Modest Reduction in CAG Repeat Length Rescues Motor Deficits but Not Purkinje Cell Pathology and Gliosis in Spinocerebellar Ataxia Type 1 Mice
Abstract
1. Introduction
2. Methods and Materials
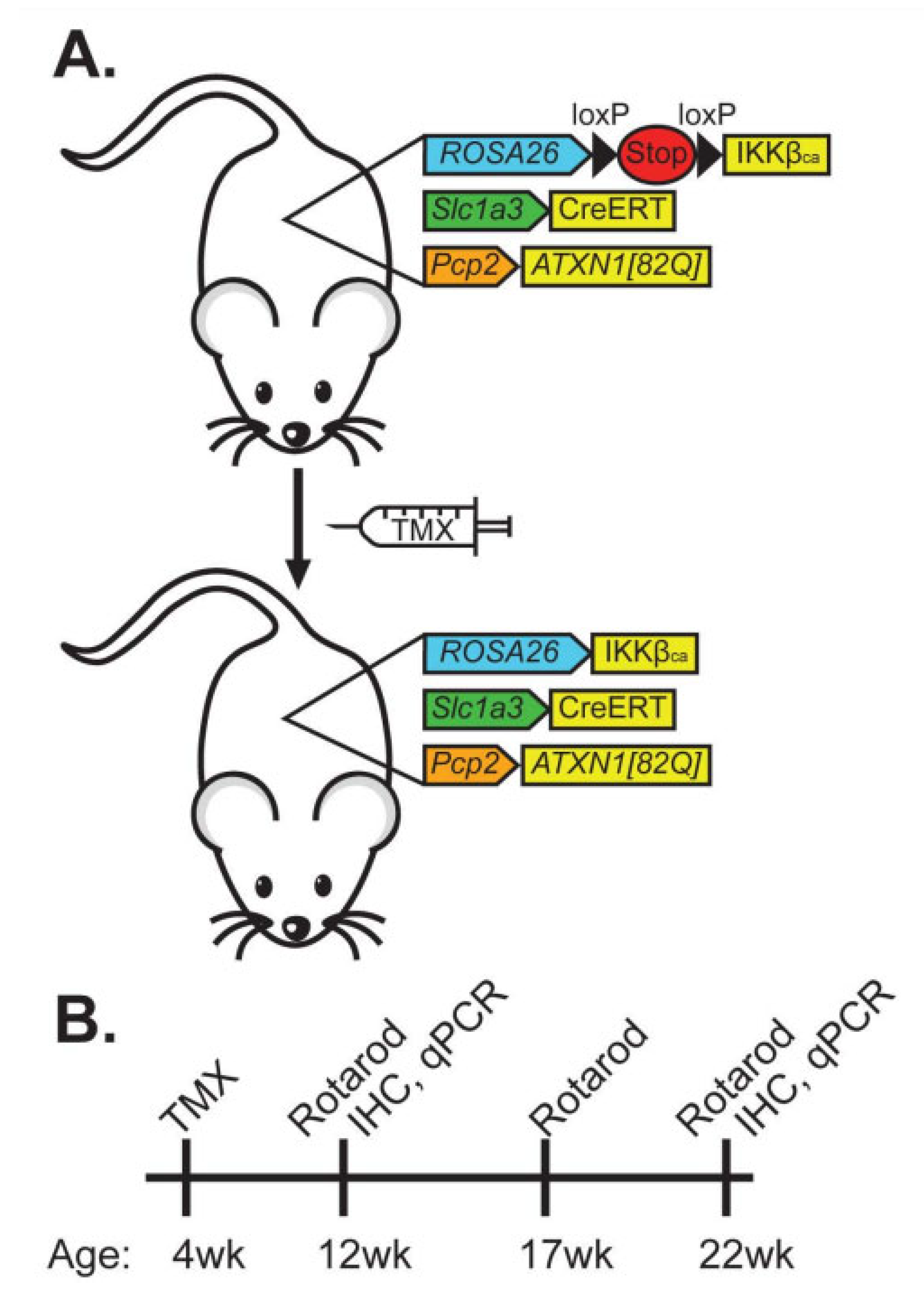
2.1. Generation of ATXN1[82Q];IKKβCA;Slc1a3-CreERT Mice
2.2. Repeat Sequencing
2.3. Rotarod Analysis
2.4. Immunofluorescent (IF) Staining
2.5. Quantitative Analysis of Immunofluorescent Staining
2.6. RT-qPCR
2.7. Statistical Analysis
2.8. Data Availability
3. Results
3.1. Creation of Mice with Conditional, Astroglia-Selective, and TMX-Dependent Expression of Constitutively Active IKKβ
3.2. CAG Repeat Length in Our ATXN1[82Q] Mice Shortened from 82 to 71 Repeats
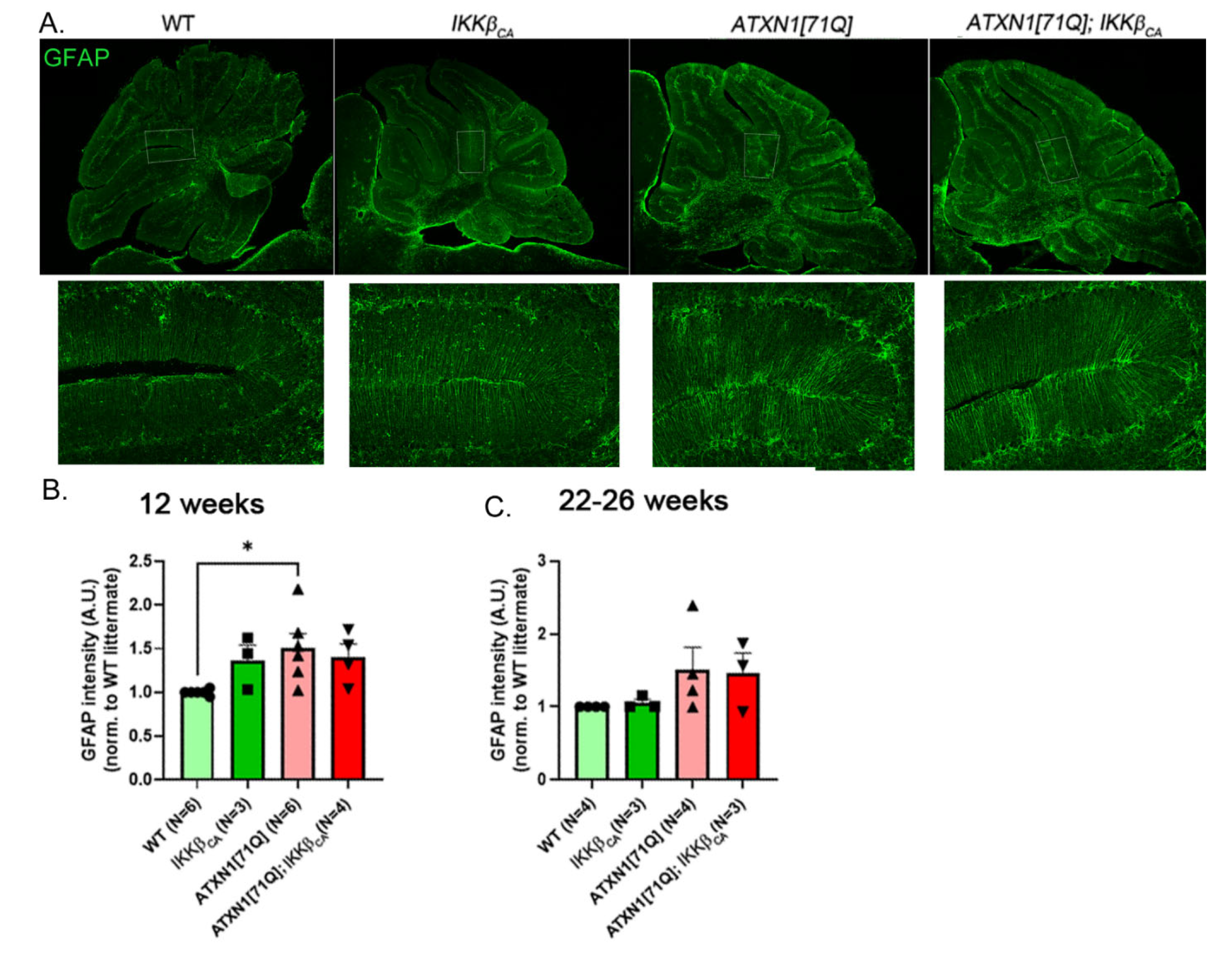
3.3. Expression of IKKβCA Does Not Significantly Alter GFAP Reactivity in Bergmann Glia
3.4. 71 CAG Repeats Is Not Sufficient to Cause Motor Deficits
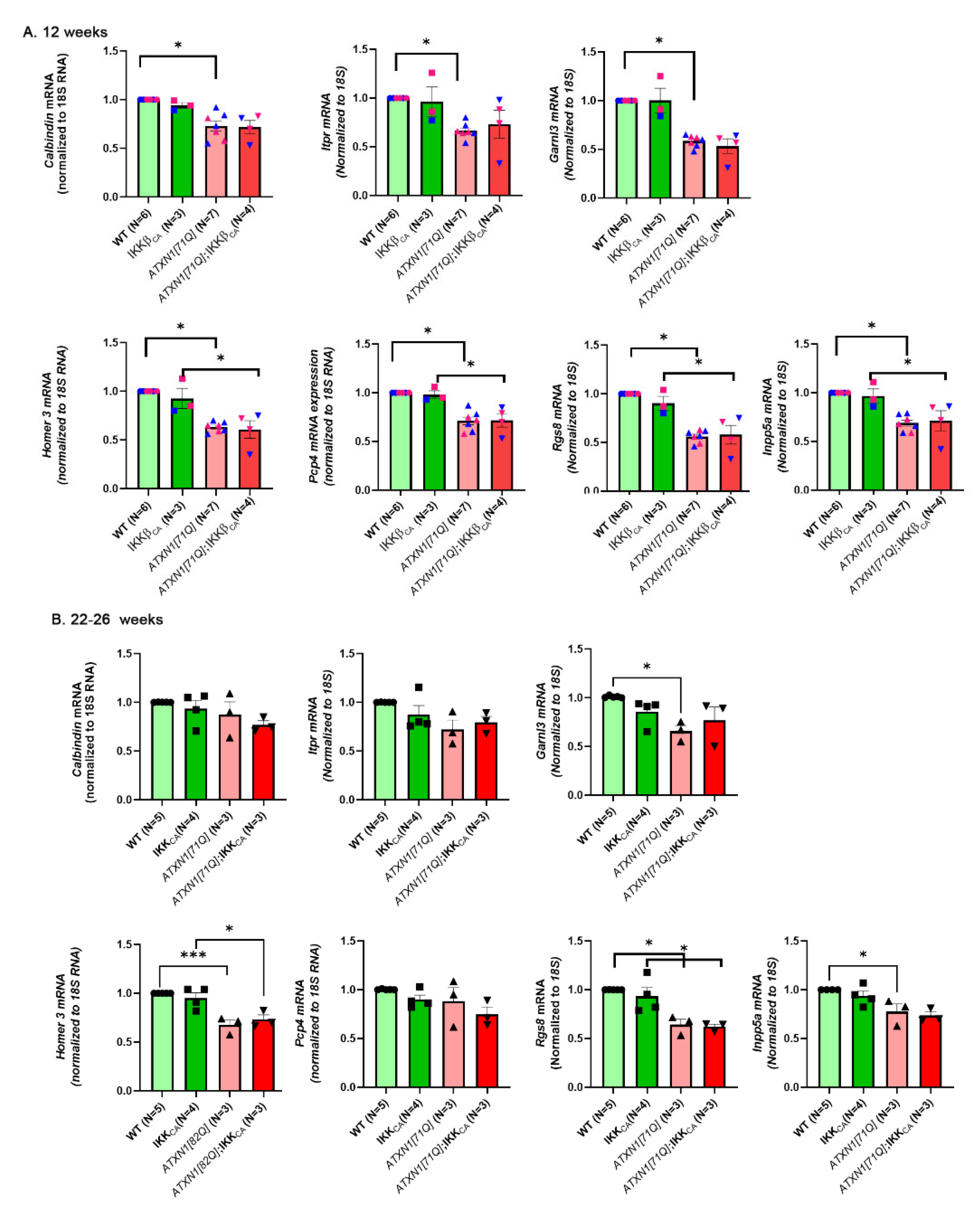
3.5. Pathology of Purkinje Neurons Is Still Detectable in ATXN1[71Q] Mice
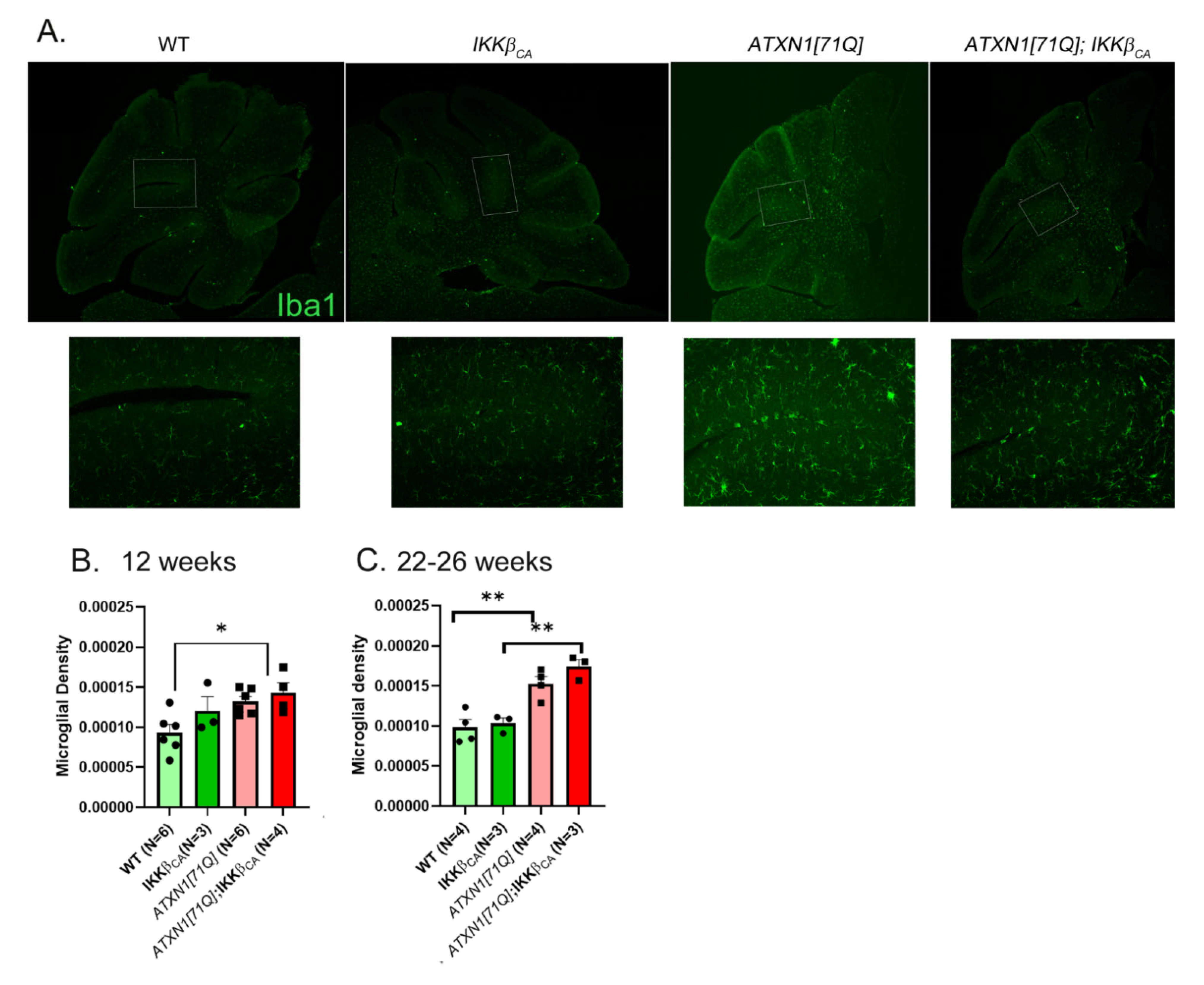
3.6. Activation of Astroglial NF-κB Signaling Early in Disease Slightly Increases Microglial Density
4. Discussion
5. Main Points
- Further activating the NF-κB pathway in astrocytes is not beneficial in Purkinje cell-specific transgenic Spinocerebellar ataxia type 1 mice.
- Modest reduction in CAG repeat length rescues motor deficits but not Purkinje cell pathology in Spinocerebellar ataxia type 1 mice.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zoghbi, H.Y.; Orr, H.T. Pathogenic mechanisms of a polyglutamine-mediated neurodegenerative disease, Spinocerebellar ataxia type 1. J. Biol. Chem. 2009, 284, 7425–7429. [Google Scholar] [CrossRef] [PubMed]
- Banfi, S.; Servadio, A.; Chung, M.; Kwiatkowski, T.J.; McCall, A.E.; Duvick, L.A.; Shen, Y.; Roth, E.J.; Orr, H.T.; Zoghbi, H.Y. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat. Genet. 1994, 7, 513–520. [Google Scholar] [CrossRef]
- Orr, H.; Chung, M.; Banfi, S.; Kwiatkowski, T.J.; Servadio, A.; Beaudet, A.; McCall, A.; Duvick, L.; Ranum, L.; Zoghbi, H. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993, 4, 221–226. [Google Scholar] [CrossRef]
- Matilla-Dueñas, A.; Goold, R.; Giunti, P. Clinical, genetic, molecular, and pathophysiological insights into spinocerebellar ataxia type 1. Cerebellum 2008, 7, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Pollack, M.S.; Lyons, S.L.A.; Ferrell, T.R.E.; Daiger, S.P.; Beaudet, A.L. Spinocerebellar Ataxia: Variable Age of Onset and Linkage to Human Leukocyte Antigen in a Large Kindred. Ann. Neurol. 1988, 23, 580–584. [Google Scholar] [CrossRef]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- Diallo, A.; Jacobi, H.; Tezenas du Montcel, S.; Klockgether, T. Natural history of most common spinocerebellar ataxia: A systematic review and meta-analysis. J. Neurol. 2021, 268, 2749–2756. [Google Scholar] [CrossRef]
- Jacobi, H.; Bauer, P.; Giunti, P.; Labrum, R.; Klockgether, T. The natural history of spinocerebellar. Neurology 2011, 3974, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Donato, S.D.; Mariotti, C.; Taroni, F. Spinocerebellar Ataxia Type 1. In Handbook of Clinical Neurology, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 103. [Google Scholar] [CrossRef]
- Genis, D.; Matilla, T.; Volpini, V.; Rosell, J.; Dávalos, A.; Ferrer, I.; Molins, A.; Estivill, X. Clinical, neuropathologic, and genetic studies of a large spinocerebellar ataxia type 1 (SCA1) kindred: (CAG)n expansion and early premonitory signs and symptoms. Neurology 1995, 45, 24–30. [Google Scholar] [CrossRef]
- Diallo, A.; Jacobi, H.; Cook, A.; Labrum, R.; Durr, A.; Brice, A.; Charles, P.; Marelli, C.; Mariotti, C.; Nanetti, L.; et al. Survival in patients with spinocerebellar ataxia types 1, 2, 3, and 6 (EUROSCA): A longitudinal cohort study. Lancet Neurol. 2018, 17, 327–334. [Google Scholar] [CrossRef]
- Jacobi, H.; du Montcel, S.T.; Bauer, P.; Giunti, P.; Cook, A.; Labrum, R.; Parkinson, M.H.; Durr, A.; Brice, A.; Charles, P.; et al. Long-term disease progression in spinocerebellar ataxia types 1, 2, 3, and 6: A longitudinal cohort study. Lancet Neurol. 2015, 14, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Rüb, U.; Bürk, K.; Timmann, D.; den Dunnen, W.; Seidel, K.; Farrag, K.; Brunt, E.; Heinsen, H.; Egensperger, R.; Bornemann, A.; et al. Spinocerebellar ataxia type 1 (SCA1): New pathoanatomical and clinico-pathological insights. Neuropathol. Appl. Neurobiol. 2012, 38, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Seidel, K.; Siswanto, S.; Brunt, E.R.P. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012, 124, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Servadio, A.; Koshy, B.; Armstrong, D.; Antalffy, B.; Orr, H.T.; Zoghbi, H.Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat. Genet. 1995, 10, 94–98. [Google Scholar] [CrossRef]
- Cerrato, V. Cerebellar astrocytes: Much more than passive bystanders in ataxia pathophysiology. J. Clin. Med. 2020, 9, 757. [Google Scholar] [CrossRef]
- Yamada, K.; Watanabe, M. Cytodifferentiation of Bergmann glia and its relationship with Purkinje cells. Anat. Sci. Int./Jpn. Assoc. Anat. 2002, 77, 94–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Q.; Wang, W.; Takano, T.; Nedergaard, M. Bergmann glia modulate cerebellar Purkinje cell bistability via Ca2+−dependent K+ uptake. Proc. Natl. Acad. Sci. USA 2012, 109, 7911–7916. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef]
- Escartin, C.; Carmignoto, G.; Agarwal, A.; Allen, N.J.; Araque, A.; Barbeito, L.; Quintana, F.J.; Ransohoff, R.M.; Riquelme-perez, M.; Robel, S.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Pekny, M.; Wilhelmsson, U.; Pekna, M. The dual role of astrocyte activation and reactive gliosis. Neurosci. Lett. 2014, 565, 30–38. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Phatnani, H.; Maniatis, T. Astrocytes in Neurodegenerative Disease. Cold Spring Harb. Perspect. Biol. 2015, 7, a020628. [Google Scholar] [CrossRef]
- Barres, B.A. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron 2008, 60, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, H.; Polymenidou, M.; Cleveland, D.W. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009, 187, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Diaz-Castro, B.; Looger, L.L.; Baljit, S. Khakh Dysfunctional Calcium and Glutamate Signaling in Striatal Astrocytes from Huntington’s Disease Model Mice. J. Neurosci. 2016, 36, 3453–3470. [Google Scholar] [CrossRef]
- Sheeler, C.; Rosa, J.; Ferro, A.; Mcadams, B.; Borgenheimer, E.; Cvetanovic, M. Glia in Neurodegeneration: The Housekeeper, the Defender and the Perpetrator. Int. J. Mol. Sci. 2020, 21, 9188. [Google Scholar] [CrossRef]
- Yamanaka, K.; Chun, S.J.; Boillee, S.; Fujimori-Tonou, N.; Yamashita, H.; Gutmann, D.H.; Takahashi, R.; Misawa, H.; Cleveland, D.W. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008, 11, 251–253. [Google Scholar] [CrossRef]
- Cvetanovic, M.; Ingram, M.; Orr, H.; Opal, P. Early activation of microglia and astrocytes in mouse models of spinocerebellar ataxia type 1. Neuroscience 2015, 289, 289–299. [Google Scholar] [CrossRef]
- Ferro, A.; Qu, W.; Lukowicz, A.; Svedberg, D.; Johnson, A.; Cvetanovic, M. Inhibition of NF-κB signaling in IKKβ F/F;LysM Cre mice causes motor deficits but does not alter pathogenesis of Spinocerebellar ataxia type 1. PLoS ONE 2018, 13, e0200013. [Google Scholar] [CrossRef]
- Burright, E.N.; Clark, B.H.; Servadio, A.; Matilla, T.; Feddersen, R.M.; Yunis, W.S.; Duvick, L.; Zoghbi, H.Y.; Orr, H.T. SCA1 transgenic mice: A model for neurodegeneration caused by an expanded CAG trinucleotide repeat. Cell 1995, 82, 937–948. [Google Scholar] [CrossRef]
- Kim, J.H.; Lukowicz, A.; Qu, W.; Johnson, A.; Cvetanovic, M. Astroglia contribute to the pathogenesis of spinocerebellar ataxia Type 1 ( SCA1 ) in a biphasic, stage-of-disease specific manner. Glia 2018, 66, 1972–1987. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human Astrocytes: Secretome Profiles of Cytokines and Chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.Y.; Chen, Y.C.; Chen, H.M.; Tu, P.H.; Chern, Y. A critical role of astrocyte-mediated nuclear factor-κB-dependent inflammation in huntington’s disease. Hum. Mol. Genet. 2013, 22, 1826–1842. [Google Scholar] [CrossRef] [PubMed]
- Mincheva-Tasheva, S.; Soler, R.M. NF- B Signaling Pathways: Role in Nervous System Physiology and Pathology. Neuroscience 2012, 19, 175–194. [Google Scholar] [CrossRef]
- Delhase, M.; Hayakawa, M.; Chen, Y.; Karin, M. Positive and negative regulation of IkappaB kinase activity through IKKbeta subunit phosphorylation. Science 1999, 284, 309–314. [Google Scholar] [CrossRef]
- Dunn, S.L.; Young, E.A.; Hall, M.D.; McNulty, S. Activation of astrocyte intracellular signaling pathways by interleukin-1 in rat primary striatal cultures. Glia 2002, 37, 31–42. [Google Scholar] [CrossRef]
- Zandi, E.; Rothwarf, D.M.; Delhase, M.; Hayakawa, M.; Karin, M. The IkB Kinase Complex (IKK) Contains Two Kinase Subunits, IKKa and IKKb, Necessary for IkB Phosphorylation and NF-kB Activation. Cell 1997, 91, 243–252. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Zhong, H.; May, M.J.; Jimi, E.; Ghosh, S. The phosphorylation status of nuclear NF-kB determines its association with CBP/p300 or HDAC-1. Mol. Cell 2002, 9, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Bracchi-Ricard, V.; Hu, W.-H.; Frydel, B.; Bramwell, A.; Karmally, S.; Green, E.J.; Bethea, J.R. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J. Exp. Med. 2005, 202, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Persaud, T.; Hu, X.; Karmally, S.; Shestopalov, V.I.; Dvoriantchikova, G.; Ivanov, D.; Nathanson, L.; Barnum, S.R.; Bethea, J.R. Transgenic Inhibition of Astroglial NF-κB Improves Functional Outcome in Experimental Autoimmune Encephalomyelitis by Suppressing Chronic Central Nervous System Inflammation. J. Immunol. 2009, 182, 2628–2640. [Google Scholar] [CrossRef]
- Fu, E.S.; Ping, Y.; Sagen, J.; Candiotti, K.A.; Morton, P.D.; Liebl, D.J.; Bethea, J.R.; Brambilla, R. Transgenic inhibition of glial NF-kappa B reduces pain behavior and inflammation after peripheral nerve injury. Pain 2010, 148, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Litvinchuk, A.; Chiang, A.C.-A.; Aithmitti, N.; Jankowsky, J.L.; Zheng, H. Astrocyte-Microglia Cross Talk through Complement Activation Modulates Amyloid Pathology in Mouse Models of Alzheimer’s Disease. J. Neurosci. 2016, 36, 577–589. [Google Scholar] [CrossRef]
- Sasaki, Y.; Derudder, E.; Hobeika, E.; Pelanda, R.; Reth, M.; Rajewsky, K.; Schmidt-Supprian, M. Canonical NF-??B Activity, Dispensable for B Cell Development, Replaces BAFF-Receptor Signals and Promotes B Cell Proliferation upon Activation. Immunity 2006, 24, 729–739. [Google Scholar] [CrossRef]
- Ebner, B.A.; Ingram, M.A.; Barnes, J.A.; Duvick, L.A.; Frisch, J.L.; Clark, H.B.; Zoghbi, H.Y.; Ebner, T.J.; Orr, H.T. Purkinje Cell Ataxin-1 Modulates Climbing Fiber Synaptic Input in Developing and Adult Mouse Cerebellum. J. Neurosci. 2013, 33, 5806–5820. [Google Scholar] [CrossRef]
- Qu, W.; Johnson, A.; Kim, J.H.; Lukowicz, A.; Svedberg, D.; Cvetanovic, M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J. Neuroinflamm. 2017, 14, 107. [Google Scholar] [CrossRef]
- Clark, H.B.; Burright, E.N.; Yunis, W.S.; Larson, S.; Wilcox, C.; Hartman, B.; Matilla, A.; Zoghbi, H.Y.; Orr, H.T. Purkinje Cell Expression of a Mutant Allele of SCA1 in Transgenic Mice Leads to Disparate Effects on Motor Behaviors, Followed by a Progressive Cerebellar Dysfunction and Histological Alterations. J. Neurosci. 1997, 17, 7385–7395. [Google Scholar] [CrossRef]
- Serra, H.G.; Duvick, L.; Zu, T.; Carlson, K.; Stevens, S.; Jorgensen, N.; Lysholm, A.; Burright, E.; Zoghbi, H.Y.; Clark, H.B.; et al. ROR a-Mediated Purkinje Cell Development Determines Disease Severity in Adult SCA1 Mice. Cell 2006, 127, 697–708. [Google Scholar] [CrossRef]
- Xia, H.; Mao, Q.; Eliason, S.L.; Harper, S.Q.; Martins, I.H.; Orr, H.T.; Paulson, H.L.; Yang, L.; Kotin, R.M.; Davidson, B.L. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat. Med. 2004, 10, 816–820. [Google Scholar] [CrossRef]
- Duvick, L.; Barnes, J.; Ebner, B.; Agrawal, S.; Andresen, M.; Lim, J.; Giesler, G.J.; Zoghbi, H.Y.; Orr, H.T. SCA1-like disease in mice expressing wild-type Ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron 2010, 67, 929–935. [Google Scholar] [CrossRef]
- Klement, I.A.; Skinner, P.J.; Kaytor, M.D.; Yi, H.; Hersch, S.M.; Clark, H.B.; Zoghbi, H.Y.; Orr, H.T. Ataxin-1 Nuclear Localization and Aggregation: Role in Polyglutamine-Induced Disease in SCA1 Transgenic Mice. Cell 1998, 95, 41–53. [Google Scholar] [CrossRef]
- Lei, Z.; Yue, Y.; Stone, S.; Wu, S.; Lin, W. NF-kB activation accounts for the cytoprotective effects of PERK activation on oligodendrocytes during EAE. J. Neurosci. 2020, 40, 6444–6456. [Google Scholar] [CrossRef]
- Madisen, L.; Garner, A.R.; Shimaoka, D.; Chuong, A.S.; Klapoetke, N.C.; Li, L.; van der Bourg, A.; Niino, Y.; Egolf, L.; Monetti, C.; et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 2015, 85, 942–958. [Google Scholar] [CrossRef]
- Paukert, M.; Agarwal, A.; Cha, J.; Doze, V.A.; Kang, J.U.; Bergles, D.E. Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 2014, 82, 1263–1270. [Google Scholar] [CrossRef]
- Gennarino, V.; Singh, R.K.; White, J.J.; De Maio, A.; Han, K.; Kim, J.Y.; Jafar-Nejad, P.; Di Ronza, A.; Kang, H.; Sayegh, L.S.; et al. Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels. Cell 2015, 160, 1087–1098. [Google Scholar] [CrossRef]
- Mellesmoen, A.; Sheeler, C.; Ferro, A.; Rainwater, O.; Cvetanovic, M. Brain Derived Neurotrophic Factor ( BDNF ) Delays Onset of Pathogenesis in Transgenic Mouse Model of Spinocerebellar Ataxia Type 1 (SCA1). Front. Cell Neurosci. 2019, 12, 509. [Google Scholar] [CrossRef]
- Aguzzi, A.; Barres, B.A.; Bennett, M.L. Microglia: Scapegoat, saboteur, or something else? Science 2013, 339, 156–161. [Google Scholar] [CrossRef]
- Prinz, M.; Mildner, A. Microglia in the CNS: Immigrants from another world. Glia 2011, 59, 177–187. [Google Scholar] [CrossRef]
- Chen, S.H.; Oyarzabal, E.A.; Sung, Y.F.; Chu, C.H.; Wang, Q.; Chen, S.L.; Lu, R.B.; Hong, J.S. Microglial regulation of immunological and neuroprotective functions of astroglia. Glia 2015, 63, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Lian, H.; Yang, L.; Cole, A.; Sun, L.; Chiang, A.C.; Fowler, S.W.; Shim, D.J.; Rodriguez-Rivera, J.; Taglialatela, G.; Jankowsky, J.L.; et al. NFκB-Activated Astroglial Release of Complement C3 Compromises Neuronal Morphology and Function Associated with Alzheimer’s Disease. Neuron 2015, 85, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Lattke, M.; Reichel, S.N.; Magnutzki, A.; Abaei, A.; Rasche, V.; Walther, P.; Calado, D.P.; Ferger, B.; Wirth, T.; Baumann, B. Transient IKK2 activation in astrocytes initiates selective non-cell-autonomous neurodegeneration. Mol. Neurodegener. 2017, 12, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ouali Alami, N.; Schurr, C.; Olde Heuvel, F.; Tang, L.; Li, Q.; Tasdogan, A.; Kimbara, A.; Nettekoven, M.; Ottaviani, G.; Raposo, C.; et al. NF-κB activation in astrocytes drives a stage-specific beneficial neuroimmunological response in ALS. EMBO J. 2018, 37, e98697. [Google Scholar] [CrossRef]



| Primer Name | Gene Group | PrimeTime or Custom | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|---|---|
| Calbindin | Magenta | Custom | AAG-GCT-TTT-GAG-TTA-TAT-GAT-CAG | TTC-TTC-TCA-CAC-AGA-TCT-TTC-AGC |
| PCP4 | Magenta | Custom | CCA-ACG-GAA-AAG-ACA-AGA-CG | TGT-CGA-TAT-CAA-ATT-CTT-CTT-GGA |
| Homer3 | Magenta | Custom | TGA-AGA-AGA-TGC-TGT-CAG-AAG-G | CTG-TCC-TGA-AGC-GCG-AAG |
| RGS8 | Magenta | Custom | CTG-TCA-CAC-AAA-TCA-GAC-TCC-TG | TGC-TTC-TTC-CGT-GGA-GAG-TC |
| ITPR | Magenta | Custom | GAA-GGC-ATC-TTT-GGA-GGA-AGT | ACC-CTG-AGG-AAG-GTT-CTG-C |
| Inpp5a | Magenta | Custom | ATT-CGG-ACA-CTT-TGG-AGA-GC | CCT-TTT-CTT-GAC-CAT-TTG-CAC |
| Garnl3 | Magenta | Custom | TCA-TGA-AGC-CGT-GTG-TGC | CAG-GGA-TGG-GAG-GTC-ATC |
| 18S rRNA | Reference gene (used in all experiments) | Custom | AGT-CCC-TGC-CCT-TTG-TAC-ACA | CGA-TCC-GAG-GGC-CTC-ACT-A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilliat, S.; Rosa, J.-G.; Benjamin, G.; Sbrocco, K.; Lin, W.; Cvetanovic, M. Modest Reduction in CAG Repeat Length Rescues Motor Deficits but Not Purkinje Cell Pathology and Gliosis in Spinocerebellar Ataxia Type 1 Mice. Neuroglia 2023, 4, 52-68. https://doi.org/10.3390/neuroglia4010005
Gilliat S, Rosa J-G, Benjamin G, Sbrocco K, Lin W, Cvetanovic M. Modest Reduction in CAG Repeat Length Rescues Motor Deficits but Not Purkinje Cell Pathology and Gliosis in Spinocerebellar Ataxia Type 1 Mice. Neuroglia. 2023; 4(1):52-68. https://doi.org/10.3390/neuroglia4010005
Chicago/Turabian StyleGilliat, Stephen, Juao-Guilherme Rosa, Genevieve Benjamin, Kaelin Sbrocco, Wensheng Lin, and Marija Cvetanovic. 2023. "Modest Reduction in CAG Repeat Length Rescues Motor Deficits but Not Purkinje Cell Pathology and Gliosis in Spinocerebellar Ataxia Type 1 Mice" Neuroglia 4, no. 1: 52-68. https://doi.org/10.3390/neuroglia4010005
APA StyleGilliat, S., Rosa, J.-G., Benjamin, G., Sbrocco, K., Lin, W., & Cvetanovic, M. (2023). Modest Reduction in CAG Repeat Length Rescues Motor Deficits but Not Purkinje Cell Pathology and Gliosis in Spinocerebellar Ataxia Type 1 Mice. Neuroglia, 4(1), 52-68. https://doi.org/10.3390/neuroglia4010005






