New Development of Disaster-Related and Tropical Infectious Diseases Control
Abstract
1. Introduction
2. Studies of Disaster-Related Infectious Disease
2.1. Infectious Disease Itself Is a Disaster
2.2. Infectious Diseases Caused by Disaster in Global Warming
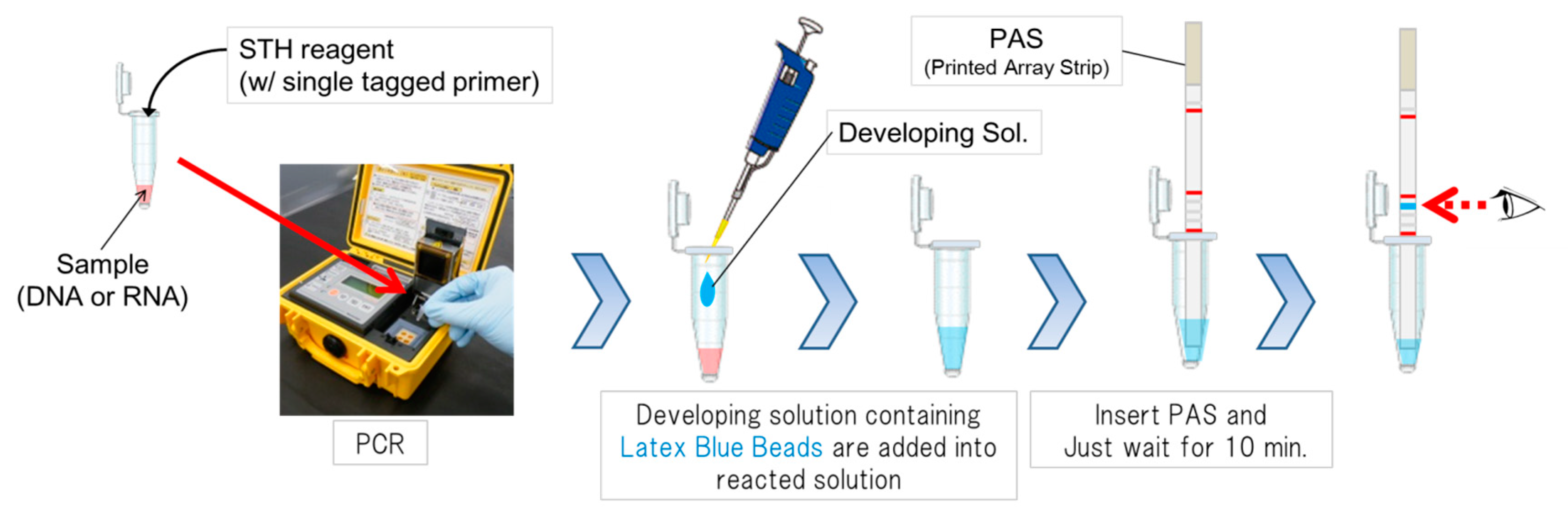
2.3. STH-PAS as Point-Of-Care Testing for Disaster-Related Infectious diseases
2.4. MCP Proteins as Severity Markers
2.5. Attempts to Controls MCP Proteins
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shibahara, S. Revisiting the March 11, 2011 earthquake and tsunami: Resilience and restoration. Tohoku J. Exp. Med. 2012, 226, 1–2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hattori, T.; Chagan-Yasutan, H.; Shiratori, B.; Egawa, S.; Izumi, T.; Kubo, T.; Nakajima, C.; Suzuki, Y.; Niki, T.; Alisjahbana, B.; et al. Development of point-of-care testing for disaster-related infectious diseases. Tohoku J. Exp. Med. 2016, 238, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Leaning, J.; Guha-Sapir, D. Natural disasters, armed conflict, and public health. N. Engl. J. Med. 2013, 369, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D. Natural Disasters; Routledge: Abingdon, UK, 2018. [Google Scholar]
- Watson, J.T.; Gayer, M.; Connolly, M.A. Epidemics after natural disasters. Emerg. Infect. Dis. 2007, 13, 1–5. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Ding, G.; Liu, Q.; Jiang, B. Identifying flood-related infectious diseases in Anhui Province, China: A spatial and temporal analysis. Am. J. Trop. Med. Hyg. 2016, 94, 741–749. [Google Scholar] [CrossRef]
- Pascapurnama, D.N.; Murakami, A.; Chagan-Yasutan, H.; Hattori, T.; Sasaki, H.; Egawa, S. Prevention of tetanus outbreak following natural disaster in indonesia: Lessons learned from previous disasters. Tohoku J. Exp. Med. 2016, 238, 219–227. [Google Scholar] [CrossRef]
- Rossiello, M.R.; Szema, A. Health effects of climate change-induced wildfires and heatwaves. Cureus 2019, 11, 4771. [Google Scholar] [CrossRef]
- Hutchins, D.A.; Jansson, J.K.; Remais, J.V.; Rich, V.I.; Singh, B.K.; Trivedi, P. Climate change microbiology—Problems and perspectives. Nat. Rev. Microbiol. 2019, 17, 391–396. [Google Scholar] [CrossRef]
- Liu-Helmersson, J.; Rocklov, J.; Sewe, M.; Brannstrom, A. Climate change may enable Aedes aegypti infestation in major European cities by 2100. Environ. Res. 2019, 172, 693–699. [Google Scholar] [CrossRef]
- Filho, W.L.; Scheday, S.; Boenecke, J.; Gogoi, A.; Maharaj, A.; Korovou, S. Climate change, health and mosquito-borne diseases: Trends and Implications to the pacific Region. Int. J. Environ. Res. Public Health 2019, 16, 5114. [Google Scholar] [CrossRef]
- Usuzawa, M.; Telan, E.O.; Kawano, R.; Dizon, C.S.; Alisjahbana, B.; Ashino, Y.; Egawa, S.; Fukumoto, M.; Izumi, T.; Ono, Y.; et al. Awareness of disaster reduction frameworks and risk perception of natural disaster: A questionnaire survey among Philippine and Indonesian health care personnel and public health students. Tohoku J. Exp. Med. 2014, 233, 43–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, R.A.; McMichael, A.J. Social and environmental risk factors in the emergence of infectious diseases. Nat. Med. 2004, 10, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Mee, P.; Wagner, R.G.; Gomez-Olive, F.X.; Kabudula, C.; Kahn, K.; Madhavan, S.; Collinson, M.; Byass, P.; Tollman, S.M. Changing use of traditional healthcare amongst those dying of HIV related disease and TB in rural South Africa from 2003–2011: A retrospective cohort study. BMC Complement. Altern. Med. 2014, 14, 504. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.R.; Moll, A.; Sturm, A.W.; Pawinski, R.; Govender, T.; Lalloo, U.; Zeller, K.; Andrews, J.; Friedland, G. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006, 368, 1575–1580. [Google Scholar] [CrossRef]
- Lawn, S.D.; Myer, L.; Bekker, L.G.; Wood, R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: Impact on treatment outcomes and implications for tuberculosis control. AIDS 2006, 20, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, U.R.; Leano, P.S.; Chagan-Yasutan, H.; Shiratori, B.; Saitoh, H.; Ashino, Y.; Suzuki, Y.; Hattori, T.; Telan, E.F. Frequent detection of anti-tubercular-glycolipid-IgG and -IgA antibodies in healthcare workers with latent tuberculosis infection in the Philippines. Clin. Dev. Immunol. 2012, 2012, 610707. [Google Scholar] [CrossRef] [PubMed]
- Senoputra, M.A.; Shiratori, B.; Hasibuan, F.M.; Koesoemadinata, R.C.; Apriani, L.; Ashino, Y.; Ono, K.; Oda, T.; Matsumoto, M.; Suzuki, Y.; et al. Diagnostic value of antibody responses to multiple antigens from Mycobacterium tuberculosis in active and latent tuberculosis. Diagn. Microbiol. Infect. Dis. 2015, 83, 278–285. [Google Scholar] [CrossRef]
- Ross, A.G.; Ditangco, R.A.; Belimac, J.G.; Olveda, R.M.; Mercado, E.S.; Chau, T.N.; Crowe, S.M. The dire sexual health crisis among MSM in the Philippines: An exploding HIV epidemic in the absence of essential health services. Int. J. Infect. Dis. 2015, 37, 6–8. [Google Scholar] [CrossRef]
- Devi, S. Stigma, politics, and an epidemic: HIV in the Philippines. Lancet 2019, 394, 2139–2140. [Google Scholar] [CrossRef]
- Lawn, S.D.; Butera, S.T.; Folks, T.M. Contribution of immune activation to the pathogenesis and transmission of human immunodeficiency virus type 1 infection. Clin. Microbiol. Rev. 2001, 14, 753–777. [Google Scholar] [CrossRef]
- Aurin, T.H.; Munshi, S.K.; Kamal, S.M.; Rahman, M.M.; Hossain, M.S.; Marma, T.; Rahman, F.; Noor, R. Molecular approaches for detection of the multi-drug resistant tuberculosis (MDR-TB) in Bangladesh. PLoS ONE 2014, 9, e99810. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Kobayashi, N.; Nagai, H.; Chagan-Yasutan, H.; Telan, E.; Solante, M.B. Nationwide HIV-, MDR-TB survey in Japan and collaborative study in the Philippines. Int. J. Mycobacteriol. 2016, 5, 18–19. [Google Scholar] [CrossRef] [PubMed]
- Solante, M.B.; Chagan-Yasutan, H.; Hattori, T.; Leano, S.; Saludar, N.R.; Gartin, A.M.C.G.; Soolinger, D.V.; Telan, E. High rates of human immunodeficiency virus and drug resistance in tuberculosis patients in Manila, Philippines. Biotechnol. Res. J. 2017, 1, 157–162. [Google Scholar] [CrossRef]
- Tupasi, T.E.; Garfin, A.M.; Kurbatova, E.V.; Mangan, J.M.; Orillaza-Chi, R.; Naval, L.C.; Balane, G.I.; Basilio, R.; Golubkov, A.; Joson, E.S.; et al. Factors associated with loss to follow-up during treatment for multidrug-resistant tuberculosis, the philippines, 2012–2014. Emerg. Infect. Dis. 2016, 22, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, B.; Leano, S.; Nakajima, C.; Chagan-Yasutan, H.; Niki, T.; Ashino, Y.; Suzuki, Y.; Telan, E.; Hattori, T. Elevated OPN, IP-10, and neutrophilia in loop-mediated isothermal amplification confirmed tuberculosis patients. Mediat. Inflamm. 2014, 2014, 513263. [Google Scholar] [CrossRef] [PubMed]
- Phelan, J.E.; Lim, D.R.; Mitarai, S.; de Sessions, P.F.; Tujan, M.A.A.; Reyes, L.T.; Medado, I.A.P.; Palparan, A.G.; Naim, A.N.M.; Jie, S.; et al. Mycobacterium tuberculosis whole genome sequencing provides insights into the Manila strain and drug-resistance mutations in the Philippines. Sci. Rep. 2019, 9, 9305. [Google Scholar] [CrossRef]
- Su, G.L. Correlation of climatic factors and dengue incidence in Metro Manila, Philippines. Ambio 2008, 37, 292–294. [Google Scholar] [CrossRef]
- Sumi, A.; Telan, E.F.; Chagan-Yasutan, H.; Piolo, M.B.; Hattori, T.; Kobayashi, N. Effect of temperature, relative humidity and rainfall on dengue fever and leptospirosis infections in Manila, the Philippines. Epidemiol. Infect. 2017, 145, 78–86. [Google Scholar] [CrossRef]
- Bravo, L.; Roque, V.G.; Brett, J.; Dizon, R.; L’Azou, M. Epidemiology of dengue disease in the Philippines (2000–2011): A systematic literature review. PLoS Negl. Trop. Dis. 2014, 8, 3027. [Google Scholar] [CrossRef]
- The Lancet Infectious, D. Infectious disease crisis in the Philippines. Lancet Infect. Dis. 2019, 19, 1265. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Ndhlovu, L.C.; Lacuesta, T.L.; Kubo, T.; Leano, P.S.; Niki, T.; Oguma, S.; Morita, K.; Chew, G.M.; Barbour, J.D.; et al. Galectin-9 plasma levels reflect adverse hematological and immunological features in acute dengue virus infection. J. Clin. Virol. 2013, 58, 635–640. [Google Scholar] [CrossRef] [PubMed]
- Liles, V.R.; Pangilinan, L.S.; Daroy, M.L.G.; Dimamay, M.T.A.; Reyes, R.S.; Bulusan, M.K.; Dimamay, M.P.S.; Luna, P.A.S.; Mercado, A.; Bai, G.; et al. Evaluation of a rapid diagnostic test for detection of dengue infection using a single-tag hybridization chromatographic-printed array strip format. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 515–521. [Google Scholar] [CrossRef]
- Bornstein, P.; Sage, E.H. Matricellular proteins: Extracellular modulators of cell function. Curr. Opin. Cell Biol. 2002, 14, 608–616. [Google Scholar] [CrossRef]
- Elola, M.T.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vasta, G.R.; Rabinovich, G.A. Galectins: Matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol. Life Sci. 2007, 64, 1679–1700. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E.; Sage, E.H. Revisiting the matricellular concept. Matrix Biol. 2014, 37, 1–14. [Google Scholar] [CrossRef]
- Zhu, C.; Anderson, A.C.; Schubart, A.; Xiong, H.; Imitola, J.; Khoury, S.J.; Zheng, X.X.; Strom, T.B.; Kuchroo, V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005, 6, 1245–1252. [Google Scholar] [CrossRef]
- Katoh, S.; Ishii, N.; Nobumoto, A.; Takeshita, K.; Dai, S.Y.; Shinonaga, R.; Niki, T.; Nishi, N.; Tominaga, A.; Yamauchi, A.; et al. Galectin-9 inhibits CD44-hyaluronan interaction and suppresses a murine model of allergic asthma. Am. J. Respir. Crit. Care Med. 2007, 176, 27–35. [Google Scholar] [CrossRef]
- Madireddi, S.; Eun, S.Y.; Lee, S.W.; Nemcovicova, I.; Mehta, A.K.; Zajonc, D.M.; Nishi, N.; Niki, T.; Hirashima, M.; Croft, M. Galectin-9 controls the therapeutic activity of 4-1BB-targeting antibodies. J. Exp. Med. 2014, 211, 1433–1448. [Google Scholar] [CrossRef]
- Nobumoto, A.; Nagahara, K.; Oomizu, S.; Katoh, S.; Nishi, N.; Takeshita, K.; Niki, T.; Tominaga, A.; Yamauchi, A.; Hirashima, M. Galectin-9 suppresses tumor metastasis by blocking adhesion to endothelium and extracellular matrices. Glycobiology 2008, 18, 735–744. [Google Scholar] [CrossRef]
- Niki, T.; Tsutsui, S.; Hirose, S.; Aradono, S.; Sugimoto, Y.; Takeshita, K.; Nishi, N.; Hirashima, M. Galectin-9 is a high affinity IgE-binding lectin with anti-allergic effect by blocking IgE-antigen complex formation. J. Biol. Chem. 2009, 284, 32344–32352. [Google Scholar] [CrossRef]
- Lv, K.; Xu, W.; Wang, C.; Niki, T.; Hirashima, M.; Xiong, S. Galectin-9 administration ameliorates CVB3 induced myocarditis by promoting the proliferation of regulatory T cells and alternatively activated Th2 cells. Clin. Immunol. 2011, 140, 92–101. [Google Scholar] [CrossRef]
- PB, J.R.; Schreiber, T.H.; Rajasagi, N.K.; Suryawanshi, A.; Mulik, S.; Veiga-Parga, T.; Niki, T.; Hirashima, M.; Podack, E.R.; Rouse, B.T. TNFRSF25 agonistic antibody and galectin-9 combination therapy controls herpes simplex virus-induced immunoinflammatory lesions. J. Virol. 2012, 86, 10606–10620. [Google Scholar] [CrossRef]
- Kadowaki, T.; Morishita, A.; Niki, T.; Hara, J.; Sato, M.; Tani, J.; Miyoshi, H.; Yoneyama, H.; Masaki, T.; Hattori, T.; et al. Galectin-9 prolongs the survival of septic mice by expanding Tim-3-expressing natural killer T cells and PDCA-1+ CD11c+ macrophages. Crit. Care 2013, 17, 284. [Google Scholar] [CrossRef]
- Sehrawat, S.; Suryawanshi, A.; Hirashima, M.; Rouse, B.T. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: Shifting the balance toward regulators. J. Immunol. 2009, 182, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Mengshol, J.A.; Golden-Mason, L.; Arikawa, T.; Smith, M.; Niki, T.; McWilliams, R.; Randall, J.A.; McMahan, R.; Zimmerman, M.A.; Rangachari, M.; et al. A crucial role for Kupffer cell-derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS ONE 2010, 5, 9504. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Liao, Y.; Wang, Y.; Liu, C.; Zhu, X.; Chen, Z.K.; Sun, Z. Tim-3 ligand galectin-9 reduces IL-17 level and accelerates Klebsiella pneumoniae infection. Cell Immunol. 2011, 269, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Vega-Carrascal, I.; Bergin, D.A.; McElvaney, O.J.; McCarthy, C.; Banville, N.; Pohl, K.; Hirashima, M.; Kuchroo, V.K.; Reeves, E.P.; McElvaney, N.G. Galectin-9 signaling through TIM-3 is involved in neutrophil-mediated Gram-negative bacterial killing: An effect abrogated within the cystic fibrosis lung. J. Immunol. 2014, 192, 2418–2431. [Google Scholar] [CrossRef]
- Elahi, S.; Niki, T.; Hirashima, M.; Horton, H. Galectin-9 binding to Tim-3 renders activated human CD4+ T cells less susceptible to HIV-1 infection. Blood 2012, 119, 4192–4204. [Google Scholar] [CrossRef]
- Abdel-Mohsen, M.; Chavez, L.; Tandon, R.; Chew, G.M.; Deng, X.; Danesh, A.; Keating, S.; Lanteri, M.; Samuels, M.L.; Hoh, R.; et al. Human Galectin-9 Is a Potent Mediator of HIV Transcription and Reactivation. PLoS Pathog. 2016, 12, e1005677. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sada-Ovalle, I.; Beladi, S.; Anderson, A.C.; Dardalhon, V.; Hotta, C.; Kuchroo, V.K.; Behar, S.M. Tim3 binding to galectin-9 stimulates antimicrobial immunity. J. Exp. Med. 2010, 207, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Dembele, B.P.; Chagan-Yasutan, H.; Niki, T.; Ashino, Y.; Tangpukdee, N.; Shinichi, E.; Krudsood, S.; Kano, S.; Hattori, T. Plasma levels of Galectin-9 reflect disease severity in malaria infection. Malar. J. 2016, 15, 403. [Google Scholar] [CrossRef] [PubMed]
- Chagan-Yasutan, H.; Lacuesta, T.L.; Ndhlovu, L.C.; Oguma, S.; Leano, P.S.; Telan, E.F.; Kubo, T.; Morita, K.; Uede, T.; Dimaano, E.M.; et al. Elevated levels of full-length and thrombin-cleaved osteopontin during acute dengue virus infection are associated with coagulation abnormalities. Thromb. Res. 2014, 134, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Bai, G.; Motoda, H.; Ozuru, R.; Chagan-Yasutan, H.; Hattori, T.; Matsuba, T. Synthesis of a cleaved form of osteopontin by THP-1 cells and Its alteration by phorbol 12-Myristate 13-Acetate and BCG infection. Int. J. Mol. Sci. 2018, 19, 418. [Google Scholar] [CrossRef]
- Nau, G.J.; Guilfoile, P.; Chupp, G.L.; Berman, J.S.; Kim, S.J.; Kornfeld, H.; Young, R.A. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc. Natl. Acad. Sci. USA 1997, 94, 6414–6419. [Google Scholar] [CrossRef] [PubMed]
- Hasibuan, F.M.; Shiratori, B.; Senoputra, M.A.; Chagan-Yasutan, H.; Koesoemadinata, R.C.; Apriani, L.; Takahashi, Y.; Niki, T.; Alisjahbana, B.; Hattori, T. Evaluation of matricellular proteins in systemic and local immune response to Mycobacterium tuberculosis infection. Microbiol. Immunol. 2015, 59, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, B.; Zhao, J.; Okumura, M.; Chagan-Yasutan, H.; Yanai, H.; Mizuno, K.; Yoshiyama, T.; Idei, T.; Ashino, Y.; Nakajima, C.; et al. Immunological roles of elevated plasma levels of matricellular proteins in Japanese patients with pulmonary tuberculosis. Int. J. Mol. Sci. 2016, 18. [Google Scholar] [CrossRef]
- Niki, T.; Fujita, K.; Rosen, H.; Hirashima, M.; Masaki, T.; Hattori, T.; Hoshino, K. Plasma Galectin-9 Concentrations in Normal and Diseased Condition. Cell Physiol. Biochem. 2018, 50, 1856–1868. [Google Scholar] [CrossRef]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef]
- Klement, J.D.; Paschall, A.V.; Redd, P.S.; Ibrahim, M.L.; Lu, C.; Yang, D.; Celis, E.; Abrams, S.I.; Ozato, K.; Liu, K. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J. Clin. Investig. 2018, 128, 5549–5560. [Google Scholar] [CrossRef]
- Bai, G.; Matsuba, T.; Kikuchi, H.; Chagan-Yasutan, H.; Motoda, H.; Ozuru, R.; Yamada, O.; Oshima, Y.; Hattori, T. Inhibition of inflammatory-molecule synthesis in THP-1 cells stimulated with phorbol 12-myristate 13-acetate by brefelamide derivatives. Int. Immunopharmacol. 2019, 75, 105831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yamada, O.; Kida, S.; Murase, S.; Hattori, T.; Oshima, Y.; Kikuchi, H. Downregulation of PD-L1 by amide analogues of brefelamide: Alternatives to antibody-based cancer immunotherapy. Exp. Ther. Med. Press 2020. [Google Scholar] [CrossRef]




| Subtype | Reaction ※1 | Copy Numbers ※2 (Copy/mL) |
|---|---|---|
| DENV-1 | 0.034 | 7.3 |
| DENV-2 | 0.084 | 18.4 |
| DENV-3 | 0.032 | 6.9 |
| DENV-4 | 0.080 | 17.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.; Niki, T.; Kikuchi, H.; Sumi, A.; Kobayashi, N.; Haruyama, T.; Zhang, J.; Chagan-Yasutan, H.; Hattori, T. New Development of Disaster-Related and Tropical Infectious Diseases Control. Reports 2020, 3, 5. https://doi.org/10.3390/reports3010005
Bai G, Niki T, Kikuchi H, Sumi A, Kobayashi N, Haruyama T, Zhang J, Chagan-Yasutan H, Hattori T. New Development of Disaster-Related and Tropical Infectious Diseases Control. Reports. 2020; 3(1):5. https://doi.org/10.3390/reports3010005
Chicago/Turabian StyleBai, Gaowa, Toshiro Niki, Haruhisa Kikuchi, Ayako Sumi, Nobuyuki Kobayashi, Takahiro Haruyama, Jing Zhang, Haorile Chagan-Yasutan, and Toshio Hattori. 2020. "New Development of Disaster-Related and Tropical Infectious Diseases Control" Reports 3, no. 1: 5. https://doi.org/10.3390/reports3010005
APA StyleBai, G., Niki, T., Kikuchi, H., Sumi, A., Kobayashi, N., Haruyama, T., Zhang, J., Chagan-Yasutan, H., & Hattori, T. (2020). New Development of Disaster-Related and Tropical Infectious Diseases Control. Reports, 3(1), 5. https://doi.org/10.3390/reports3010005






