A Novel Case of Recurrent Mucinous Borderline Ovarian Tumor: Early Relapse and Fatal Outcome
Abstract
:1. Introduction
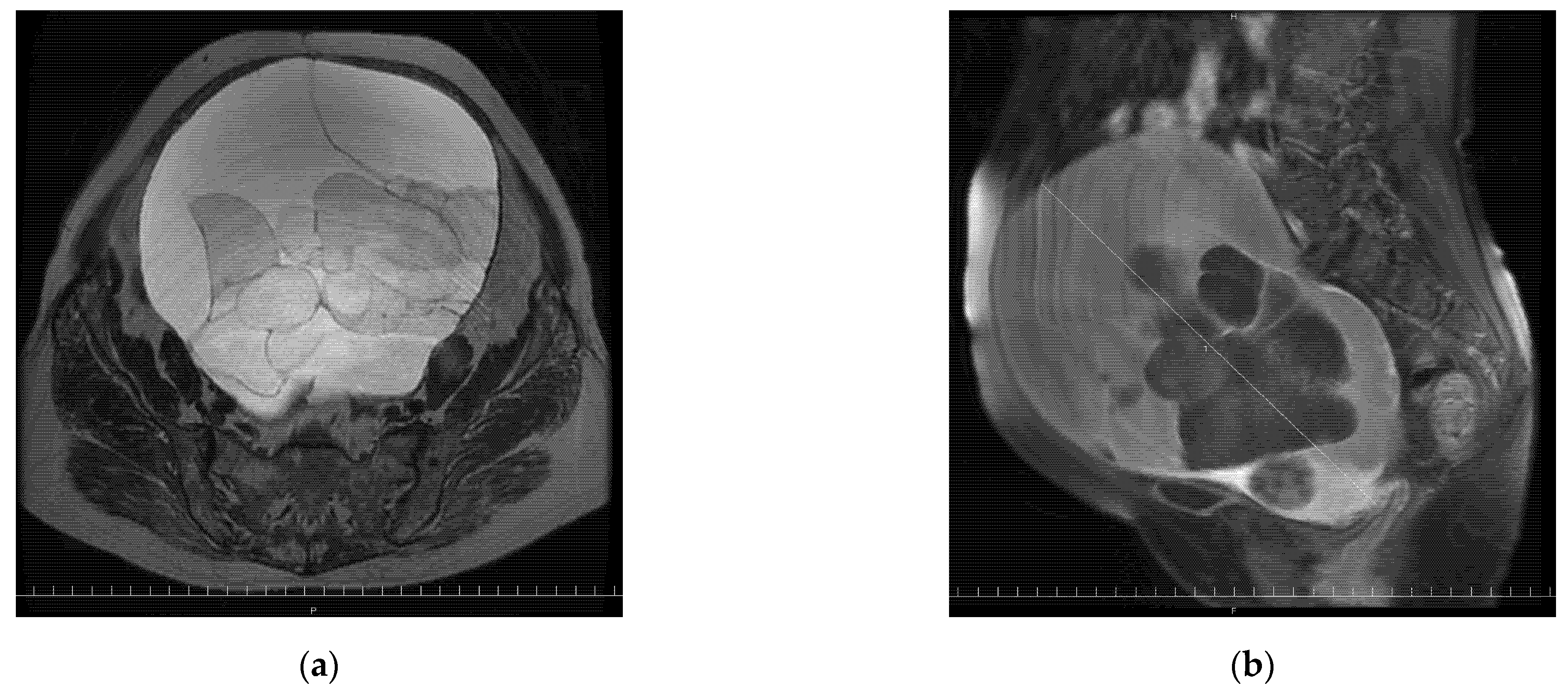
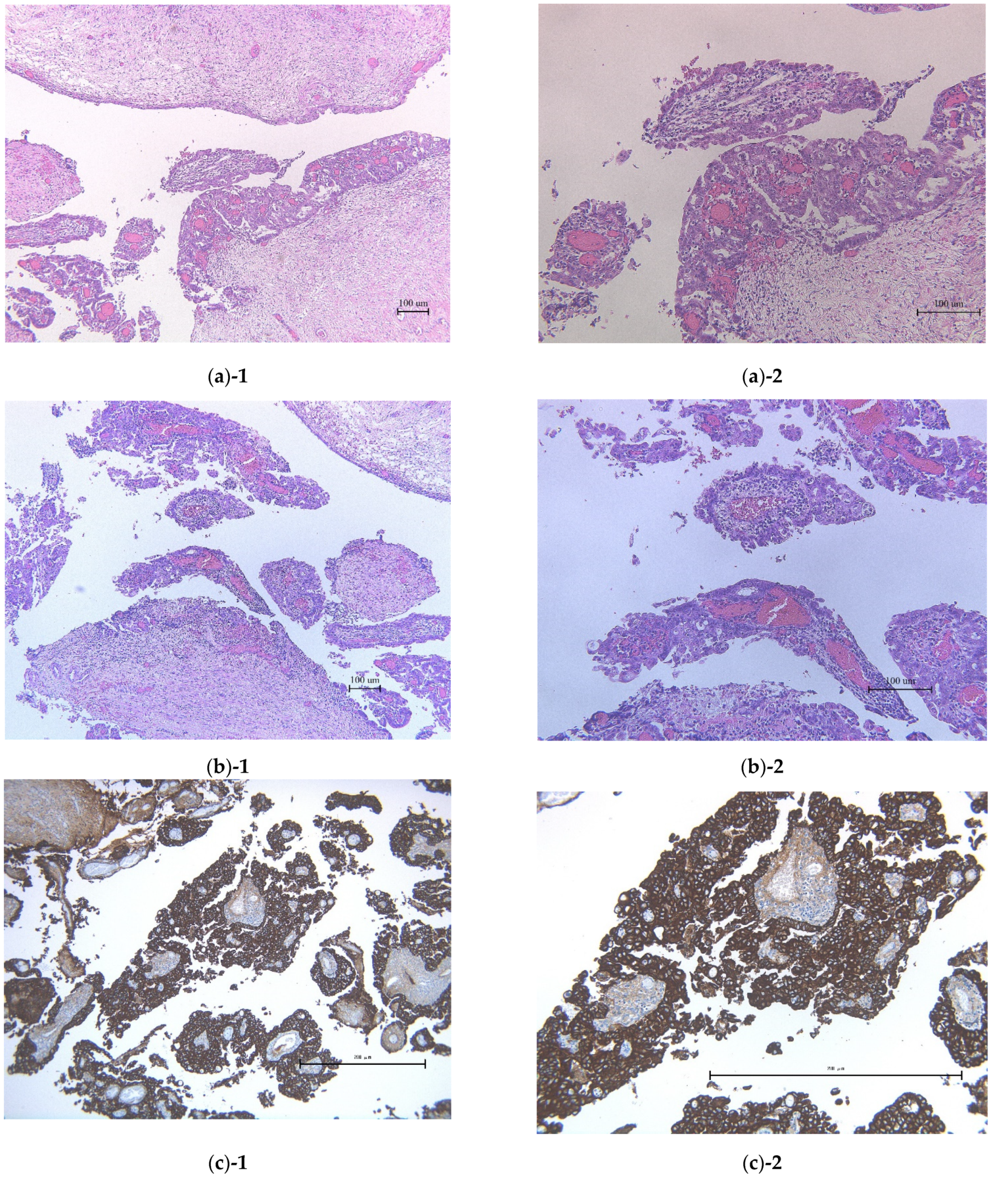
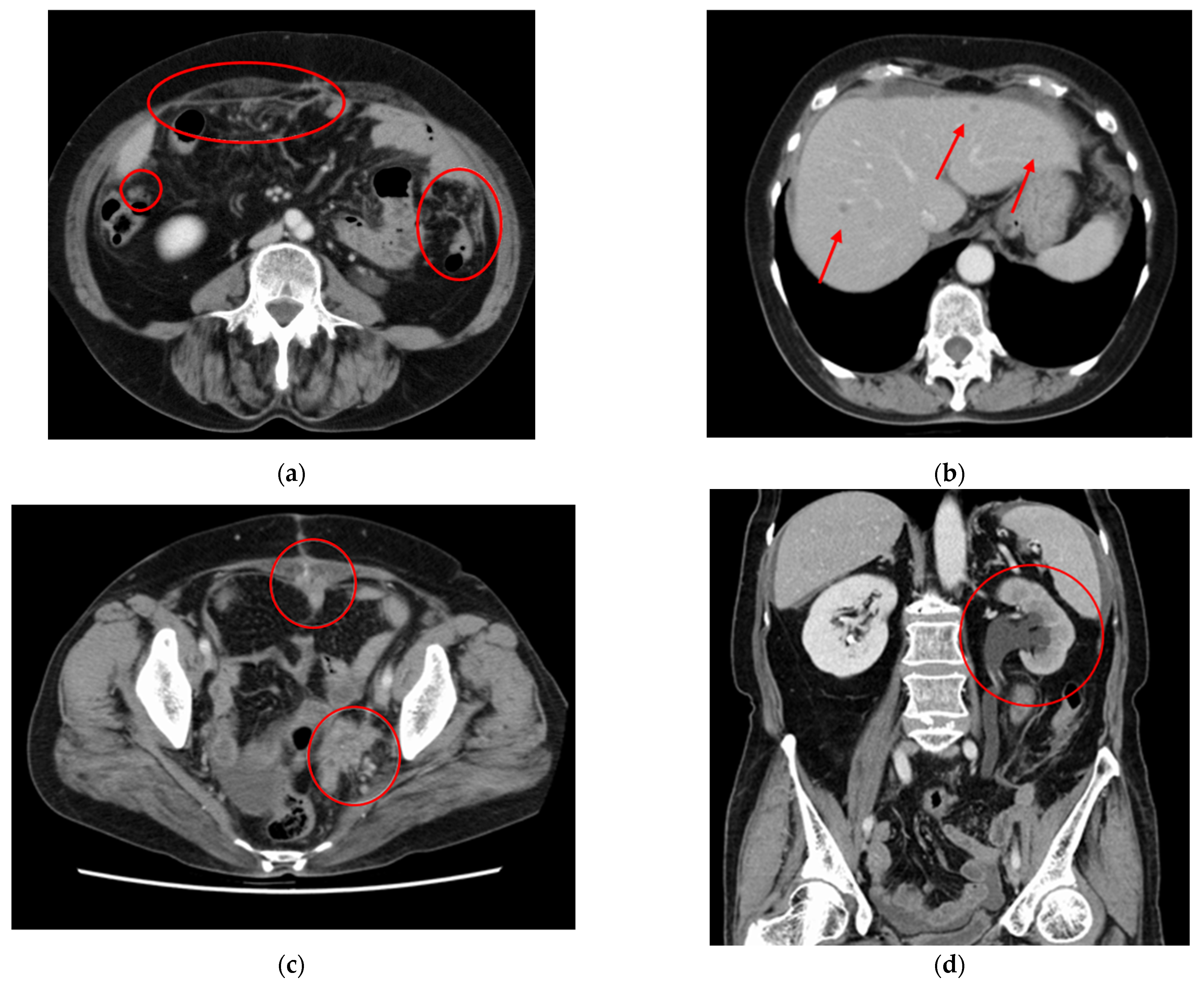
2. Case Presentation Section
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2017, 470, 125–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavassolo, F.A.; Devilee, P. (Eds.) WHO Classification of Tumors: Pathology and Genetics of the Tumors of the Breast and Female Genital Organs. IARC Press: Lyon, France, 2003; pp. 113–202. [Google Scholar]
- Japan Society of Obstetrics and Gynecology, The Japanese Society of Pathology. The General Rules for Clinical and Pathological Management of Ovarian Tumor, Fallopian Tube Cancer, and Primary Peritoneal Cancer Pathological Edition, 1 ed; Kanehara Shuppan: Kyoto, Japan, 2016; pp. 15–65. [Google Scholar]
- Japan Society of Gynecologic Oncology. Guidelines for Treatment of Ovarian Cancer, Fallopian Tube Cancer and Primary Peritoneal Cancer; Japan Society of Gynecologic Oncology (JSGO): Kyoto, Japan, 2020; pp. 159–173. [Google Scholar]
- Song, T.; Lee, Y.-Y.; Choi, C.H.; Kim, T.-J.; Lee, J.-W.; Bae, D.-S.; Kim, B.-G. Histologic distribution of borderline ovarian tumors worldwide: A systematic review. J Gynecol. Oncol. 2013, 24, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagase, S.; Ohta, T.; Takahashi, F.; Yaegashi, N.; Board members of the 2020 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: Annual patient report for 2017 and annual treatment report for 2012. J. Obstet. Gynaecol. Res. 2021, 47, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Obermair, A.; Hiebl, S. Laparoscopy in the treatment of ovarian tumours of low malignant potential. Aust. N. Z. J. Obs. Gynaecol. 2007, 47, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Nagase, S.; Ohta, T.; Takahashi, F.; Enomoto, T. The 2017 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: Annual patients report for 2015 and annual treatment report for 2010. J. Obstet. Gynaecol. Res. 2018, 45, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, K.; Machida, H.; Mandelbaum, R.S.; Grubbs, B.H.; Roman, L.D.; Sood, A.K.; Gershenson, D.M. Mucinous borderline ovarian tumor versus invasive well-differentiated mucinous ovarian cancer: Difference in characteristics and outcomes. Gynecol. Oncol. 2019, 153, 230–237. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Ewald-Riegler, N.; Du Bois, O.; Harter, P. Borderline tumors of the ovary-a systematic review. Geburtshilfe Und Frauenheilkd. 2009, 69, 807–833. [Google Scholar] [CrossRef]
- Åkeson, M.; Zetterqvist, B.-M.; Dahllöf, K.; Jakobsen, A.-M.; Brännström, M.; Horvath, G. Population-based cohort follow-up study of all patients operated for borderline ovarian tumor in western Sweden during an 11-year period. Int. J. Gynecol. Cancer 2008, 18, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.L.; Clement, P.B.; Chercover, D.J.; Sornarajah, T.; Gilks, C.B. Early recurrence of ovarian serous borderline tumor as high-grade carcinoma: A report of two cases. Int. J. Gynecol. Pathol. 2004, 23, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Nakata, S.; Kasai, M.; Deguchi, M. Ovarian carcinoma development after laparoscopically-assisted ovarian cystectomy for a mucinous cystic tumor of borderline malignancy. Jpn. J. Gynecol. Obstet. Endosc. 2008, 24, 322–325. [Google Scholar] [CrossRef]
- Ma, J.-W.; Miao, Y.; Liang, C.-N.; Wang, N.; Jiang, B.; Wang, Q.-Y.; Kang, J.; Hou, G.; Yin, Y. Malignant transformation of a borderline ovarian tumor with pulmonary and pleural metastases after years of latency: A case report and literature review. Front Med. 2020, 7, 571348. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Nagtegaal, I.D.; Overbeek, L.I.; Flucke, U.; Massuger, L.F.; Bulten, J. A patient with a noninvasive mucinous ovarian borderline tumor presenting with late pleural metastases. Int. J. Gynecol. Pathol. 2015, 34, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, A.; Strada, I.; Di Marcoberardino, B.; Maccarini, L.R.; Pozzati, F.; Rossi, M.; Biglia, N.; De Iaco, P. Clinical significance of microinvasion in borderline ovarian tumors and its impact on surgical management. Int. J. Gynecol. Cancer 2012, 22, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Ushijima, K.; Kawano, K.; Tsuda, N.; Nishio, S.; Terada, A.; Kato, H.; Tasaki, K.; Matsukuma, K. Epithelial borderline ovarian tumor: Diagnosis and treatment strategy. Obstet. Gynecol. Sci. 2015, 58, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morice, P.; Uzan, C.; Fauvet, R.; Gouy, S.; Duvillard, P.; Darai, E. Borderline ovarian tumour: Pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. Lancet Oncol. 2012, 13, e103–e115. [Google Scholar] [CrossRef]
- Trillsch, F.; Mahner, S.; Woelber, L.; Vettorazzi, E.; Reuss, A.; Ewald-Riegler, N.; de Gregorio, N.; Fotopoulou, C.; Schmalfeldt, B.; Burges, A.; et al. Age-dependent differences in borderline ovarian tumours (BOT) regarding clinical characteristics and outcome: Results from a sub-analysis of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) ROBOT study. Ann. Oncol. 2014, 25, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Hannan, A.; Sheikh, F.; Syed, A.A.; Siddiqui, N. Borderline tumors of the ovary: A clinicopathological study. Pak. J. Med. Sci. 2017, 33, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Ronnett, B.M.; Kajdacsy-Balla, A.; Gilks, C.B.; Merino, M.J.; Silva, E.; Werness, B.A.; Young, R.H. Mucinous borderline ovarian tumors: Points of general agreement and persistent controversies regarding nomenclature, diagnostic criteria, and behavior. Hum. Pathol. 2004, 35, 949–960. [Google Scholar] [CrossRef] [PubMed]
- Moroney, M.R.; Post, M.D.; Berning, A.A.; Sheeder, J.; Corr, B.R. An evaluation of frozen section and lymph node dissection results for mucinous ovarian tumors. Int. J. Gynecol. Cancer 2018, 28, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; Lhommé, C.; Pautier, P.; Duvillard, P.; Morice, P. Safety of simple cystectomy in patients with unilateral mucinous borderline tumors. Fertil. Steril. 2006, 85, 1510.e1–1510.e4. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, K.; Nakayama, K.; Nakamura, A.; Hadano, N.; Kurose, S.; Razia, S.; Aoki, S.; Kyo, S. A Novel Case of Recurrent Mucinous Borderline Ovarian Tumor: Early Relapse and Fatal Outcome. Reports 2022, 5, 15. https://doi.org/10.3390/reports5020015
Nakagawa K, Nakayama K, Nakamura A, Hadano N, Kurose S, Razia S, Aoki S, Kyo S. A Novel Case of Recurrent Mucinous Borderline Ovarian Tumor: Early Relapse and Fatal Outcome. Reports. 2022; 5(2):15. https://doi.org/10.3390/reports5020015
Chicago/Turabian StyleNakagawa, Kyoko, Kentaro Nakayama, Akiho Nakamura, Nagisa Hadano, Sonomi Kurose, Sultana Razia, Showa Aoki, and Satoru Kyo. 2022. "A Novel Case of Recurrent Mucinous Borderline Ovarian Tumor: Early Relapse and Fatal Outcome" Reports 5, no. 2: 15. https://doi.org/10.3390/reports5020015
APA StyleNakagawa, K., Nakayama, K., Nakamura, A., Hadano, N., Kurose, S., Razia, S., Aoki, S., & Kyo, S. (2022). A Novel Case of Recurrent Mucinous Borderline Ovarian Tumor: Early Relapse and Fatal Outcome. Reports, 5(2), 15. https://doi.org/10.3390/reports5020015




