Abstract
Chronic itch (CI) is an unpleasant skin sensation accompanied by an intense scratching desire that lasts 6 weeks or longer. Despite the high prevalence and negative impact on affected individuals and a huge healthcare burden, CI mechanisms are only partially understood, and consequently, treatment of CI remains sub-optimal. The complexity of CI treatment also stems from the comorbid existence of persistent itch with other somatic and psychological disorders. Etiologies of CI are multiple and diverse, although CI is often a result of dermatologically related conditions such as atopic dermatitis and psoriasis. Unfolding the pathophysiology of CI can provide possibilities for better therapy. Itch signaling is complex and neurons and non-neuronal cells play a role. This review focuses on recent findings on the role of glial cells in itch. Central glia (astrocytes and microglia) and peripheral glia (satellite glial cells and Schwann cells) are found to contribute to the development or persistence of itch. Hence, glial modulation has been proposed as a potential option in CI treatment. In experimental models of itch, the blockade of signal transducer and the activator of transcription (STAT) 3-mediated reactive astrogliosis have been shown to suppress chronic itch. Administration of a microglial inhibitor, minocycline, has also been demonstrated to suppress itch-related microglial activation and itch. In sensory ganglia, gap-junction blockers have successfully blocked itch, and hence, gap-junction-mediated coupling, with a potential role of satellite glial cells have been proposed. This review presents examples of glial involvement in itch and opportunities and challenges of glial modulation for targeting itch.
1. Introduction
Chronic itch (CI) is a common medical condition that is highly disturbing for the affected patients and their families and poses a burden to the healthcare system and healthcare economy [1]. However, it has remained poorly controlled, partially due to a complex pathogenesis that is not yet fully understood. The complex neurophysiology of itch is gradually being unfolded. Recent investigations have resulted in the identification of cellular and molecular aspects of itch-specific neuronal signaling pathways [2]. In parallel, the potential role of non-neuronal cells, glial cells, in the development and maintenance of itch has attracted great scientific attention [3]. This scientific curiosity is fueled by the fact that current therapeutic strategies are only partially effective, and hence, the identification of novel targets is warranted [4]. In this line, glial modulation seems to have the potential for targeting itch [5]. Evidence shows that both peripheral and central glia contribute to the processing of itch [3]. Interestingly, the timing and type of contribution among these cells are found to be different [6]. Experimental results also show that glial modulation can potentially be a novel target for itch modulation [7]. This focused review aims to present what is known about the contribution of astrocytes, microglia, satellite glial cells (SGCs), and Schwann cells (SCs) in the development or persistence of itch and how glial modulation could be a potential future target in the therapeutic options for CI. Since glial modulation is not a challenge-free approach, limitations and future perspectives are also presented.
2. Chronic Itch, Risk Factors, and Underlying Mechanisms
2.1. Chronic Itch Epidemiology and Etiology
Chronic itch (CI), or chronic pruritus, is an unpleasant skin sensation accompanied by a desire to scratch that persists for more than 6 weeks [8]. CI is a common and irritating symptom with an up to 20% lifetime prevalence and is known to be associated with a diverse range of dermatological and non-dermatological medical conditions [1]. Cutaneous conditions that accompany itch include but are not limited to psoriasis, atopic dermatitis, and lichen planus [9]. Although itch in dermatological conditions is highly prevalent, and hence, more studied [1], persistent itch also appears as a problematic symptom in chronic renal disease, chronic hepatobiliary conditions, diabetes mellitus, hypothyroidism, and some malignancies [10]. In fact, dermatologic, systemic, neurologic, psychogenic, mixed, or unknown etiologies are all considered to underlie CI [11] (Table 1). As a consequence, a diverse range of clinical settings and clinicians receive patients with CI. CI poses a highly negative impact on affected individuals and their families, and dramatically reduces various domains of life quality [12].

Table 1.
Various itch etiologies and examples of potential mechanisms.
Table 1 depicts the major etiological aspects proposed in the literature [13] that most likely underlie chronic itch.
Its complex pathogenesis, together with numerous contributing factors, has made CI a medical challenge to treat, and independent of the type of therapy, a care gap exists, with insufficient itch relief [1,14]. Recent investigations in pruritus and antipruritic have revealed a number of mechanistic aspects of CI and, consequently, new possibilities for novel therapeutic options [15,16].
2.2. Proposed Mechanisms Underlying Chronic Itch
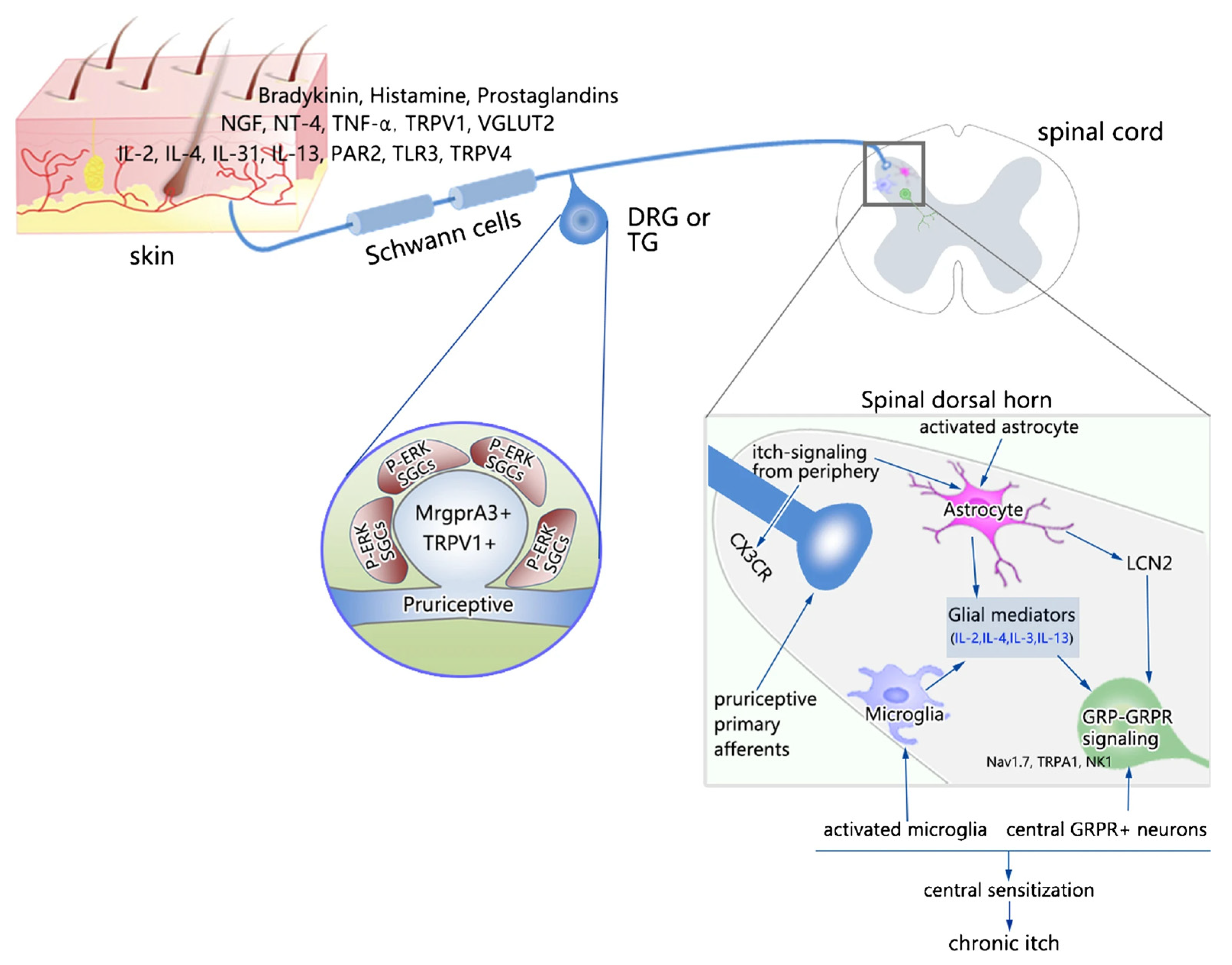
In the complex pathogenesis of CI, major contributing elements are neuronal, immune, and non-neuronal cells [17,18,19]. The role of immune cells and their cross-talk with neurons has been the subject of growing interest, and immune therapy of CI has been explored [5,20]. For example, in atopic dermatitis, cross-talk between the nervous system, cutaneous immune system, and keratinocytes is proposed [21]. Itch pathways in the peripheral nervous system (PNS) and central nervous system (CNS) have been reviewed in great detail in excellent recent reviews summarizing our understanding of both physiological and pathological itch [4,5,22,23], including the sensitization that occurs in the itch circuitry at both the peripheral and central nervous systems [24]. For example, TLRs that are expressed in neurons and glial and immune cells have been implicated in itch [25,26]. Consequently, these advancements have resulted in the opening of new therapeutic options for CI [15]. Figure 1 depicts an overview of proposed peripheral and central mechanisms of sensitization of itch processing [24]. It is suggested that the release of inflammatory and immune modulators leads to the activation of pruriceptive neurons and peripheral glia, followed by long-lasting alterations in neuronal sensitivities and the activation of central glial cells.
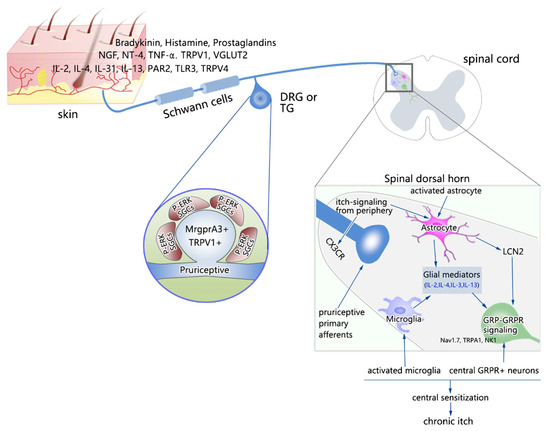
Figure 1.
Peripheral and central mechanisms of sensitization of itch processing.
Abbreviations: BDNF, brain-derived neurotrophic factor; CX3CR, C-X-C motif chemokine receptor 3; GRP, gastrin-releasing peptide; GRPR, gastrin-releasing peptide receptor; LCN2, lipocalin-2; NK1, neurokinin 1; NT-4, neurotrophin-4; PAR2, protease-activated receptor 2; PGE2, prostaglandin E2; TLR3, Toll-like receptor 3; TLR4, Toll-like receptor 4; TNF-α, tumor necrosis factor-α; TRPA1, transient receptor potential ankyrin 1; TRPV1, transient receptor potential vanilloid 1; TRPV4, transient receptor potential vanilloid 4; VGLUT2, vesicular glutamate transporter 2. This figure is reused from [24] with permission granted by Springer Nature (License Number 5438930655438).
2.3. Risk Factors for Chronic Itch
The development and persistence of itch in CI conditions have been associated with numerous factors [25] that are categorized into three larger domains: predisposing factors, triggering factors, and maintaining and/or aggravating factors. Interestingly, these factors can act separately or in combination. A link has been proposed between these factors to underly or, at least in part, explain the transition from acute to chronic states of itch [25]. Table 2 summarizes these categories with a few examples.

Table 2.
Various factors and examples suggested to play roles in acute, chronic, and transition from acute to chronic itch.
Since the focus of this review is on glial cells’ involvement in CI, in the following sections, the nervous system’s glial cells are presented first, and thereafter, the roles of these cells in the development and persistence of CI are presented with examples.
3. Glial Cells of the Nervous System
Glial cells are non-neuronal components of the nervous system and are broadly divided into the glial residents of the PNS and the glial residents of the CNS [27]. These cells [28] are critical to maintaining nervous system homeostasis, but they also have an important role in signal transductions and immune response regulation in the CNS and PNS. Glia is currently recognized as cells with a diverse, important, and dynamic range of functions [28].
Glial cells of the CNS include astrocytes, microglia, oligodendrocytes, and radial glia. Each of these cells takes on a distinct task in the nervous system, for example, roles in homeostasis and synaptic transmission [29]. Advancement in the understanding of glial cell function and interactions between glial cells and neuron–glia help to better understand the physiological and pathological conditions of the nervous system [29].
In the peripheral nervous system, specialized cells are generated during neuronal development. These cells include satellite glial cells (SGCs) that are located in the sensory and autonomic ganglia, olfactory ensheathing cells, Schwann cells, and enteric glia [30]. SGCs are presented with a unique morphological feature where they wrap around neuronal cell bodies shaping a complete envelope [31]. This unit is formed in sensory and autonomic ganglia and allows for close interaction between SGCs and neurons in the PNS [31]. Neuroglia types [32] are presented in Table 3.

Table 3.
Neuroglia types.
It is becoming clearer that the perturbation of glial function leads to a range of pathological conditions, including nervous system disorders, for example, Parkinson’s and Alzheimer’s diseases [33], but also chronic pain [34]. Although central glia (microglia and astrocytes) have been studied extensively to understand their roles in the initiation and maintenance of chronic pain [34], the role of peripheral glia, SGCs, and SCs in chronic pain has also attracted scientific attention [24]. Discoveries in this field have opened up the therapeutic promise of targeting the treatment of chronic pain focusing on both neuronal and glial modulations [35]. Pain and itch are well-known to have a large overlap in terms of common mediators, receptors, and signaling pathways [24]. In line with this, the involvement of glial cells in the PNS and CNS in persistent itch was proposed [3,6,36], and consequently, the possibility of glial modulation for CI has emerged and been discussed [37].
In the below sections, examples have been outlined of the involvement of glial cells in itch, and according to the identified potential mechanisms, therapeutic potentials are also proposed.
4. Glial Cells and Itch
4.1. Astrocytes in Itch
Astrocytes play a key role in the CNS to maintain homeostasis, and their role in brain pathologies, such as neurological and neuropsychiatric disorders, has recently been reviewed [36,38]. Astrocytes comprise a large cell population that accounts for up to 40% of glial cells in the CNS [39]. Traditionally, these cells were known as principal cells to maintain structure and environmental support for neurons, but currently, it is widely accepted that astrocytes have critical roles in neural processes [40,41]. Astrocytes are coupled by gap junctions [42], where they are capable of exchanging ions and small cytosolic components. These cells express the glial fibrillary acidic protein (GFAP), which is a marker of their activation [43]. These cells are also adjacent to the cerebral blood vessels, and because of this feature, astrocytes are involved in the regulation of blood flow during neuronal activation [44]. It is reported that in the human brain, an astrocyte can contact up to 2 million synapses [45].
It is proposed that astrocytes might drive pathogenesis via neuroinflammatory mechanisms [46]. This mechanism has been discussed for various CNS disorders [47,48]. The role of astrocytes in chronic pain has been noted earlier, and hence, has been investigated more [49]; however, evidence is accumulating about the involvement of astrocytes in CI [20,50]. Consequently, targeting the pathological contribution of astrocytes, in particular spinal astrocytes [20], has opened up new strategies for the treatment of CI [3,37,51].
4.1.1. Activation of Spinal Astrocytes in the Itch–Scratch Cycle
Long-term scratching and itching behaviors have been reported in mouse models of dermatitis (both atopic and contact dermatitis). In parallel, in the dermatomes of lesioned areas, long-term activation of spinal astrocytes has been found [36,37]. It has been shown that when the scratching is prevented, spinal astrogliosis can be suppressed [50]. This finding emphasizes the role of scratching in astrogliosis in CI. In addition, when an astroglial inhibitor, l- α-aminoadipate, was administered intrathecally, CI was diminished in a mouse model of dry skin injury [52]. This finding was also confirmatory for the contribution of spinal astroglia in CI.
Another finding comes from the observations that severe CI is present in patients with cutaneous T cell lymphoma (CTCL), and in a mouse model of CTCL184, persistent astrogliosis could be identified to link the observation in patients with a modeled problem [53]. These findings suggest that astrocytes play an essential role in CI. It remains to be determined if the activation of astrocytes will be sufficient to provoke scratching behavior [4,5].
4.1.2. Targeting Astrocytes in Itch
In the development and maintenance of CI, an important receptor is TLR4, expressed in spinal astrocytes [52]. Another target is STAT3, which has been found activated in astroglia in CI, [54]. This has been confirmed by the disruption of STAT3 in astroglia, for example, by pharmacological inhibition that was able to suppress CI in an experimental mouse model [54]. It has been reported that upregulation of the innate immune factor lipocalin-2 (LCN2), which is a STAT3-dependent process, might be the key to the underlying mechanism [54,55]. LCN2 is secreted from astrocytes and can exacerbate itch evoked by spinal injection of gastrin-releasing peptide (GRP). The itch circuitry of GRP-GRPR in the spinal cord has been known [56].
The IL-33 receptor (ST2) is also upregulated in the spinal cord astrocytes, and it is suggested to play a role in CI [57]. The spinal IL-33–ST2 signaling pathway is proposed to be responsible for CI through the activation of the JAK2–STAT3 cascade [58] in spinal astrocytes.
Itch is also related to mechanical senses [57,59], and the role of PIEZO1 that transduces mechanical itch in mice is currently under further investigation [60]. PIEZO1 is expressed in mice, and it is a functional ion channel protein that is sensitive to mechanical pressure. Expression of this receptor has been reported in two types of sensory neurons that were already implicated in chemical-evoked itch, for example, after the application of histamine [60]. Evidence shows that over-expression of PIEZO1 in mice results in hypersensitivity to itch stimuli, and pharmacological blockade of PIEZO1 can subside scratching in mice [60]. We only partially know about the potential role of astrocytes in mechanical itch. So far, as it is compared with the chemical itch, no particular role has been presented for astrocytes in mechanical itch [61]. However, spinal astrocytes are demonstrated with a role in alloknesis [52]. For example, dry skin results in alloknesis that can be blocked pharmacologically by an astroglial inhibitor or a TLR4 antagonist. This confirms that astrocytes are potentially involved in mechanically induced itch via the TLR signaling pathway.
Dry skin also leads to the overexpression of spinal CXCR3 and its ligand CXCL10, and CI in this condition can be suppressed by the administration of a CXCR3 antagonist or by knocking out CXCR3 [62]. These observations show the importance of cytokines and chemokines in promoting CI through the activation of astrocytes [20]. Collectively, studies [36,37] show that manipulations of STAT3 and TLR4 in astrocytes via various techniques, such as pharmacological tools or genetic ablation, can reduce CI [6]. In contrast, these manipulations do not affect acute itch that is provoked by histaminergic and non-histaminergic stimulation. Experimental evidence shows that L-α-aminoadipate could not block scratching evoked by chloroquine or histamine [50]. In addition, L-α-aminoadipate could subside acetone/ether/water (AEW)-evoked CI and alloknesis but did not affect acute itch [52]. Therefore, in CI, targeting astrocytic molecules might be a promising target for therapeutic purposes.
4.1.3. Clinical Implication of Targeting Astrocytes in Itch
We still do not have a piece of clinical evidence for reactive astrocytes in patients with CI, for example, in those with atopic dermatitis. In addition, astroglial activation is seen in both chronic pain and CI, and any overlapping mechanism needs further investigation. Only a few studies are looking into a distinction when these two conditions occur separately, i.e., in chronic pain and CI [36,50]. This is particularly valuable as sensitization occurs in both conditions [24,26]. Perhaps combined studies where we can study both itch and pain can help in understanding the pathogenesis of co-occurrence and the development of novel therapeutic agents. It is worth mentioning that the interaction of chronic pain and itch is only partially understood, and a complex mechanism at a different level of neuraxis is proposed [63,64].
4.2. Microglia in Itch
The role of spinal microglia in neuropathic pain [65,66,67] has been investigated to a larger extent than CI [68]. Below examples are provided from the studies that investigated the role of microglia in itch and how its modulation can influence itch.
In a mouse model of CI induced by repeated applications of 2, 4-dinitrofluorobenzene (DNFB) [69], long-term scratching lasting 7 days after the final dose became evident. Spinal microglia activation was also recorded in this study, and both behavioral and spinal microglial activation was blocked by a microglial inhibitor. The authors [69] demonstrated that this effect was at least in part mediated via increased signaling in the p38 MAPK pathway that was reversed by a p38 inhibitor. Another signaling pathway that was investigated in this study was the fractalkine/CX3CR1 signaling pathway that was activated in the DNFB-induced pruritus model [68]. Antiserum against CX3CR1 or FKN could inhibit p38 activity and suppress scratching [69]. These findings presented that microglia contribute to prolonged itch via FKN/CX3CR1/p38MAPK pathways [69]. This pathway is interestingly involved in chronic pain with the activation of the FKN/CX3CR1 in neuron–microglia communication [70].
In a mouse model of psoriasis-induced CI [68], 5% imiquimod (IMQ) cream was used daily on the shaved skin for 7 days, and a higher expression of a microglial marker (Iba1, ionized calcium-binding adaptor molecule-1) was observed [68]. The administration of minocycline in this model suppressed itch and microglial activation [68]. The authors reported a sex-related response in the model with higher scratch and alloknesis scores in females. IMQ is a Toll-like receptor 7 (TLR7) agonist, and it has been shown that TLR7 mediates pruritus [71]. The sex-dependent response phenomenon in itch needs further investigation to identify whether it is mediated via the differential expression of TLR7 in sensory neurons or glia [68]. Chen et al. [72] have shown that microglia regulate inflammatory and neuropathic pain in male mice, but astrocytes regulate neuropathic pain in both male and female mice [72].
It has been reported that the expression of P2X4R in microglial cells can be manipulated by pharmacological agents and genetics that can influence itch behaviors [36]. The pattern of microglial activation in chronic pain and CI might look similar [3], but interestingly, there is no injury in terms of glial activation in itch. It is important to recognize how CI and microglial activation occur and if a distinct mechanism is evident in this regard between CI and pain. Massive activation of microglia is also known to occur after nerve injury and contributes to neuropathic pain [73]. Activated microglia can strongly influence neuronal and astroglial functions [74]. This is important information that can help us understand chronicity in itch and pain and the potential distinct mechanisms in these two phenomena. In addition, one may consider targeting microglia using inhibitors of TLRs, ATP receptors, cytokines, and MAP kinases. However, potential side effects are expected, especially after long-term treatment [75]. A number of strategies have been proposed to control abnormal microglial activation and return to homeostasis, for example, SPMs and CB2 agonists, cell therapies, and neuromodulation [75].
Iba1, the microglial activation marker, was not upregulated in the chronic phase of atopic dermatitis and dry skin models in mice [76]. Interestingly, a short-term activation was seen where microglia became activated within 30 to 60 min after the administration of some compounds known to exert itch (pruritogens). It has been suggested that this transient activation might be a result of the p38 mitogen-activated protein kinase. When a microglial inhibitor was administered intrathecally, it could only inhibit itch in the acute phase of the atopic dermatitis model (Day 3) [76]. Short-term upregulation of Iba1 in the dry skin model was observed on Days 1–3 but not 5. In addition, in the psoriasis model, microglial activation has been observed, where it could also be reversed by intrathecal administration of minocycline leading to itching inhibition.
These observations collectively point to short-term versus long-term activation of central glia, and while microglia activation is mainly seen in the short-term or transient (acute) phase of itch, astrocytes remain active longer and can be the drive of CI [76]. However, more investigation is required to reveal itch-related signaling and microglia’s role and also to test the effect of selective inhibitors of microglia to block the early phase of itch models [76].
4.3. Satellite Glial Cells in Itch
SGC is the most abundant glial cell in the sensory ganglia. SGCs enwrap neuronal cell bodies entirely and provide a supportive and functional unit [77]. Their roles are diverse, and a number of nervous system disorders are reported to have a link to SGCs [3,31,78,79]. Pain has been studied more extensively than itch in this regard [79,80,81,82]. SGCs also regulate the immune system via phagocytosis, the release of inflammatory substances (e.g., prostaglandins, IL-6, and TNF-α), and T-cell response regulation [83]. SGCs express different receptors [84]. One example is the expression of transient receptor potential ankyrin 1, which is sensitized in neuropathic and inflammatory pain [84]. Mitterreiter et al. presented that SGCs express TLR [83]. TLR signaling between neurons and SGCs has been demonstrated in sensory ganglia following nerve injury. Neuroinflammation also plays a critical role in neuropathic pain [85]. Interestingly, in diabetes mellitus, both pain and itch are present, and both have been linked to inflammation via several mechanisms, including the activation of glial cells [85]. Therefore, targeting neuroinflammation can serve as a potential treatment for both chronic neuropathic pain and CI. Further studies are needed to provide more information on the mechanisms and treatment options.
4.3.1. SGCs in Cholestatic Itch
A new study [86] investigated cell responses to lysophosphatidic acid (LPA) 18:1 in a dorsal root ganglia culture and reported high activation of SGCs compared with neuronal activation. Schwann cells also reacted to LPA 18:1. LPA has long been suggested to play a critical role in cholestatic itch [11]. These new observations led the investigators to propose that neuronal responsiveness is a consequence of glial activation and the existence of functional crosstalk between the neurons and LPA-activated glial cells that can be considered as an essential mechanism underlying cholestatic itch [87,88].
Another study [89] proposed the concept of a cholestatic itch. Increased levels of bile acids and bilirubin have been reported in plasma samples of patients with cholestasis. These substances can be precipitated and activate MRGPRX4 for triggering itch pathways [89]. It has been shown that MRGPRX4 receptors are expressed in human dorsal root ganglion neurons and are co-expressed with the itch receptor HRH1. Bile acids activate MRGPRX4 receptors. A positive correlation has been reported between cholestatic itch and plasma bile acids levels in itchy patients. Taken together, these data suggest that MRGPRX4 is likely to underlie cholestatic itch [89]. However, the role of SGCs needs further investigation in this signaling pathway. In addition, high-level expression of TGR5 (an itch receptor in humans) in SGCs has been reported. TGR5 is known to mediate bile acid-induced itch [90], but the SGC-related mechanisms are not known. Interestingly, TGR5 is not expressed in hDRG neurons [89]. Therefore, the role of SGCs in cholestatic itch needs further investigation. Patients with liver diseases also suffer from CI, but the involvement of neuroglia remains largely unknown.
4.3.2. SGCs in Trigeminal Itch
A recent itch study in mice [81] has reported alterations in SGCs and neurons in trigeminal ganglia (TG). The authors investigated if any abnormal neuronal activity takes place in the sensory ganglia following the induction of itch. The idea was formed based on previous pain studies and alterations in sensory ganglia in both neurons and SGCs. A mouse model of itch was, therefore, established by repeated applications (11 days) of 2,4,6-trinitro-1-chlorobenzene (TNCB) to the external ear, and neuronal TG and SGC alterations were studied [81]. Behavioral results showed that the model evoked itch, and the scratching behavior in animals treated with TNCB was significantly higher compared with the control group. The scratching was maintained several days after the last treatment confirming a chronic state of itch. Following the administration of TNCB, immunostaining showed about 35% greater activation in SGCs marked with a glial fibrillary acidic protein marker and an increase in gap-junction-mediated coupling. Gap junction blockers could suppress scratching. A higher response of SGCs to ATP was also demonstrated by calcium imaging. This study [81] collectively demonstrated several alterations in TG, including SGC activation and an elevated response to ATP. Interestingly, SGC coupling, which has been previously found to increase in response to pain, did not show any change in this itch model. [81].
4.4. Schwann Cells in Itch
The role of Schwann cells (SCs) in itch has been less investigated [3,91]. A recent study investigated the role of SCs in cholestatic itch [92]. SCs express receptors for LPA, and in cultured SCs, LPA has been shown to activate these cells [91] and potentially underlie cholestatic pruritus, similar to what has been explained above with SGCs’ contribution to cholestatic itch. SCs also express TRPA1 and TRPV1 [93,94], which are proposed to contribute to the itch–scratch cycle. Scratching could damage epidermal nerve endings and activate SCs for the repair process. However, this could alter the neuronal transmission and trigger itch. For example, factors secreted by SCs during the repair process could influence adjacent cells and neurons and promote itch signaling. Our knowledge is limited, but it has been reported that SCs can secrete inflammatory substances (e.g., TNF-α, IL-1, IL-4, IL-6). Evidence also shows distinct SC alterations in atopic dermatitis and psoriasis during degeneration and repair [91]. Therefore, SCs have the potential to become involved in itch signaling, but further investigation is required [91].
5. Concluding Remarks and Future Perspectives
Chronic itch (CI) affects millions of people worldwide and requires proper treatment. Currently, available therapies are suboptimal, which might partially be a result of an incomplete understanding of CI. Under CI conditions, there are severe and abnormal itch responses, such as sensitization for itch and the vicious itch–scratch cycle. These phenomena lead to further exacerbation of the CI condition.
The itch sensation is a result of the interaction between itch mediators (exogenous and endogenous) or pruritogens, and itch receptors, where a trigger signal is generated and transmitted through peripheral afferent nerve fibers of the PNS to CNS leading to further processing in higher cortical centers and final itch sensation perception. A total of 13 receptor groups that are identified are involved in conveying cutaneous itching [95]. The major categories are G-protein-coupled receptors (GPCR) signaling, non-GPCR signaling, and ion channel signaling pathway. The majority of the itch receptors are GPCR and approximately 35% of all approved drugs target GPCRs. Toll-like receptors (TLRs), interleukin receptors (ILRs), and mechanosensitive purinoceptors have been studied for itch with a new wave of interest. Notably, a lack of Piezo2 signaling is suggested to underlie the conversion of touch into a sensation of itch and contribute to mechanical-induced itch.
The role of glial cells in itch has attracted much scientific attention, and recent studies have shown that astrocytes and microglia of the CNS, SGCs, and SCs of the PNS are involved. The pattern of activation and timing of activation of these cells are different. For example, evidence shows that microglia may contribute to the early phase of itch or acute itch rather than the chronic phase of itch. However, astrocytes are involved in the chronic status or maintenance of itch [6]. Less is known about the timing of SCs’ and SGCs’ activation and their link to the consequent timing and pattern of activation of central glia. In addition, glial–glial signaling is less studied than glial–neuronal signaling. Therefore, further studies are required to deepen our understanding of glial roles and glial–glial and glial–neuronal communication within the CNS and PNS to elucidate the mechanisms underlying pathologic itch. Another open research area for further investigation is the elucidation of mechanisms underlying the transition from an acute to a chronic state of itch. This would help in the identification of preventive strategies.
Clarification of glial cells’ role in itch has naturally raised attention to the possibilities of targeting these cells for therapeutic purposes. Experimental data show promising results in itch models. Some researchers have also explored the modulation of glial cells [96] for other disorders that can inspire the itch research field. However, targeting glial cells is not a challenge-free strategy because maintaining their ordinary roles is necessary for nervous system structure and function. Therefore, glial modulation itself has become an active research field. Advancement in technology allows various novel methods to emerge, which can help in studying glial modulation. For example, optogenetics, the use of DREADDS (designer receptors exclusively activated by designer drugs) [97], and graphene–glial interfaces are new technologies that can revolutionize research in understanding glia–glia and glia–neuron interactions. It is expected that these and many more new-generation glial technologies and therapies that can potentially benefit CI will soon become available.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflict of interest.
References
- Weisshaar, E. Itch: A Global Problem? Front. Med. 2021, 8, 665575. [Google Scholar] [CrossRef]
- Schmelz, M. How Do Neurons Signal Itch? Front. Med. 2021, 8, 643006. [Google Scholar] [CrossRef]
- Andersen, H.H.; Arendt-Nielsen, L.; Gazerani, P. Glial Cells are Involved in Itch Processing. Acta Derm. Venereol. 2016, 96, 723–727. [Google Scholar] [CrossRef]
- Yang, H.; Chen, W.; Zhu, R.; Wang, J.; Meng, J. Critical Players and Therapeutic Targets in Chronic Itch. Int. J. Mol. Sci. 2022, 23, 9935. [Google Scholar] [CrossRef]
- Cevikbas, F.; Lerner, E.A. Physiology and Pathophysiology of Itch. Physiol. Rev. 2020, 100, 945–982. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Tsuda, M. Spinal glial cells in itch modulation. Pharmacol. Res. Perspect. 2021, 9, e00754. [Google Scholar] [CrossRef]
- Ji, R.-R. Third Special Issue on Mechanisms of Pain and Itch. Neurosci. Bull. 2022, 38, 339–341. [Google Scholar] [CrossRef]
- Ständer, S. Classification of Itch. Curr. Probl. Dermatol. 2016, 50, 1–4. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yosipovitch, G. Itching as a systemic disease. J. Allergy Clin. Immunol. 2019, 144, 375–380. [Google Scholar] [CrossRef]
- Kremer, A.E.; Mettang, T.; Weisshaar, E. Non-dermatological Challenges of Chronic Itch. Acta Derm. Venereol. 2020, 100, adv00025. [Google Scholar] [CrossRef]
- Warlich, B.; Fritz, F.; Osada, N.; Bruland, P.; Stumpf, A.; Schneider, G.; Dugas, M.; Pfleiderer, B.; Ständer, S. Health-Related Quality of Life in Chronic Pruritus: An Analysis Related to Disease Etiology, Clinical Skin Conditions and Itch Intensity. Dermatology 2015, 231, 253–259. [Google Scholar] [CrossRef]
- Schneider, G.; Ständer, S.; Kahnert, S.; Pereira, M.P.; Mess, C.; Huck, V.; Agelopoulos, K.; Frank, G.; Schneider, S.W. Biological and psychosocial factors associated with the persistence of pruritus symptoms: Protocol for a prospective, exploratory observational study in Germany (individual project of the Interdisciplinary SOMACROSS Research Unit [RU 5211]). BMJ Open 2022, 12, e060811. [Google Scholar] [CrossRef]
- Pereira, M.P.; Zeidler, C.; Storck, M.; Agelopoulos, K.; Philipp-Dormston, W.G.; Zink, A.; Ständer, S. Challenges in Clinical Research and Care in Pruritus. Acta Derm. Venereol. 2020, 100, adv00028. [Google Scholar] [CrossRef] [PubMed]
- Fowler, E.; Yosipovitch, G. A New Generation of Treatments for Itch. Acta Derm. Venereol. 2020, 100, adv00027. [Google Scholar] [CrossRef] [PubMed]
- McEwen, M.W.; Fite, E.M.; Yosipovitch, G.; Patel, T. Drugs on the Horizon for Chronic Pruritus. Dermatol. Clin. 2018, 36, 335–344. [Google Scholar] [CrossRef]
- Rasband, M.N. Glial Contributions to Neural Function and Disease. Mol. Cell. Proteom. 2016, 15, 355–361. [Google Scholar] [CrossRef]
- Zuchero, J.B.; Barres, B.A. Glia in mammalian development and disease. Development 2015, 142, 3805–3809. [Google Scholar] [CrossRef]
- Wang, F.; Kim, B.S. Itch: A Paradigm of Neuroimmune Crosstalk. Immunity 2020, 52, 753–766. [Google Scholar] [CrossRef]
- Ji, R.R. Neuroimmune interactions in itch: Do chronic itch, chronic pain, and chronic cough share similar mechanisms? Pulm. Pharmacol. Ther. 2015, 35, 81–86. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis: An expanding therapeutic pipeline for a complex disease. Nat. Rev. Drug Discov. 2022, 21, 21–40. [Google Scholar] [CrossRef]
- Mack, M.R.; Kim, B.S. The Itch-Scratch Cycle: A Neuroimmune Perspective. Trends Immunol. 2018, 39, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Kim, H.J.; Back, S.K.; Na, H.S. Common and discrete mechanisms underlying chronic pain and itch: Peripheral and central sensitization. Pflügers Arch. -Eur. J. Physiol. 2021, 473, 1603–1615. [Google Scholar] [CrossRef]
- Dong, X.; Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef]
- Jin, S.-Y.; Wang, F. Sensitization Mechanisms of Chronic Itch. Int. J. Dermatol. Venereol. 2019, 2, 211–215. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Ho, M.S.; Zorec, R.; Parpura, V. The Concept of Neuroglia. Adv. Exp. Med. Biol. 2019, 1175, 1–13. [Google Scholar] [CrossRef]
- Losada-Perez, M. Glia: From ‘just glue’ to essential players in complex nervous systems: A comparative view from flies to mammals. J. Neurogenet. 2018, 32, 78–91. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef]
- Sinegubov, A.; Andreeva, D.; Burzak, N.; Vasyutina, M.; Murashova, L.; Dyachuk, V. Heterogeneity and Potency of Peripheral Glial Cells in Embryonic Development and Adults. Front. Mol. Neurosci. 2022, 15, 737949. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Butt, A.M. Neuroglia: Definition, Classification, Evolution, Numbers, Development. In Glial Physiology and Pathophysiology; Wiley: New York, NY, USA, 2013; Chapter 3; pp. 73–104. [Google Scholar]
- Hanslik, K.L.; Marino, K.M.; Ulland, T.K. Modulation of Glial Function in Health, Aging, and Neurodegenerative Disease. Front. Cell Neurosci. 2021, 15, 718324. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, C.R.; Andriessen, A.S.; Chen, G.; Wang, K.; Jiang, C.; Maixner, W.; Ji, R.-R. Central Nervous System Targets: Glial Cell Mechanisms in Chronic Pain. Neurotherapeutics 2020, 17, 846–860. [Google Scholar] [CrossRef]
- Li, T.; Chen, X.; Zhang, C.; Zhang, Y.; Yao, W. An update on reactive astrocytes in chronic pain. J. Neuroinflamm. 2019, 16, 140. [Google Scholar] [CrossRef]
- Tsuda, M. Modulation of Pain and Itch by Spinal Glia. Neurosci. Bull. 2018, 34, 178–185. [Google Scholar] [CrossRef]
- Tsuda, M. Spinal dorsal horn astrocytes: New players in chronic itch. Allergol. Int. 2017, 66, 31–35. [Google Scholar] [CrossRef]
- Hanani, M.; Verkhratsky, A. Satellite Glial Cells and Astrocytes, a Comparative Review. Neurochem. Res. 2021, 46, 2525–2537. [Google Scholar] [CrossRef]
- Westergard, T.; Rothstein, J.D. Astrocyte Diversity: Current Insights and Future Directions. Neurochem. Res. 2020, 45, 1298–1305. [Google Scholar] [CrossRef]
- Liu, X.; Ying, J.; Wang, X.; Zheng, Q.; Zhao, T.; Yoon, S.; Yu, W.; Yang, D.; Fang, Y.; Hua, F. Astrocytes in Neural Circuits: Key Factors in Synaptic Regulation and Potential Targets for Neurodevelopmental Disorders. Front. Mol. Neurosci. 2021, 14, 729273. [Google Scholar] [CrossRef]
- Chiareli, R.A.; Carvalho, G.A.; Marques, B.L.; Mota, L.S.; Oliveira-Lima, O.C.; Gomes, R.M.; Birbrair, A.; Gomez, R.S.; Simão, F.; Klempin, F.; et al. The Role of Astrocytes in the Neurorepair Process. Front. Cell. Dev. Biol. 2021, 9, 665795. [Google Scholar] [CrossRef]
- Stephan, J.; Eitelmann, S.; Zhou, M. Approaches to Study Gap Junctional Coupling. Front. Cell. Neurosci. 2021, 15, 640406. [Google Scholar] [CrossRef] [PubMed]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- MacVicar, B.A.; Newman, E.A. Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect Biol. 2015, 7, a020388. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Eroglu, C. Cell Biology of Astrocyte-Synapse Interactions. Neuron 2017, 96, 697–708. [Google Scholar] [CrossRef]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Lu, H.-J.; Gao, Y.-J. Astrocytes in Chronic Pain: Cellular and Molecular Mechanisms. Neurosci. Bull. 2022. [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Green, D.; Dong, X. Supporting itch: A new role for astrocytes in chronic itch. Nat. Med. 2015, 21, 841–842. [Google Scholar] [CrossRef]
- Liu, T.; Han, Q.; Chen, G.; Huang, Y.; Zhao, L.X.; Berta, T.; Gao, Y.J.; Ji, R.R. Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 2016, 157, 806–817. [Google Scholar] [CrossRef]
- Ottevanger, R.; van Beugen, S.; Evers, A.W.M.; Willemze, R.; Vermeer, M.H.; Quint, K.D. Itch in patients with cutaneous T-cell lymphoma as a quality of life indicator. JAAD Int. 2022, 9, 57–64. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Koga, K.; Tozaki-Saitoh, H.; Kohro, Y.; Toyonaga, H.; Yamaguchi, C.; Hasegawa, A.; Nakahara, T.; Hachisuka, J.; Akira, S.; et al. STAT3-dependent reactive astrogliosis in the spinal dorsal horn underlies chronic itch. Nat. Med. 2015, 21, 927–931. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Cao, T.; Jin, L.; Li, B.; Fang, H.; Zhang, J.; Zhang, Y.; Hu, J.; Wang, G. Increased Lipocalin-2 Contributes to the Pathogenesis of Psoriasis by Modulating Neutrophil Chemotaxis and Cytokine Secretion. J. Investig. Dermatol. 2016, 136, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Yamagata, R.; Kohno, K.; Yamane, T.; Shiratori-Hayashi, M.; Kohro, Y.; Tozaki-Saitoh, H.; Tsuda, M. Sensitization of spinal itch transmission neurons in a mouse model of chronic itch requires an astrocytic factor. J. Allergy Clin. Immunol. 2020, 145, 183–191.e110. [Google Scholar] [CrossRef]
- Du, L.; Hu, X.; Yang, W.; Yasheng, H.; Liu, S.; Zhang, W.; Zhou, Y.; Cui, W.; Zhu, J.; Qiao, Z.; et al. Spinal IL-33/ST2 signaling mediates chronic itch in mice through the astrocytic JAK2-STAT3 cascade. Glia 2019, 67, 1680–1693. [Google Scholar] [CrossRef]
- Fairlie-Clarke, K.; Barbour, M.; Wilson, C.; Hridi, S.U.; Allan, D.; Jiang, H.R. Expression and Function of IL-33/ST2 Axis in the Central Nervous System Under Normal and Diseased Conditions. Front. Immunol. 2018, 9, 2596. [Google Scholar] [CrossRef]
- Sakai, K.; Akiyama, T. New insights into the mechanisms behind mechanical itch. Exp. Dermatol. 2020, 29, 680–686. [Google Scholar] [CrossRef]
- Hill, R.Z.; Loud, M.C.; Dubin, A.E.; Peet, B.; Patapoutian, A. PIEZO1 transduces mechanical itch in mice. Nature 2022, 607, 104–110. [Google Scholar] [CrossRef]
- Shiratori-Hayashi, M.; Tsuda, M. Role of reactive astrocytes in the spinal dorsal horn under chronic itch conditions. J. Pharmacol. Sci. 2020, 144, 147–150. [Google Scholar] [CrossRef]
- Jing, P.B.; Cao, D.L.; Li, S.S.; Zhu, M.; Bai, X.Q.; Wu, X.B.; Gao, Y.J. Chemokine Receptor CXCR3 in the Spinal Cord Contributes to Chronic Itch in Mice. Neurosci. Bull. 2018, 34, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ji, R.R. New insights into the mechanisms of itch: Are pain and itch controlled by distinct mechanisms? Pflugers Arch. 2013, 465, 1671–1685. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Kuwaki, T.; Kashiwadani, H. Hypothalamic orexinergic neurons modulate pain and itch in an opposite way: Pain relief and itch exacerbation. J. Physiol. Sci. 2022, 72, 21. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, S.I.; Itokazu, T.; Nishibe, M.; Yamashita, T. Neuroplasticity related to chronic pain and its modulation by microglia. Inflamm. Regen. 2022, 42, 15. [Google Scholar] [CrossRef] [PubMed]
- Pottorf, T.S.; Rotterman, T.M.; McCallum, W.M.; Haley-Johnson, Z.A.; Alvarez, F.J. The Role of Microglia in Neuroinflammation of the Spinal Cord after Peripheral Nerve Injury. Cells 2022, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M. Microglia-Mediated Regulation of Neuropathic Pain: Molecular and Cellular Mechanisms. Biol. Pharm. Bull. 2019, 42, 1959–1968. [Google Scholar] [CrossRef]
- Xu, Z.; Qin, Z.; Zhang, J.; Wang, Y. Microglia-mediated chronic psoriatic itch induced by imiquimod. Mol. Pain 2020, 16, 1744806920934998. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, J.; Hu, R.; Sun, Y.; Ma, Y.; Chen, Z.; Jiang, H. Microglia are involved in pruritus induced by DNFB via the CX3CR1/p38 MAPK pathway. Cell Physiol. Biochem. 2015, 35, 1023–1033. [Google Scholar] [CrossRef]
- Silva, R.; Malcangio, M. Fractalkine/CX(3)CR(1) Pathway in Neuropathic Pain: An Update. Front. Pain Res. 2021, 2, 684684. [Google Scholar] [CrossRef]
- Liu, T.; Xu, Z.Z.; Park, C.K.; Berta, T.; Ji, R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010, 13, 1460–1462. [Google Scholar] [CrossRef]
- Chen, G.; Luo, X.; Qadri, M.Y.; Berta, T.; Ji, R.R. Sex-Dependent Glial Signaling in Pathological Pain: Distinct Roles of Spinal Microglia and Astrocytes. Neurosci. Bull. 2018, 34, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Gwak, Y.S.; Hulsebosch, C.E.; Leem, J.W. Neuronal-Glial Interactions Maintain Chronic Neuropathic Pain after Spinal Cord Injury. Neural. Plast 2017, 2017, 2480689. [Google Scholar] [CrossRef]
- Garland, E.F.; Hartnell, I.J.; Boche, D. Microglia and Astrocyte Function and Communication: What Do We Know in Humans? Front. Neurosci. 2022, 16, 824888. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, Y.-Q.; Qadri, Y.J.; Serhan, C.N.; Ji, R.-R. Microglia in Pain: Detrimental and Protective Roles in Pathogenesis and Resolution of Pain. Neuron 2018, 100, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Wittekindt, M.; Kaddatz, H.; Joost, S.; Staffeld, A.; Bitar, Y.; Kipp, M.; Frintrop, L. Different Methods for Evaluating Microglial Activation Using Anti-Ionized Calcium-Binding Adaptor Protein-1 Immunohistochemistry in the Cuprizone Model. Cells 2022, 11, 1723. [Google Scholar] [CrossRef] [PubMed]
- Hanani, M. Satellite glial cells in sensory ganglia: From form to function. Brain Res. Brain Res. Rev. 2005, 48, 457–476. [Google Scholar] [CrossRef]
- Hanani, M. How Is Peripheral Injury Signaled to Satellite Glial Cells in Sensory Ganglia? Cells 2022, 11, 512. [Google Scholar] [CrossRef]
- Gazerani, P. Satellite Glial Cells in Pain Research: A Targeted Viewpoint of Potential and Future Directions. Front. Pain Res. 2021, 2, 646068. [Google Scholar] [CrossRef]
- Andreeva, D.; Murashova, L.; Burzak, N.; Dyachuk, V. Satellite Glial Cells: Morphology, functional heterogeneity, and role in pain. Front. Cell Neurosci. 2022, 16, 1019449. [Google Scholar] [CrossRef]
- Cohen, M.; Feldman-Goriachnik, R.; Hanani, M. Satellite Glial Cells and Neurons in Trigeminal Ganglia Are Altered in an Itch Model in Mice. Cells 2022, 11, 886. [Google Scholar] [CrossRef]
- Costa, F.A.L.; Neto, F.L.M. Satellite glial cells in sensory ganglia: Its role in pain. Braz. J. Anesthesiol. Engl. Ed. 2015, 65, 73–81. [Google Scholar] [CrossRef]
- Mitterreiter, J.G.; Ouwendijk, W.J.D.; van Velzen, M.; van Nierop, G.P.; Osterhaus, A.; Verjans, G. Satellite glial cells in human trigeminal ganglia have a broad expression of functional Toll-like receptors. Eur. J. Immunol. 2017, 47, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Itson-Zoske, B.; Cai, Y.; Qiu, C.; Pan, B.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Satellite glial cells in sensory ganglia express functional transient receptor potential ankyrin 1 that is sensitized in neuropathic and inflammatory pain. Mol. Pain 2020, 16, 1744806920925425. [Google Scholar] [CrossRef]
- Fang, X.X.; Wang, H.; Song, H.L.; Wang, J.; Zhang, Z.J. Neuroinflammation Involved in Diabetes-Related Pain and Itch. Front. Pharmacol. 2022, 13, 921612. [Google Scholar] [CrossRef] [PubMed]
- Robering, J.W.; Gebhardt, L.; Wolf, K.; Kühn, H.; Kremer, A.E.; Fischer, M.J.M. Lysophosphatidic acid activates satellite glia cells and Schwann cells. Glia 2019, 67, 999–1012. [Google Scholar] [CrossRef]
- Vander Does, A.; Levy, C.; Yosipovitch, G. Cholestatic Itch: Our Current Understanding of Pathophysiology and Treatments. Am. J. Clin. Dermatol. 2022, 23, 647–659. [Google Scholar] [CrossRef]
- Patel, S.P.; Vasavda, C.; Ho, B.; Meixiong, J.; Dong, X.; Kwatra, S.G. Cholestatic pruritus: Emerging mechanisms and therapeutics. J. Am. Acad. Dermatol. 2019, 81, 1371–1378. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, T.; Liu, S.; Wu, Q.; Johnson, O.; Wu, Z.; Zhuang, Z.; Shi, Y.; Peng, L.; He, R.; et al. MRGPRX4 is a bile acid receptor for human cholestatic itch. Elife 2019, 8, e48431. [Google Scholar] [CrossRef]
- Alemi, F.; Kwon, E.; Poole, D.P.; Lieu, T.; Lyo, V.; Cattaruzza, F.; Cevikbas, F.; Steinhoff, M.; Nassini, R.; Materazzi, S.; et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J. Clin. Investig. 2013, 123, 1513–1530. [Google Scholar] [CrossRef]
- Bray, E.R.; Chéret, J.; Yosipovitch, G.; Paus, R. Schwann cells as underestimated, major players in human skin physiology and pathology. Exp. Dermatol. 2020, 29, 93–101. [Google Scholar] [CrossRef]
- Langedijk, J.; Beuers, U.H.; Oude Elferink, R.P.J. Cholestasis-Associated Pruritus and Its Pruritogens. Front. Med. 2021, 8, 639674. [Google Scholar] [CrossRef] [PubMed]
- Maglie, R.; Souza Monteiro de Araujo, D.; Antiga, E.; Geppetti, P.; Nassini, R.; De Logu, F. The Role of TRPA1 in Skin Physiology and Pathology. Int. J. Mol. Sci. 2021, 22, 3065. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Kahremany, S.; Hofmann, L.; Gruzman, A.; Cohen, G. Advances in Understanding the Initial Steps of Pruritoceptive Itch: How the Itch Hits the Switch. Int. J. Mol. Sci. 2020, 21, 4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Stieger, K.C.; Kozai, T.D.Y. Challenges and opportunities of advanced gliomodulation technologies for excitation-inhibition balance of brain networks. Curr. Opin. Biotechnol. 2021, 72, 112–120. [Google Scholar] [CrossRef]
- Peeters, L.M.; Missault, S.; Keliris, A.J.; Keliris, G.A. Combining designer receptors exclusively activated by designer drugs and neuroimaging in experimental models: A powerful approach towards neurotheranostic applications. Br. J. Pharmacol. 2020, 177, 992–1002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).