Tomotaka Ishii, Shuhei Arima, Shinji Shibuya, Masayuki Honda, Reina Sasaki-Tanaka, Ryota Masuzaki, Shini Kanezawa, Masahiro Ogawa, and et al. 2022. "Liver Transplantation from a Human Leukocyte Antigen-Matched Sibling Donor: Effectiveness of Direct-Acting Antiviral Therapy against Hepatitis C Virus Infection" Reports 5, no. 4: 49. https://doi.org/10.3390/reports5040049
APA StyleKanda, T., Matsumoto, N., Ishii, T., Arima, S., Shibuya, S., Honda, M., Sasaki-Tanaka, R., Masuzaki, R., Kanezawa, S., Ogawa, M., Yamazaki, S., Aramaki, O., Kogure, H., & Okamura, Y. (2022). Liver Transplantation from a Human Leukocyte Antigen-Matched Sibling Donor: Effectiveness of Direct-Acting Antiviral Therapy against Hepatitis C Virus Infection. Reports, 5(4), 49. https://doi.org/10.3390/reports5040049








