An FGFR1-Altered Intramedullary Thoracic Tumor with Unusual Clinicopathological Features: A Case Report and Literature Review
Abstract
1. Introduction
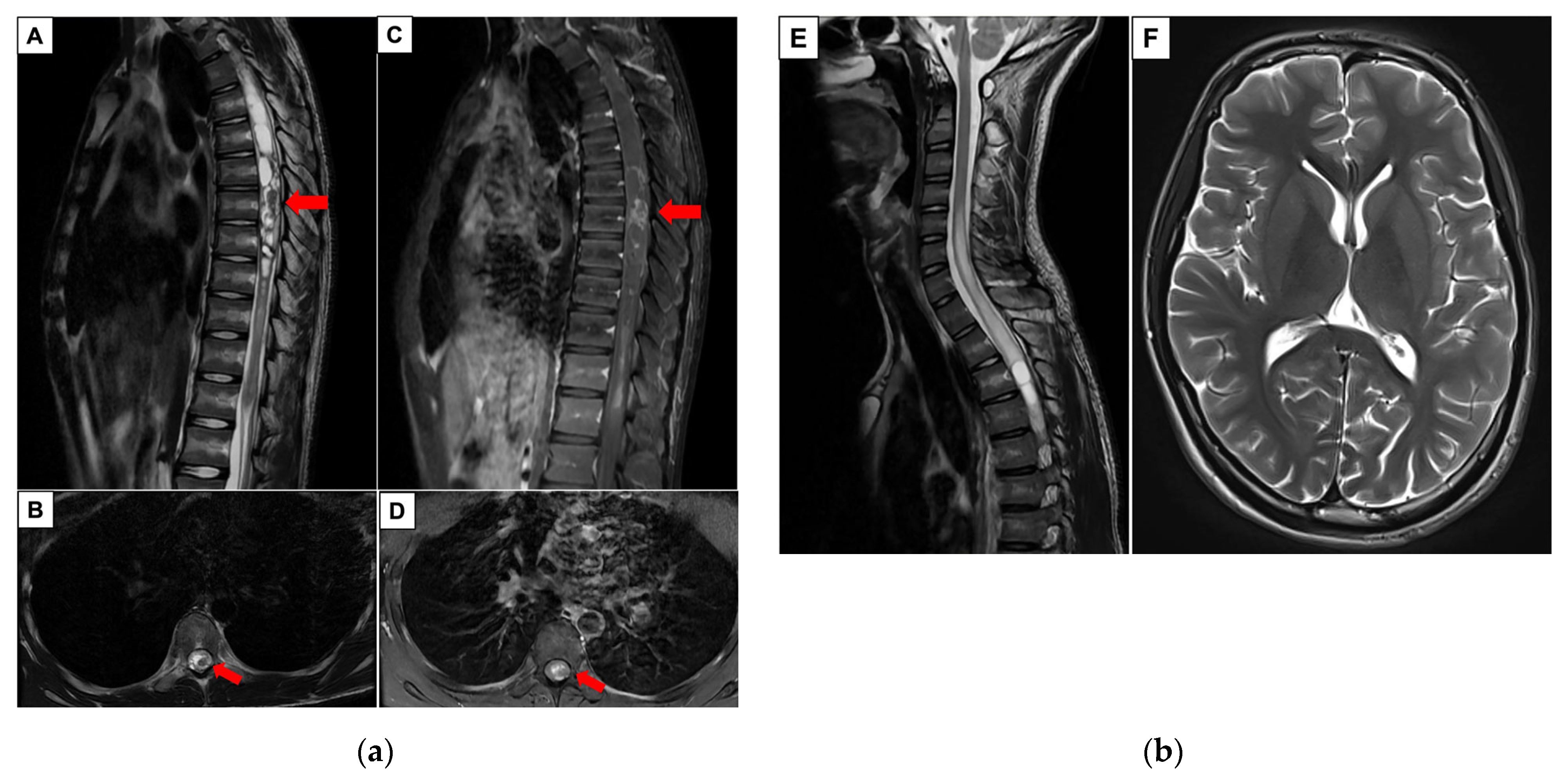
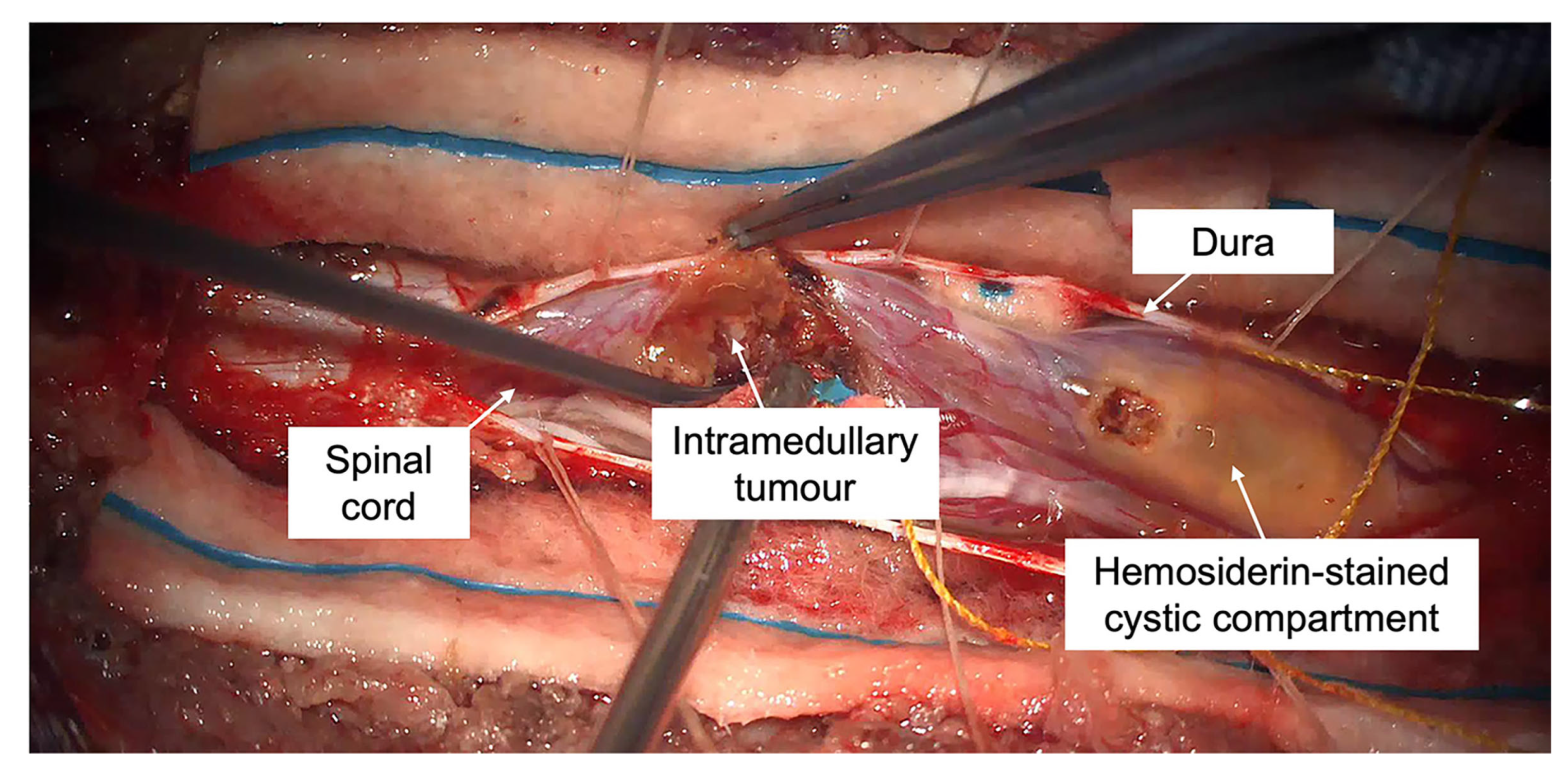
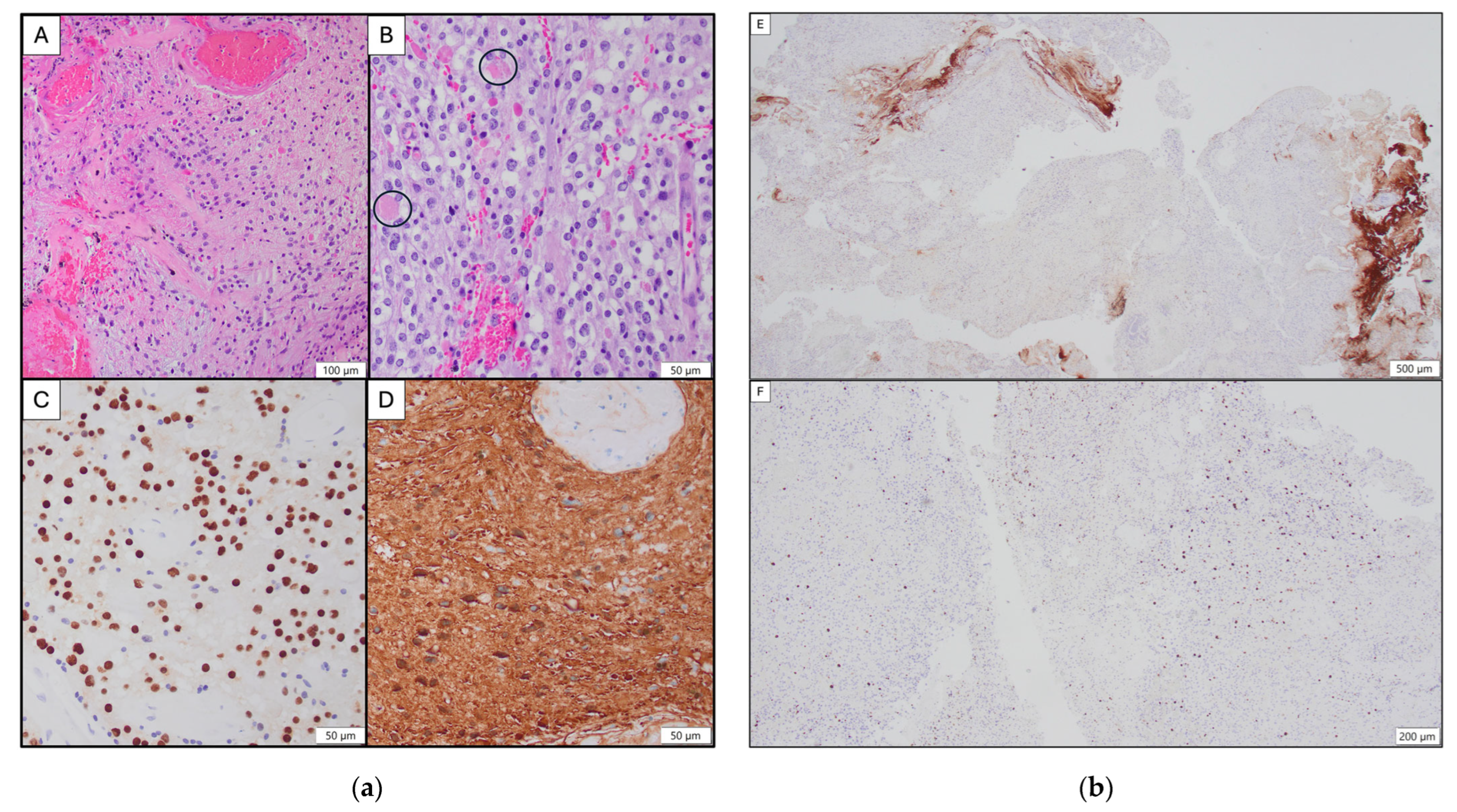
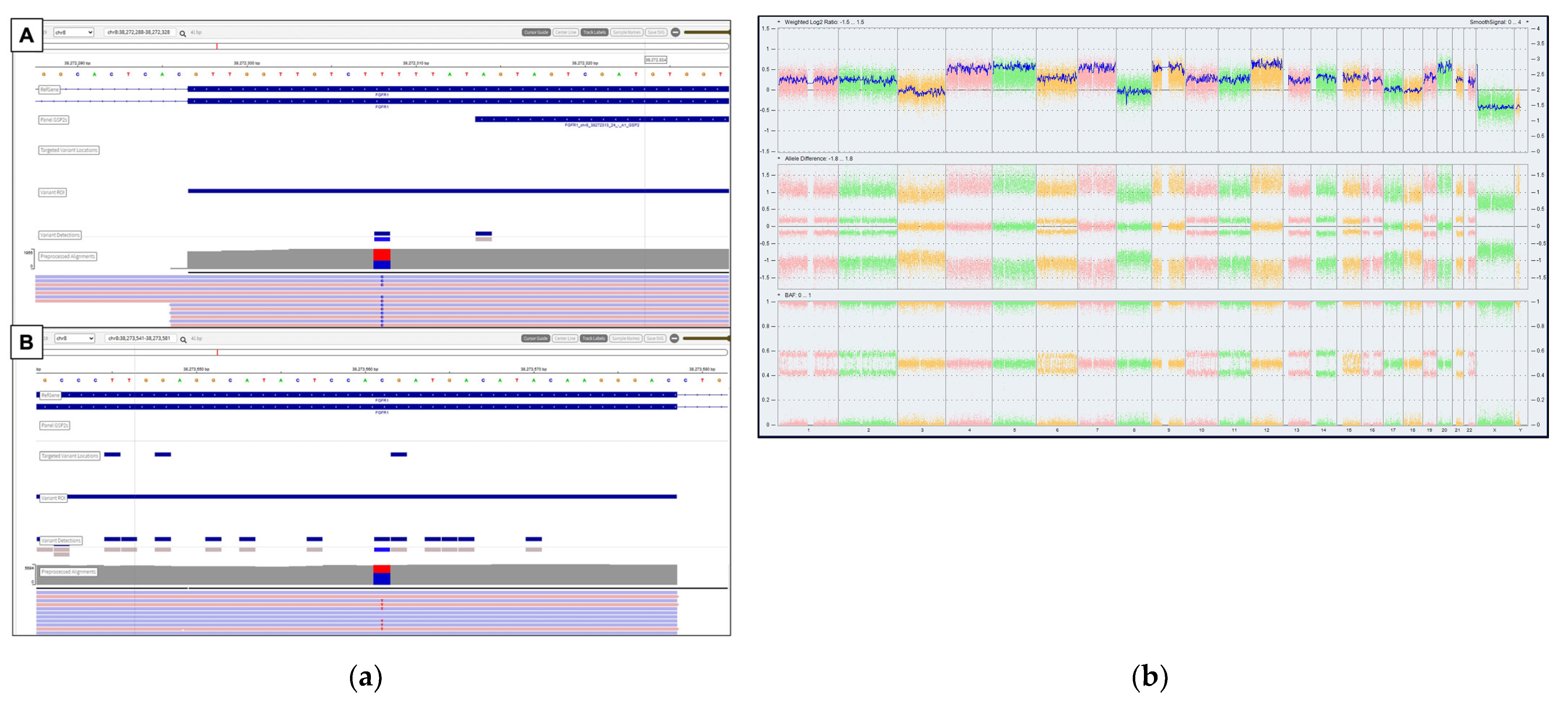
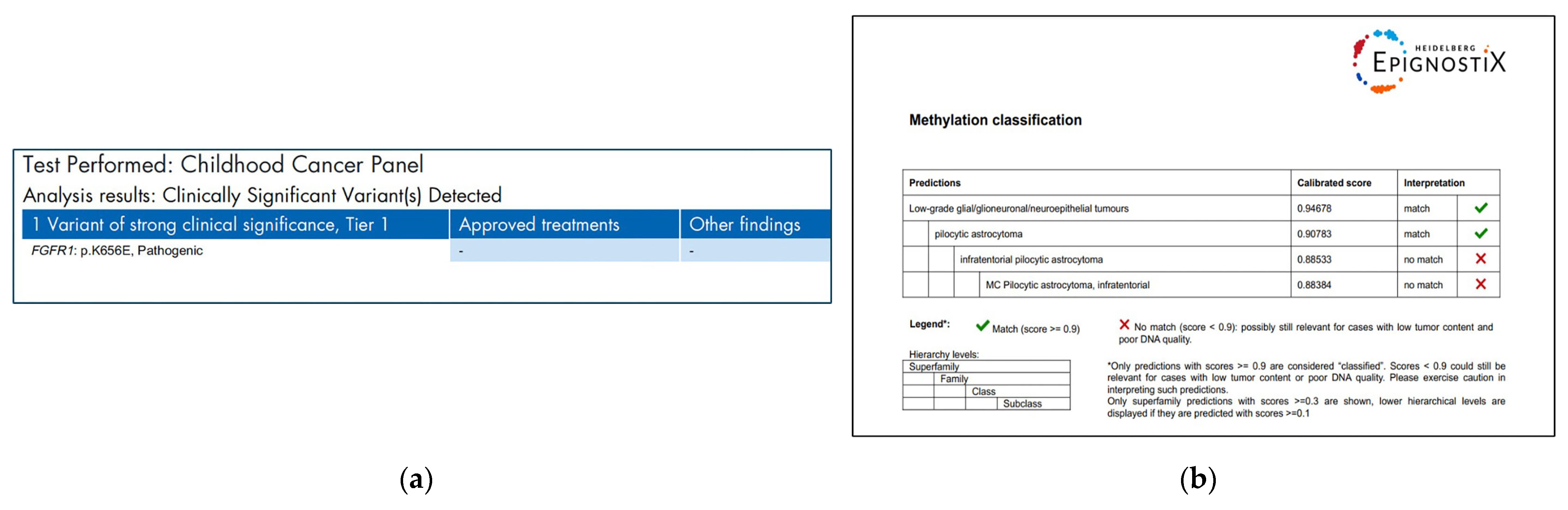
2. Case Report
3. Discussion
3.1. Pediatric Spinal Gliomas in the Era of WHO 2021 Classification
3.2. Overview of FGFR Alterations in Pediatric Primary CNS Tumors
3.3. Targeting FGFR in Pediatric Primary CNS Tumors
3.4. Study Reflections and Future Directions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caroli, E.; Salvati, M.; Ferrante, L. Spinal glioblastoma with brain relapse in a child: Clinical considerations. Spinal Cord. 2005, 43, 565–567. [Google Scholar] [CrossRef]
- Huisman, T.A. Pediatric tumors of the spine. Cancer Imaging 2009, 9, S45–S48. [Google Scholar] [CrossRef]
- Kumar, N.; Tan, W.L.B.; Wei, W.; Vellayappan, B.A. An overview of the tumors affecting the spine-inside to out. Neurooncol. Pract. 2020, 7, i10–i17. [Google Scholar] [CrossRef]
- Zuo, W.; He, Y.; Li, W.; Wu, H.; Zhao, Z.; Zhang, Y.; Chen, S.; Yin, Y. Landscape of FGF/FGFR Alterations in 12,372 Chinese Cancer Patients. J. Cancer 2020, 11, 6695–6699. [Google Scholar] [CrossRef]
- Katoh, M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat. Rev. Clin. Oncol. 2019, 16, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M.; Nakagama, H. FGF receptors: Cancer biology and therapeutics. Med. Res. Rev. 2014, 34, 280–300. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Schlessinger, J. Cell signaling by receptor tyrosine kinases. Cell 2010, 141, 1117–1134. [Google Scholar] [CrossRef]
- Dono, A.; El Achi, H.; Bundrant, B.E.; Goli, P.S.; Zhu, P.; Ozkizilkaya, H.I.; Esquenazi, Y.; Ballester, L.Y. Infiltrating gliomas with FGFR alterations: Histologic features, genetic alterations, and potential clinical implications. Cancer Biomark. Sect. A Dis. Markers 2023, 36, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Chioni, A.M.; Grose, R.P. Biological Significance and Targeting of the FGFR Axis in Cancer. Cancers 2021, 13, 5681. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.M.; Ekert, P.G.; Fleuren, E.D.G. Biological and clinical implications of FGFR aberrations in paediatric and young adult cancers. Oncogene 2023, 42, 1875–1888. [Google Scholar] [CrossRef]
- Picca, A.; Sansone, G.; Santonocito, O.S.; Mazzanti, C.M.; Sanson, M.; Di Stefano, A.L. Diffuse Gliomas with FGFR3-TACC3 Fusions: Oncogenic Mechanisms, Hallmarks, and Therapeutic Perspectives. Cancers 2023, 15, 5555. [Google Scholar] [CrossRef]
- Métais, A.; Bouchoucha, Y.; Kergrohen, T.; Dangouloff-Ros, V.; Maynadier, X.; Ajlil, Y.; Carton, M.; Yacoub, W.; Saffroy, R.; Figarella-Branger, D.; et al. Pediatric spinal pilocytic astrocytomas form a distinct epigenetic subclass from pilocytic astrocytomas of other locations and diffuse leptomeningeal glioneuronal tumours. Acta Neuropathol. 2023, 145, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Blackham, K.A.; Passalacqua, M.A.; Sandhu, G.S.; Gilkeson, R.C.; Griswold, M.A.; Gulani, V. Applications of Time-Resolved MR Angiography. Am. J. Roentgenol. 2011, 196, W613–W620. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.J.L.; Tey, M.L.; Seow, W.T.; Low, D.C.Y.; Chang, K.T.E.; Ng, L.P.; Looi, W.S.; Wong, R.X.; Tan, E.E.K.; Low, S.Y.Y. Intraoperative Fluorescein Sodium in Pediatric Neurosurgery: A Preliminary Case Series from a Singapore Children’s Hospital. NeuroSci 2023, 4, 54–64. [Google Scholar] [CrossRef]
- Ryall, S.; Tabori, U.; Hawkins, C. Pediatric low-grade glioma in the era of molecular diagnostics. Acta Neuropathol. Commun. 2020, 8, 30. [Google Scholar] [CrossRef]
- Fontebasso, A.M.; Shirinian, M.; Khuong-Quang, D.A.; Bechet, D.; Gayden, T.; Kool, M.; De Jay, N.; Jacob, K.; Gerges, N.; Hutter, B.; et al. Non-random aneuploidy specifies subgroups of pilocytic astrocytoma and correlates with older age. Oncotarget 2015, 6, 31844–31856. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Sill, M.; Schrimpf, D.; Patel, A.; Sturm, D.; Jäger, N.; Sievers, P.; Schweizer, L.; Banan, R.; Reuss, D.; Suwala, A.; et al. Advancing CNS tumor diagnostics with expanded DNA methylation-based classification. medRxiv 2025. [Google Scholar] [CrossRef]
- AlRayahi, J.; Alwalid, O.; Mubarak, W.; Maaz, A.U.R.; Mifsud, W. Pediatric Brain Tumors in the Molecular Era: Updates for the Radiologist. Semin. Roentgenol. 2023, 58, 47–66. [Google Scholar] [CrossRef]
- Wilson, P.E.; Oleszek, J.L.; Clayton, G.H. Pediatric spinal cord tumors and masses. J. Spinal Cord. Med. 2007, 30 (Suppl. S1), S15–S20. [Google Scholar] [CrossRef]
- Shweikeh, F.; Quinsey, C.; Murayi, R.; Randle, R.; Nuño, M.; Krieger, M.D.; Patrick Johnson, J. Treatment patterns of children with spine and spinal cord tumors: National outcomes and review of the literature. Childs Nerv. Syst. 2017, 33, 1357–1365. [Google Scholar] [CrossRef]
- Tobin, M.K.; Geraghty, J.R.; Engelhard, H.H.; Linninger, A.A.; Mehta, A.I. Intramedullary spinal cord tumors: A review of current and future treatment strategies. Neurosurg. Focus FOC 2015, 39, E14. [Google Scholar] [CrossRef]
- Kalayci, M.; Cagavi, F.; Gül, Ş.; Yenidünya, S.; Açikgöz, B. Intramedullary spinal cord metastases: Diagnosis and treatment—An illustrated review. Acta Neurochir. 2005, 146, 1347–1354, discussion 1354. [Google Scholar] [CrossRef]
- Catran, A.J.; Jalil, M.F.A. Haemorrhagic intramedullary spinal cord lesion. J. Clin. Neurosci. 2018, 48, 85. [Google Scholar] [CrossRef]
- Kaufman, B.A.; Park, T.S. Congenital spinal cord astrocytomas. Child’s Nerv. Syst. 1992, 8, 389–393. [Google Scholar] [CrossRef]
- Gutierrez, F.; Oi, S.; McLone, D. Intraspinal tumors in children: Clinical review, surgical results and follow-up in 51 cases. Concepts Pediatr. Neurosurg. 1983, 4, 1983-405. [Google Scholar]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, 5th edition: Central Nervous System Tumours, 5th ed.; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2021; p. 568. [Google Scholar]
- Collins, V.P.; Jones, D.T.; Giannini, C. Pilocytic astrocytoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Hutter, B.; Jäger, N.; Korshunov, A.; Kool, M.; Warnatz, H.J.; Zichner, T.; Lambert, S.R.; Ryzhova, M.; Quang, D.A.; et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat. Genet. 2013, 45, 927–932. [Google Scholar] [CrossRef]
- Stepien, N.; Mayr, L.; Schmook, M.T.; Raimann, A.; Dorfer, C.; Peyrl, A.; Azizi, A.A.; Schramm, K.; Haberler, C.; Gojo, J. Feasibility and antitumour activity of the FGFR inhibitor erdafitnib in three paediatric CNS tumour patients. Pediatr. Blood Cancer 2024, 71, e30836. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Conklin, H.M.; Huang, S.; Srivastava, D.; Sanford, R.; Ellison, D.W.; Merchant, T.E.; Hudson, M.M.; Hoehn, M.E.; Robison, L.L.; et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011, 13, 223–234. [Google Scholar] [CrossRef]
- Bandopadhayay, P.; Bergthold, G.; London, W.B.; Goumnerova, L.C.; Morales La Madrid, A.; Marcus, K.J.; Guo, D.; Ullrich, N.J.; Robison, N.J.; Chi, S.N.; et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer 2014, 61, 1173–1179. [Google Scholar] [CrossRef]
- Upadhyaya, S.A.; Ghazwani, Y.; Wu, S.; Broniscer, A.; Boop, F.A.; Gajjar, A.; Qaddoumi, I. Mortality in children with low-grade glioma or glioneuronal tumors: A single-institution study. Pediatr. Blood Cancer 2018, 65, e26717. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef]
- Bale, T.A. FGFR- gene family alterations in low-grade neuroepithelial tumors. Acta Neuropathol. Commun. 2020, 8, 21. [Google Scholar] [CrossRef]
- Yvone, G.M.; Breunig, J.J. Pediatric low-grade glioma models: Advances and ongoing challenges. Front. Oncol. 2024, 13, 1346949. [Google Scholar] [CrossRef]
- Ryall, S.; Zapotocky, M.; Fukuoka, K.; Nobre, L.; Guerreiro Stucklin, A.; Bennett, J.; Siddaway, R.; Li, C.; Pajovic, S.; Arnoldo, A.; et al. Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas. Cancer Cell 2020, 37, 569–583.e565. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M. FGFs, heparan sulfate and FGFRs: Complex interactions essential for development. BioEssays 2000, 22, 108–112. [Google Scholar] [CrossRef]
- Turner, N.; Grose, R. Fibroblast growth factor signalling: From development to cancer. Nat. Rev. Cancer 2010, 10, 116–129. [Google Scholar] [CrossRef]
- Du, S.; Zhang, Y.; Xu, J. Current progress in cancer treatment by targeting FGFR signaling. Cancer Biol. Med. 2023, 20, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Helsten, T.; Elkin, S.; Arthur, E.; Tomson, B.N.; Carter, J.; Kurzrock, R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin. Cancer Res. 2016, 22, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Friesel, R.E.; Maciag, T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal transduction. FASEB J. 1995, 9, 919–925. [Google Scholar] [CrossRef]
- Lazo De La Vega, L.; Comeau, H.; Sallan, S.; Al-Ibraheemi, A.; Gupta, H.; Li, Y.Y.; Tsai, H.K.; Kang, W.; Ward, A.; Church, A.J.; et al. Rare FGFR Oncogenic Alterations in Sequenced Pediatric Solid and Brain Tumors Suggest FGFR Is a Relevant Molecular Target in Childhood Cancer. JCO Precis. Oncol. 2022, 6, e2200390. [Google Scholar] [CrossRef]
- Morin, E.; Apfelbaum, A.A.; Sturm, D.; Ayoub, G.; DiGiacomo, J.; Bahadur, S.; Chandarana, B.; Power, P.C.; Cusick, M.M.; Novikov, D.; et al. A diverse landscape of FGFR alterations and co-mutations defines novel therapeutic strategies in pediatric low-grade gliomas. bioRxiv 2024, 2024.08.27.609922. [Google Scholar] [CrossRef]
- Louis, D.N.; Aldape, K.D.; Capper, D.; Gianni, C.; Horbinski, C.M.; Ng, H.K.; Perry, A.; Reifenberger, G.; Sarkar, C.; Soffietti, R.; et al. Glioblastoma, IDH-wildtype. In Central Nervous System Tumours, 5th ed.; WHO Classification of Tumours Series; WHO Classification of Tumours Editorial Board, Ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6. [Google Scholar]
- Gonzalez-Vega, M.; Lebert, B.M.; Campion, S.; Wagner, A.; Aguilar-Bonilla, A.; Smith, A.A. Fibroblast growth factor receptor 1 gene mutation as a potential risk factor for spontaneous intracranial hemorrhage in pediatric low-grade glioma patients. Neurooncol Adv. 2024, 6, vdae074. [Google Scholar] [CrossRef] [PubMed]
- Campion, S.; Aguilar-Bonilla, A.E.; Elbabaa, S.; Smith, A.A. LGG-31. Pediatric low-grade gliomas with FGFR1 mutations and spontaneous hemorrhage: Case series. Neuro-Oncology 2022, 24, i95. [Google Scholar] [CrossRef]
- Ishi, Y.; Yamaguchi, S.; Hatanaka, K.C.; Okamoto, M.; Motegi, H.; Kobayashi, H.; Terasaka, S.; Houkin, K. Association of the FGFR1 mutation with spontaneous hemorrhage in low-grade gliomas in pediatric and young adult patients. J. Neurosurg. 2021, 134, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.P.; Svrckova, P.; Cowan, F.; Chong, W.K.; Mankad, K. Intracranial hemorrhage in neonates: A review of etiologies, patterns and predicted clinical outcomes. Eur. J. Paediatr. Neurol. 2018, 22, 690–717. [Google Scholar] [CrossRef]
- Lee, C.; Chen, R.; Sun, G.; Liu, X.; Lin, X.; He, C.; Xing, L.; Liu, L.; Jensen, L.D.; Kumar, A.; et al. VEGF-B prevents excessive angiogenesis by inhibiting FGF2/FGFR1 pathway. Signal Transduct. Target. Ther. 2023, 8, 305. [Google Scholar] [CrossRef]
- Jones, D.T.; Kocialkowski, S.; Liu, L.; Pearson, D.M.; Bäcklund, L.M.; Ichimura, K.; Collins, V.P. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008, 68, 8673–8677. [Google Scholar] [CrossRef]
- Lassaletta, A.; Zapotocky, M.; Bouffet, E. Chemotherapy in pediatric low-grade gliomas (PLGG). Childs Nerv. Syst. 2024, 40, 3229–3239. [Google Scholar] [CrossRef]
- Nguyen, T.; Mueller, S.; Malbari, F. Review: Neurological Complications From Therapies for Pediatric Brain Tumors. Front. Oncol. 2022, 12, 853034. [Google Scholar] [CrossRef]
- Sturm, D.; Capper, D.; Andreiuolo, F.; Gessi, M.; Kölsche, C.; Reinhardt, A.; Sievers, P.; Wefers, A.K.; Ebrahimi, A.; Suwala, A.K.; et al. Multiomic neuropathology improves diagnostic accuracy in pediatric neuro-oncology. Nat. Med. 2023, 29, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yue, L.; Leng, Q.; Chang, C.; Gan, C.; Ye, T.; Cao, D. Targeting FGFR for cancer therapy. J. Hematol. Oncol. 2024, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Kommalapati, A.; Tella, S.H.; Borad, M.; Javle, M.; Mahipal, A. FGFR Inhibitors in Oncology: Insight on the Management of Toxicities in Clinical Practice. Cancers 2021, 13, 2968. [Google Scholar] [CrossRef]
- Sahm, F.; Brandner, S.; Bertero, L.; Capper, D.; French, P.J.; Figarella-Branger, D.; Giangaspero, F.; Haberler, C.; Hegi, M.E.; Kristensen, B.W.; et al. Molecular diagnostic tools for the World Health Organization (WHO) 2021 classification of gliomas, glioneuronal and neuronal tumors; an EANO guideline. Neuro Oncol. 2023, 25, 1731–1749. [Google Scholar] [CrossRef]
- Jagasia, S.; Tasci, E.; Zhuge, Y.; Camphausen, K.; Krauze, A.V. Cost Matrix of Molecular Pathology in Glioma-Towards AI-Driven Rational Molecular Testing and Precision Care for the Future. Biomedicines 2022, 10, 3029. [Google Scholar] [CrossRef]
| Year/Authors | Patient Number | Age (Years) | Tumor Location | Molecular Alteration |
|---|---|---|---|---|
| 2021/Ishi et al. [48] | 4 | 2 to 19 | 3 hypothalamus and 1 thalamus | 1 FGFR1 p.K656E, 2 FGFR1 p.K656E + p.D652G, and 1 FGFR1 p.N546K |
| 2022/Campion et al. [47] | 4 | Not available | 1 brainstem, 1 optic pathway, and 2 suprasellar | All FGFR1 p.N546K |
| 2024/Gonzalez-Vega et al. [46] | 5 | 1.4 to 17 | 1 hypothalamus, 3 optic pathway, and 1 suprasellar | 4 FGFR1 p.N546K and 1 FGFR1 Dup.Exon9-18 |
| 2025/our case | 1 | 17 | Intramedullary thoracic spine | FGFR1 p.K656E and V561M |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aw, S.J.; Goh, J.Y.; Esguerra, J.M.; Tan, T.S.E.; Tan, E.E.K.; Low, S.Y.Y. An FGFR1-Altered Intramedullary Thoracic Tumor with Unusual Clinicopathological Features: A Case Report and Literature Review. Neuroglia 2025, 6, 39. https://doi.org/10.3390/neuroglia6040039
Aw SJ, Goh JY, Esguerra JM, Tan TSE, Tan EEK, Low SYY. An FGFR1-Altered Intramedullary Thoracic Tumor with Unusual Clinicopathological Features: A Case Report and Literature Review. Neuroglia. 2025; 6(4):39. https://doi.org/10.3390/neuroglia6040039
Chicago/Turabian StyleAw, Sze Jet, Jian Yuan Goh, Jonis M. Esguerra, Timothy S. E. Tan, Enrica E. K. Tan, and Sharon Y. Y. Low. 2025. "An FGFR1-Altered Intramedullary Thoracic Tumor with Unusual Clinicopathological Features: A Case Report and Literature Review" Neuroglia 6, no. 4: 39. https://doi.org/10.3390/neuroglia6040039
APA StyleAw, S. J., Goh, J. Y., Esguerra, J. M., Tan, T. S. E., Tan, E. E. K., & Low, S. Y. Y. (2025). An FGFR1-Altered Intramedullary Thoracic Tumor with Unusual Clinicopathological Features: A Case Report and Literature Review. Neuroglia, 6(4), 39. https://doi.org/10.3390/neuroglia6040039






