Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Data Collection
2.3. Statistical Analysis
3. Results
Clinical Approaches
4. Discussion
Study Limitations and Strong Points
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, D.S.A.; Hamid, L.R.; Ali, A.; Salam, R.A.; Zuberi, N.; Lassi, Z.S.; Das, J.K. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2021, 21, 801. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Kumar, S.; Sharma, S.S. SARS-CoV-2 prevalence and maternal-perinatal outcomes among pregnant women admitted for delivery: Experience from COVID-19-dedicated maternity hospital in Jammu, Jammu and Kashmir (India). J. Med. Virol. 2021, 93, 5505–5514. [Google Scholar] [CrossRef] [PubMed]
- Diriba, K.; Awulachew, E.; Getu, E. The effect of coronavirus infection (SARS-CoV-2, MERS-CoV, and SARS-CoV) during pregnancy and the possibility of vertical maternal-fetal transmission: A systematic review and meta-analysis. Eur. J. Med. Res. 2020, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Ayed, A.; Embaireeg, A.; Benawadh, A.; Al-Fouzan, W.; Hammoud, M.; Al-Hathal, M.; Alzaydai, A.; Ahmad, A.; Ayed, M. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID-19 in Kuwait. BMC Pregnancy Childbirth 2020, 20, 754. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.; Baía, I.; Domingues, R.; Barros, H. Pregnancy and Breastfeeding During COVID-19 Pandemic: A Systematic Review of Published Pregnancy Cases. Front. Public Health 2020, 8, 558144. [Google Scholar] [CrossRef]
- Gupta, V.; Yadav, Y.; Sharma, R.; Mishra, M.; Ambedkar, D.; Gupta, V. Maternal and Perinatal Outcomes of Hospitalized COVID-19 Positive Pregnant Women. Cureus 2022, 14, e21817. [Google Scholar] [CrossRef]
- Mirbeyk, M.; Saghazadeh, A.; Rezaei, N. A systematic review of pregnant women with COVID-19 and their neonates. Arch. Gynecol. Obstet. 2021, 304, 5–38. [Google Scholar] [CrossRef]
- González, R.; García-Otero, L.; Pons-Duran, C.; Marbán-Castro, E.; Goncé, A.; Llurba, E.; Gil, M.D.M.; Rodríguez-Zambrano, M.Á.; Chen, H.; Ramírez, M.; et al. Hydroxychloroquine efficacy and safety in preventing SARS-CoV-2 infection and COVID-19 disease severity during pregnancy (COVID-Preg): A structured summary of a study protocol for a randomised placebo controlled trial. Trials 2020, 21, 607. [Google Scholar] [CrossRef]
- Novoa, R.H.; Quintana, W.; Llancarí, P.; Urbina-Quispe, K.; Guevara-Ríos, E.; Ventura, W. Maternal clinical characteristics and perinatal outcomes among pregnant women with coronavirus disease 2019. A systematic review. Travel Med. Infect. Dis. 2021, 39, 101919. [Google Scholar] [CrossRef]
- Adhikari, E.H.; Moreno, W.; Zofkie, A.C.; MacDonald, L.; McIntire, D.D.; Collins, R.R.J.; Spong, C.Y. Pregnancy Outcomes Among Women with and Without Severe Acute Respiratory Syndrome Coronavirus 2 Infection. JAMA Netw. Open 2020, 3, e2029256. [Google Scholar] [CrossRef] [PubMed]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Gabrieli, D.; Cahen-Peretz, A.; Shimonovitz, T.; Marks-Garber, K.; Amsalem, H.; Kalish, Y.; Lavy, Y.; Walfisch, A. Thromboembolic events in pregnant and puerperal women after COVID-19 lockdowns: A retrospective cohort study. Int. J. Gynaecol. Obstet. 2021, 155, 95–100. [Google Scholar] [CrossRef]
- Zitiello, A.; Grant, G.E.; Ben Ali, N.; Feki, A. Thrombocytopaenia in pregnancy: The importance of differential diagnosis during the COVID-19 pandemic. J. Matern. Fetal Neonatal. Med. 2022, 35, 2414–2416. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.J.; Schapero, M.; Iverson, R.; Yarrington, C.D. Obstetric Hemorrhage Risk Associated with Novel COVID-19 Diagnosis from a Single-Institution Cohort in the United States. Am. J. Perinatol. 2020, 37, 1411–1416. [Google Scholar] [CrossRef]
- Tehrani, H.A.; Darnahal, M.; Vaezi, M.; Haghighi, S. COVID-19 associated thrombotic thrombocytopenic purpura (TTP); A case series and mini-review. Int. Immunopharmacol. 2021, 93, 107397. [Google Scholar] [CrossRef]
- Moses, M.L.; Kazzi, N.G.; Lee, L. Severe Thrombocytopenia in a Pregnant Patient with Asymptomatic COVID-19 Infection: A Case Report. Cureus 2021, 13, e12990. [Google Scholar] [CrossRef]
- Makatsariya, A.D.; Slukhanchuk, E.V.; Bitsadze, V.O.; Khizroeva, J.K.H.; Tretyakova, M.V.; Tsibizova, V.I.; Elalamy, I.; Gris, J.C.; Grandone, E.; Makatsariya, N.A.; et al. Thrombotic microangiopathy, DIC-syndrome and COVID-19: Link with pregnancy prothrombotic state. J. Matern. Fetal Neonatal. Med. 2022, 35, 2536–2544. [Google Scholar] [CrossRef]
- Makatsariya, A.; Slukhanchuk, E.; Bitsadze, V.; Khizroeva, J.; Tretyakova, M.; Tsibizova, V.; Dobryakov, A.; Elalamy, I.; Gris, J.C. COVID-19, neutrophil extracellular traps and vascular complications in obstetric practice. J. Perinat. Med. 2020, 48, 985–994. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048–2058. [Google Scholar] [CrossRef]
- Kotlar, B.; Gerson, E.; Petrillo, S.; Langer, A.; Tiemeier, H. The impact of the COVID-19 pandemic on maternal and perinatal health: A scoping review. Reprod. Health 2021, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Jevtic, S.D.; Malinowski, A.K.; Othman, M.; Kadir, R.A.A. Physician experiences in management of COVID-19-associated coagulopathy in pregnancy: Communication from the ISTH SSC Subcommittee on Women’s Health Issues in Thrombosis and Haemostasis. J. Thromb. Haemost. 2021, 19, 2539–2545. [Google Scholar] [CrossRef] [PubMed]
- Servante, J.; Swallow, G.; Thornton, J.G.; Myers, B.; Munireddy, S.; Malinowski, A.K.; Othman, M.; Li, W.; O’Donoghue, K.; Walker, K.F. Haemostatic and thrombo-embolic complications in pregnant women with COVID-19: A systematic review and critical analysis. BMC Pregnancy Childbirth 2021, 21, 108. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, B.; Yuan, T.; Chen, X.; Ao, Y.; Fitzpatrick, T.; Li, P.; Zhou, Y.; Lin, Y.F.; Duan, Q.; et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J. Infect. 2020, 80, 656–665. [Google Scholar] [CrossRef]
- Kadir, R.A.; Kobayashi, T.; Iba, T.; Erez, O.; Thachil, J.; Kazi, S.; Malinowski, A.K.; Othman, M. COVID-19 coagulopathy in pregnancy: Critical review, preliminary recommendations, and ISTH registry-Communication from the ISTH SSC for Women’s Health. J. Thromb. Haemost. 2020, 18, 3086–3098. [Google Scholar] [CrossRef]
- Giespers, S.; Goh, E.; Kew, T.; Allotey, J.; Brizuela, V.; Kara, E.; Kunst, H.; Bonet, M.; Thangaratinam, S.; Chatterjee, S.; et al. Treatment of COVID-19 in pregnant women: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 120–128. [Google Scholar] [CrossRef]
- Schwartz, D.A. An Analysis of 38 Pregnant Women With COVID-19, Their Newborn Infants, and Maternal-Fetal Transmission of SARS-CoV-2: Maternal Coronavirus Infections and Pregnancy Outcomes. Arch. Pathol. Lab. Med. 2020, 144, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chu, H.; Pei, Y.V.; Zhang, J. Laboratory Effects of COVID-19 Infection in Pregnant Women and Their Newborns: A Systematic Review and Meta-Analysis. Front. Glob. Womens Health 2021, 2, 647072. [Google Scholar] [CrossRef]
- Pettirosso, E.; Giles, M.; Cole, S.; Rees, M. COVID-19 and pregnancy: A review of clinical characteristics, obstetric outcomes and vertical transmission. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, 640–659. [Google Scholar] [CrossRef]
- Mullins, E.; Evans, D.; Viner, R.M.; O’Brien, P.; Morris, E. Coronavirus in pregnancy and delivery: Rapid review. Ultrasound Obstet. Gynecol. 2020, 55, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Levy, A.; Yagil, Y.; Bursztyn, M.; Barkalifa, R.; Scharf, S.; Yagil, C. ACE2 expression and activity are enhanced during pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1953–R1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todros, T.; Masturzo, B.; De Francia, S. COVID-19 infection: ACE2, pregnancy and preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 253, 330. [Google Scholar] [CrossRef] [PubMed]
- Illi, B.; Vasapollo, B.; Valensise, H.; Totta, P. SARS-CoV-2, Endothelial Dysfunction, and the Renin-Angiotensin System (RAS): A Potentially Dangerous Triad for the Development of Pre-Eclampsia. Reprod. Med. 2021, 2, 95–106. [Google Scholar] [CrossRef]
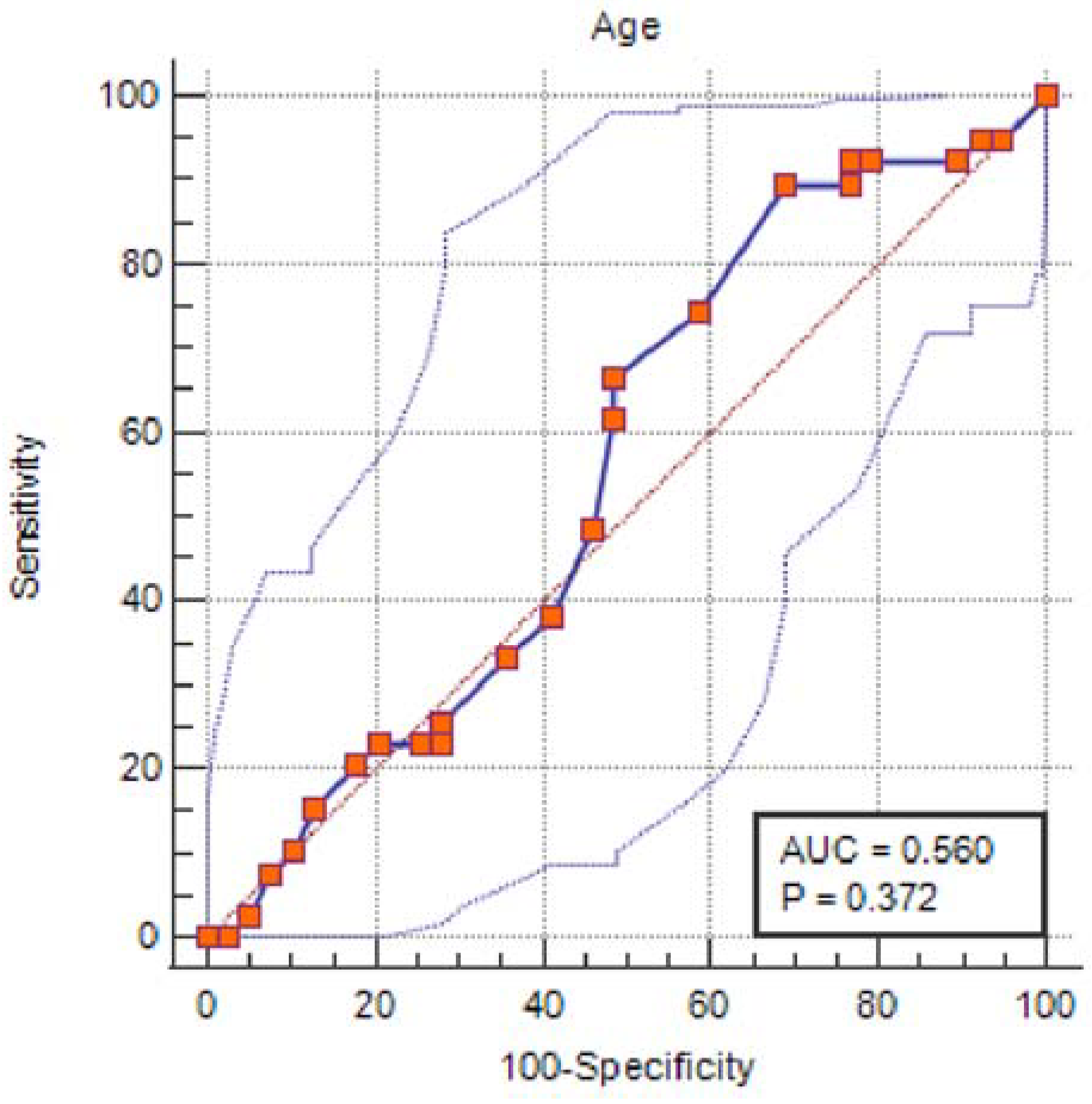
| Control (n = 39) | COVID-19 (n = 39) | ||||
|---|---|---|---|---|---|
| Mean ± SD (Min.–Max.) | Median (Q1–Q3) | Mean ± SD (Min.–Max.) | Median (Q1–Q3) | p | |
| Age | 30.05 ± 6.79 | 28.9 ± 5.76 | 0.421 | ||
| (17–41) | (18–41) | ||||
| Gestation | 2.77 ± 1.94 | 2 (1–3) | 2.18 ± 1.17 | 2 (1–3) | 0.305 |
| (1–9) | (1–6) | ||||
| Parity | 2.15 ± 1.6 | 2 (1–3) | 1.67 ± 1.13 | 2 (1–2) | 0.208 |
| (0–6) | (0–6) | ||||
| Normal Range | Control (n = 39) | COVID-19 (n = 39) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD (Min.–Max.) | Median (Q1–Q3) | Mean ± SD (Min.–Max.) | Median (Q1–Q3) | p | ||
| Leukocytes | 4.00–10.00 × 109/L | 12.16 ± 3.26 (7.19–21.9) | 11.98 (9.74–13.81) | 11.34 ± 4.07 (4.08–22.21) | 10.77 (8.67–13.33) | 0.168 |
| Neutrophils | 2–7 × 109/L | 8.91 ± 3 (4.16–18.71) | 8.49 (6.48–10.72) | 8.66 ± 3.63 (2.45–18.88) | 7.9 (6.2–10.11) | 0.442 |
| Lymphocyte | 1.0–4.1 × 109/L | 3.59 ± 3.7 (1.41–17.3) | 2.39 (1.86–3.1) | 1.93 ± 0.83 (0.37–3.91) | 1.96 (1.45–2.38) | 0.002 |
| Red blood cells | 4.7–6.1 million cells/mcL | 4.21 ± 0.48 (3.39–5.38) | 4.16 (3.92–4.54) | 4.24 ± 0.37 (3.42–4.89) | 4.19 (3.94–4.52) | 0.756 |
| Hemoglobin | 11.5–16 g/dL | 11.2 ± 1.18 (8.6–13.5) | 11.5 (10.4–12.1) | 11.86 ± 1.25 (8.4–14.3) | 11.8 (11.2–2.7) | 0.021 |
| Hematocrit | 35–48 % | 36.28 ± 3.71 (28.5–43.4) | 36.5 (33.7–38.5) | 37.58 ± 3.3 (29.2–44.9) | 37.7 (35.5–39.4) | 0.105 |
| Platelets | 150–450 × 109/L | 246.62 ± 75.86 (101–420) | 261 (185–307) | 245.54 ± 83.65 (57–484) | 242 (185–307) | 0.953 |
| International Normalized Ratio | 0.8–1.2 | 0.97 ± 0.12 (0.77–1.24) | 0.99 (0.88–1.07) | 0.99 ± 0.13 (0.8–1.2) | 0.98 (0.88–1.13) | 0.558 |
| Creatinine | 0.50–0.90 mg/dL | 1.9 ± 8.23 (0.37–52) | 0.56 (0.52–0.67) | 2.51 ± 12.08 (0.38–76) | 0.57 (0.5–0.64) | 0.708 |
| Urea | 16–43 mg/dL | 19.51 ± 7 (11.19–48.78) | 17.99 (15.08–22.5) | 18.71 ± 6.16 (8.87–35.62) | 19.24 (13.41–22.3) | 0.881 |
| Uric acid | 2.3–6.10 mg/dL | 3.88 ± 1.1 (2.3–6.69) | 3.67 (3.01–4.62) | 4.2 ± 0.86 (2.94–6.69) | 4.08 (3.55–4.91) | 0.157 |
| AST | 0.00–31.00 U/L | 19.41 ± 7.33 (11–43) | 17 (15–22) | 26.23 ± 15.4 (12–98) | 22 (18–31) | 0.005 |
| ALT | 0.00–34.00 U/L | 15.85 ± 8.74 (4–50) | 14 (11–19) | 21.21 ± 24.65 (6–126) | 13 (10–19) | 0.952 |
| Glucose | 60–115 mg/dL | 89.54 ± 15.77 (66–163) | 87 (80–98) | 91.74 ± 15.31 (71–134) | 92 (80–98) | 0.535 |
| C Reactive Protein | 0.00–5.00 mg/L | 10.34 ± 11.41 (2.07–73.2) | 7.38 (4.93–12.71) | 18.77 ± 31.37 (0.05–150.87) | 8.48 (4.31–15.5) | 0.932 |
| Lactate dehydrogenase | 0.00–247.00 U/L | 94.13 ± 79.04 (11–260) | 65 (26–154) | 227.67 ± 82.6 (110.8–418.9) | 216.2 (144.3–262.6) | <0.0001 |
| Fibrinogen | 180–450 mg/dL | 466.19 ± 111.16 (301.6–903.2) | 451 (405.5–484.4) | 396.25 ± 138.09 (79.1–658) | 357.8 (309.5–506.6) | 0.005 |
| Ferritin | 18–160 ng/mL | 78.49 ± 53.9 (11.05–160) | 97 (19–127) | 40.26 ± 67.14 (5.92–424.18) | 22.2 (12.2–49.99) | <0.0001 |
| Control | COVID-19 | Total | p | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| Antiviral | No | 39 (100) | 34 (87.2) | 73 (93.6) | 0.055 |
| Yes | 0 (0) | 4 (10.3) | 4 (5.1) | ||
| Refused | 0 (0) | 1 (2.6) | 1 (1.3) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Anticoagulant | No | 37a (94.9) | 2b (5.1) | 39 (50) | <0.0001 |
| Yes | 2a (5.1) | 37b (94.9) | 39 (50) | ||
| Total | 39 (1) | 39 (1) | 78 (1) | ||
| Dexamethasone | No | 38a (97.4) | 20b (51.3) | 58 (74.4) | <0.0001 |
| Yes | 1a (2.6) | 19b (48.7) | 20 (25.6) | ||
| Total | 39 (100) | 39(100) | 78 (100) | ||
| Uterotonic | No | 5a (12.8) | 15b (38.5) | 20 (25.6) | 0.01 |
| Yes | 34a (87.2) | 24b(61.5) | 58 (74.4) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Antibiotics | No | 12a (30.8) | 4b (10.3) | 16 (20.5) | 0.025 |
| Yes | 27a (69.2) | 35b (89.7) | 62 (79.5) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Anti-inflammatory and Analgesics | No | 5 (12.8) | 4 (10.3) | 9 (11.5) | 0.99 |
| Yes | 34 (87.2) | 35 (89.7) | 69 (88.5) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Vitamins | No | 31a (79.5) | 5b (12.8) | 36 (46.2) | <0.0001 |
| Yes | 8a (20.5) | 34b (87.2) | 42 (53.8) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Hemostatics | No | 39 (100) | 39 (100) | 78 (100) | |
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Blood products | No | 39 (100) | 38 (97.4) | 77 (98.7) | 0.99 |
| Yes | 0 (0) | 1 (2.6) | 1 (1.3) | ||
| Total | 39 (100) | 39 (100) | 78 (100) |
| Control (n = 39) | COVID-19 (n = 39) | ||||
|---|---|---|---|---|---|
| Mean ± SD (Min.–Max.) | Median (Q1–Q3) | Mean ± SD (Min.–Max.) | Median (Q1–Q3) | p | |
| Days of hospitalization | 4.18 ± 1.39 (2–9) | 4 (4–5) | 5.03 ± 2.32 (2–13) | 5 (3–7) | 0.056 |
| Birth weight (grams) | 3363.44 ± 520.03 (2550–4450) | 3265 (2925–3700) | 3199.69 ± 477.35 (2180–4200) | 3200 (2900–3450) | 0.408 |
| APGAR score | 7.64 ± 3.38 (0–10) | 9 (8–10) | 7.41 ± 3.24 (0–10) | 9 (8–9) | 0.197 |
| Control | COVID-19 | Total | p | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | |||
| IUGR | No | 39 (100) | 37 (94.9) | 76 (97.4) | 0.494 |
| Yes | 0 (0) | 2 (5.1) | 2 (2.6) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Preeclampsia | No | 38 (97.4) | 38a (97.4) | 76(97.4) | 0.99 |
| Yes | 1 (2.6) | 1 (2.6) | 2 (2.6) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Premature Birth | No | 38 (97.4) | 36 (92.3) | 74 (94.9) | 0.615 |
| Yes | 1 (2.6) | 3 (7.7) | 4 (5.1) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| Miscarriage | No | 37 (0.949) | 37 (0.949) | 74 (0.949) | 0.99 |
| Yes | 2 (0.051) | 2 (0.051) | 4 (0.051) | ||
| Total | 39 (100) | 39 (100) | 78 (100) | ||
| C-Section | No | 24a (75) | 14b (43.8) | 38 (59.4) | 0.011 |
| Yes | 8a (25) | 18b (56.3) | 26 (40.6) | ||
| Total | 32 (100) | 32 (100) | 64 (100) | ||
| Vaginal Birth | No | 8a (25) | 18b (56.3) | 26 (40.6) | 0.011 |
| Yes | 24a (75) | 14b (43.8) | 38 (59.4) | ||
| Total | 32 (100) | 32 (100) | 64 (100) | ||
| Neonatal Asphyxia | No | 31a (96.9) | 22b (68.8) | 53 (82.8) | 0.003 |
| Yes | 1a (3.1) | 10b (31.3) | 11 (17.2) | ||
| Total | 32 (100) | 32 (100) | 64 (100) | ||
| Gender | Male | 2 (28.6) | 19 (59.4) | 21 (53.8) | 0.215 |
| Female | 5 (71.4) | 13 (40.6) | 18 (46.2) | ||
| Total | 7 (100) | 32 (100) | 39 (100) | ||
| Neonatal COVID-19 | No | 32 (100) | 32 (100) | 64 (100) | |
| Total | 32 (100) | 32 (100) | 64 (100) | ||
| Fetal/Neonatal Death | No | 32 (82.10) | 32 (82.10) | 64 (82.10) | |
| Total | 32 (82.10) | 32 (82.10) | 64 (82.10) |
| Control n (%) | COVID-19 n (%) | Total n (%) | p | ||
|---|---|---|---|---|---|
| Smoking | No | 34(87.2) | 33(84.6) | 67(85.9) | 0.745 |
| Yes | 5(12.8) | 6(15.4) | 11(14.1) | ||
| Total | 39(100) | 39(100) | 78(100) | ||
| Obesity | No | 35(89.7) | 37(94.9) | 72(92.3) | 0.675 |
| Yes | 4(10.3) | 2(5.1) | 6(7.7) | ||
| Total | 39(100) | 39(100) | 78(100) | ||
| Comorbidities | No | 37(94.9) | 35(89.7) | 72(92.3) | 0.675 |
| Yes | 2(5.1) | 4(10.3) | 6(7.7) | ||
| Total | 39(100) | 39(100) | 78(100) | ||
| Diabetes | No | 37(94.9) | 36(92.3) | 73(93.6) | 0.99 |
| Yes | 2(5.1) | 3(7.7) | 5(6.4) | ||
| Total | 39(100) | 39(100) | 78(100) |
| Variables | B | S.E. | Wald | p | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Smoke (No/Yes) | −0.35 | 0.673 | 0,27 | 0.603 | 0.705 | 0.189 | 2.634 |
| Obesity (No/Yes) | 1.604 | 1.175 | 1.864 | 0.172 | 4.974 | 0.497 | 49.767 |
| Comorbidities (No/Yes) | −22.841 | 40,192.95 | 0 | 0.99 | 0 | 0 | . |
| Diabetes (No/Yes) | 22.071 | 40,192.95 | 0 | 0.99 | 3.85 × 109 | 0 | . |
| Constant | −0.519 | 1.481 | 0.123 | 0.726 | 0.595 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitranovici, M.I.; Chiorean, D.M.; Oală, I.E.; Petre, I.; Cotoi, O.S. Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania. Reports 2022, 5, 27. https://doi.org/10.3390/reports5030027
Mitranovici MI, Chiorean DM, Oală IE, Petre I, Cotoi OS. Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania. Reports. 2022; 5(3):27. https://doi.org/10.3390/reports5030027
Chicago/Turabian StyleMitranovici, Melinda Ildiko, Diana Maria Chiorean, Ioan Emilian Oală, Izabella Petre, and Ovidiu Simion Cotoi. 2022. "Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania" Reports 5, no. 3: 27. https://doi.org/10.3390/reports5030027
APA StyleMitranovici, M. I., Chiorean, D. M., Oală, I. E., Petre, I., & Cotoi, O. S. (2022). Evaluation of the Obstetric Patient: Pregnancy Outcomes during COVID-19 Pandemic—A Single-Center Retrospective Study in Romania. Reports, 5(3), 27. https://doi.org/10.3390/reports5030027









