How Does Maternal Immune Activity Affect Fetal Survival and Brain Development? The Critical Roles of IL-17A and Microglia
Abstract
1. Introduction
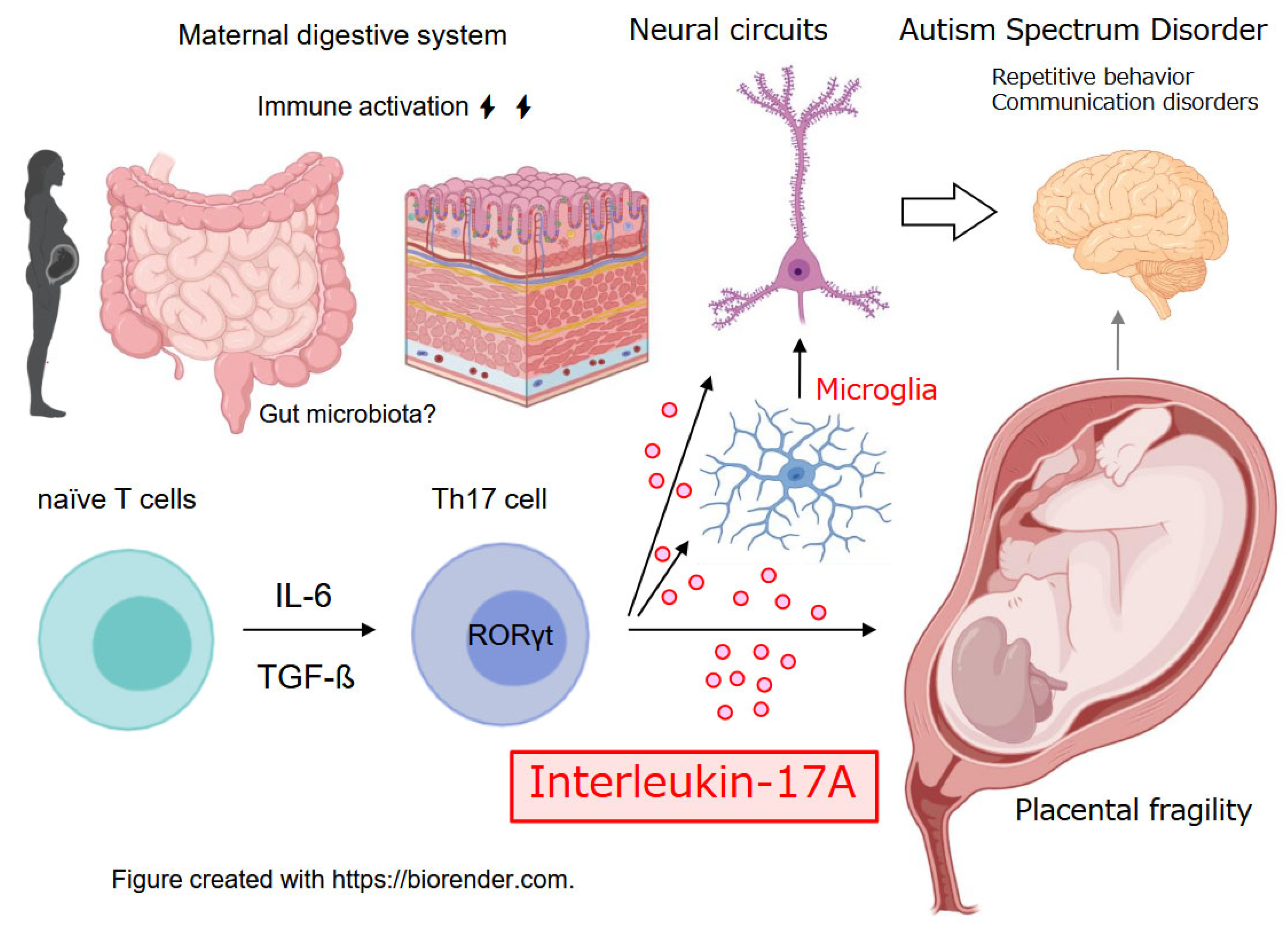
2. Placental IL-17A/IL-17RA Axis: A Fault Line in Pregnancy Immune Homeostasis
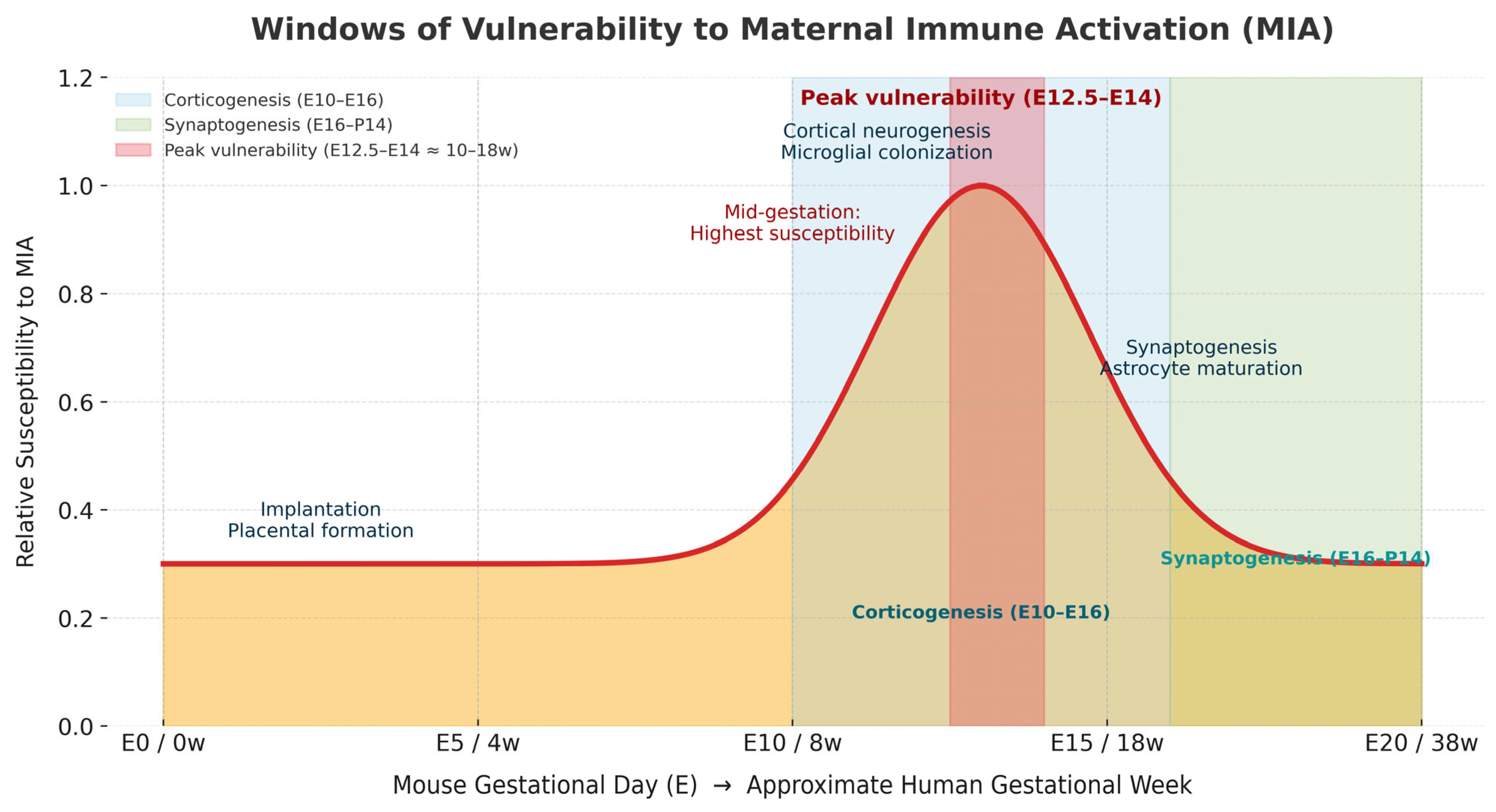
3. Critical Windows of Susceptibility
4. Microglial Spatiotemporal Dynamics
5. Complement Pathway and Synaptic Pruning
6. Overview of Maternal Immune Activation (MIA)
7. Comparative Roles of IL-17A, IL-6, and TNF-α in Maternal Immune Activation
| Model | Trigger/Agent | Primary Receptor/Axis | Gestational Window (Rodent) | Dose/Route (Guideline) | Maternal Acute Readouts | Placental/Fetal Readouts | Core Offspring Phenotypes | Key Cytokines/Pathways | Strengths | Limitations | Standardization Notes | Key Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly(I:C) | dsRNA analog (viral mimic) | TLR3 → IL-6/IL-17A axis | E9.5–E13.5 (lab-dependent) | i.p.; strain/batch-dependent (see guideline) | Fever-like response, weight loss, serum cytokine surge | ↑ Resorption rate (at higher doses), placental inflammation | ↓ Social interaction, ↑ repetitive behaviors, sensorimotor gating changes | IL-6, IL-17A, chemokines | High reproducibility; rapid viral-mimic induction | Batch/dose/window-sensitive phenotypes | Report batch, dose, gestational window | [2,36] |
| LPS | Endotoxin (bacterial mimic) | TLR4 → IL-1β/TNF-α axis | E9.5–E13.5 | i.p./s.c.; high dose near term increases fetal lethality | Fever, weight loss, inflammatory cytokines ↑ | Preterm/abortion risk ↑, placental inflammation | Anxiety-/depression-like, learning/social changes | IL-1β, TNF-α | Bacterial infection mimic | Dose-dependent maternal toxicity; variability | Detail dose and route per guideline | [2,36] |
| Live influenza/infection | Influenza virus (example) | Multi-PRR, systemic inflammatory axis | Typically mid-gestation | Intranasal; BSL compliance | Fever, weight change, serum cytokines | Placental inflammation, fetal growth/viability impact | ↓ Social attention, communication changes | IL-6, TNF-α, others | Highest clinical relevance | Pathogen/variant and site differences | Specify inoculum, timing, strain | [5,40] |
| Maternal IL-6 injection | Recombinant IL-6 | IL-6R/gp130 → STAT3 | E12.5 ± | i.p.; single/repeat per report | Acute cytokine rise; minimal maternal behavior change | Directly engages fetal neurodevelopmental pathways | Social/sensory phenotypes | IL-6 | Pathogen-independent causality | Simplifies physiology; limits external validity | Clarify dose/timing rationale | [3,41] |
| Direct fetal IL-17A | Recombinant IL-17A (fetal intracerebral) | IL-17RA/RC → NF-κB/MAPK | E13 ± (corticogenesis) | Intraventricular (stereotaxic) | Minimal maternal load | Microglial activation/relocation; circuit disruption | Sensory/social phenotypes (study-dependent) | IL-17A | Direct fetal brain causality | Invasive; translational limits | Detail surgical conditions/operator skill | [7,16] |
| PRIMA-17 | Maternal IL-17RA deficiency + IL-17A excess | Embryo-restricted IL-17A signaling | Mid-gestation (E12.5 ±) | Genetic × immunologic combination | Low dependence on maternal cytokine peaks | Placental adhesion ↓; fetal loss ↑; circuit defects | ASD-like (social ↓, repetitive ↑) | IL-17A/IL-17RA | Fetal-selective inference; reduces confounders | Complex to build; inter-site reproducibility | Protocol sharing/registration | [42] |
| NHP MIA | Poly(I:C) or live pathogen | Multi-PRR/IL-6–IL-17 axis | Mid-gestation (species-appropriate) | i.v./i.m./intranasal (site protocol) | Temp/weight/cytokines longitudinal | Placental function and brain growth imaging; behavior | ↓ social attention/interactions | IL-6, IL-17A etc. | Closer to human behavior | High cost; small N; ethics | Protocol transparency; shared metrics | [43,44] |
| Clinical corollaries | Natural infection in pregnancy | Pathogen-dependent; systemic inflammatory axis | All trimesters (severity/pathogen-dependent) | EHR/lab/inpatient data | Fever; CRP/cytokines | Perinatal outcomes; placental pathology | Neurodevelopmental follow-up (e.g., ASD) | IL-6/IL-17A etc. | Max clinical validity | Confounding (comorbidity/access) | Rigorous epidemiologic adjustment | [5,45] |
| Human brain organoids | IL-6/IL-17A exposure; conditioned media | Cytokine receptors → downstream signaling | In vitro developmental model | Concentration/exposure optimization | — | Genotype-dependent cellular responses | Microcircuit/lineage phenotypes | IL-6/IL-17A | Test human-specific mechanisms | Does not recreate maternal–placental–fetal axis | Register dose/time/reproducibility | [46] |
8. MIA and the Risk of Fetal Loss
9. Relationship Between Miscarriage and Neurodevelopmental Disorders
10. Effects of MIA on Fetal Brain Development
11. DOHaD and MIA
12. Clinical Implications and Future Directions
- Critical windows of susceptibility. Pinpoint gestational stages at most significant risk to guide targeted prevention and timing of interventions [23].
- Gene–environment interplay. Identify genetic backgrounds that confer heightened vulnerability to MIA and leverage these insights for individualized prevention and treatment [44].
- Therapeutic mitigation. Develop and test strategies to blunt MIA sequelae, including IL-17A blockade and microglia-modulating drugs [42,50]. Together, these findings suggest that interventions targeting IL-17A signaling in the fetus may represent a promising strategy for preventing neurodevelopmental abnormalities. The PRIMA-17 paradigm underscores that IL-17A alone, without confounding cytokines such as IL-6, is sufficient to reprogram neurodevelopment [23].
- Translation to humans. Rigorously evaluate the extent to which animal-model findings generalize to human pregnancy and neurodevelopment [51].
- Co-exposures and context. Map interactions between MIA and other environmental factors—nutrition, psychosocial stress, and chemical exposures—to capture real-world complexity [49].
13. Sex-Differential Vulnerability to MIA
14. Cross-Species Translation: NHP and Human Multi-Omics at the Maternal–Fetal Interface
15. Neurovascular Unit and the BBB in MIA
16. Reporting Standards for MIA (Checklist)
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASD | Autism spectrum disorder. |
| CDC | U.S. Centers for Disease Control and Prevention. |
| CD68 | Cluster of differentiation 68 (marker of activated microglia/macrophages). |
| DOHaD | Developmental Origins of Health and Disease. |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders. |
| IL-1β | Interleukin-1 beta. |
| IL-6 | Interleukin-6. |
| IL-17A | Interleukin-17A. |
| IL-17RA | Interleukin-17 receptor A. |
| LPS | Lipopolysaccharide. |
| MIA | Maternal immune activation. |
| Poly (I:C) | Polyinosinic–polycytidylic acid (viral dsRNA mimetic). |
| RORγt | Retinoic acid receptor-related orphan receptor gamma t. |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2. |
| Tg | Transgenic. |
| Th17 | T helper 17 (cell). |
| TGF-β | Transforming growth factor beta. |
| TLR3 | Toll-like receptor 3. |
| TNF-α | Tumor necrosis factor alpha. |
References
- Mor, G.; Cardenas, I. The immune system in pregnancy: A unique complexity. Am. J. Reprod. Immunol. 2010, 63, 425–433. [Google Scholar] [CrossRef]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Thompson, C. The infectious origins of stillbirth. Am. J. Obstet. Gynecol. 2003, 189, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Fell, D.B.; Savitz, D.A.; Kramer, M.S.; Gessner, B.D.; Katz, M.A.; Knight, M.; Luteijn, J.; Marshall, H.; Bhat, N.; Gravett, M.; et al. Maternal influenza and birth outcomes: Systematic review of comparative studies. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 48–59. [Google Scholar] [CrossRef]
- Di Mascio, D.; Khalil, A.; Saccone, G.; Rizzo, G.; Buca, D.; Liberati, M.; Vecchiet, J.; Nappi, L.; Scambia, G.; Berghella, V.; et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2020, 2, 100107. [Google Scholar] [CrossRef]
- Walker, C.K.; Krakowiak, P.; Baker, A.; Hansen, R.L.; Ozonoff, S.; Hertz-Picciotto, I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015, 169, 154–162. [Google Scholar] [CrossRef]
- Tome, S.; Sasaki, T.; Takahashi, S.; Takei, Y. Elevated maternal retinoic acid-related orphan receptor-γt enhances the effect of polyinosinic–polycytidylic acid in inducing fetal loss. Exp. Anim. 2019, 68, 491–497. [Google Scholar] [CrossRef]
- Choi, G.B.; Yim, Y.S.; Wong, H.; Kim, S.; Kim, H.; Kim, S.V.; Hoeffer, C.A.; Littman, D.R.; Huh, J.R. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 2016, 351, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Yousef, H.; Czupalla, C.J.; Lee, D.; Chen, M.B.; Burke, A.N.; Zera, K.A.; Zandstra, J.; Berber, E.; Lehallier, B.; Mathur, V.; et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nat. Med. 2019, 25, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Ostrem, B.E.L.; Domínguez-Iturza, N.; Stogsdill, J.A.; Faits, T.; Kim, K.; Levin, J.Z.; Arlotta, P. Fetal brain response to maternal inflammation requires microglia. Development 2024, 151, dev202252. [Google Scholar] [CrossRef]
- Ozaki, K.; Kato, D.; Ikegami, A.; Hashimoto, A.; Sugio, S.; Guo, Z.; Shibushita, M.; Tatematsu, T.; Haruwaka, K.; Moorhouse, A.J.; et al. Maternal immune activation induces sustained changes in fetal microglia motility. Sci. Rep. 2020, 10, 21378. [Google Scholar] [CrossRef]
- Das Sarma, J.; Ciric, B.; Marek, R.; Sadhukhan, S.; Caruso, M.L.; Shafagh, J.; Fitzgerald, D.C.; Shindler, K.S.; Rostami, A. Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J. Neuroinflammation 2009, 6, 14. [Google Scholar] [CrossRef]
- Kang, Z.; Wang, C.; Zepp, J.; Wu, L.; Sun, K.; Zhao, J.; Chandrasekharan, U.; E DiCorleto, P.; Trapp, B.D.; Ransohoff, R.M.; et al. Act1 mediates IL-17–induced EAE pathogenesis selectively in NG2+ glial cells. Nat. Neurosci. 2013, 16, 1401–1408. [Google Scholar] [CrossRef]
- Sasaki, T.; Tome, S.; Takei, Y. Intraventricular IL-17A administration activates microglia and alters their localization in the mouse embryo cerebral cortex. Mol. Brain 2020, 13, 93. [Google Scholar] [CrossRef]
- Thion, M.S.; Ginhoux, F.; Garel, S. Microglia and early brain development: An intimate journey. Science 2018, 362, 185–189. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Martínez-Cerdeño, V.; Noctor, S.C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 2013, 33, 4216–4233. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Buklijas, T. A conceptual framework for the developmental origins of health and disease. J. Dev. Orig. Health Dis. 2010, 1, 6–18. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Developmental origins of health and disease—Global public health implications. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bilbo, S.D.; Schwarz, J.M. Early-life programming of later-life brain and behavior: A critical role for the immune system. Front. Behav. Neurosci. 2009, 3, 14. [Google Scholar] [CrossRef]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, I.; Finsen, B.; Zimmer, J.; González, B.; Castellano, B. Development of microglia in the postnatal rat hippocampus. Hippocampus 1998, 8, 458–474. [Google Scholar] [CrossRef]
- Fox, C.J.; Russell, K.I.; Wang, Y.T.; Christie, B.R. Contribution of NR2A and NR2B NMDA subunits to bidirectional synaptic plasticity in the hippocampus in vivo. Hippocampus 2006, 16, 907–915. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood–brain barrier disruption in CNS inflammatory disease. J. Clin. Investig. 2012, 122, 2454–2468. [Google Scholar] [CrossRef]
- Chen, X.; Oppenheim, J.J. Th17 cells and Tregs: Unlikely allies. J. Leukoc. Biol. 2014, 95, 723–731. [Google Scholar] [CrossRef]
- Okubo, Y.; Mera, T.; Wang, L.; Faustman, D.L. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci. Rep. 2013, 3, 3153. [Google Scholar] [CrossRef] [PubMed]
- Nedoszytko, B.; Lange, M.; Sokołowska-Wojdyło, M.; Renke, J.; Trzonkowski, P.; Sobjanek, M.; Szczerkowska-Dobosz, A.; Niedoszytko, M.; Górska, A.; Romantowski, J.; et al. The role of regulatory T cells and genes involved in their differentiation in pathogenesis of selected inflammatory and neoplastic skin diseases. Part I: Treg properties and functions. Postepy Dermatol. Alergol. 2017, 34, 285–294. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T.; et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017, 14, 204. [Google Scholar] [CrossRef]
- Prajeeth, C.K.; Löhr, K.; Floess, S.; Zimmermann, J.; Ulrich, R.; Gudi, V.; Beineke, A.; Baumgärtner, W.; Müller, M.; Huehn, J.; et al. Effector molecules released by Th1 but not Th17 cells drive an M1 response in microglia. Brain Behav. Immun. 2014, 37, 248–259. [Google Scholar] [CrossRef]
- Engelhardt, B.; Ransohoff, R.M. Capture, crawl, cross: The T cell code to breach the blood–brain barriers. Trends Immunol. 2012, 33, 579–589. [Google Scholar] [CrossRef]
- Cipollini, V.; Anrather, J.; Orzi, F.; Iadecola, C. Th17 and cognitive impairment: Possible mechanisms of action. Front. Neuroanat. 2019, 13, 95. [Google Scholar] [CrossRef]
- Rostami, A.; Ciric, B. Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination. J. Neurol. Sci. 2013, 333, 76–87. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia sculpt postnatal neural circuits in an activity- and complement-dependent manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Kentner, A.C.; Bilbo, S.D.; Brown, A.S.; Hsiao, E.Y.; McAllister, A.K.; Meyer, U. Maternal immune activation: Reporting guidelines to improve the rigor, reproducibility, and transparency of the MIA model. Neuropsychopharmacology 2019, 44, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Vento-Tormo, R.; Efremova, M.; Botting, R.A.; Turco, M.Y.; Vento-Tormo, M.; Meyer, K.B.; Park, J.-E.; Stephenson, E.; Polański, K.; Goncalves, A.; et al. Single-cell reconstruction of the early maternal–fetal interface in humans. Nature 2018, 563, 347–353. [Google Scholar] [CrossRef]
- Iwata, S.; Hyugaji, M.; Soga, Y.; Morikawa, M.; Sasaki, T.; Takei, Y. Gene expression of psychiatric disorder-related kinesin superfamily proteins (Kifs) is potentiated in alternatively activated primary cultured microglia. BMC Res. Notes 2025, 18, 44. [Google Scholar] [CrossRef]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood–brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef]
- Mednick, S.A.; Machon, R.A.; Huttunen, M.O.; Bonett, D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 1988, 45, 189–192. [Google Scholar] [CrossRef]
- Wu, W.L.; Hsiao, E.Y.; Yan, Z.; Mazmanian, S.K.; Patterson, P.H. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain Behav. Immun. 2017, 62, 11–23. [Google Scholar] [CrossRef]
- Andruszewski, D.; Uhlfelder, D.C.; Desiato, G.; Regen, T.; Waisman, A.; Mufazalov, I.A. Embryo-restricted responses to maternal IL-17A promote neurodevelopmental disorders in mouse offspring. Mol. Psychiatry 2025, 30, 1585–1593. [Google Scholar] [CrossRef]
- Bauman, M.D.; Iosif, A.-M.; Smith, S.E.P.; Bregere, C.; Amaral, D.G.; Patterson, P.H. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiatry 2014, 75, 332–341. [Google Scholar] [CrossRef]
- Machado, C.J.; Whitaker, A.M.; Smith, S.E.; Patterson, P.H.; Bauman, M.D. Maternal immune activation in nonhuman primates alters social attention in juvenile offspring. Biol. Psychiatry 2015, 77, 823–832. [Google Scholar] [CrossRef]
- Atladóttir, H.O.; Thorsen, P.; Østergaard, L.; Schendel, D.E.; Lemcke, S.; Abdallah, M.; Parner, E.T. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 2010, 40, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Paşca, A.M.; Sloan, S.A.; Clarke, L.E.; Tian, Y.; Makinson, C.D.; Huber, N.; Kim, C.H.; Park, J.-Y.; O’Rourke, N.A.; Nguyen, K.D.; et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 2015, 12, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Lacey, H.A.; Baker, P.N.; Crocker, I.P. E-cadherin in the assessment of aberrant placental cytotrophoblast turnover in pregnancies complicated by pre-eclampsia. Histochem. Cell Biol. 2005, 124, 499–506. [Google Scholar] [CrossRef]
- Rinkenberger, J.L.; Werb, Z. The labyrinthine placenta. Nat. Genet. 2000, 25, 248–250. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood–brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex differences in autism spectrum disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Hanamsagar, R.; Bilbo, S.D. Sex differences in neurodevelopmental and neurodegenerative disorders: Focus on microglial function and neuroinflammation. J. Steroid Biochem. Mol. Biol. 2016, 160, 127–133. [Google Scholar] [CrossRef] [PubMed]
| Sample Source | Biomarker/Panel | Mechanistic Link (Summary) | Evidence Tier | Primary Translational Use Case | Key Refs |
|---|---|---|---|---|---|
| Maternal blood | IL-6 (±IL-1β, TNF-α), IL-17A | Core MIA cytokines; placental IL-6 signaling shapes fetal brain; IL-17A linked to cortical malformations | Strong | Prenatal inflammation risk stratification; trial eligibility; longitudinal monitoring | [3,7,41] |
| Maternal blood | Treg/Th17 balance markers (e.g., IL-10, TGF-β ↔ IL-17A) | Tolerance vs. inflammation; tunes pregnancy maintenance and MIA susceptibility | Supportive | Resilience stratification; immunophenotyping for interventions | [26,28] |
| Maternal blood | VEGF-A | With IL-17A, disrupts endothelial tight junctions; impacts barrier integrity | Supportive | Surrogate for barrier fragility; vascular response monitoring | [25,39,49] |
| Placenta (pathology/molecular) | E-cadherin (trophoblast adhesion), VEGF-A | Loss weakens labyrinth architecture; vascular factors reshape microenvironment | Strong/Supportive | Histopathologic anchor; evaluation of prior inflammation | [25,38] |
| Placenta (single-cell) | Immune/trophoblast ligand–receptor interactions (incl. IL-17A/IL-17RA) | Maps immune–epithelial/vascular crosstalk at the human maternal–fetal interface | Strong | Cross-species triangulation; pathway nomination | [37] |
| Cord blood/Neonatal serum | IL-6, IL-17A | Reflects in utero inflammatory exposure; early-life immune programming | Supportive | Early-life stratification; baseline for follow-up | [3,7] |
| Maternal clinical context | Infection requiring hospitalization | Pregnancy infections requiring hospitalization associate with increased ASD risk | Strong | Risk stratification; covariate control in analyses | [45] |
| Neonatal/infant functional | EEG, eye-tracking, social gaze metrics | Downstream functional readouts of circuit impact | Supportive/Emerging | Pre-intervention screening; predictive marker development | [36] |
| Mechanistic bridge (preclinical → clinical) | NVU/BBB dysfunction signals (IL-17A-, VEGF-A-mediated) | IL-17A induces tight junction breakdown; VEGF-A increases permeability | Strong/Supportive | Target selection; biomarker prioritization | [39,49] |
| Model-to-human triangulation | PRIMA-17, NHP, human organoids | Conserved IL-17A axis and convergent circuit phenotypes across models | Strong/Supportive | External validation; bridge from discovery to validation | [42,44,46] |
| Domain | Items to Report | Rationale |
|---|---|---|
| Animal information | Species, strain, substrain; supplier; sex; age; housing (light/dark cycle, enrichment, cage density); microbiological status (SPF, gnotobiotic, conventional) | Baseline characteristics influence immune response and neurodevelopmental outcomes. |
| Breeding and pregnancy | Mating scheme (timed mating, plug check, IVF/ET use); parity of dams; gestational staging method; number of dams per group | Accurate pregnancy staging is critical for reproducibility. |
| MIA induction | Inducing agent (poly(I:C), LPS, influenza, recombinant cytokines, genetic model); source, lot, purity; dose and concentration; administration route (i.p., i.v., s.c., intranasal, intraventricular); timing (gestational day); injection schedule (single vs. repeated) | Variability in agent and timing strongly affects outcomes. |
| Maternal readouts | Acute sickness behaviors (weight loss, hypothermia, mobility); maternal cytokine levels (IL-6, IL-17A, TNF-α); clinical chemistry (CRP, glucose); mortality | Confirms effective immune activation and safety margins. |
| Pregnancy outcomes | Implantation number, resorption rate, stillbirths; litter size; sex ratio; placental weight; fetal weight; E-cadherin or VEGF-A expression; histopathology (labyrinthine structure) | Links maternal immune state with placental/fetal health. |
| Fetal and neonatal measures | Fetal brain cytokines; microglial morphology/motility; apoptosis markers; neonatal survival; growth curves; developmental milestones (eye opening, reflexes) | Establishes early-life impact of MIA. |
| Offspring phenotypes | Behavioral domains (social, repetitive, anxiety-like, cognition, sensory gating); neuroanatomy (cortical layering, interneuron markers); electrophysiology (EEG, evoked potentials) | Provides translational readouts relevant to ASD/SCZ. |
| Randomization and blinding | Random assignment of dams; blinding of experimenters for behavioral testing and histology | Minimizes bias. |
| Statistical considerations | Litter effects accounted for; power analysis; number of dams vs. pups reported separately; attrition documented | Ensures appropriate inference and reproducibility. |
| Ethical and reporting standards | ARRIVE guideline adherence; approval numbers from ethics committees; compliance with MIA reporting guideline [36] | Transparency, reproducibility, and comparability. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubo, A.; Kamiya, S.; Sanaka, S.; Nakamura, K.; Kishi, K.; Sasaki, T. How Does Maternal Immune Activity Affect Fetal Survival and Brain Development? The Critical Roles of IL-17A and Microglia. Neuroglia 2025, 6, 45. https://doi.org/10.3390/neuroglia6040045
Kubo A, Kamiya S, Sanaka S, Nakamura K, Kishi K, Sasaki T. How Does Maternal Immune Activity Affect Fetal Survival and Brain Development? The Critical Roles of IL-17A and Microglia. Neuroglia. 2025; 6(4):45. https://doi.org/10.3390/neuroglia6040045
Chicago/Turabian StyleKubo, Asumi, Sara Kamiya, Sae Sanaka, Kenyu Nakamura, Kyoko Kishi, and Tetsuya Sasaki. 2025. "How Does Maternal Immune Activity Affect Fetal Survival and Brain Development? The Critical Roles of IL-17A and Microglia" Neuroglia 6, no. 4: 45. https://doi.org/10.3390/neuroglia6040045
APA StyleKubo, A., Kamiya, S., Sanaka, S., Nakamura, K., Kishi, K., & Sasaki, T. (2025). How Does Maternal Immune Activity Affect Fetal Survival and Brain Development? The Critical Roles of IL-17A and Microglia. Neuroglia, 6(4), 45. https://doi.org/10.3390/neuroglia6040045







