Abstract
Primary intraosseous squamous cell carcinoma (PIOSCC) is a rare and aggressive malignancy arising exclusively within the jaws, without any initial connection with the oral mucosa. The etiology and the epidemiology are unclear due to the rarity of the disease, and there is no current universally accepted staging or treatment protocol. Clinically, the posterior mandible is the most affected site, and common symptoms are swelling and pain. The diagnosis is often difficult either because it requires stringent criteria to be satisfied or because of the absence of a pathognomonic histological pattern. Aggressive surgery is the first-choice treatment, often followed by radiotherapy. The lymph nodal status seems to be the most important factor influencing the prognosis, which is usually poor, with a 5-year survival rate ranging from 30% to 40%. In the present article, we report an unusual case of cystogenic PIOSCC interesting the anterior mandible of a young 34-year-old male, which came to our attention after complaint about recurrent infective episodes affecting a dentigerous cyst (impacted lower canine) discovered ten years before. The age, site, and extension are uncommon. Extensive surgical treatment with fibula free flap reconstruction, adjuvant therapy, and salvage surgery was carried out. The patient was disease-free at a 31-month follow-up.
1. Introduction
Primary intraosseous squamous cell carcinoma (PIOSCC) is a rare malignancy, presumably arising from odontogenic epithelium [1]. Its proper definition has been a challenge over time, and the name has changed multiple times over the last 50 years (Table 1). Eventually, it has been defined as a squamous cell carcinoma arising from odontogenic tissues (such as odontogenic epithelium residues, odontogenic cysts or tumors) within the jaws and without any initial connection with the oral mucosa [2]. Tumors originating from salivary glands, from maxillary antrum, from the oral cavity or any metastatic malignancy have also to be excluded to meet the diagnostic criteria. The 2005 World Health Organization (WHO) classification highlighted three different subcategories, based on the origin of the neoplasm: solid subtype, subtypes originating from odontogenic cysts squamous cell carcinoma (SCC) associated with other benign epithelial odontogenic tumors [3].

Table 1.
Changes in the definition and classification of the PIOSCC over the years, with related authors.
Due to its rarity and the lack of knowledge about this neoplasm, a prognostic classification has not been made. Nevertheless, clinical and radiological evaluations provide adequate diagnostic panorama and proper surgical planning. Chronic inflammation seems to be the only established risk factor, even though tobacco and alcohol might be involved as well [2,12,13,14]. Although there is not an internationally approved protocol, the therapeutic options include surgical excision, radiotherapy, chemotherapy, or a combination of these [1,15,16]. Prognostic factors are the lymph nodal involvement and the histological grading [2,17]. The overall 5-year survival rate ranges from 30% to 40% [6]. In order to gain further knowledge and information on this rare tumor, we reported the particular case of a young male affected by a PIOSCC derived from the transformation of a pre-existing dentigerous cyst and unusually located in the anterior mandible. Moreover, recurrent infection is the clinical presentation in our patient even if this symptom was rarely described as a manifestation of PIOSCC (Table 2). Following accurate clinical staging and virtual surgical planning, bilateral neck dissection and composite anterior mandibular resection were carried out, along with immediate reconstruction with an osteo-septo-cutaneous free fibular flap. Adjuvant chemoradiation, salvage surgery, and intensive follow-up were performed, and currently, the patient is disease-free at a 3-year follow-up.

Table 2.
Patient treatment history.
2. Case Presentation Section
Due to the retrospective nature of this study, it was granted an exemption by the Institutional Review Board of the University Hospital of Modena, Italy. All procedures performed involving the human participant were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The patient consented to publication of this report and clinical photographs.
A 34-year old male presented to a private dental outpatient clinic with pus drainage and swelling in the III quadrant of the oral cavity, suggesting a dental infection. Collecting the medical history, the patient referred that 10 years before in an orthopantomography (OPG), an incidental finding of a radiotransparent lesion was documented (Figure 1). The mandible radiolucency was related to an impacted 33 dental element (left lower canine), a fact that had been compatible with the diagnosis of a dentigerous cyst (Figure 1). The patient was aware of the presence of this cystic lesion, but he had refused to remove it despite the dentist’s advice. For purulent discharge, the patient was treated with an antibiotic therapy (ceftriaxone 1g IM, 1 infiltration/day, for 1 week) prescribed by the private dentist. A cone-beam computed tomography (CBCT) of the mandible was also performed; no atypical radiologic signs were revealed (Figure 2). Nevertheless, a second episode of infection developed two months later, and it was treated again with antibiotic therapy prescribed by the private dentist (3 cycles of amoxicillin/clavulanate 1g per os) followed by ceftriaxone (1g IM, 1 infiltration/day, for 1 week) that we prescribed after our consultation for poor clinical response to previous treatment. The following month the patient had a new infective episode and was hospitalized in our department for further diagnostic investigations.
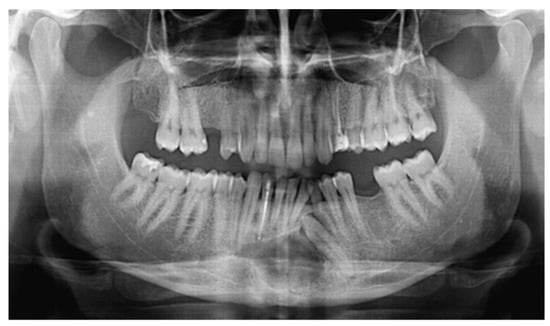
Figure 1.
During a routine orthopantomography (2007) performed 10 years before PIOSCC diagnosis, an impacted left lower canine, and a related incidental radiolucent cystic lesion was detected, compatible with the diagnosis of a dentigerous cyst.
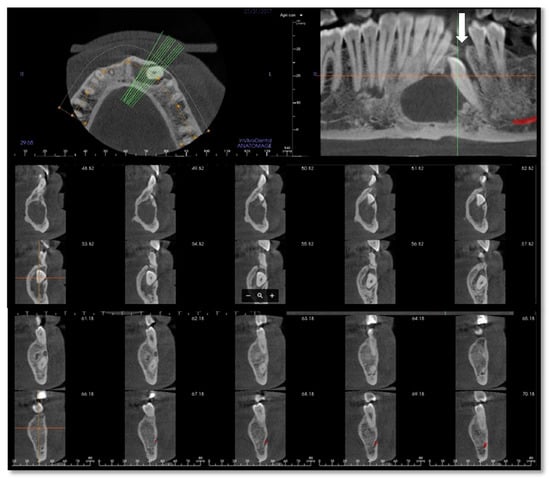
Figure 2.
Cone-beam computed tomography (CBCT) of the mandible performed in January 2017 after the first infective episode and showing no atypical radiologic findings. The osteolytic alveolar defect (white arrow) has led to the recurrent oral discharge.
The clinical examination did not highlight any comorbidity. The physical examination showed a mild swelling of the left anterior mandible (Figure 3); there were no palpable lymphadenopathies. No mucosal ulceration in the inferior oral vestibule, neither oral fistula was detectable; no purulent discharge (Figure 4a) occurred after the last antibiotic therapy. The patient was a smoker (3.6 pack/year) and stopped his smoking habit 1 year before the hospitalization. He drank beer occasionally.
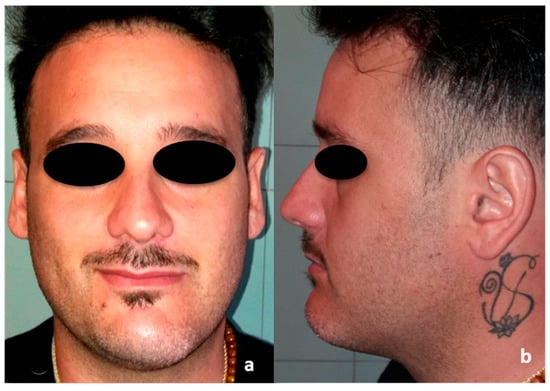
Figure 3.
Preoperative pictures. (a) Frontal view; (b) side view.
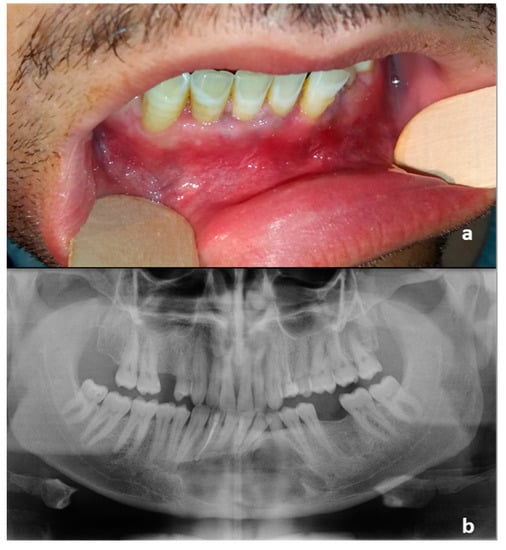
Figure 4.
(a) Intraoral preoperative picture. The fistula was not noticeable after antibiotic therapy. No ulceration of the oral mucosa was detectable; (b) Orthopantomography following the diagnostic biopsy and lower canine extraction.
Considering the long permanence of the cystic lesion and the documented benign radiological aspect over time (irregular but well-defined margins) (Figure 1 and Figure 2), the moderate entity of the swelling and the absence of lateral cervical lymphadenopathies on physical examination, we initially decided to adopt a conservative approach with a surgical enucleation of the neoformation in general anesthesia. In particular, the integrity of the oral vestibular mucosa and the adherent gingiva, despite the edemas, convinced us for this therapeutic approach. At the beginning of the procedure and after the single oral vestibule incision, the lardaceous aspect of the tissue was noted, and frozen section diagnoses were performed, afterward confirmed by a definitive diagnosis of moderately differentiated infiltrating squamous cell carcinoma (Figure 5). We suspended the planned surgical enucleation. The patient was awakened and informed.
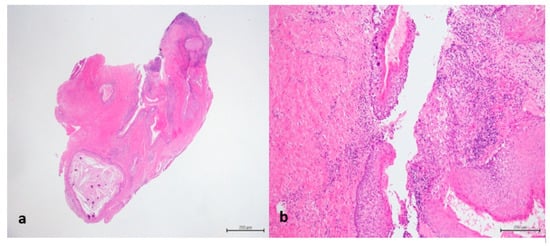
Figure 5.
(a) Dentigerous cyst lined by epithelium (H&E staining, 12.5× magnification); (b) In detail transition between normal epithelium and carcinoma in situ (H&E staining, 100× magnification).
For oncological staging, the patient had orthopantomography, preoperative CT and MRI (Figure 6) of the head and neck, CT of the thorax, all with and without contrast, ultrasound echography of the neck and abdomen, a rhino-pharyngo-laryngeal fibroscopy and an angio-CT of the inferior limbs. No secondary lesions nor lymph node invasion were detected in preoperative staging.
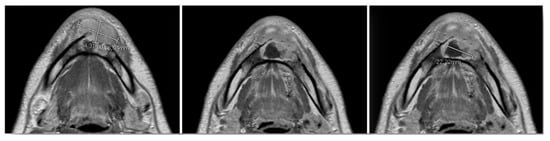
Figure 6.
Preoperative MRI, multiple axial views. Vestibular extraosseous spread of the tumor was evident.
Virtual surgical planning software ProPlan CMF (Depuy Synthes, Solothurn, Switzerland, and Materialise, Leuven, Belgium) was used for detailed planning of reconstruction with vascularized fibular flap. Planning began with conversion into three-dimensional (3D) virtual models of maxillofacial and lower extremities CT data (Figure 7). The surgeon determined virtually the resection margins and localization of the optimal angles for mandibular osteotomies, used for producing the osteotomy guides. Fibula osteotomy guides were also prepared, following virtual mandibular reconstruction and automatic adjustment of the fibula segments length and osteotomy angles (Figure 8).
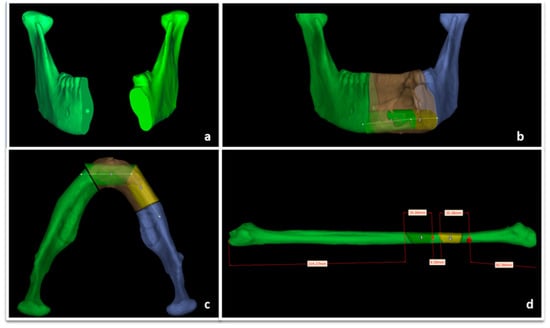
Figure 7.
ProPlan CMF (Depuy Synthes, Solothurn, Switzerland, and Materialise, Leuven, Belgium) preoperative surgical planning of mandibular resection vascularized fibular flap reconstruction. (a) Mandibular defect; (b) mandibular reconstruction planning, coronal view; (c) mandibular reconstruction planning, axial view; (d) fibular segmentation planning.
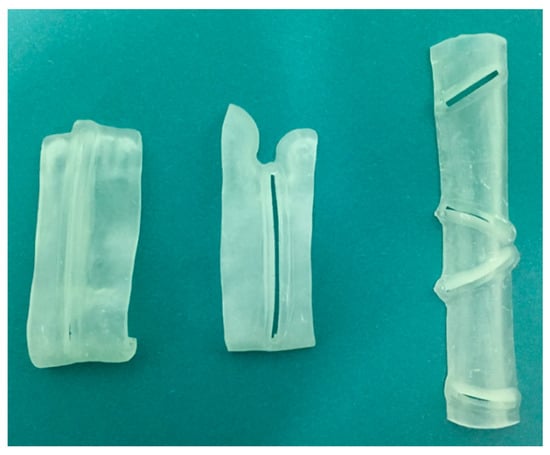
Figure 8.
Osteotomy cutting guides.
Three weeks after the biopsy, a temporary tracheotomy, a segmental mandibulectomy with piezosurgical device [21] (Type C defect according to Jewer et al. [18] and Type IB according to Cordeiro [19]), a bilateral elective neck dissection, and a free osteo-septo-cutaneous fibula flap reconstruction were carried out [20].
The visor flap approach to the tumor was chosen, with a short extension in the mental skin to better control the subcutaneous skin of the cheek (Figure 9) (Video 1 in supplementary file).
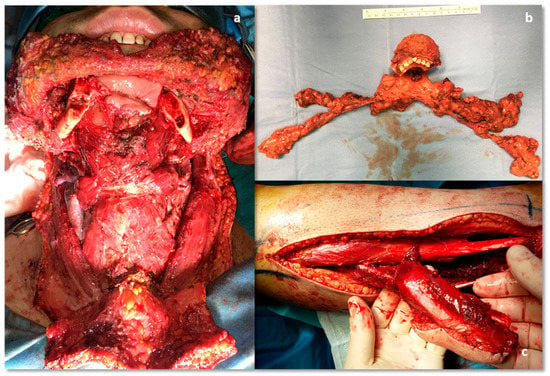
Figure 9.
Intraoperative pictures. (a) Mandibular defect; (b) segmental mandibulectomy and bilateral. Lymph node elective neck dissection, en-bloc excision; (c) harvested fibular flap, donor site.
Because of the large extraosseous spread of the tumor, the choice of sparing the cheek skin had to be verified through multiple frozen sections of the subcutis tissue (Figure 6) (Video 1 in supplementary file). During the operation, 19 intra-operative frozen sections of the soft tissues were performed in order to preserve as much skin as possible with the aims in this young man of obtaining both surgical radicality, aesthetic, and functional results; the examination report eventually documented the absence of carcinoma in all samples. Then the cutting guides were secured in the planned position without interfering with the tumor; the osteotomies were carried out using a piezosurgical device [21] through the cutting slots, and the en block tumor was safely removed (Video 1 in supplementary file).
On gross examination, the lesion appeared greyish, with an ill-defined surface, measuring about 6.5 cm × 5 cm. Histopathological findings resulted in a G2 (moderately differentiated) squamous cell carcinoma infiltrating the mandible (Figure 10b). Mandibular bone infiltration measured 5 mm, with mucosa and subepithelial connective tissue involvement. There was no evidence of vascular or perineural invasion, and tumor-free surgical margins were eventually confirmed. All 99 dissected lymph nodes were negative. The postoperative course was uneventful, and the patient was discharged from the hospital 21 days after surgery (Figure 11).
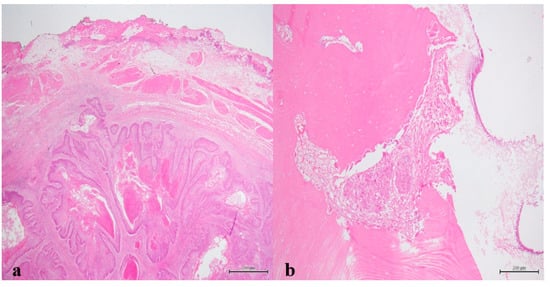
Figure 10.
(a) Peripheral palisading islands of various sizes consisting of moderately-differentiated squamous cells within the connective tissue (H&E staining, 12.5× magnification); (b) Squamous cells tumor island invading bone (H&E staining, 100× magnification).
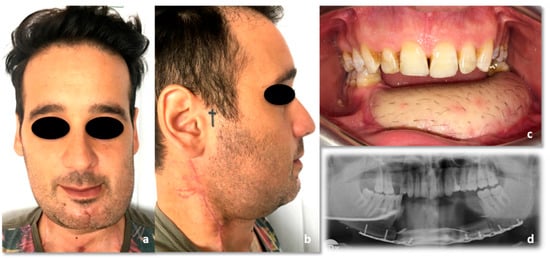
Figure 11.
Forty-five-days post-operative condition. (a) Frontal view; (b) side view; (c) intraoral picture; (d) Rx orthopantomography.
The patient’s case was debated at our local head and neck cancer multidisciplinary team meeting to plan adjuvant radiotherapy. After the third RT cycle (two months after the surgery), the patient developed an aching swelling in the left cheek, even though he had already started the treatment. Given the worsening of the symptoms following the antibiotic therapy, an ultrasound examination was performed, demonstrating a 3 cm diameter hypo-anechoic avascular lesion anteriorly to the left masseter muscle. An echo-guided biopsy was performed, and the histological exam showed the presence of G3 squamous cell carcinoma fragments. A salvage surgical excision was performed, completely removing the lymph node with clear margins, although extracapsular spread was documented in the final report. Given this new finding, a further head and neck cancer multidisciplinary team meeting made an indication for radiotherapy restart together with cisplatin-based chemotherapy at a dose of 40 mg/m2/day per cycle, which were successfully completed (Figure 12). The patient ended its surgical and radiochemotherapy oncological treatment within 6 months from the initial diagnosis (Table 2).
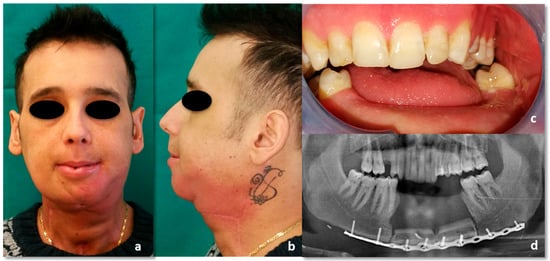
Figure 12.
Post-radiochemoterapy follow-up. (a) Frontal view; (b) side view; (c) intraoral picture; (d) CT, coronal view.
The patient has had a follow-up every 3 months ever since. No tumor recurrence was detected at the 31-month follow-up. His actual quality of life is good, and the patient is pretty satisfied with the aesthetic and functional outcomes, including facial nerve function that has remained entirely normal (Figure 13) (Video 2 in supplementary file). He only referred a minimal temporo-mandibular joint discomfort two-years after treatment, treated successfully with a functional approach. The next therapeutic step should be the infiltration of the radio-treated tissues with autologous fat, in order to improve the lower face contour and the skin texture.
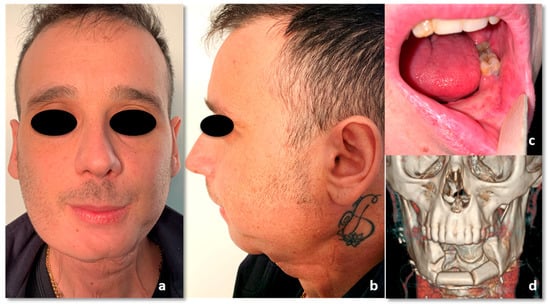
Figure 13.
Thirty-one-months follow-up. (a) Frontal view. (b) side view. (c) intraoral picture. (d) 3D CT reconstruction.
3. Discussion
PIOSCC is a rare malignant neoplasm arising exclusively within the jaws, defined as a squamous cell carcinoma having no initial connection with the oral mucosa and originating from odontogenic epithelium residues or an odontogenic cyst or tumor [2]. Since Loos first described it in 1913 as Intra-alveolar Carcinoma of the jaw [4], several terms have been adopted over time for this tumor (Table 1) and, only in 2005, WHO defined it as Primary intraosseous squamous cell carcinoma [22].
According to this classification [3], it is possible to distinguish three subcategories of PIOSCC (Table 1):
- ▪
- The solid type that invades marrow spaces and induces osseous resorption (carcinomas arising de novo);
- ▪
- Squamous cancers arising from the lining of odontogenic cysts, divided into two different subtypes:
- Keratocystic odontogenic tumor,
- Odontogenic cysts (such as residual periapical cysts, root cysts, dentigerous cyst, and lateral periodontal cyst);
- ▪
- SCC associated with other benign epithelial odontogenic tumors.
According to this classification [3], our case can be cataloged as PIOSCC subtype 2B. Due to its rarity, the epidemiology of PIOSCC is not well known, and only approximately 200 cases have been reported in the literature [23]. The peak incidence of PIOSCC is in the fifth-sixth decade (mean 54 years), ranging from 4 to 81 years, although it is exceptional in the first two decades [2]. A male:female ratio of 2.5:1 has been observed by many authors [2,24]. The mandible (79%) is affected more frequently than the maxilla (21%), especially the posterior portion, while the anterior locations are very rare [1,2,14,22,25,26]. Therefore, the unusual location of the lesion in the anterior mandible in our patient (Table 2) could have led to misdiagnosis and delay in the proper management. The exact etiopathogenetic process is currently unknown. The most accredited theory states that PIOSCC derives from the malignant transformation of odontogenic epithelium remnants within the jaws, that can be identified in three main sources: epithelial cells rests of Malassez, remnants of the dental lamina and the reduced enamel epithelium [2,12,14]. Many authors suggest that chronic inflammation (caused by repeated traumatisms or by frequent infections) might be the main predisposing factor: reactive oxygen metabolites’ formation would induce damage to DNA, proteins, and cell membranes, leading to the blockage of apoptosis and a consequent uncontrolled proliferation [14]. Therefore, tobacco and alcohol might play a role in this process because they increase cellular oxidative stress, even though a clear connection has not been established [13], unlike oral SCC. Referring to PIOSCC cystogenic type, the presence of keratinization in the lining epithelium of a cyst might increase the risk of malignant transformation [27].
Histologically, there is not a PIOSCC pathognomonic pattern [2]: the tissue specimens may vary from well to poorly-differentiated tumors, with or without keratinization [2,16]. Generally, PIOSCC has similar and often indistinguishable characteristics from those of a conventional oral SCC, such as islands or cords of neoplastic squamous epithelium in fibrous connective tissue with varying degrees of diffuse infiltration of lymphocytes [1,2]. However, there are some features that might suggest an odontogenic origin of the neoplasm and guide the diagnosis, as observed by Huang et al. in nearly half of the cases analyzed:
- ▪
- an alveolar pattern of the tumor nests in a prominent stroma,
- ▪
- clear cell components,
- ▪
- palisading arrangement of the peripheral basal cells [2].
The nuclei of these cells are often oriented away from the basement membrane, and foci of central necrosis could be found [26]. Obviously, a pre-operative biopsy is mandatory [14].
More frequently, PIOSCCs subtype 2B are well-to-moderately differentiated neoplasms, and both the pre-operative lesion biopsy and the definitive histological examination of our patient (Table 3) are consistent with these findings [14].

Table 3.
Diagnostic criteria of PIOSCC.
Clinical features of PIOSCC are really non-specific. Patients most commonly present with jaw swelling and pain, and paraesthesia or numbness of the mandibular nerve are frequent as well [1,2,24,28]. It should be noted that early-stage PIOSCC might frequently be asymptomatic or might cause mild dental disorders, then leading to diagnostic delay [1]. As a consequence, a lymph-node invasion may already be present when the biopsy is performed, determining a worse prognostic situation [23]. The localization has to be taken into account as well, considering that PIOSCC mainly develops in posterior mandible regions [1,2,14,22,25]. Finally, recurrent episodes of oral infections without response to adequate antibiotic therapy should be investigated, especially in patients with a previous history of cysts, even though this is not a typical PIOSCC manifestation [28]. Nevertheless, recurrent infection episodes involved a documented dentigerous cyst in our patient, that did not respond to the antibiotic therapy.
This clinical history and the biopsy report have led us to consider a PIOSCC diagnosis, despite the unusual localization and the absence of lymph node metastasis (Table 3).
The radiological appearance of PIOSCC is variable, ranging from well-defined benign-like masses to ill-defined osteolytic lesions [12,14]. The most common imaging presentation is a radiolucent cup or dish-shaped osteolytic bone lesion, followed by diffuse and poorly defined borders appearance known as ‘moth-eaten’ radiographic pattern [24]. Kaffe et al. proposed that the presence of indistinct margins without a sclerotic outline may be an important peculiarity of PIOSCC [29] (Figure 2). Another significant radiograph characteristic is the lack of root resorption, presumably suggesting that the tumor invasion occurs along the path of least resistance, resulting in a unique pattern known as ‘floating-teeth,’ which is rarely seen in benign odontogenic cysts or tumors [2] (Figure 2). Radiopaque foci corresponding to calcifications or dentinoid structures and ground-glass opacity (possibly mimicking fibrous dysplasia or ossifying fibroma) have also been reported by some authors [12,30].
In our case, orthopantomography (Figure 1) and CT (Figure 2) showed a uniloculated 38 × 14 mm osteolytic area affecting the chin symphysis and extending in the left paramedian region. The lesion interrupted the vestibular bone cortex but spared the lingual one.
MRI with gadolinium-contrast represents a second-level examination essential for tumor staging, and in our case, the extent of the lesion through the anterior cortex erosion in the soft tissues of the chin was documented (Figure 6).
PIOSCC differential diagnosis is challenging. Gardner et al., and later Shambhulingappa et al., proposed some diagnostic criteria that have to fulfilled [31,32]:
- ▪
- Absence of ulcers in the oral mucosa during the examination, except if associated with a trauma or a recent tooth extraction
- ▪
- Exclusion of metastatic SCC from the distant primary tumor through the demonstration of a clear chest x-ray at the time of diagnosis and with a minimum follow-up of six months
- ▪
- Histological evidence of SCC
The identification of direct transition from the benign epithelial lining of a pre-existing cyst to an SCC differentiates PIOSCC cystogenic type from the solid one [31]. In our case, this last criterion was also fulfilled (Figure 5b).
Nevertheless, it has to be noted that PIOSCC usually has a 2 to 36-week diagnostic delay, making it difficult to fulfill all of the above-mentioned criteria [33]. Such a significant delay implies that often the PIOSCC has already invaded the oral mucosa, leading to a critical analysis of the patient’s clinical history, radiologic, and histologic findings. On a practical basis, it is possible to diagnose the PIOSCC when there is the presence of histological evidence of SCC and at least one of the following conditions:
- ▪
- absence of mucosal lesion and metastasis,
- ▪
- presence of mucosal lesion but histological identification of an underlying odontogenic cyst.
In our case, there was a 10-year diagnostic delay that has led to recurrent purulent discharge from an oral fistula, no more noticeable after antibiotic therapy at the moment of PIOSCC diagnosis (Figure 4a).
PIOSCC is not included in the AJCC classification, and there is not an internationally approved therapeutic protocol [34]. The staging of PIOSCC is difficult. Firstly, PIOSCC cannot be classified as a primary bone tumor despite the intraosseous occurrence; secondly, the international oral squamous cell carcinoma classification is not applicable because all PIOSCCs should be classified T4 regardless of the size, due to the intimate contact with the bone marrow [2,35].
To conclude, TNM staging for the oral cavity is not applicable to PIOSCC. Since PIOSCC is an aggressive malignant tumor, the recommended treatment is a wide radical surgical excision together with elective neck dissection due to eventual lymph node involvement [1,15,16]. Adjuvant radiation therapy is often recommended, while the role of chemotherapy is still questionable [1,23]. A multidisciplinary oncologic management of these cases is definitely indicated.
The treatment we adopted for our patient was a segmental mandibulectomy together with a bilateral elective lymph node (levels I-III) neck dissection (Figure 9). This aggressive surgical approach was selected because of the extension and localization (anterior mandible) of the lesion with consequent bilateral lymphatic neck drainage, even though there were not lymphadenopathies on the clinical and radiological findings at the time of the diagnosis (cN0). The subsequent development of a lymphatic metastasis a few months after the surgery suggests that this aggressive surgical approach was actually the best choice. Given the aggressiveness of the lesion, the worsening of its histological grading, and the young age of the patient, indication for combined radio-chemotherapeutic adjuvant treatment was also made.
The mandibular defect was reconstructed using an osteo-septo-cutaneous free fibula flap since it allows to rebuild large defects with both functional and aesthetic satisfying results [20]. The virtual surgical planning performed was useful, mainly due to the patient’s young age.
The prognosis for PIOSCC is poor in the majority of cases. Thomas et al., reviewing 33 cases of PIOSCC, observed a two-year overall survival of 67% [24], while Shear et al. reported a five-year survival rate ranging from 30% to 40% [6]. The main negative prognostic factors of PIOSCC are nodal involvement, high histological grade, and advanced N classification [17]. The lymph node status is universally accepted as the most important determinant influencing both recurrence and survival. In our case, the patient had no nodal metastases at the time of diagnosis. The higher survival of patients treated with adjuvant radiotherapy (Table 4), the perimandibular soft tissue involvement, and the patient’s good performance status led us to recommend adjuvant radiotherapy. Nevertheless, after two radiotherapy sessions, our patient underwent lymph nodal excision due to the occurrence of a G3 SCC lesion in a masseteric lymph node, demonstrating the oncological aggressiveness of the disease. This event prompted us to indicate a combined radiochemotherapy treatment.

Table 4.
Lymph node status at the time of diagnosis, treatment, and reconstruction modalities by various authors.
4. Conclusions
Primary intraosseous squamous cell carcinoma is a rare and hardly recognizable tumor. The diagnostic delay could range from a few weeks to 18 months [36].
A long history of recurrent inflammation or infection close to the malignancy makes it difficult to get accurate T staging. The perimandibular soft tissue involvement is related to PIOSCC aggressiveness and highlights the need for oncological adjuvant treatment. Prompt surgical healing is needed for the early start of adjuvant radiotherapy: in lower jaw localization, this goal is more easily attainable if bony free flap reconstruction is considered [20,37].
The patient’s young age justifies surgical planning for the preservation of aesthetics and functional structures; in particular, the cheek skin was preserved on the basis of the absence of disease in multiple intraoperative frozen section biopsies. Early recurrence was detected and treated with salvage surgery, followed by adjuvant radiochemotherapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/2571-841X/3/2/12/s1, Video 1: main surgical steps of PIOSCC resection. Video 2: facial nerve function at a 31-month follow-up.
Author Contributions
Conceptualization, A.A. and S.N.; methodology, A.P.; software, A.A.; validation, A.A., L.C., and R.N.; formal analysis, S.N.; investigation, M.P.; data curation, M.d.B. and G.B.; writing—original draft preparation, M.d.B.; writing—review and editing, A.P.; visualization, M.d.B.; supervision, A.A.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Praetorius, F. Primary Intraosseous Squamous Cell Carcinoma. In Surgical Pathology of the Head and Neck; Barnes, L., Ed.; CRC Press: New York, NY, USA, 2008; Volume 3, pp. 1297–1301. ISBN 9780429075469. [Google Scholar]
- Huang, J.W.; Luo, H.Y.; Li, Q.; Li, T.J. Primary intraosseous squamous cell carcinoma of the jaws: Clinicopathologic presentation and prognostic factors. Arch. Pathol. Lab. Med. 2009, 133, 1834–1840. [Google Scholar]
- Eversole, L.R.; Siar, C.H.; Van Der Waal, I. Primary intraosseous squamous cell carcinomas. In World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors; Barnes, L., Eveson, J.W., Reichart, P., Sidransky, D., Eds.; IARC Press: Lyon, France, 2005; pp. 290–291. [Google Scholar]
- Morrison, R.; Deeley, T.J. Intra-alveolar carcinoma of the jaw. Treatment by supervoltage radiotherapy. Br. J. Radiol. 1962, 35, 321–326. [Google Scholar] [CrossRef]
- Willis, R.A. Pathology of Tumours; Butterworth & Co., Ed.; CV Mosby Co.: London, UK, 1948. [Google Scholar]
- Shear, M. Primary intra-alveolar epidermoid carcinoma of the jaw. J. Pathol. 1969, 97, 645–651. [Google Scholar] [CrossRef]
- Pindborg, J.J.; Kramer, I.R.H.; Torloni, H. Histological Typing of Odontogenic Tumours, Jaw Cysts and Allied Lesions.; Geneva World Health Organization: Geneva, Switzerland, 1971. [Google Scholar]
- Kramer, I.R.H.; Pindborg, J.J.; Shear, M. Histological Typing of Odontogenic Tumors, 2nd ed.; World Health Organization, Ed.; Springer: Heidelberg, Germany, 1992. [Google Scholar]
- Elzay, R.P. Primary intraosseous carcinoma of the jaws. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 299–303. [Google Scholar] [CrossRef]
- Slootweg, P.J.; Müller, H. Malignant ameloblastoma or ameloblastic carcinoma. Oral Surg. Oral Med. Oral. Pathol. 1984, 57, 168–176. [Google Scholar] [CrossRef]
- Waldron, C.A.; Mustoe, T.A. Primary intraosseous carcinoma of the mandible with probable origin in an odontogenic cyst. Oral Surg. Oral. Med. Oral Pathol. 1989, 67, 716–724. [Google Scholar] [CrossRef]
- Lopes Dias, J.; Borges, A.; Lima Rego, R. Primary intraosseous squamous cell carcinoma of the mandible: A case with atypical imaging features. BJR Case Rep. 2016, 2, 20150276. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Mittal, S.; Gupta, D.K. Primary intraosseous squamous cell carcinoma arising in odontogenic cysts: An insight in pathogenesis. J. Oral Maxillofac. Surg. 2013, 71, e7-14. [Google Scholar] [CrossRef] [PubMed]
- Bodner, L.; Manor, E.; Shear, M.; van der Waal, I. Primary intraosseous squamous cell carcinoma arising in an odontogenic cyst: A clinicopathologic analysis of 116 reported cases. J. Oral Pathol. Med. 2011, 40, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Chen, Y.; Gokavarapu, S.; Cao, W.; Ji, T. Primary intraosseous squamous cell carcinoma of the mandible: Locoregional control and survival is significantly reduced if the tumour is more than 4cm in size. Br. J. Oral Maxillofac. Surg. 2018, 56, 48–53. [Google Scholar] [CrossRef]
- Thakur, A.; Tupkari, J.V.; Joy, T.; Gogri, A.A. Primary intraosseous squamous cell carcinoma—A rare odontogenic malignancy. J. Oral Maxillofac. Pathol. 2017, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Shen, H.; Qi, X.; Wang, Z.; Wang, Y.; Hu, Q.; Han, W. Prognostic factors of primary intraosseous squamous cell carcinoma (PIOSCC): A retrospective review. PLoS ONE 2016, 11, e0153646. [Google Scholar]
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; Zuker, R.M.; Rosen, I.B.; Gullane, P.J.; Rotstein, L.E.; Freeman, J.E. Orofacial and mandibular reconstruction with the iliac crest free flap: A review of 60 cases and a new method of classification. Plast. Reconstr. Surg. 1989, 84, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, P.G.; Henderson, P.W.; Matros, E. A 20-year experience with 202 segmental mandibulectomy defects: A defect classification system, algorithm for flap selection, and surgical outcomes. Plast. Reconstr. Surg. 2018, 141, 571e–581e. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, L.; Anesi, A.; Negrello, S. Mandible: Lateral, Hemimandibular, Anterior; Springer International Publishing: Cham, Switzerland, 2019; pp. 27–38. [Google Scholar]
- Anesi, A.; Ferretti, M.; Cavani, F.; Salvatori, R.; Bianchi, M.; Russo, A.; Chiarini, L.; Palumbo, C. Structural and ultrastructural analyses of bone regeneration in rabbit cranial osteotomy: Piezosurgery versus traditional osteotomes. J. Cranio Maxillofac. Surg. 2018, 46, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Eversole, L.R.; Sabes, W.R.; Rovin, S. Aggressive growth and neoplastic potential of odontogenic cysts. With special reference to central epidermoid and mucoepidermoid carcinomas. Cancer 1975, 35, 270–282. [Google Scholar] [CrossRef]
- Cariati, P.; Fernandez, A.B.M.; Tara, M.P.D.P.; Solis, J.F.; Lara, I.M. Primary intraosseous odontogenic squamous cell carcinoma of the mandible. J. Oral Maxillofac. Pathol. 2017, 21, 182. [Google Scholar] [CrossRef]
- Thomas, G.; Pandey, M.; Mathew, A.; Abraham, E.K.; Francis, A.; Somanathan, T.; Iype, E.M.; Sebastian, P.; Nair, M.K. Primary intraosseous carcinoma of the jaw: Pooled analysis of world literature and report of two new cases. Int. J. Oral Maxillofac. Surg. 2001, 30, 349–355. [Google Scholar] [CrossRef]
- Tamgadge, S.; Tamgadge, A.; Modak, N.; Bhalerao, S. Primary intraosseous squamous cell carcinoma arising from an odontogenic keratocyst: A case report and literature review. Ecancermedicalscience 2013, 7, 316. [Google Scholar]
- Reichart, P.A.; Philipsen, H.P. Odontogenic Tumors and Allied Lesions; Quintessence Publishing Co., Ltd.: London, UK, 2004. [Google Scholar]
- Bereket, C.; Bekçioğlu, B.; Koyuncu, M.; Şener, İ.; Kandemir, B.; Türer, A. Intraosseous carcinoma arising from an odontogenic cyst: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e445-9. [Google Scholar] [CrossRef]
- Susan, M.; Charles, A. Primary intraosseous squamous carcinoma. Int. J. Oral Maxillofac. Surg. 1991, 20, 362–365. [Google Scholar]
- Kaffe, I.; Ardekian, L.; Peled, M.; Machtey, E.; Laufer, D. Radiological features of primary intra-osseous carcinoma of the jaws. Analysis of the literature and report of a new case. Dentomaxillofac. Radiol. 1998, 27, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, H.; Katase, N.; Matsumura, T.; Hara, M.; Yanagi, Y.; Nagatsuka, H.; Iida, S.; Asaumi, J.-I. Solid-type primary intraosseous squamous cell carcinoma of the mandible: A case report with histopathological and imaging features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e71-7. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.F. A survey of odontogenic cysts and their relationship to squamous cell carcinoma. J. Can. Dent. Assoc. 1975, 3, 161. [Google Scholar]
- Shambhulingappa, P.; Sheikh, S.; Puri, N.; Jindal, S.K. Primary intraosseous carcinoma of mandible: An update on review of literature with a case report. J. Clin. Exp. Dent. 2010, 2, 2–5. [Google Scholar] [CrossRef]
- McGowan, R.H. Primary intra-alveolar carcinoma. A difficult diagnosis. Br. J. Oral Surg. 1980, 18, 259–265. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Berlin, Germany, 2017; ISBN 9783319406176. [Google Scholar]
- Woolgar, J.A.; Triantafyllou, A.; Ferlito, A.; Devaney, K.O.; Lewis, J.S.; Rinaldo, A.; Slootweg, P.J.; Barnes, L. Intraosseous carcinoma of the jaws: A clinicopathologic review. Part III: Primary intraosseous squamous cell carcinoma. Head Neck 2013, 35, 906–909. [Google Scholar] [CrossRef]
- To, E.H.W.; Brown, J.S.; Ward-Booth, R.P.; Avery, B.S. Primary intraosseous carcinoma of the jaws. Three new cases and a review of the literature. Br. J. Oral Maxillofac. Surg. 1991, 29, 19–25. [Google Scholar] [CrossRef]
- Boyd, J.B.; Mulholland, R.S.; Davidson, J.; Gullane, P.J.; Rotstein, L.E.; Brown, D.H.; Freeman, J.E.; Irish, J.C. The Free Flap and Plate in Oromandibular Reconstruction: Long-Term Review and Indications. Plast. Reconstr. Surg. 1995, 95, 1018–1028. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).