The Dual Role of Astrocytes in CNS Homeostasis and Dysfunction
Abstract
1. Introduction
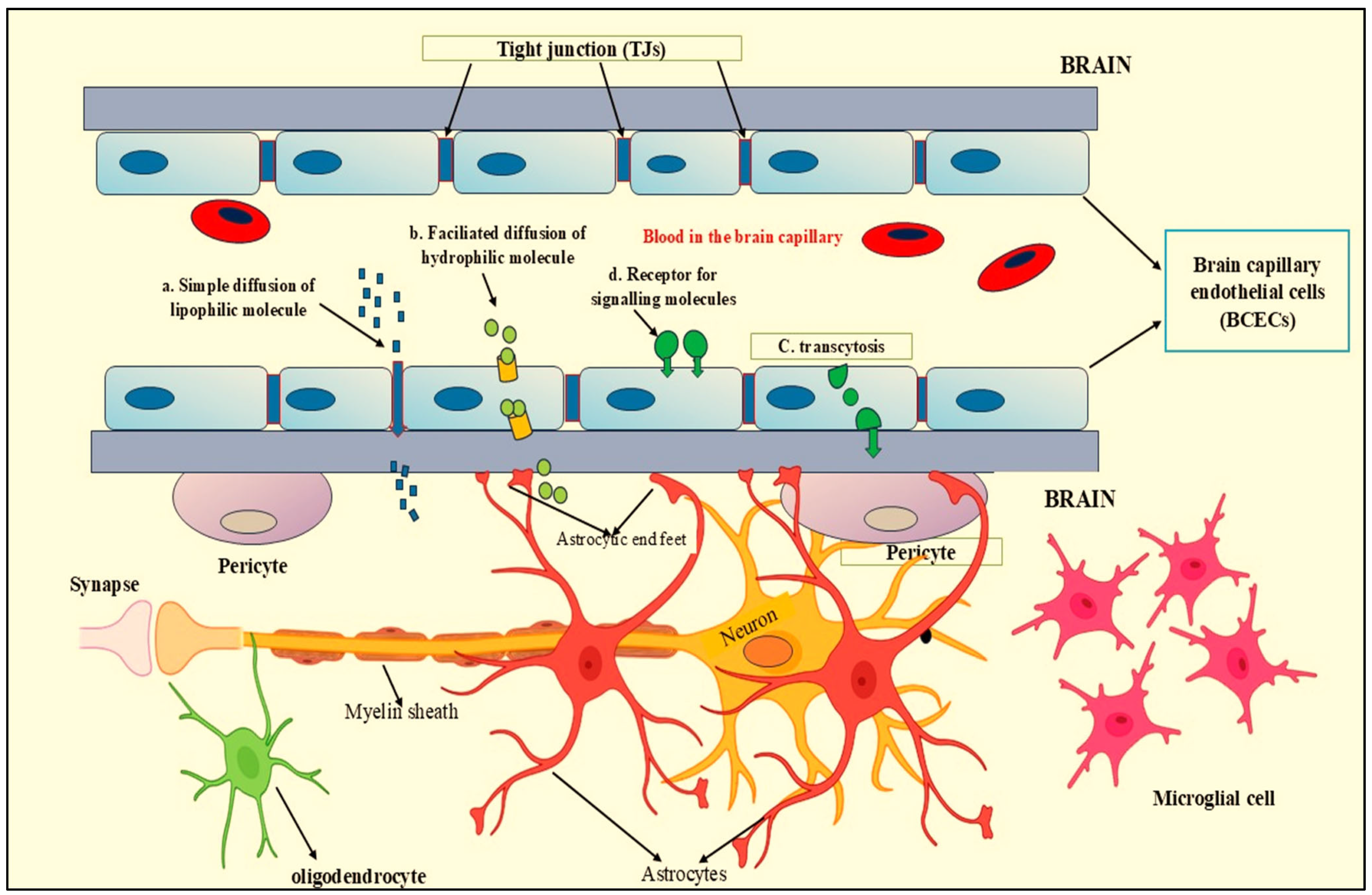
2. The Role of AQP4 in Maintaining Water Homeostasis Within the Brain
Astrocyte–Microglia Interaction
3. Role of Astrocyte in the Central Nervous System
3.1. Astrocyte Function in Healthy CNS
Astrocytes Are Multifunctional Glial Cells That Play Key Roles in Maintaining CNS Homeostasis
- Interaction with Synapse
- Uptake:
- ➢
- Ions like K+ and H2O
- ➢
- Neurotransmitters: Glutamate, GABA, Glycine, D-serine
- Release:
- ➢
- Energy substrates (e.g., lactate)
- ➢
- Transmitter precursors (e.g., glutamine)
- ➢
- Neurotransmitters (e.g., glutamate)
- ➢
- Purines (ATP, adenosine)
- ➢
- Growth factors (e.g., BDNF, TNF-α)
- ➢
- Neurosteroids and other signalling molecules
- Interaction with the Node of Ranvier
- Provides energy substrates
- Other potential, less understood supportive functions
- Interaction with Blood Vessels
- Uptake:
- ➢
- Glucose and H2O from blood
- Release:
- ➢
- Vasoactive agents:
- ▪
- PGE (Prostaglandin E), NO (Nitric Oxide)—cause dilation
- ▪
- AA (Arachidonic Acid)—causes contraction
- Gap Junction Communication
- Transfers ions like K+ and Ca2+ between astrocytes for buffering and signalling [51].
3.2. Astrocytes and BBB Formation and Maintenance
3.3. Astrocytes Control the Formation of Functional Synapses
3.4. Astrocyte in Sleep
4. Role of Astrocytes in Diseased Conditions
4.1. Role of Astrocytes in Parkinson’s Disease
4.2. Role of Astrocytes in Alzheimer’s Disease
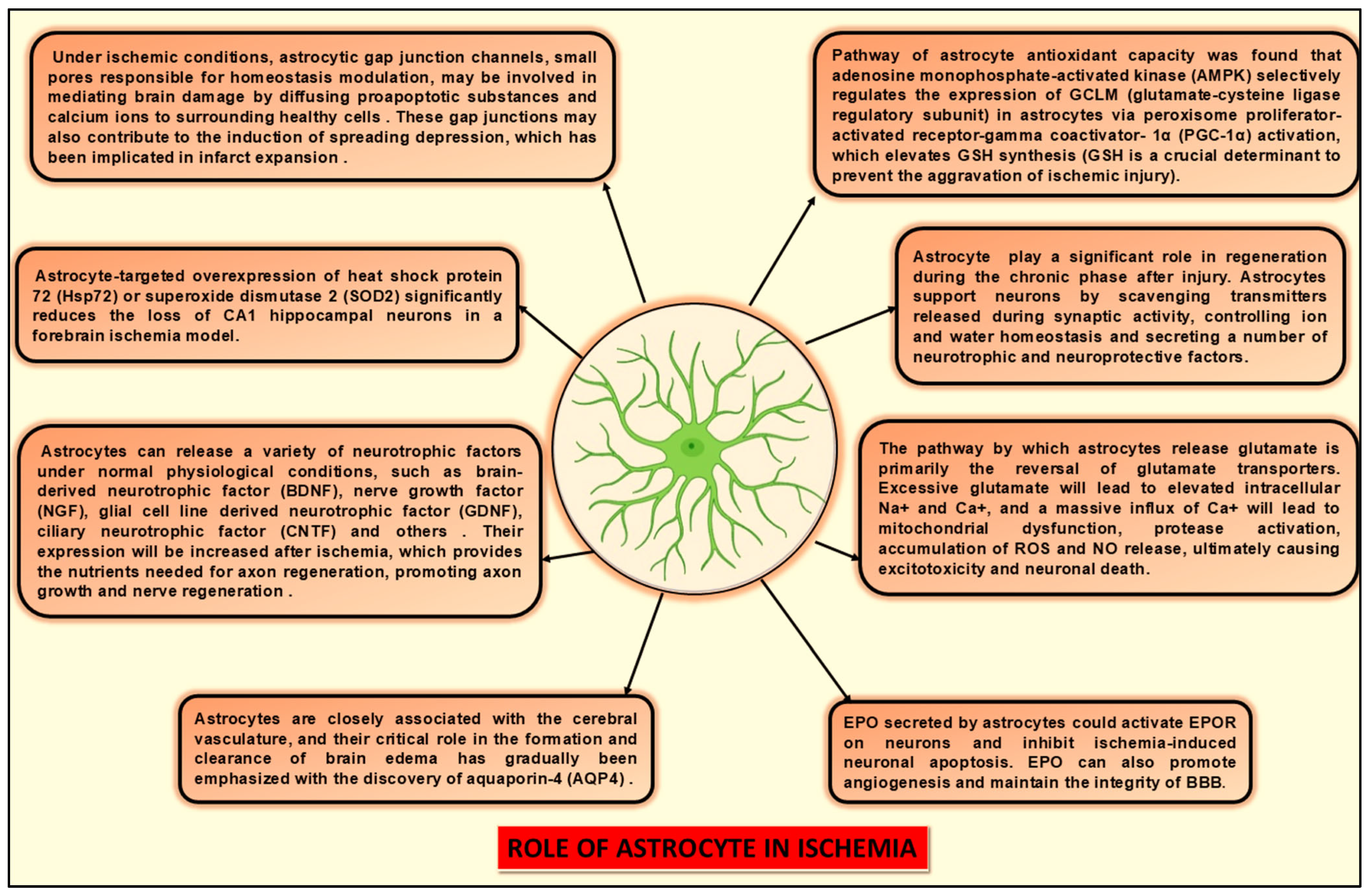
4.3. Role of Astrocytes in Ischemia
4.4. Astrocyte in Multiple Sclerosis
4.5. Astrocyte in Amyotrophic Lateral Sclerosis (ALS)
4.6. Astrocyte in Epilepsy
5. Key Signalling Pathways and Molecular Target
5.1. Glucagon-like Peptide 1 (GLP-1)
5.2. αB-Crystallin
5.3. Toll-like Receptor (TLR)3
5.4. Triggering Receptor Expressed on Myeloid Cells 2 (TREM2)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| AA | Arachidonic Acid |
| AD | Alzheimer’s disease |
| AGEs | Advanced glycation end-products |
| ALS | Amyotrophic lateral sclerosis |
| AMPA | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate |
| ApoE | Apolipoprotein E |
| APP | Amyloid precursor proteins |
| AQP4 | Aquaporin 4 |
| ARE | Antioxidant response element |
| Atg7 | Autophagy–related 7 gene |
| BBB | Blood–brain barrier |
| BCECs | Brain capillary endothelial cells |
| C1q | Conserved protein domain |
| CLN5 | Claudin-5 |
| CNS | Central nervous system |
| COMT | Catechol-O-methyl transferase |
| CSF | Cerebrospinal fluid |
| Cx43 | Connexin43 |
| DsRNA | Double-stranded RNA |
| EAE | Experimental autoimmune encephalomyelitis |
| ECM | Extracellular matrix |
| GFAP | Glial fibrillary acidic protein |
| GLP-1 | Glucagon-like peptide 1 |
| GPx | Glutathione peroxidase |
| GS | Glymphatic system |
| HIF1 | Hypoxia inducible factor-1 |
| HMGB1 | High mobility group box 1 |
| Hsp72 | Heat shock protein 72 |
| IFN | Interferon |
| IL-1α | Interleukin -1 alpha |
| iNOS | Inducible nitric oxide synthase |
| IRF3 | Interferon regulatory factor 3 |
| ISF | Interstitial fluid |
| LIF | Leukaemia inhibitory factor |
| LPS | Lipopolysaccharide |
| MANF | Mesencephalic astrocyte-derived neurotrophic factor |
| MAO-B | Monoamine oxidase-B |
| MAPKs | Mitogen-activated protein kinases |
| miRNA-146a | microRNA-146a. |
| MMPs | Matrix metalloproteinases |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| mRNA | Messenger RNA |
| MS | Multiple sclerosis |
| MyD88 | Myeloid differentiation primary-response protein 88 |
| NF-κβ | Nuclear factor κβ |
| NGF | Nerve growth factor |
| NO | Nitric Oxide |
| NT-4 | Neurotrophin-4 |
| NVU | Neurovascular unit |
| OAPs | Orthogonal arrays of particles |
| PD | Parkinson’s disease |
| PGE | Prostaglandin E |
| RAGE | Receptor for advanced glycation end products |
| ROS | Reactive oxygen species |
| S100B | S100 calcium-binding protein B |
| SOD2 | Superoxide dismutase 2 |
| SPs | Senile plaques |
| TJs | Tight junctions |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TRAF | TNF receptor-associated factor |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| TSP | Thrombospondins |
| VEGF-A | Vascular endothelial growth factor A |
References
- Verkhratsky, A.; Zorec, R.; Parpura, V. Stratification of astrocytes in healthy and diseased brain. Brain Pathol. 2017, 27, 629–644. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef] [PubMed]
- Stanca, S.; Rossetti, M.; Bongioanni, P. Astrocytes as Neuroimmunocytes in Alzheimer’s Disease: A Biochemical Tool in the Neuron–Glia Crosstalk along the Pathogenetic Pathways. Int. J. Mol. Sci. 2023, 24, 13880. [Google Scholar] [CrossRef] [PubMed]
- Batiuk, M.Y.; Martirosyan, A.; Wahis, J.; de Vin, F.; Marneffe, C.; Kusserow, C.; Koeppen, J.; Viana, J.F.; Oliveira, J.F.; Voet, T.; et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020, 11, 1220. [Google Scholar] [CrossRef]
- Cerrato, V.; Turrini, G.; Vitali, I.; Xiong, B.; Solanelles-Farré, L.; Lopes, A.; Magrinelli, E.; Bocchi, R.; Götz, M.; Fischer-Sternjak, J.; et al. A single-cell transcriptomic atlas maps cerebellar astrocyte diversity and uncovers the transcriptional code underlying their maturation trajectories. Cosmetics 2025. [Google Scholar] [CrossRef]
- Cha, J.; Zeng, P.; Zong, H.; Zhao, J.; Chen, J.; Zuo, H.; Zhang, B.; Shi, C.; Li, J.; Hua, Q.; et al. Single-cell RNA sequencing of neonatal cortical astrocytes reveals versatile cell clusters during astrocyte-neuron conversion. Mol. Biol. Rep. 2025, 52, 189. [Google Scholar] [CrossRef]
- Khakh, B.S.; Sofroniew, M.V. Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 2015, 18, 942–952. [Google Scholar] [CrossRef]
- Khodadadei, F.; Arshad, R.; Morales, D.M.; Gluski, J.; Marupudi, N.I.; McAllister, J.P.; Limbrick, D.D.; Harris, C.A. The effect of A1 and A2 reactive astrocyte expression on hydrocephalus shunt failure. Fluids Barriers CNS 2022, 19, 78. [Google Scholar] [CrossRef]
- Ding, Z.-B.; Song, L.-J.; Wang, Q.; Kumar, G.; Yan, Y.-Q.; Ma, C.-G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Liddelow, S.A.; Chakraborty, C.; Münch, A.E.; Heiman, M.; Barres, B.A. Normal aging induces A1-like astrocyte reactivity. Proc. Natl. Acad. Sci. USA 2018, 115, E1896–E1905. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Rennels, M.L.; Gregory, T.F.; Blaumanis, O.R.; Fujimoto, K.; Grady, P.A. Evidence for a ‘Paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985, 326, 47–63. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Maugeri, R.; Schiera, G.; Di Liegro, C.; Fricano, A.; Iacopino, D.; Di Liegro, I. Aquaporins and Brain Tumors. Int. J. Mol. Sci. 2016, 17, 1029. [Google Scholar] [CrossRef]
- Díaz-Castro, B.; Robel, S.; Mishra, A. Astrocyte Endfeet in Brain Function and Pathology: Open Questions. Annu. Rev. Neurosci. 2023, 46, 101–121. [Google Scholar] [CrossRef]
- Bloch, O.; Manley, G.T. The role of aquaporin-4 in cerebral water transport and edema. Neurosurg. Focus 2007, 22, 1–7. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Shen, Y.; Zhu, W.; Wang, Y.; Wang, J.; Zhang, N.; Li, C.; Xie, L.; Wu, Q. Moxibustion improves hypothalamus Aqp4 polarization in APP/PS1 mice: Evidence from spatial transcriptomics. Front. Aging Neurosci. 2023, 15, 1069155. [Google Scholar] [CrossRef]
- Mueller, S.M.; McFarland White, K.; Fass, S.B.; Chen, S.; Shi, Z.; Ge, X.; Engelbach, J.A.; Gaines, S.H.; Bice, A.R.; Vasek, M.J.; et al. Evaluation of gliovascular functions of AQP4 readthrough isoforms. Front. Cell. Neurosci. 2023, 17, 1272391. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Mizushima, N.; Kiyooka, M.; Ohsumi, M.; Ueno, T.; Ohsumi, Y.; Kominami, E. Apg7p/Cvt2p: A Novel Protein-activating Enzyme Essential for Autophagy. Mol. Biol. Cell 1999, 10, 1367–1379. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, J.-Y.; Li, Y.; Ban, M.; Sun, Q.; Wang, H.-J.; Zhao, D.; Tong, P.-G.; Wang, L.; Wang, K.-J.; et al. Endothelial depletion of Atg7 triggers astrocyte–microvascular disassociation at blood–brain barrier. J. Cell Biol. 2023, 222, e202103098. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Kelly, J.R.; Morales, J.E.; Sun, R.C.; De, A.; Burkin, D.J.; McCarty, J.H. The alpha7 integrin subunit in astrocytes promotes endothelial blood–brain barrier integrity. Development 2023, 150, dev201356. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric Glia Regulate Intestinal Barrier Function and Inflammation Via Release of S-Nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Verkman, A.S.; Phuan, P.; Asavapanumas, N.; Tradtrantip, L. Biology of AQP4 and Anti-AQP4 Antibody: Therapeutic Implications for NMO. Brain Pathol. 2013, 23, 684–695. [Google Scholar] [CrossRef]
- Tradtrantip, L.; Zhang, H.; Saadoun, S.; Phuan, P.; Lam, C.; Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Anti–Aquaporin-4 monoclonal antibody blocker therapy for neuromyelitis optica. Ann. Neurol. 2012, 71, 314–322. [Google Scholar] [CrossRef]
- Duan, T.; Tradtrantip, L.; Phuan, P.-W.; Bennett, J.L.; Verkman, A.S. Affinity-matured ‘aquaporumab’ anti-aquaporin-4 antibody for therapy of seropositive neuromyelitis optica spectrum disorders. Neuropharmacology 2020, 162, 107827. [Google Scholar] [CrossRef]
- Verkman, A.S.; Smith, A.J.; Phuan, P.; Tradtrantip, L.; Anderson, M.O. The aquaporin-4 water channel as a potential drug target in neurological disorders. Expert Opin. Ther. Targets 2017, 21, 1161–1170. [Google Scholar] [CrossRef]
- Sun, M.; You, H.; Hu, X.; Luo, Y.; Zhang, Z.; Song, Y.; An, J.; Lu, H. Microglia–Astrocyte Interaction in Neural Development and Neural Pathogenesis. Cells 2023, 12, 1942. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Bao, J.; Bai, Q.; Wang, G. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Chen, J.; Xu, S.; Wang, L.; Liu, X.; Liu, G.; Tan, Q.; Li, W.; Zhang, S.; Du, Y. Refining the interactions between microglia and astrocytes in Alzheimer’s disease pathology. Neuroscience 2025, 573, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cai, X.; Shi, B.; Mo, Y.; Zhang, J.; Luo, W.; Yu, B.; Li, X. Mechanisms and Therapeutic Prospects of Microglia-Astrocyte Interactions in Neuropathic Pain Following Spinal Cord Injury. Mol. Neurobiol. 2025, 62, 4654–4676. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.; Gharagozloo, M.; Simard, C.; Gris, D. Astrocytes maintain glutamate homeostasis in the cns by controlling the balance between glutamate uptake and release. Cells 2019, 8, 184. [Google Scholar] [CrossRef] [PubMed]
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437. [Google Scholar] [CrossRef]
- Farmer, W.T.; Murai, K. Resolving astrocyte heterogeneity in the CNS. Front. Cell. Neurosci. 2017, 11, 300. [Google Scholar] [CrossRef]
- Benveniste, E.N. Cytokine Actions in the Central Nervous System. Cytokine Growth Factor Rev. 1998, 9, 259–275. [Google Scholar] [CrossRef]
- Haydon, P.G. Neuroglial networks: Neurons and glia talk to each other. Curr. Biol. 2000, 10, R712–R714. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Bush, T.G.; Blumauer, N.; Kruger, L.; Mucke, L.; Johnson, M.H. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res. 1999, 835, 91–95. [Google Scholar] [CrossRef]
- Bush, T.G.; Puvanachandra, N.; Horner, C.H.; Polito, A.; Ostenfeld, T.; Svendsen, C.N.; Mucke, L.; Johnson, M.H.; Sofroniew, M. V Leukocyte Infiltration, Neuronal Degeneration, and Neurite Outgrowth after Ablation of Scar-Forming, Reactive Astrocytes in Adult Transgenic Mice. Neuron 1999, 23, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Endo, F.; Kasai, A.; Soto, J.S.; Yu, X.; Qu, Z.; Hashimoto, H.; Gradinaru, V.; Kawaguchi, R.; Khakh, B.S. Molecular basis of astrocyte diversity and morphology across the CNS in health and disease. Science 2022, 378, eadc9020. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.A.; Jaronen, M.; Covacu, R.; Zandee, S.E.J.; Scalisi, G.; Rothhammer, V.; Tjon, E.C.; Chao, C.C.; Kenison, J.E.; Blain, M.; et al. Environmental Control of Astrocyte Pathogenic Activities in CNS Inflammation. Cell 2019, 176, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.L.; Staddon, J.M. The cell biology of the blood-brain barrier. Annu. Rev. Neurosci. 1999, 22, 11–28. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Moulson, A.J.; Squair, J.W.; Franklin, R.J.M.; Tetzlaff, W.; Assinck, P. Diversity of Reactive Astrogliosis in CNS Pathology: Heterogeneity or Plasticity? Front. Cell. Neurosci. 2021, 15, 703810. [Google Scholar] [CrossRef]
- Mi, H.; Haeberle, H.; Barres, B.A. Induction of Astrocyte Differentiation by Endothelial Cells. J. Neurosci. 2001, 21, 1538–1547. [Google Scholar] [CrossRef]
- Das, S.; Li, Z.; Noori, A.; Hyman, B.T.; Serrano-Pozo, A. Meta-analysis of mouse transcriptomic studies supports a context-dependent astrocyte reaction in acute CNS injury versus neurodegeneration. J. Neuroinflammation 2020, 17, 227. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Saviuk, M.; Vedunova, M.V. Necroptosis in CNS diseases: Focus on astrocytes. Front. Aging Neurosci. 2023, 14, 1016053. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Farahani, R.; Pina-Benabou, M.H.; Kyrozis, A.; Siddiq, A.; Barradas, P.C.; Chiu, F.C.; Cavalcante, L.A.; Lai, J.C.K.; Stanton, P.K.; Rozental, R. Alterations in metabolism and gap junction expression may determine the role of astrocytes as “good Samaritans” or executioners. Glia 2005, 50, 351–361. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood–Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood–Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef]
- Zidarič, T.; Gradišnik, L.; Velnar, T. Astrocytes and human artificial blood-brain barrier models. Bosn. J. Basic Med. Sci. 2022, 22, 651. [Google Scholar] [CrossRef]
- Zamproni, L.N.; Gökçe, B.; Venckute Larsson, J.; Ceballos-Torres, A.; Gram, M.; Porcionatto, M.A.; Herland, A. Unraveling the influence of astrocytes on endothelial cell transcription: Towards understanding blood-brain barrier in vitro models’ dynamics. Brain Res. Bull. 2025, 224, 111328. [Google Scholar] [CrossRef]
- Ye, Q.; Jo, J.; Wang, C.-Y.; Oh, H.; Zhan, J.; Choy, T.J.; Kim, K.I.; D’Alessandro, A.; Reshetnyak, Y.K.; Jung, S.Y.; et al. Astrocytic Slc4a4 regulates blood-brain barrier integrity in healthy and stroke brains via a CCL2-CCR2 pathway and NO dysregulation. Cell Rep. 2024, 43, 114193. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Pivoriūnas, A. Astroglia support, regulate and reinforce brain barriers. Neurobiol. Dis. 2023, 179, 106054. [Google Scholar] [CrossRef] [PubMed]
- Toth, A.E.; Klepe, A.; Lipka, D.V.; Goldeman, C.; Brodin, B.; Nielsen, M.S. SorLA in astrocytes regulates blood-brain barrier integrity. Front. Drug Deliv. 2023, 2, 1082689. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.A.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef]
- Blanco-Suarez, E.; Liu, T.F.; Kopelevich, A.; Allen, N.J. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 2018, 100, 1116–1132. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflammation 2019, 16, 140. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Dossi, E.; Vasile, F.; Rouach, N. Human astrocytes in the diseased brain. Brain Res. Bull. 2018, 136, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Lyon, K.A.; Allen, N.J. From Synapses to Circuits, Astrocytes Regulate Behavior. Front. Neural Circuits 2022, 15, 786293. [Google Scholar] [CrossRef]
- Li, Y.; Que, M.; Wang, X.; Zhan, G.; Zhou, Z.; Luo, X.; Li, S. Exploring Astrocyte-Mediated Mechanisms in Sleep Disorders and Comorbidity. Biomedicines 2023, 11, 2476. [Google Scholar] [CrossRef]
- Que, M.; Li, Y.; Wang, X.; Zhan, G.; Luo, X.; Zhou, Z. Role of astrocytes in sleep deprivation: Accomplices, resisters, or bystanders? Front. Cell. Neurosci. 2023, 17, 1188306. [Google Scholar] [CrossRef]
- Sriram, S.; Carstens, K.; Dewing, W.; Fiacco, T.A. Astrocyte regulation of extracellular space parameters across the sleep-wake cycle. Front. Cell. Neurosci. 2024, 18, 1401698. [Google Scholar] [CrossRef]
- Haydon, P.G. Astrocytes and the modulation of sleep. Curr. Opin. Neurobiol. 2017, 44, 28–33. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Sieber, B.-A.; Manzino, L.; Sonsalla, P.K. Some features of the nigrostriatal dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse. Mol. Chem. Neuropathol. 1989, 10, 171–183. [Google Scholar] [CrossRef]
- Dervan, A.G.; Meshul, C.K.; Beales, M.; McBean, G.J.; Moore, C.; Totterdell, S.; Snyder, A.K.; Meredith, G.E. Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson’s disease. Exp. Neurol. 2004, 190, 145–156. [Google Scholar] [CrossRef]
- Mallajosyula, J.K.; Kaur, D.; Chinta, S.J.; Rajagopalan, S.; Rane, A.; Nicholls, D.G.; Di Monte, D.A.; Macarthur, H.; Andersen, J.K. MAO-B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS ONE 2008, 3, e1616. [Google Scholar] [CrossRef]
- Tong, J.; Ang, L.C.; Williams, B.; Furukawa, Y.; Fitzmaurice, P.; Guttman, M.; Boileau, I.; Hornykiewicz, O.; Kish, S.J. Low levels of astroglial markers in Parkinson’s disease: Relationship to α-synuclein accumulation. Neurobiol. Dis. 2015, 82, 243–253. [Google Scholar] [CrossRef]
- Mirza, B.; Hadberg, H.; Thomsen, P.; Moos, T. The absence of reactive astrocytosis is indicative of a unique inflammatory process in Parkinson’s disease. Neuroscience 1999, 95, 425–432. [Google Scholar] [CrossRef]
- Gu, X.-L.; Long, C.-X.; Sun, L.; Xie, C.; Lin, X.; Cai, H. Astrocytic expression of Parkinson’s disease-related A53T α-synuclein causes neurodegeneration in mice. Mol. Brain 2010, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Knott, C.; Stern, G.; Kingsbury, A.; Welcher, A.; Wilkin, G. Elevated glial brain-derived neurotrophic factor in Parkinson’s diseased nigra. Parkinsonism Relat. Disord. 2002, 8, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-F.H.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A Glial Cell Line-Derived Neurotrophic Factor for Midbrain Dopaminergic Neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef] [PubMed]
- Petrova, P.S.; Raibekas, A.; Pevsner, J.; Vigo, N.; Anafi, M.; Moore, M.K.; Peaire, A.E.; Shridhar, V.; Smith, D.I.; Kelly, J.; et al. MANF: A New Mesencephalic, Astrocyte-Derived Neurotrophic Factor with Selectivity for Dopaminergic Neurons. J. Mol. Neurosci. 2003, 20, 173–188. [Google Scholar] [CrossRef]
- Chen, P.-S.; Peng, G.-S.; Li, G.; Yang, S.; Wu, X.; Wang, C.-C.; Wilson, B.; Lu, R.-B.; Gean, P.-W.; Chuang, D.-M.; et al. Valproate protects dopaminergic neurons in midbrain neuron/glia cultures by stimulating the release of neurotrophic factors from astrocytes. Mol. Psychiatry 2006, 11, 1116–1125. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, L.; Wang, T.; Wei, S.-J.; Gao, H.-M.; Liu, J.; Wilson, B.; Liu, B.; Zhang, W.; Kim, H.-C.; et al. 3-Hydroxymorphinan is neurotrophic to dopaminergic neurons and is also neuroprotective against LPS-induced neurotoxicity. FASEB J. 2005, 19, 1–25. [Google Scholar] [CrossRef]
- Poli, G. ROS and Parkinson’s Disease: A View to a Kill. In Free Radicals in Brain Pathophysiology; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ishida, Y.; Nagai, A.; Kobayashi, S.; Kim, S.U. Upregulation of protease-activated receptor-1 in astrocytes in Parkinson disease: Astrocyte-mediated neuroprotection through increased levels of glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2006, 65, 66–77. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J. Bioenerg. Biomembr. 2009, 41, 517–521. [Google Scholar] [CrossRef]
- Lee, J.; Li, J.; Johnson, D.A.; Stein, T.D.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Johnson, J.A. Nrf2, a multi-organ protector? FASEB J. 2005, 19, 1061–1066. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Morton, M.R.; Pickett, C.B. The antioxidant responsive element. Activation by oxidative stress and identification of the DNA consensus sequence required for functional activity. J. Biol. Chem. 1991, 266, 11632–11639. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-C.; Vargas, M.R.; Pani, A.K.; Smeyne, R.J.; Johnson, D.A.; Kan, Y.W.; Johnson, J.A. Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc. Natl. Acad. Sci. USA 2009, 106, 2933–2938. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, R.; Fusco, R.; Cuzzocrea, S. Astrocytes: Role and functions in brain pathologies. Front. Pharmacol. 2019, 10, 1114. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Muzikansky, A.; Gómez-Isla, T.; Growdon, J.H.; Betensky, R.A.; Frosch, M.P.; Hyman, B.T. Differential relationships of reactive astrocytes and microglia to fibrillar amyloid deposits in alzheimer disease. J. Neuropathol. Exp. Neurol. 2013, 72, 462–471. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell 1993, 75, 1039–1042. [Google Scholar] [CrossRef]
- Pimplikar, S.W. Reassessing the amyloid cascade hypothesis of Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2009, 41, 1261–1268. [Google Scholar] [CrossRef]
- Yankner, B.A.; Duffy, L.K.; Kirschner, D.A. Neurotrophic and Neurotoxic Effects of Amyloid β Protein: Reversal by Tachykinin Neuropeptides. Science 1990, 250, 279–282. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Olabarria, M.; Chvatal, A.; Verkhratsky, A. Astroglia in dementia and Alzheimer’s disease. Cell Death Differ. 2009, 16, 378–385. [Google Scholar] [CrossRef]
- Nagele, R.G.; D’Andrea, M.R.; Lee, H.; Venkataraman, V.; Wang, H.-Y. Astrocytes accumulate Aβ42 and give rise to astrocytic amyloid plaques in Alzheimer disease brains. Brain Res. 2003, 971, 197–209. [Google Scholar] [CrossRef]
- Hatten, M.E.; Liem, R.K.H.; Shelanski, M.L.; Mason, C.A. Astroglia in CNS injury. Glia 1991, 4, 233–243. [Google Scholar] [CrossRef]
- Olabarria, M.; Noristani, H.N.; Verkhratsky, A.; Rodríguez, J.J. Concomitant astroglial atrophy and astrogliosis in a triple transgenic animal model of Alzheimer’s disease. Glia 2010, 58, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Wang, K.-C.; Tarnawski, M.; Lach, B. Microglial cells are the driving force in fibrillar plaque formation, whereas astrocytes are a leading factor in plaque degradation. Acta Neuropathol. 2000, 100, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Kurt, M.A.; Davies, D.C.; Kidd, M. β-Amyloid Immunoreactivity in Astrocytes in Alzheimer’s Disease Brain Biopsies: An Electron Microscope Study. Exp. Neurol. 1999, 158, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Wyss-Coray, T.; Loike, J.D.; Brionne, T.C.; Lu, E.; Anankov, R.; Yan, F.; Silverstein, S.C.; Husemann, J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 2003, 9, 453–457. [Google Scholar] [CrossRef]
- Koistinaho, M.; Lin, S.; Wu, X.; Esterman, M.; Koger, D.; Hanson, J.; Higgs, R.; Liu, F.; Malkani, S.; Bales, K.R.; et al. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-β peptides. Nat. Med. 2004, 10, 719–726. [Google Scholar] [CrossRef]
- Vincent, V.A.M.; Tilders, F.J.H.; Van Dam, A.-M. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: A role for transforming growth factor ? Glia 1997, 19, 190–198. [Google Scholar] [CrossRef]
- DeWitt, D.A.; Perry, G.; Cohen, M.; Doller, C.; Silver, J. Astrocytes Regulate Microglial Phagocytosis of Senile Plaque Cores of Alzheimer’s Disease. Exp. Neurol. 1998, 149, 329–340. [Google Scholar] [CrossRef]
- Johnstone, M.; Gearing, A.J.; Miller, K.M. A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced. J. Neuroimmunol. 1999, 93, 182–193. [Google Scholar] [CrossRef]
- Rossi, D.; Brambilla, L.; Valori, C.F.; Crugnola, A.; Giaccone, G.; Capobianco, R.; Mangieri, M.; Kingston, A.E.; Bloc, A.; Bezzi, P.; et al. Defective Tumor Necrosis Factor-α-dependent Control of Astrocyte Glutamate Release in a Transgenic Mouse Model of Alzheimer Disease. J. Biol. Chem. 2005, 280, 42088–42096. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abramov, A.Y.; Canevari, L.; Duchen, M.R. Calcium signals induced by amyloid β peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1742, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Gavillet, M.; Bélanger, M.; Laroche, T.; Viertl, D.; Lashuel, H.A.; Magistretti, P.J. Amyloid-β Aggregates Cause Alterations of Astrocytic Metabolic Phenotype: Impact on Neuronal Viability. J. Neurosci. 2010, 30, 3326–3338. [Google Scholar] [CrossRef] [PubMed]
- Petito, C.K.; Morgello, S.; Felix, J.C.; Lesser, M.L. The Two Patterns of Reactive Astrocytosis in Postischemic Rat Brain. J. Cereb. Blood Flow Metab. 1990, 10, 850–859. [Google Scholar] [CrossRef]
- Norenberg, M.D. The Astrocyte in Liver Disease. In Personality and Individual Differences; Elsevier: Amsterdam, The Netherlands, 1981; pp. 303–352. [Google Scholar]
- Anderson, M.F.; Blomstrand, F.; Blomstrand, C.; Eriksson, P.S.; Nilsson, M. Astrocytes and Stroke: Networking for Survival? Neurochem. Res. 2003, 28, 293–305. [Google Scholar] [CrossRef]
- Fawcett, J.W.; Asher, R. The glial scar and central nervous system repair. Brain Res. Bull. 1999, 49, 377–391. [Google Scholar] [CrossRef]
- Ayata, C.; Ropper, A.H. Ischaemic brain oedema. J. Clin. Neurosci. 2002, 9, 113–124. [Google Scholar] [CrossRef]
- Budd, S.L.; Lipton, S.A. Calcium tsunamis: Do astrocytes transmit cell death messages via gap junctions during ischemia? Nat. Neurosci. 1998, 1, 431–432. [Google Scholar] [CrossRef]
- Rouach, N.; Avignone, E.; Même, W.; Koulakoff, A.; Venance, L.; Blomstrand, F.; Giaume, C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell 2002, 94, 457–475. [Google Scholar] [CrossRef]
- Largo, C.; Cuevas, P.; Herreras, O. Is glia disfunction the initial cause of neuronal death in ischemic penumbra? Neurol. Res. 1996, 18, 445–448. [Google Scholar] [CrossRef]
- Saito, R.; Graf, R.; Hübel, K.; Fujita, T.; Rosner, G.; Heiss, W.-D. Reduction of Infarct Volume by Halothane: Effect on Cerebral Blood Flow or Perifocal Spreading Depression-Like Depolarizations. J. Cereb. Blood Flow Metab. 1997, 17, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.J.; MacVicar, B. Connexin and pannexin hemichannels of neurons and astrocytes. Channels 2008, 2, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Siushansian, R.; Bechberger, J.F.; Cechetto, D.F.; Hachinski, V.C.; Naus, C.C.G. Connexin43 null mutation increases infarct size after stroke. J. Comp. Neurol. 2001, 440, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Håberg, A.; Qu, H.; Sæther, O.; Unsgård, G.; Haraldseth, O.; Sonnewald, U. Differences in Neurotransmitter Synthesis and Intermediary Metabolism between Glutamatergic and GABAergic Neurons during 4 Hours of Middle Cerebral Artery Occlusion in the Rat: The Role of Astrocytes in Neuronal Survival. J. Cereb. Blood Flow Metab. 2001, 21, 1451–1463. [Google Scholar] [CrossRef]
- Dringen, R. Metabolism and functions of glutathione in brain. Prog. Neurobiol. 2000, 62, 649–671. [Google Scholar] [CrossRef]
- Mizui, T.; Kinouchi, H.; Chan, P.H. Depletion of brain glutathione by buthionine sulfoximine enhances cerebral ischemic injury in rats. Am. J. Physiol. Circ. Physiol. 1992, 262, H313–H317. [Google Scholar] [CrossRef]
- Iwata-Ichikawa, E.; Kondo, Y.; Miyazaki, I.; Asanuma, M.; Ogawa, N. Glial Cells Protect Neurons Against Oxidative Stress via Transcriptional Up-Regulation of the Glutathione Synthesis. J. Neurochem. 1999, 72, 2334–2344. [Google Scholar] [CrossRef]
- Xu, L.; Emery, J.F.; Ouyang, Y.; Voloboueva, L.A.; Giffard, R.G. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia 2010, 58, 1042–1049. [Google Scholar] [CrossRef]
- Brosnan, C.F.; Raine, C.S. The astrocyte in multiple sclerosis revisited. Glia 2013, 61, 453–465. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Argaw, A.T.; Asp, L.; Zhang, J.; Navrazhina, K.; Pham, T.; Mariani, J.N.; Mahase, S.; Dutta, D.J.; Seto, J.; Kramer, E.G.; et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J. Clin. Invest. 2012, 122, 2454–2468. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.; Piaton, G.; Lubetzki, C. Astrocytes—Friends or foes in multiple sclerosis? Glia 2007, 55, 1300–1312. [Google Scholar] [CrossRef] [PubMed]
- Pehar, M.; Harlan, B.A.; Killoy, K.M.; Vargas, M.R. Role and Therapeutic Potential of Astrocytes in Amyotrophic Lateral Sclerosis. Curr. Pharm. Des. 2018, 23, 5010–5021. [Google Scholar] [CrossRef] [PubMed]
- Benninger, F.; Glat, M.J.; Offen, D.; Steiner, I. Glial fibrillary acidic protein as a marker of astrocytic activation in the cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 2016, 26, 75–78. [Google Scholar] [CrossRef]
- Gomes, C.; Sequeira, C.; Barbosa, M.; Cunha, C.; Vaz, A.R.; Brites, D. Astrocyte regional diversity in ALS includes distinct aberrant phenotypes with common and causal pathological processes. Exp. Cell Res. 2020, 395, 112209. [Google Scholar] [CrossRef]
- Gomes, C.; Cunha, C.; Nascimento, F.; Ribeiro, J.A.; Vaz, A.R.; Brites, D. Cortical Neurotoxic Astrocytes with Early ALS Pathology and miR-146a Deficit Replicate Gliosis Markers of Symptomatic SOD1G93A Mouse Model. Mol. Neurobiol. 2019, 56, 2137–2158. [Google Scholar] [CrossRef]
- Cunha, C.; Santos, C.; Gomes, C.; Fernandes, A.; Correia, A.M.; Sebastião, A.M.; Vaz, A.R.; Brites, D. Downregulated Glia Interplay and Increased miRNA-155 as Promising Markers to Track ALS at an Early Stage. Mol. Neurobiol. 2017, 55, 4207–4224. [Google Scholar] [CrossRef]
- Bedner, P.; Dupper, A.; Hüttmann, K.; Müller, J.; Herde, M.K.; Dublin, P.; Deshpande, T.; Schramm, J.; Häussler, U.; Haas, C.A.; et al. Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 2015, 138, 1208–1222. [Google Scholar] [CrossRef]
- Coulter, D.A.; Steinhauser, C. Role of Astrocytes in Epilepsy. Cold Spring Harb. Perspect. Med. 2015, 5, a022434. [Google Scholar] [CrossRef]
- Çarçak, N.; Onat, F.; Sitnikova, E. Astrocytes as a target for therapeutic strategies in epilepsy: Current insights. Front. Mol. Neurosci. 2023, 16, 1183775. [Google Scholar] [CrossRef] [PubMed]
- Tsybina, Y.; Kastalskiy, I.; Kazantsev, V.B.; Hramov, A.E.; Gordleeva, S. Beyond the neuron: Unveiling the role of reactive astrocytes in epileptic seizure dynamics through self-organized bistability. Comput. Biol. Med. 2025, 197, 110980. [Google Scholar] [CrossRef] [PubMed]
- Hayatdavoudi, P.; Hosseini, M.; Hajali, V.; Hosseini, A.; Rajabian, A. The role of astrocytes in epileptic disorders. Physiol. Rep. 2022, 10, e15239. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Gonzalez-Reyes, R.E.; Rubiano, M.G. Astrocyte’s RAGE: More Than Just a Question of Mood. Cent. Nerv. Syst. Agents Med. Chem. 2018, 18, 39–48. [Google Scholar] [CrossRef]
- Li, L.; Acioglu, C.; Heary, R.F.; Elkabes, S. Role of astroglial toll-like receptors (TLRs) in central nervous system infections, injury and neurodegenerative diseases. Brain. Behav. Immun. 2021, 91, 740–755. [Google Scholar] [CrossRef]
- Dostal, C.R.; Carson Sulzer, M.; Kelley, K.W.; Freund, G.G.; McCusker, R.H. Glial and tissue-specific regulation of Kynurenine Pathway dioxygenases by acute stress of mice. Neurobiol. Stress 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Smith, D.G.; Kerr, S.J.; Smythe, G.A.; Kapoor, V.; Armati, P.J.; Brew, B.J. Characterisation of kynurenine pathway metabolism in human astrocytes and implications in neuropathogenesis. Redox Rep. 2000, 5, 108–111. [Google Scholar] [CrossRef]
- Guillemin, G.J.; Kerr, S.J.; Smythe, G.A.; Smith, D.G.; Kapoor, V.; Armati, P.J.; Croitoru, J.; Brew, B.J. Kynurenine pathway metabolism in human astrocytes: A paradox for neuronal protection. J. Neurochem. 2001, 78, 842–853. [Google Scholar] [CrossRef]
- Ting, K.K.; Brew, B.; Guillemin, G. The involvement of astrocytes and kynurenine pathway in Alzheimer’s disease. Neurotox. Res. 2007, 12, 247–262. [Google Scholar] [CrossRef]
- Bao, Y.; Jiang, L.; Chen, H.; Zou, J.; Liu, Z.; Shi, Y. The Neuroprotective Effect of Liraglutide is Mediated by Glucagon-Like Peptide 1 Receptor-Mediated Activation of cAMP/PKA/CREB Pathway. Cell. Physiol. Biochem. 2015, 36, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Iwai, T.; Ito, S.; Tanimitsu, K.; Udagawa, S.; Oka, J.-I. Glucagon-like peptide-1 inhibits LPS-induced IL-1β production in cultured rat astrocytes. Neurosci. Res. 2006, 55, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Bułdak, Ł.; Machnik, G.; Skudrzyk, E.; Bołdys, A.; Okopień, B. The impact of exenatide (a GLP-1 agonist) on markers of inflammation and oxidative stress in normal human astrocytes subjected to various glycemic conditions. Exp. Ther. Med. 2019, 17, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kam, T.-I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.-S.; Kwon, S.-H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Spielman, L.J.; Gibson, D.L.; Klegeris, A. Incretin hormones regulate microglia oxidative stress, survival and expression of trophic factors. Eur. J. Cell Biol. 2017, 96, 240–253. [Google Scholar] [CrossRef]
- Batista, A.F.; Bodart-Santos, V.; De Felice, F.G.; Ferreira, S.T. Neuroprotective Actions of Glucagon-Like Peptide-1 (GLP-1) Analogues in Alzheimer’s and Parkinson’s Diseases. CNS Drugs 2019, 33, 209–223. [Google Scholar] [CrossRef]
- Hampton, D.W.; Amor, S.; Story, D.; Torvell, M.; Bsibsi, M.; van Noort, J.M.; Chandran, S. HspB5 Activates a Neuroprotective Glial Cell Response in Experimental Tauopathy. Front. Neurosci. 2020, 14, 574. [Google Scholar] [CrossRef]
- Wenzel, T.J.; Kwong, E.; Bajwa, E.; Klegeris, A. Resolution-Associated Molecular Patterns (RAMPs) as Endogenous Regulators of Glia Functions in Neuroinflammatory Disease. CNS Neurol. Disord. Drug Targets 2020, 19, 483–494. [Google Scholar] [CrossRef]
- Guo, Y.; Liang, P.; Lu, S.; Chen, R.; Yin, Y.; Zhou, J. Extracellular αB-crystallin modulates the inflammatory responses. Biochem. Biophys. Res. Commun. 2019, 508, 282–288. [Google Scholar] [CrossRef]
- Bsibsi, M.; Persoon-Deen, C.; Verwer, R.W.H.; Meeuwsen, S.; Ravid, R.; Van Noort, J.M. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 2006, 53, 688–695. [Google Scholar] [CrossRef]
- Mielcarska, M.B.; Bossowska-Nowicka, M.; Gregorczyk-Zboroch, K.P.; Wyżewski, Z.; Szulc-Dąbrowska, L.; Gieryńska, M.; Toka, F.N. Syk and Hrs Regulate TLR3-Mediated Antiviral Response in Murine Astrocytes. Oxid. Med. Cell. Longev. 2019, 2019, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cudaback, E.; Breyer, R.M.; Montine, K.S.; Keene, C.D.; Montine, T.J. Eicosanoid receptor subtype-mediated opposing regulation of TLR-stimulated expression of astrocyte glial-derived neurotrophic factor. FASEB J. 2012, 26, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, X.; Zhao, D.; Pan, L.; Huang, C.; Guo, L.; Lu, Q.; Wang, J. TLR 3 ligand Poly IC Attenuates Reactive Astrogliosis and Improves Recovery of Rats after Focal Cerebral Ischemia. CNS Neurosci. Ther. 2015, 21, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sloan, S.A.; Clarke, L.E.; Caneda, C.; Plaza, C.A.; Blumenthal, P.D.; Vogel, H.; Steinberg, G.K.; Edwards, M.S.B.; Li, G.; et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 2016, 89, 37–53. [Google Scholar] [CrossRef]
- Chen, S.; Peng, J.; Sherchan, P.; Ma, Y.; Xiang, S.; Yan, F.; Zhao, H.; Jiang, Y.; Wang, N.; Zhang, J.H.; et al. TREM2 activation attenuates neuroinflammation and neuronal apoptosis via PI3K/Akt pathway after intracerebral hemorrhage in mice. J. Neuroinflamm. 2020, 17, 168. [Google Scholar] [CrossRef]
- Rosciszewski, G.; Cadena, V.; Murta, V.; Lukin, J.; Villarreal, A.; Roger, T.; Ramos, A.J. Toll-Like Receptor 4 (TLR4) and Triggering Receptor Expressed on Myeloid Cells-2 (TREM-2) Activation Balance Astrocyte Polarization into a Proinflammatory Phenotype. Mol. Neurobiol. 2017, 55, 3875–3888. [Google Scholar] [CrossRef]
- Hoane, M.R.; Pierce, J.L.; Holland, M.A.; Birky, N.D.; Dang, T.; Vitek, M.P.; McKenna, S.E. The Novel Apolipoprotein E–Based Peptide COG1410 Improves Sensorimotor Performance and Reduces Injury Magnitude following Cortical Contusion Injury. J. Neurotrauma 2007, 24, 1108–1118. [Google Scholar] [CrossRef]
- Wei, M.-D.; Lan, Y.-X.; Lu, K.; Wang, Y.; Chen, W.-Y. Knockdown of astrocytic TREM2 in the hippocampus relieves cognitive decline in elderly male mice. Behav. Brain Res. 2021, 397, 112939. [Google Scholar] [CrossRef]









| Receptors | Cell Types Expressing the Receptor | Receptor Agonist | Effect of Immune-Activated Astrocytes | Protective Function of Induced Astrocytes | References |
|---|---|---|---|---|---|
| GLPR1 | Astrocytes Microglia Neuron | GLP-1, exenatide, liraglutide | Inhibit IL-1β and TNF | Upregulate glutathione peroxidase | [144,145,147] |
| TLR2, TLR1, CD14 | Astrocytes Microglia Neuron | αB-Crystallin | Inhibit IL-1β and IL-6 | Upregulate LIF, NGF, BDNF | [149,151] |
| TLR3 | Astrocytes Microglia Neuron | mRNA, Poly (I: C) | Inhibit IL-6, downregulate GFAP | Upregulate neurotrophin-4, LIF, BDNF, GDNF, IL-10, IFN-β | [154,155] |
| TREM2 | Astrocytes Microglia Neuron | Lipids, apolipoprotein E, COG1410 | Inhibit IL-1β and TNF, downregulate GFAP | Not determined | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiwari, A.; Rout, S.; Deep, P.; Sahu, C.; Samal, P.K. The Dual Role of Astrocytes in CNS Homeostasis and Dysfunction. Neuroglia 2025, 6, 38. https://doi.org/10.3390/neuroglia6040038
Tiwari A, Rout S, Deep P, Sahu C, Samal PK. The Dual Role of Astrocytes in CNS Homeostasis and Dysfunction. Neuroglia. 2025; 6(4):38. https://doi.org/10.3390/neuroglia6040038
Chicago/Turabian StyleTiwari, Aarti, Satyabrata Rout, Prasanjit Deep, Chandan Sahu, and Pradeep Kumar Samal. 2025. "The Dual Role of Astrocytes in CNS Homeostasis and Dysfunction" Neuroglia 6, no. 4: 38. https://doi.org/10.3390/neuroglia6040038
APA StyleTiwari, A., Rout, S., Deep, P., Sahu, C., & Samal, P. K. (2025). The Dual Role of Astrocytes in CNS Homeostasis and Dysfunction. Neuroglia, 6(4), 38. https://doi.org/10.3390/neuroglia6040038





