The Role of Oral Microbiota and Glial Cell Dynamics in Relation to Gender in Cardiovascular Disease Risk
Abstract
1. Introduction
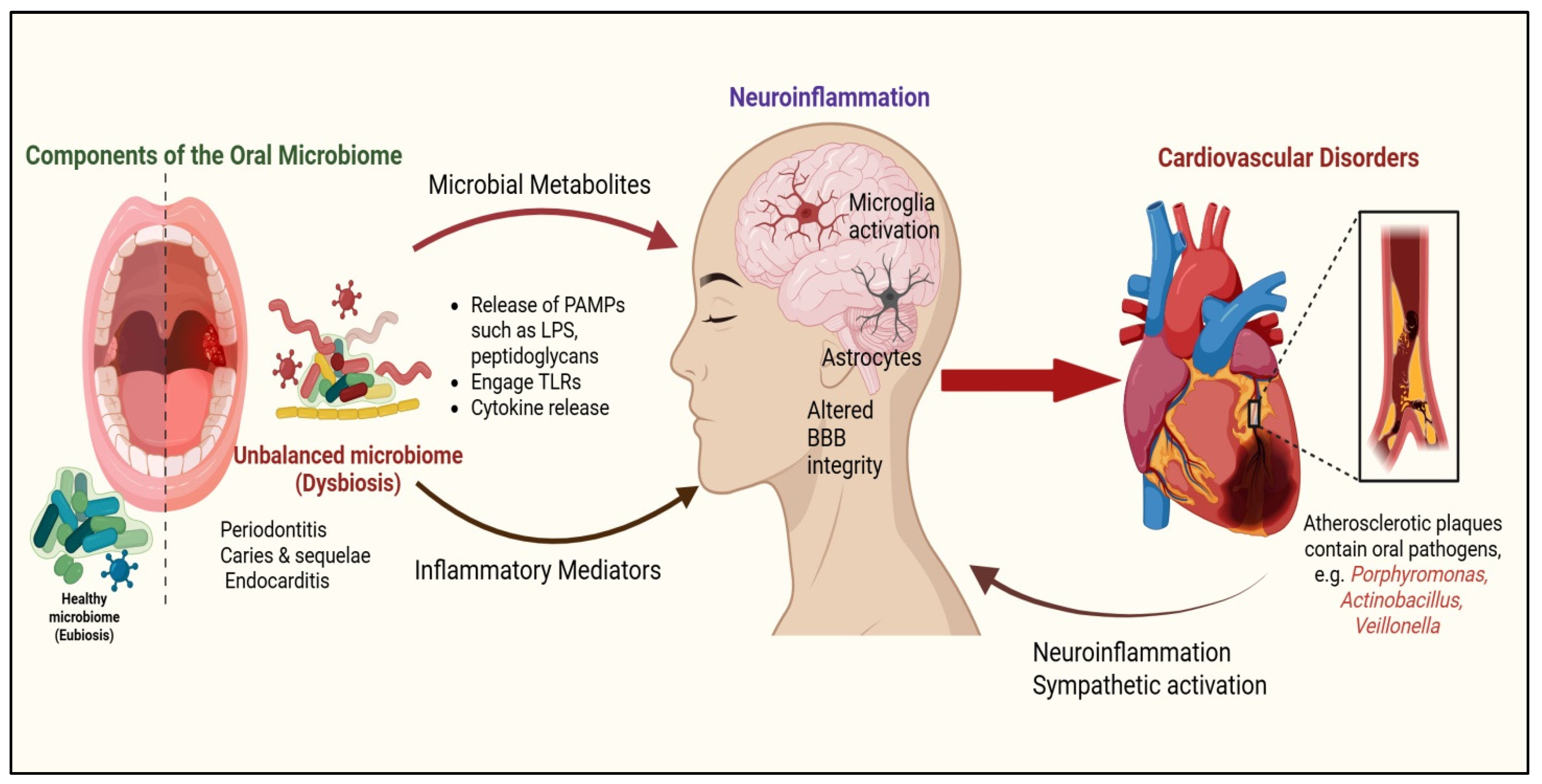
1.1. Oral Microbiota, Glial Interaction, and Their Significance in Health and Disease
1.1.1. Overview of the Oral Microbiota
1.1.2. Oral Microbiota and Gliotransmission: Implications for Systemic Health
1.2. CVD: Risk Factors and Impact of Oral Dysbiosis on Pathophysiology
1.3. Rationale for Exploring Glial Cells in Oral Dysbiosis–CVD Link
2. Gender Differences in CVD Risk
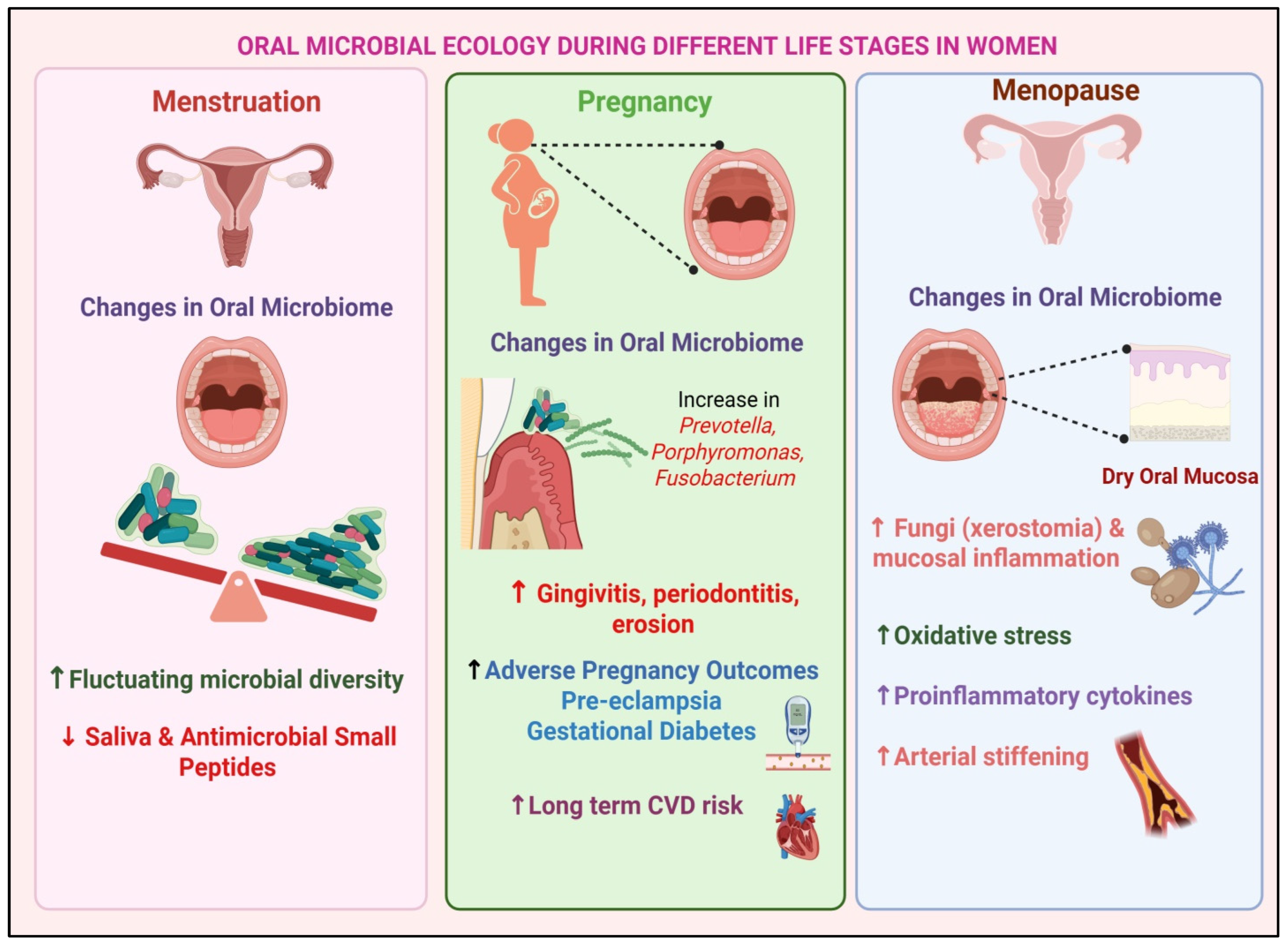
3. Gender-Specific Features of Oral Microbiota
4. Glial Dynamics in Cardiovascular Disease
4.1. Microglial Activation via LPS, Blood–Brain Barrier Disruption, and the Paraventricular Nucleus (PVN)
4.2. Role of Astrocytes in the Rostral Ventrolateral Medulla (RVLM) and Microglial Priming
4.3. Microglial Priming and Its Contribution to Neurogenic Hypertension
5. Impact of Oral Microbiota on Cardiovascular Health
5.1. Oral Microbiota Dysbiosis: Driving Inflammation, Endothelial Dysfunction, and Atherosclerosis
5.2. Gender-Specific Links Between Oral Microbiota and Cardiovascular Risk Markers
6. Mechanisms Underlying Gender Differences in the Oral Microbiota–CVD Axis
6.1. Potential Mechanisms Through Which Gender-Specific Factors Modulate the Relationship Between Oral Microbiota and CVD Risk
6.2. Hormonal Modulation, Immune Responses, and Gene–Environment Interactions
6.3. Clinical and Experimental Trials Targeting the Oral–Brain–Heart Axis
7. Clinical Implications and Future Directions
7.1. Sex-Specific Oral–Cardiovascular Prevention: Microbiota- and Glial-Marker-Guided Screening and Early Intervention
7.2. Bridging Cardiology and Dentistry: Neuroinflammatory Diagnostics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular Disease |
| PAMPs | Pathogen-Associated Molecular Patterns |
| LPS | Lipopolysaccharide |
| TLR | Toll Like Receptors |
| ILs | Interleukins |
| CVOs | Circumventricular Organs |
| CNS | Central Nervous System |
| BBB | Blood–Brain Barrier |
| PVN | Paraventricular Nucleus |
| RVLM | Rostral Ventrolateral Medulla |
| GABA | Gamma-Aminobutyric acid |
| NTS | Nucleus Tractus Solitarius |
| CRP | C-Reactive Protein |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| VCAM-1 | Vascular Cell Adhesion Molecule 1 |
| LDL | Low-Density Lipoprotein |
| HDL | High-Density Lipoprotein |
| CHD | Coronary Heart Disease |
| PCOS | Polycystic Ovarian Syndrome |
| APOs | Adverse Pregnancy Outcomes |
| OLP | Oral Lichen Planus |
| HRT | Hormone Replacement Therapy |
| cIMT | Carotid Intima Media Thickness |
| GWAS | Genome-Wide Association Study |
| NLRP3 | NLR family pyrin domain containing 3 |
| PAD | Peptidyl Arginine Deiminase |
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Handelsman, J. Beyond the Venn diagram: The hunt for a core microbiome. Environ. Microbiol. 2012, 14, 4–12. [Google Scholar] [CrossRef]
- Jenkinson, H.F. Beyond the oral microbiome. Environ. Microbiol. 2011, 13, 3077–3087. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Keijser, B.J.F.; Zaura, E.; Huse, S.M.; van der Vossen, J.M.B.M.; Schuren, F.H.J.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. Dis. Primers 2017, 3, 17038. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, H.; Zhao, L. PAMPs and DAMPs as the Bridge Between Periodontitis and Atherosclerosis: The Potential Therapeutic Targets. Front. Cell Dev. Biol. 2022, 10, 856118. [Google Scholar] [CrossRef]
- Mallard, C. Innate immune regulation by toll-like receptors in the brain. ISRN Neurol. 2012, 2012, 701950. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Cheng, G.; Hardy, M. Gut microbiome, short-chain fatty acids, alpha-synuclein, neuroinflammation, and ROS/RNS: Relevance to Parkinson’s disease and therapeutic implications. Redox Biol. 2024, 71, 103092. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Bridging the brain and gut: Neuroimmune mechanisms of neuroinflammation and therapeutic insights. Front. Cell. Neurosci. 2025, 19, 1590002. [Google Scholar] [CrossRef]
- Hanani, M. Satellite glial cells in sympathetic and parasympathetic ganglia: In search of function. Brain Res. Rev. 2010, 64, 304–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Pan, W.; Xu, Y.; Zhang, J.; Wan, J.; Jiang, H. Microglia-Mediated Neuroinflammation: A Potential Target for the Treatment of Cardiovascular Diseases. J. Inflamm. Res. 2022, 15, 3083–3094. [Google Scholar] [CrossRef] [PubMed]
- Colombari, E.; Sato, M.A.; Cravo, S.L.; Bergamaschi, C.T.; Campos, R.R.J.; Lopes, O.U. Role of the medulla oblongata in hypertension. Hypertension 2001, 38 Pt 2, 549–554. [Google Scholar] [CrossRef]
- Suarez-Roca, H.; Mamoun, N.; Sigurdson, M.I.; Maixner, W. Baroreceptor Modulation of the Cardiovascular System, Pain, Consciousness, and Cognition. Compr. Physiol. 2021, 11, 1373–1423. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Wang, X.; Tong, L.; Chen, G.; Zhou, S.; Zhang, H.; Liu, H.; Lu, W.; Wang, G.; et al. Microglia-derived TNF-α contributes to RVLM neuronal mitochondrial dysfunction via blocking the AMPK-Sirt3 pathway in stress-induced hypertension. J. Neuroinflamm. 2023, 20, 137. [Google Scholar] [CrossRef]
- Ilievski, V. Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration, and amyloid beta production in mice. PLoS ONE 2018, 13, 204941. [Google Scholar] [CrossRef]
- Pritchard, A.B.; Fabian, Z.; Lawrence, C.L.; Morton, G.; Crean, S.; Alder, J.E. An Investigation into the Effects of Outer Membrane Vesicles and Lipopolysaccharide of Porphyromonas Gingivalis on Blood-Brain Barrier Integrity, Permeability, and Disruption of Scaffolding Proteins in a Human in Vitro Model. J. Alzheimers Dis. 2022, 86, 343–364. [Google Scholar] [CrossRef]
- Gabarrini, G. The peptidylarginine deiminase of P. gingivalis is a virulence factor that modulates the immune response and contributes to dysbiosis. J. Clin. Periodontol. 2018, 49, 736–747. [Google Scholar]
- Nakayama, M.; Ohara, N. Molecular Mechanisms of Porphyromonas Gingivalis -Host Cell Interaction on Periodontal Diseases. Jpn. Dent. Sci. Rev. 2017, 53, 134–140. [Google Scholar] [CrossRef]
- Tariq, M.B.; Lee, J.; McCullough, L.D. Sex Differences in the Inflammatory Response to Stroke. Semin. Immunopathol. 2023, 45, 295–313. [Google Scholar] [CrossRef]
- Lynch, M.A. Exploring Sex-Related Differences in Microglia May Be a Game-Changer in Precision Medicine. Front. Aging Neurosci. 2022, 14, 868448. [Google Scholar] [CrossRef]
- Cecil, J.D.; Sirisaengtaksin, N.; O’Brien-Simpson, N.M.; Krachler, A.M. Outer membrane vesicle-host cell interactions. Microbiol. Spectr. 2019, 7, 10-1128. [Google Scholar] [CrossRef]
- Lai, D.; Ma, W.; Wang, J.; Zhang, L.; Shi, J.; Lu, C.; Gu, X. Immune Infiltration and Diagnostic Value of Immune-related Genes in Periodontitis Using Bioinformatics Analysis. J. Periodontal. Res. 2023, 58, 369–380. [Google Scholar] [CrossRef]
- de Ceglia, R.; Ledonne, A.; Litvin, D.G.; Lind, B.L.; Carriero, G.; Latagliata, E.C.; Bindocci, E.; Di Castro, M.A.; Savtchouk, I.; Vitali, I.; et al. Specialized Astrocytes Mediate Glutamatergic Gliotransmission in the CNS. Nature 2023, 622, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, M.; Li, H.; Gao, D.; Zhao, L.; Zhu, M. Glial cells improve Parkinson’s disease by modulating neuronal function and regulating neuronal ferroptosis. Front. Cell Dev. Biol. 2024, 12, 1510897. [Google Scholar] [CrossRef]
- Ferreira-Neto, H.C.; Antunes, V.R.; Stern, J.E. ATP stimulates rat hypothalamic sympathetic neurons by enhancing AMPA receptor-mediated currents. J. Neurophysiol. 2015, 114, 159–169. [Google Scholar] [CrossRef]
- Li, D.P.; Chen, S.R.; Pan, H.L. Adenosine inhibits paraventricular pre-sympathetic neurons through ATP-dependent potassium channels. J. Neurochem. 2010, 113, 530–542. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Z.; Geng, B.; Cai, J. The Bidirectional Signal Communication of Microbiota-Gut-Brain Axis in Hypertension. Int. J. Hypertens. 2021, 2021, 8174789. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- White-Al Habeeb, N.M.A.; Higgins, V.; Wolska, A.; Delaney, S.R.; Remaley, A.T.; Beriault, D.R. The Present and Future of Lipid Testing in Cardiovascular Risk Assessment. Clin. Chem. 2023, 69, 456–469. [Google Scholar] [CrossRef]
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef]
- Tang, C.; Pang, T.; Dang, C.; Liang, H.; Wu, J.; Shen, X.; Wang, L.; Luo, R.; Lan, H.; Zhang, P. Correlation between the Cardiometabolic Index and Arteriosclerosis in Patients with Type 2 Diabetes Mellitus. BMC Cardiovasc. Disord. 2024, 24, 186. [Google Scholar] [CrossRef]
- Gomez-Delgado, F.; Torres-Peña, J.D.; Gutierrez-Lara, G.; Romero-Cabrera, J.L.; Perez-Martinez, P. Artificial sweeteners and cardiovascular risk. Curr. Opin. Cardiol. 2023, 38, 344–351. [Google Scholar] [CrossRef]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The importance of healthy lifestyle behaviors in the prevention of cardiovascular disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; An, H.J.; Seo, Y.G. The Relationship between Breakfast and Sleep and Cardiovascular Risk Factors. Nutrients 2023, 15, 4596. [Google Scholar] [CrossRef]
- Billings, F. Mouth Infection as a Source of Systemic Disease. J. Am. Med. Assoc. 1914, 63, 2024–2025. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Lund Håheim, A.L. Oral anaerobe bacteria-a common risk for cardiovascular disease and mortality and some forms of cancer? Front. Oral Health 2024, 5, 1348946. [Google Scholar] [CrossRef]
- Ramírez, J.H.; Parra, B.; Gutierrez, S.; Arce, R.M.; Jaramillo, A.; Ariza, Y.; Contreras, A. Biomarkers of Cardiovascular Disease Are Increased in Untreated Chronic Periodontitis: A Case Control Study. Aust. Dent. J. 2014, 59, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Liang, J.; Li, X.; Deng, Y.; Cheng, S.; Wu, Q.; Song, W.; He, Y.; Zhu, J.; Zhang, X.; et al. Association between Oral Microbial Dysbiosis and Poor Functional Outcomes in Stroke-Associated Pneumonia Patients. BMC Microbiol. 2023, 23, 305. [Google Scholar] [CrossRef] [PubMed]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human Oral, Gut, and Plaque Microbiota in Patients with Atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4592–4598. [Google Scholar] [CrossRef]
- Liang, J.; Ren, Y.; Zheng, Y.; Lin, X.; Song, W.; Zhu, J.; Zhang, X.; Zhou, H.; Wu, Q.; He, Y.; et al. Functional Outcome Prediction of Acute Ischemic Stroke Based on the Oral and Gut Microbiota. Mol. Neurobiol. 2025, 62, 5413–5431. [Google Scholar] [CrossRef]
- Johansson, A.; Johansson, I.; Eriksson, M.; Ahrén, A.M.; Hallmans, G.; Stegmayr, B. Systemic antibodies to the leukotoxin of the oral pathogen Actinobacillus actinomycetemcomitans correlate negatively with stroke in women. Cerebrovasc. Dis. 2005, 20, 226–232. [Google Scholar] [CrossRef]
- Zhen, W.; Wang, Z.; Wang, Q.; Sun, W.; Wang, R.; Zhang, W.; Zhang, Y.; Qin, W.; Li, B.; Wang, Q.; et al. Cardiovascular Disease Therapeutics via Engineered Oral Microbiota: Applications and Perspective. iMeta 2024, 3, e197. [Google Scholar] [CrossRef]
- Tonelli, A.; Lumngwena, E.N.; Ntusi, N.A.B. The oral microbiome in the pathophysiology of cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 386–403. [Google Scholar] [CrossRef] [PubMed]
- Kerr, N.; Dietrich, D.W.; Bramlett, H.M.; Raval, A.P. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci. Ther. 2019, 25, 1308–1317. [Google Scholar] [CrossRef] [PubMed]
- Nelson, L.H.; Lenz, K.M. The immune system as a novel regulator of sex differences in brain and behavioral development. J. Neurosci. Res. 2017, 95, 447–461. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C.; Giannoni, M.; Cominelli, F.; Pizarro, T.T.; Pietropaoli, D. Meta-analysis of oral microbiome reveals sex-based diversity in biofilms during periodontitis. JCI Insight 2024, 9, e171311. [Google Scholar] [CrossRef]
- Di Giosia, P.; Passacquale, G.; Petrarca, M.; Giorgini, P.; Marra, A.M.; Ferro, A. Gender differences in cardiovascular prophylaxis: Focus on antiplatelet treatment. Pharmacol. Res. 2017, 119, 36–47. [Google Scholar] [CrossRef]
- Kim, C.; Redberg, R.F.; Pavlic, T.; Eagle, K.A. A systematic review of gender differences in mortality after coronary artery bypass graft surgery and percutaneous coronary interventions. Clin. Cardiol. 2007, 30, 491–495. [Google Scholar] [CrossRef]
- Rajendran, A.; Minhas, A.S.; Kazzi, B.; Varma, B.; Choi, E.; Thakkar, A.; Michos, E.D. Sex-Specific Differences in Cardiovascular Risk Factors and Implications for Cardiovascular Disease Prevention in Women. Atherosclerosis 2023, 384, 117269. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Hiremath, P.G.; Aversano, T.; Spertus, J.A.; Lemmon, C.C.; Naiman, D.Q.; Czarny, M.J. Sex Differences in Health Status and Clinical Outcomes After Nonprimary Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 2022, 15, e011308. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and Cardiovascular Disease: The Deep Blue Sea of Women’s Heart. Trends Cardiovasc. Med. 2020, 30, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Elder, P.; Sharma, G.; Gulati, M.; Michos, E.D. Identification of female-specific risk enhancers throughout the lifespan of women to improve cardiovascular disease prevention. Am. J. Prev. Cardiol. 2020, 2, 100028. [Google Scholar] [CrossRef] [PubMed]
- Okoth, K.; Chandan, J.S.; Marshall, T.; Thangaratinam, S.; Thomas, G.N.; Nirantharakumar, K.; Adderley, N.J. Association between the Reproductive Health of Young Women and Cardiovascular Disease in Later Life: Umbrella Review. BMJ 2020, 371, m3502. [Google Scholar] [CrossRef]
- AlHarbi, S.G.; Almushayt, A.S.; Bamashmous, S.; Abujamel, T.S.; Bamashmous, N.O. The oral microbiome of children in health and disease—A literature review. Front. Oral Health 2024, 5, 1477004. [Google Scholar] [CrossRef]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef]
- Souilhol, C.; Harmsen, M.C.; Evans, P.C.; Krenning, G. Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 2018, 114, 565–577. [Google Scholar] [CrossRef]
- Dou, Y.; Xin, J.; Zhou, P.; Tang, J.; Xie, H.; Fan, W.; Zhang, Z.; Wu, D. Bidirectional Association between Polycystic Ovary Syndrome and Periodontal Diseases. Front. Endocrinol. 2023, 14, 1008675. [Google Scholar] [CrossRef]
- Akcalı, A.; Bostanci, N.; Özçaka, Ö.; Öztürk-Ceyhan, B.; Gümüş, P.; Buduneli, N.; Belibasakis, G.N. Association between Polycystic Ovary Syndrome, Oral Microbiota and Systemic Antibody Responses. PLoS ONE 2014, 9, e108074. [Google Scholar] [CrossRef]
- González, F. Inflammation in Polycystic Ovary Syndrome: Underpinning of insulin resistance and ovarian dysfunction. Steroids 2012, 77, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Nannan, M.; Xiaoping, L.; Ying, J. Periodontal disease in pregnancy and adverse pregnancy outcomes: Progress in related mechanisms and management strategies. Front. Med. 2022, 9, 963956. [Google Scholar] [CrossRef]
- Carrillo-de-Albornoz, A.; Figuero, E.; Herrera, D.; Bascones-Martínez, A. Gingival changes during pregnancy: II. Influence of hormonal variations on the subgingival biofilm. J. Clin. Periodontol. 2010, 37, 230–240. [Google Scholar] [CrossRef]
- Han, Y.W. Fusobacterium nucleatum: A commensal-turned pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Vander Haar, E.L.; So, J.; Gyamfi-Bannerman, C.; Han, Y.W. Fusobacterium nucleatum and adverse pregnancy outcomes: Epidemiological and mechanistic evidence. Anaerobe 2018, 50, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lane-Cordova, A.D.; Khan, S.S.; Grobman, W.A.; Greenland, P.; Shah, S.J. Long-Term Cardiovascular Risks Associated With Adverse Pregnancy Outcomes: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 2106–2116. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kusiak, A.; Ossowska, A.; Grzybowska, M.E. Changes in the Oral Cavity in Menopausal Women-A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Tramice, A.; Paris, D.; Manca, A.; Guevara Agudelo, F.A.; Petrosino, S.; Siracusa, L.; Carbone, M.; Melck, D.; Raymond, F.; Piscitelli, F. Analysis of the Oral Microbiome during Hormonal Cycle and Its Alterations in Menopausal Women: The “AMICA” Project. Sci. Rep. 2022, 12, 22086. [Google Scholar] [CrossRef]
- Hildreth, K.L.; Kohrt, W.M.; Moreau, K.L. Oxidative stress contributes to large elastic arterial stiffening across the stages of the menopausal transition. Menopause 2014, 21, 624–632. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, Y.; Huang, W.; Jin, Z.; You, F.; Li, X.; Xiao, C. Direct and Indirect Effects of Estrogens, Androgens and Intestinal Microbiota on Colorectal Cancer. Front. Cell. Infect. Microbiol. 2024, 14, 1458033. [Google Scholar] [CrossRef]
- Martinelli, S.; Nannini, G.; Cianchi, F.; Coratti, F.; Amedei, A. The Impact of Microbiota-Immunity-Hormone Interactions on Autoimmune Diseases and Infection. Biomedicines 2024, 12, 616. [Google Scholar] [CrossRef]
- Johansson, T.; Karlsson, T.; Bliuc, D.; Schmitz, D.; Ek, W.E.; Skalkidou, A.; Center, J.R.; Johansson, Å. Contemporary Menopausal Hormone Therapy and Risk of Cardiovascular Disease: Swedish Nationwide Register Based Emulated Target Trial. BMJ 2024, 387, e078784. [Google Scholar] [CrossRef]
- Villa, P.; Amar, I.D.; Shachor, M.; Cipolla, C.; Ingravalle, F.; Scambia, G. Cardiovascular Risk/Benefit Profile of MHT. Medicina 2019, 55, 571. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.A.; Demerath, E.; Bittner-Eddy, P.; Costalonga, M. Placental colonization with periodontal pathogens: The potential missing link. Am. J. Obstet. Gynecol. 2019, 221, 383–392.e3. [Google Scholar] [CrossRef]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. 2015, 16, 581–606. [Google Scholar] [CrossRef]
- Yeap, B.B. Testosterone and cardiovascular disease risk. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Budoff, M.J. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA 2017, 317, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J. Risk of myocardial infarction in men receiving testosterone therapy. J. Am. Heart Assoc. 2014, 11, 23538. [Google Scholar] [CrossRef]
- Sachelarie, L.; Iman, A.E.H.; Romina, M.V.; Huniadi, A.; Hurjui, L.L. Impact of Hormones and Lifestyle on Oral Health During Pregnancy: A Prospective Observational Regression-Based Study. Medicina 2024, 60, 1773. [Google Scholar] [CrossRef]
- González-Jaranay, M.; Téllez, L.; Roa-López, A.; Gómez-Moreno, G.; Moreu, G. Periodontal status during pregnancy and postpartum. PLoS ONE 2017, 12, e0178234. [Google Scholar] [CrossRef]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef]
- Bogdan-Andreescu, C.F.; Bănățeanu, A.-M.; Albu, C.-C.; Poalelungi, C.-V.; Botoacă, O.; Damian, C.M.; Dȋră, L.M.; Albu, Ş.-D.; Brăila, M.G.; Cadar, E.; et al. Oral Mycobiome Alterations in Postmenopausal Women: Links to Inflammation, Xerostomia, and Systemic Health. Biomedicines 2024, 12, 2569. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, D.R.; Komali, G.; Jayanthi, K.; Dinesh, D.; Saikavitha, T.V. Evaluation of Salivary Flow Rate, pH and Buffer in Pre, Post & Post Menopausal Women on HRT. J. Clin. Diagn. Res. 2014, 8, 233–236. [Google Scholar]
- Bowland, G.B.; Weyrich, L.S. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front. Psychiatry 2022, 13, 810008. [Google Scholar] [CrossRef]
- Grover, V.; Jain, A.; Kapoor, A.; Malhotra, R.; Singh Chahal, G. The Gender Bender effect in Periodontal Immune Response. Endocr. Metab. Immune Disord.-Drug Targets 2016, 16, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Sangalli, L.; Souza, L.C.; Letra, A.; Shaddox, L.; Ioannidou, E. Sex as a Biological Variable in Oral Diseases: Evidence and Future Prospects. J. Dent. Res. 2023, 102, 1395–1416. [Google Scholar] [CrossRef]
- Di Spirito, F.; Amato, A.; Romano, A.; Dipalma, G.; Xhajanka, E.; Baroni, A.; Serpico, R.; Inchingolo, F.; Contaldo, M. Analysis of Risk Factors of Oral Cancer and Periodontitis from a Sex- and Gender-Related Perspective: Gender Dentistry. Appl. Sci. 2022, 12, 9135. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Auriemma, R.S.; Vetrani, C.; Cataldi, M.; Frias-Toral, E.; Pugliese, G.; Camajani, E.; Savastano, S.; Colao, A.; et al. Probiotics and Prebiotics: Any Role in Menopause-Related Diseases? Curr. Nutr. Rep. 2023, 12, 83–97. [Google Scholar] [CrossRef]
- Shi, P.; Diez-Freire, C.; Jun, J.Y.; Qi, Y.; Katovich, M.J.; Li, Q.; Sriramula, S.; Francis, J.; Sumners, C.; Raizada, M.K. Brain Microglial Cytokines in Neurogenic Hypertension. Hypertension 2010, 56, 297–303. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, H.W.; Kang, E.A.; Song, M.S.; Lee, S.Y.; Ryu, P.D. Microglial activation induced by LPS mediates excitation of neurons in the hypothalamic paraventricular nucleus projecting to the rostral ventrolateral medulla. BMB Rep. 2021, 54, 620–625. [Google Scholar] [CrossRef]
- Jing, F.; Zhang, Y.; Long, T.; He, W.; Qin, G.; Zhang, D.; Chen, L.; Zhou, J. P2Y12 Receptor Mediates Microglial Activation via RhoA/ROCK Pathway in the Trigeminal Nucleus Caudalis in a Mouse Model of Chronic Migraine. J. Neuroinflammation 2019, 16, 217. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Tan, X.; Dai, Y.; Krishnan, V.; Warner, M.; Gustafsson, J.Å. Targeting estrogen receptor β in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2013, 110, 3543–3548. [Google Scholar] [CrossRef]
- O’Connor, J.L.; Nissen, J.C. The Pathological Activation of Microglia Is Modulated by Sexually Dimorphic Pathways. Int. J. Mol. Sci. 2023, 24, 4739. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Reichel, J.M.; Han, C.; Zuniga-Hertz, J.P.; Cai, D. Astrocytic Process Plasticity and IKKβ/NF-κB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metab. 2017, 25, 1091–1102.e4. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Kamiya, T.; Tsuboi, T. Gliotransmitter Release from Astrocytes: Functional, Developmental, and Pathological Implications in the Brain. Front. Neurosci. 2015, 9, 499. [Google Scholar] [CrossRef]
- Marina, N.; Tang, F.; Figueiredo, M.; Mastitskaya, S.; Kasimov, V.; Mohamed-Ali, V.; Roloff, E.; Teschemacher, A.G.; Gourine, A.V.; Kasparov, S. Purinergic Signalling in the Rostral Ventro-Lateral Medulla Controls Sympathetic Drive and Contributes to the Progression of Heart Failure Following Myocardial Infarction in Rats. Basic Res. Cardiol. 2013, 108, 317. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Almarhoumi, R.; Alvarez, C.; Harris, T.; Tognoni, C.M.; Paster, B.J.; Carreras, I.; Dedeoglu, A.; Kantarci, A. Microglial Cell Response to Experimental Periodontal Disease. J. Neuroinflammation 2023, 20, 142. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Dange, R.B.; Agarwal, D.; Masson, G.S.; Vila, J.; Wilson, B.; Nair, A.; Francis, J. Central Blockade of TLR4 Improves Cardiac Function and Attenuates Myocardial Inflammation in Angiotensin II-Induced Hypertension. Cardiovasc. Res. 2014, 103, 17–27. [Google Scholar] [CrossRef]
- Dange, R.B.; Agarwal, D.; Teruyama, R.; Francis, J. Toll-like Receptor 4 Inhibition within the Paraventricular Nucleus Attenuates Blood Pressure and Inflammatory Response in a Genetic Model of Hypertension. J. Neuroinflammation 2015, 12, 31. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wu, Y.Y.; Huang, H.; He, C.; Li, W.Z.; Wang, H.L.; Chen, H.Q.; Yin, Y.Y. Biochanin A Attenuates LPS-Induced pro-Inflammatory Responses and Inhibits the Activation of the MAPK Pathway in BV2 Microglial Cells. Int. J. Mol. Med. 2015, 35, 391–398. [Google Scholar] [CrossRef]
- Shi, Z.; Gan, X.-B.; Fan, Z.D.; Zhang, F.; Zhou, Y.-B.; Gao, X.Y.; De, W.; Zhu, G.Q. Inflammatory Cytokines in Paraventricular Nucleus Modulate Sympathetic Activity and Cardiac Sympathetic Afferent Reflex in Rats. Acta Physiol. 2011, 203, 289–297. [Google Scholar] [CrossRef]
- Barreto, G.; Veiga, S.; Azcoitia, I.; Garcia-Segura, L.M.; Garcia-Ovejero, D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: Role of its metabolites, oestradiol and dihydrotestosterone. Eur. J. Neurosci. 2007, 25, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Crain, J.M.; Nikodemova, M.; Watters, J.J. Microglia express distinct M1 and M2 phenotypic markers in the postnatal and adult central nervous system in male and female mice. J. Neurosci. Res. 2013, 91, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.M.; Cipollone, A.; D’Ardes, D.; Di Giacomo, D.; Pignatelli, P.; Cipollone, F.; Curia, M.C.; Magni, P.; Bucci, M. Risk Factors and Immunoinflammatory Mechanisms Leading to Atherosclerosis: Focus on the Role of Oral Microbiota Dysbiosis. Microorganisms 2023, 11, 1479. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Furuta, N.; Morisaki, I.; Amano, A. Exit of intracellular Porphyromonas gingivalis from gingival epithelial cells is mediated by endocytic recycling pathway. Cell. Microbiol. 2011, 13, 677–691. [Google Scholar] [CrossRef]
- Carrion, J.; Scisci, E.; Miles, B.; Sabino, G.J.; Zeituni, A.E.; Gu, Y.; Bear, A.; Genco, C.A.; Brown, D.L.; Cutler, C.W. Microbial Carriage State of Peripheral Blood Dendritic Cells (DCs) in Chronic Periodontitis Influences DC Differentiation, Atherogenic Potential. J. Immunol. 2012, 189, 3178–3187. [Google Scholar] [CrossRef]
- Zeituni, A.E.; Jotwani, R.; Carrion, J.; Cutler, C.W. Targeting of DC-SIGN on Human Dendritic Cells by Minor Fimbriated Porphyromonas gingivalis Strains Elicits a Distinct Effector T Cell Response. J. Immunol. 2009, 183, 5694–5704. [Google Scholar] [CrossRef]
- Olsen, I.; Potempa, J. Strategies for Porphyromonas gingivalis gingipain inhibition for treatment of periodontitis and associated systemic diseases. J. Oral Microbiol. 2014, 13, 1881364. [Google Scholar]
- Kozarov, E.V.; Dorn, B.R.; Shelburne, C.E.; Dunn, W.A.; Progulske-Fox, A. Human Atherosclerotic Plaque Contains Viable Invasive Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Hofmann, M.A.; Bucciarelli, L.; Jerud, A.P.; Tucker, S.; Lu, Y.; Papapanou, P.N.; Schmidt, A.M. Oral Infection With a Periodontal Pathogen Accelerates Early Atherosclerosis in Apolipoprotein E–Null Mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1405–1411. [Google Scholar] [CrossRef]
- Jain, A.; Batista, E.L.; Serhan, C.; Stahl, G.L.; Van Dyke, T.E. Role for Periodontitis in the Progression of Lipid Deposition in an Animal Model. Infect. Immun. 2003, 71, 6012–6018. [Google Scholar] [CrossRef]
- Kelk, P.; Moghbel, N.S.; Hirschfeld, J.; Johansson, A. Aggregatibacter actinomycetemcomitans Leukotoxin Activates the NLRP3 Inflammasome and Cell-to-Cell Communication. Pathogens 2022, 11, 159. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Gordon, J.H.; Diaz-Moreno, P.; Andrews, C.A.; Shimbo, D.; Hovey, K.M.; Buck, M.J.; Wactawski-Wende, J. Oral Microbiome Is Associated With Incident Hypertension Among Postmenopausal Women. J. Am. Heart Assoc. 2022, 11, e021930. [Google Scholar] [CrossRef]
- Akhi, R.; Lavrinienko, A.; Hakula, M.; Tjäderhane, L.; Hindström, R.; Nissinen, A.; Wang, C.; Auvinen, J.; Kullaa, A.M.; Ylöstalo, P.; et al. Oral Microbiome Diversity Associates with Carotid Intima Media Thickness in Middle-Aged Male Subjects. Commun. Med. 2025, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Razavi, A.C.; Potts, K.S.; Kelly, T.N.; Bazzano, L.A. Sex, gut microbiome, and cardiovascular disease risk. Biol. Sex Differ. 2019, 10, 29. [Google Scholar] [CrossRef]
- Hezel, M.P.; Weitzberg, E. The oral microbiome and nitric oxide homoeostasis. Oral Dis. 2015, 21, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kapil, V.; Haydar, S.M.A.; Pearl, V.; Lundberg, J.O.; Weitzberg, E.; Ahluwalia, A. Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic. Biol. Med. 2013, 55, 93–100. [Google Scholar] [CrossRef]
- Bryan, N.S.; Tribble, G.; Angelov, N. Oral Microbiome and Nitric Oxide: The Missing Link in the Management of Blood Pressure. Curr. Hypertens. Rep. 2017, 19, 33. [Google Scholar] [CrossRef]
- Bondonno, C.P.; Liu, A.H.; Croft, K.D.; Considine, M.J.; Puddey, I.B.; Woodman, R.J.; Hodgson, J.M. Antibacterial Mouthwash Blunts Oral Nitrate Reduction and Increases Blood Pressure in Treated Hypertensive Men and Women. Am. J. Hypertens. 2015, 28, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Mei, X.; Mell, B.; Cheng, X.; Yeo, J.-Y.; Yang, T.; Chiu, N.; Joe, B. Beyond the Gastrointestinal Tract: Oral and Sex-Specific Skin Microbiota Are Associated with Hypertension in Rats with Genetic Disparities. Physiol Genom. 2022, 54, 242–250. [Google Scholar] [CrossRef]
- Budzyński, J.; Wiśniewska, J.; Ciecierski, M.; Kędzia, A. Association between Bacterial Infection and Peripheral Vascular Disease: A Review. Int. J. Angiol. 2016, 25, 3–13. [Google Scholar]
- Gordon, J.H.; LaMonte, M.J.; Genco, R.J.; Zhao, J.; Li, L.; Hovey, K.M.; Tsompana, M.; Buck, M.J.; Andrews, C.A.; Mcskimming, D.I.; et al. Is the Oral Microbiome Associated with Blood Pressure in Older Women? High Blood Press. Cardiovasc. Prev. 2019, 26, 217–225. [Google Scholar] [CrossRef]
- Shen, M.T.; Shahin, B.; Chen, Z.; Adami, G.R. Unexpected lower level of oral periodontal pathogens in patients with high numbers of systemic diseases. PeerJ 2023, 11, e15502. [Google Scholar] [CrossRef]
- Bernabeu, E.; Canela-Xandri, O.; Rawlik, K.; Talenti, A.; Prendergast, J.; Tenesa, A. Sex differences in genetic architecture in the UK Biobank. Nat. Genet. 2021, 53, 1283–1289. [Google Scholar] [CrossRef]
- Hartiala, J.A.; Wilson Tang, W.H.; Wang, Z.; Crow, A.L.; Stewart, A.F.R.; Roberts, R.; McPherson, R.; Erdmann, J.; Willenborg, C.; Hazen, S.L.; et al. Genome-Wide Association Study and Targeted Metabolomics Identifies Sex-Specific Association of CPS1 with Coronary Artery Disease. Nat. Commun. 2016, 7, 10558. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Tao, Z.Y.; Yu, A.L.; Yang, X.P. Testosterone enhances early cardiac remodeling after myocardial infarction, causing rupture and degrading cardiac function. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H2043–H2050. [Google Scholar] [CrossRef]
- Sluijmer, A.V.; Heineman, M.J.; De Jong, F.H.; Evers, J.L. Endocrine activity of the postmenopausal ovary: The effects of pituitary down-regulation and oophorectomy. J. Clin. Endocrinol. Metab. 1995, 80, 2163–2167. [Google Scholar]
- Carlson, L.E.; Sherwin, B.B. Higher levels of plasma estradiol and testosterone in healthy elderly men compared with age-matched women may protect aspects of explicit memory. Menopause 2000, 7, 168–177. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Malhotra, K.; Chang, J.J.; Khunger, A.; Blacker, D.; Switzer, J.A.; Goyal, N.; Hernandez, A.V.; Pasupuleti, V.; Alexandrov, A.V.; Tsivgoulis, G. Minocycline for Acute Stroke Treatment: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Neurol. 2018, 265, 1871–1879. [Google Scholar] [CrossRef]
- Kohler, E.; Prentice, D.A.; Bates, T.R.; Hankey, G.J.; Claxton, A.; van Heerden, J.; Blacker, D. Intravenous Minocycline in Acute Stroke: A Randomized, Controlled Pilot Study and Meta-Analysis. Stroke 2013, 44, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, B.; Craig, D.; Bullock, R.; Malouf, R.; Passmore, P. Statins for the treatment of dementia. Cochrane Database Syst. Rev. 2014, 2014, CD007514. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, J.A.; Bustamante, A.; Giannini, N.; Pecharroman, E.; Katsanos, A.H.; Tsivgoulis, G.; Rozanski, M.; Audebert, H.; Mondello, S.; Llombart, V.; et al. Discriminative Value of Glial Fibrillar Acidic Protein (GFAP) as a Diagnostic Tool in Acute Stroke. Individual Patient Data Meta-Analysis. J. Investig. Med. 2020, 68, 1379–1385. [Google Scholar] [CrossRef]
- Kyme, C. Serum GFAP: An early indicator of intracerebral hemorrhage in acute stroke. Nat. Clin. Pract. Neurol. 2006, 2, 237. [Google Scholar] [CrossRef]
- Hase, Y.; Ameen-Ali, K.E.; Waller, R.; Simpson, J.E.; Stafford, C.; Mahesh, A.; Ryan, L.; Pickering, L.; Bodman, C.; Hase, M.; et al. Differential Perivascular Microglial Activation in the Deep White Matter in Vascular Dementia Developed Post-stroke. Brain Pathology 2022, 32, e13101. [Google Scholar] [CrossRef]
- Ye, Z.; Cao, Y.; Miao, C.; Liu, W.; Dong, L.; Lv, Z.; Iheozor-Ejiofor, Z.; Li, C. Periodontal Therapy for Primary or Secondary Prevention of Cardiovascular Disease in People with Periodontitis. Cochrane Database Syst. Rev. 2022, 10, CD009197. [Google Scholar] [CrossRef]
- Wang, Z.; Kaplan, R.C.; Burk, R.D.; Qi, Q. The Oral Microbiota, Microbial Metabolites, and Immuno-Inflammatory Mechanisms in Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 12337. [Google Scholar] [CrossRef]
- Cheng, L.; Correia, M.L.d.G. More Evidence Links Microglia and Neuroinflammation With Hypertension. Am. J. Hypertens. 2022, 35, 787–789. [Google Scholar] [CrossRef]
- Shen, X.Z.; Li, Y.; Li, L.; Shah, K.H.; Bernstein, K.E.; Lyden, P.; Shi, P. Microglia Participate in Neurogenic Regulation of Hypertension. Hypertension 2015, 66, 309–316. [Google Scholar] [CrossRef]
- Pietropaoli, D.; Del Pinto, R.; Ferri, C.; Wright, J.T.; Giannoni, M.; Ortu, E.; Monaco, A. Poor Oral Health and Blood Pressure Control Among US Hypertensive Adults. Hypertension 2018, 72, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Romańczyk, M.; Witalińska-Łabuzek, J.; Czerniuk, M.R.; Łabuzek, K.; Filipiak, K.J. Periodontitis, Blood Pressure, and the Risk and Control of Arterial Hypertension: Epidemiological, Clinical, and Pathophysiological Aspects-Review of the Literature and Clinical Trials. Curr. Hypertens. Rep. 2021, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Du, W.; Liang, Y.; Xu, P.; Ding, Q.; Chen, X.; Jia, S.; Wang, X. An Integrated Neuroimaging-Omics Approach for the Gut-Brain Communication Pathways in Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1211979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qian, X.-H.; Hu, J.; Zhang, Y.; Lin, X.; Hai, W.; Shi, K.; Jiang, X.; Li, Y.; Tang, H.-D.; et al. Integrating TSPO PET Imaging and Transcriptomics to Unveil the Role of Neuroinflammation and Amyloid-β Deposition in Alzheimer’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2024, 51, 455–467. [Google Scholar] [CrossRef]
- Pritchard, A.B.; Crean, S.; Olsen, I.; Singhrao, S.K. Periodontitis, microbiomes and their role in Alzheimer’s disease. Front. Aging Neurosci. 2017, 9, 336. [Google Scholar] [CrossRef]
- Leblhuber, F.; Geisler, S.; Steiner, K.; Fuchs, D.; Schütz, B. Elevated fecal calprotectin in patients with Alzheimer’s dementia indicates leaky gut. J. Neural Transm. 2015, 126, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Erickson, M.A. The blood-brain barrier and the regulation of immune function in the central nervous system. Neurobiol. Dis. 2010, 37, 26–32. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Tonetti, M.S.; D’Aiuto, F.; Nibali, L.; Donald, A.; Storry, C.; Parkar, M.; Suvan, J.; Hingorani, A.D.; Vallance, P.; Deanfield, J. Treatment of Periodontitis and Endothelial Function. N. Engl. J. Med. 2007, 356, 911–920. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Blackwell, J.R.; L’Heureux, J.E.; Williams, D.W.; Smith, A.; Giezen, M.; Wylie, L.J.; Kelly, J.; Jones, A.M. Nitrate-Responsive Oral Microbiome Modulates Nitric Oxide Homeostasis and Blood Pressure in Humans. J. Am. Heart Assoc. 2018, 9, 14313. [Google Scholar] [CrossRef] [PubMed]
- Vemulapalli, A.; Mandapati, S.R.; Kotha, A.; Rudraraju, H.; Aryal, S. Prevalence of complete edentulism among US adults 65 years and older: A Behavioral Risk Factor Surveillance System study from 2012 through 2020. J. Am. Dent. Assoc. 2024, 155, 399–408. [Google Scholar] [CrossRef] [PubMed]
| Female Specific Factors | Impact on Oral Microbiota | Potential Link to CVD | References |
|---|---|---|---|
| Early Menarche | Altered estrogen levels affect oral microbial composition, favoring pathogenic bacteria (e.g., Porphyromonas, Prevotella, Fusobacterium) | Increased systemic inflammation and endothelial dysfunction | [57,58,59,60] |
| Polycystic Ovary Syndrome (PCOS) | Elevated androgen levels lead to periodontal inflammation and increased T. denticola | Chronic inflammation contributes to insulin resistance and atherosclerosis | [61,62,63] |
| Pregnancy and Multiparity | Hormonal shifts enhance Prevotella and Porphyromonas growth, worsening gingival inflammation | Increased vascular stress and systemic inflammation | [64,65] |
| Adverse Pregnancy Outcomes (e.g., Pre-eclampsia and Gestational Diabetes) | Increased F. nucleatum linked to placental inflammation and poor periodontal health | Higher long-term risk of hypertension and cardiovascular dysfunction | [64,66,67,68] |
| Menopause and Estrogen Deficiency | Loss of estrogen reduces salivary flow, leading to dysbiosis (Actinomyces increase, Lactobacillus decrease) and worsening periodontal disease | Elevated oxidative stress and pro-inflammatory cytokines contribute to arterial stiffening | [69,70,71] |
| Hormone Replacement Therapy (HRT) | Influences microbiota composition, reducing pro-inflammatory bacteria but also altering immune response | Variable impact on cardiovascular risk depending on individual metabolic profiles | [72,73,74,75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, D.; Kumar, A. The Role of Oral Microbiota and Glial Cell Dynamics in Relation to Gender in Cardiovascular Disease Risk. Neuroglia 2025, 6, 30. https://doi.org/10.3390/neuroglia6030030
Ghosh D, Kumar A. The Role of Oral Microbiota and Glial Cell Dynamics in Relation to Gender in Cardiovascular Disease Risk. Neuroglia. 2025; 6(3):30. https://doi.org/10.3390/neuroglia6030030
Chicago/Turabian StyleGhosh, Devlina, and Alok Kumar. 2025. "The Role of Oral Microbiota and Glial Cell Dynamics in Relation to Gender in Cardiovascular Disease Risk" Neuroglia 6, no. 3: 30. https://doi.org/10.3390/neuroglia6030030
APA StyleGhosh, D., & Kumar, A. (2025). The Role of Oral Microbiota and Glial Cell Dynamics in Relation to Gender in Cardiovascular Disease Risk. Neuroglia, 6(3), 30. https://doi.org/10.3390/neuroglia6030030





