Abstract
Perivascular adipose tissue (PVAT)-derived extracellular vesicles (EVs) with small exosome(s) (PVAT-dEVexos) from the descending aorta are capable of entering capillaries and systemic circulation. These PVAT-dEVexos are delivered to the central nervous system (CNS) in preclinical, obese, insulin and leptin resistant, diabetic, db/db mouse models and humans with T2DM. Once within the CNS, these exosomes are capable of traversing the blood–brain barrier and the blood-cerebrospinal fluid barrier resulting in activation of the neuroglia microglia cell(s) (aMGCs) and the formation of reactive astrocytes (rACs). The chronic peripheral inflammation in the PVAT via crown-like structures consists of activated macrophages and mast cells, which harbor peripheral adipokines, cytokines, and chemokines (pCC) in addition to the EV exosomes. These pCC are transported to the systemic circulation where they may act synergistically with the PVAT-dEVexos to amplify the activation of neuroglia and result in chronic neuroinflammation. Once activated, the MGCs and ACs will contribute to even greater neuroinflammation via central nervous cytokines/chemokines (cnsCC). Activated neuroglia results in an increase of cnsCC and the creation of a vicious cycle of ongoing chronic neuroinflammation and increased redox stress. The increase in reactive oxygen species (ROS) involves the reactive species interactome that not only include reactive oxygen but also reactive nitrogen and sulfur species wherein a vicious cycle of ROS begetting inflammation and inflammation begetting ROS develops. Thus, the CNS perceives peripheral systemic inflammation from the obese PVAT depots as an injury and a response to injury wound healing mechanism develops with activation of neuroglia, cellular remodeling, neurodegeneration, and impaired cognition.
1. Introduction
Obesity has now gained global epidemic proportions and parallels the current increasing trend of the global type 2 diabetes mellitus (T2DM) epidemic [1]. For example, in the United States approximately 60% of the population has been classified as being overweight as defined by a body mass index of ≥25 kg/m2, or obese as defined by a body mass index of ≥30 kg/m2 [1]. The incidence of T2DM has also increased in parallel with the obesity epidemic and thus, T2DM could be considered a major co-morbid condition associated with obesity [2,3,4]. Recent epidemiologic findings have shown that ~85% of individuals with T2DM are also obese and it is currently estimated that by 2025 more than 300 million people will have T2DM that is related to obesity [5]. Importantly, obesity and T2DM are associated with metabolic syndrome (MetS), which increases the risk of cerebrocardiovascular disease (CCVD). Further, CCVD includes the vasculature system, heart, and brain, which are involved in the clinical utility of the MetS and its increased morbidity and mortality by 2-fold or greater [6,7,8,9].
1.1. Adipose Tissue and Remodeling in Obesity, MetS, and T2DM
Adipose tissue (AT) may be considered a multi-depot and endocrine organ that is divided into brown adipose tissue (BAT) and white adipose tissue (WAT) in mammals and humans [10,11,12,13]. BAT is responsible for non-shivering thermogenesis and is known to stain positive for uncoupling proteins (UCP-1, 2) and WAT is known to be responsible for the storage of excess fat (free fatty acids that are taken up by WAT adipocytes and stored as triglycerides and do not stain positive for UCP-1, 2 [10,11,12,13,14]. WAT may be further defined as being either subcutaneous AT (SAT) or visceral AT (VAT) [10,11,12,13]. VAT may be further subdivided into omental, mesenteric, retroperitoneal, perinephric, epicardial, pericardial, and perivascular AT (PVAT) [11,12,13]. The VAT is known to develop an inflammatory phenotype in conditions of excess nutrient intake and is more closely associated with the development of obesity, T2DM, insulin resistance (IR), and CCVD as compared to SAT [6,7,15]. Importantly, VAT mass correlates positively with the development of IR, while SAT tissue mass does not [16,17,18]. Additionally, mesenteric VAT of obese T2DM individuals has a significant increase in leptin and a downregulation of the adiponectin gene expression with decreased adiponectin as compared to SAT [19].
VAT develops chronic low-grade inflammation (metainflammation) in obesity and T2DM, which includes the PVAT of the descending aorta. PVAT is the VAT that envelops the outermost region of the aorta and is contained within the tunica adventitia layer. There is a definite ultrastructural demarcation of the PVAT within the tunica adventitia such that some have recently defined this region as the PVAT of the tunica adiposa (the periadventitial VAT or the PVAT) [20,21,22].
AT is known to be a highly plastic organ and capable of transdifferentiation from BAT to WAT and vice versa [23]. The PVAT depot in the descending thoracic aorta in health (control mice and humans) consists of a near-complete multilocular BAT depot replete with multiple mitochondria typical of BAT. In contrast, the abdominal aorta PVAT consists of a unilocular WAT depot with a relative paucity of mitochondria as compared to the BAT depot [24]. The descending thoracic aorta undergoes near-complete transdifferentiation to WAT with marked hypertrophic unilocular adipocytes in the obese, diabetic db/db mouse. Thus, it was very interesting that BAT of descending thoracic aorta in control models underwent transdifferentiation from BAT to that of a markedly enhanced volume of unilocular WAT (Figure 1) [20].
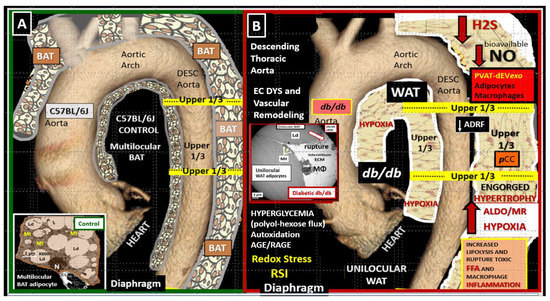
Figure 1.
The perivascular adipose tissue (PVAT) of descending aorta in the obese, diabetic db/db model undergoes transdifferentiation from multilocular brown adipose tissue (BAT) to unilocular white adipose tissue (WAT). (A) demonstrates the normal PVAT depot, which consists of multilocular brown adipose tissue (BAT) in control models (insert). (B) depicts the transdifferentiation from BAT to WAT with hypertrophic unilocular adipocytes that are prone to rupture and secrete multiple cytokines/chemokines and PVAT-derived small extracellular vesicles exosomes (PVAT-dEVexo) in the obese, diabetic db/db mice (insert). Interestingly, the transdifferentiation of BAT in controls to WAT in the db/db models is associated with aortic vascular stiffing. This modified image is published with permission by CC 4.0 [20]. ADRF = adipose- derived relaxing factor; AGE/RAGE = advanced glycation end products and their receptor for AGE; ALDO = aldosterone; DESC = descending aorta; Dys = dysfunction; EC = endothelial cell(s); ECM = extracellular matrix; FFA = free fatty acids; H2S = hydrogen sulfide; MR = mineralocorticoid receptor; NO = nitric oxide; OS = oxidative stress; pCC = peripheral cytokines/chemokines; RSI = reactive species interactome.
Our group and various colleagues have been extremely fortunate to have had the opportunity to examine multiple models of obesity, insulin resistance (IR), leptin resistance (LR), and models with no measurable leptin such as the BTBR ob/ob, with MetS and diabetes over the past decade. The data obtained from these studies have allowed for the creation of Venn diagrams to compare these multiple models and how they overlap (Figure 2) [14,20,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
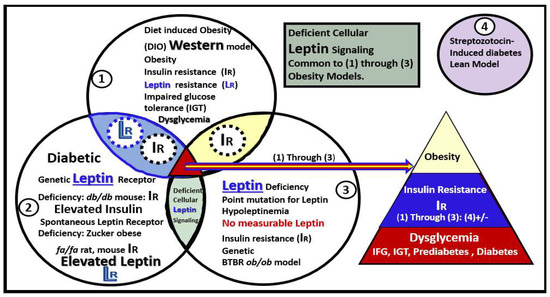
Figure 2.
Venn diagrams depicting multiple models in sets/circles (1 through 3) of brain remodeling. The model most studied was the obese, insulin-, and leptin-resistant (IR and LR, respectively) db/db mouse model at 20 weeks of age (circle 2) [14,20,25,26,30,31,32,33,34,35,36,37,38]. Furthermore, the Zucker fa/fa rat (circle 2) [25,26,27], diet-induced obesity (DIO) Western (circle 1) [25,26,28,31], and most recently the novel BTBR ob/ob mouse (circle 3) [39] in addition to the streptozotocin-induced diabetic model (circle 4-upper right colored in purple) [29] were studied. Note the overlapping circles depicting IR and LR between circles 1 and 2 (light blue ellipse) and only IR between circles 1 and 3 that share IR (yellow ellipse). Importantly, note that where all three circles (1–3) of the Venn diagram intersect the arrow points to the common features of obesity, IR, dysglycemia, prediabetes, and manifest diabetes (multicolored triangle lower-right). Only the db/db, Zucker obese fa/fa, and the Western models share in common LR. Deficient cellular leptin signaling is common in all models studied (circles 1–3). This modified image is provided with permission by CC 4.0 [39]. IFG = impaired fasting glucose; IGT = impaired glucose tolerance; IR = insulin resistance; LR = leptin resistance.
Notably, these above studies allowed for the current transmission electron microscopic (TEM) observations of small PVAT-derived EVexosomes (PVAT-dEVexos) in the unilocular engorged rupture-prone WAT of the descending thoracic aorta and macrophages that create crown-like structures in the PVAT of obese, diabetic db/db models.
1.2. EVexosomes in VAT and PVAT Are Capable of Interorgan Signaling to the Brain and Result in Neuroglial Activation
The history of extracellular vesicle exosomes (EVexos) is rich and this field of research has greatly expanded since they were first discovered in reticulocytes by Harding et al., and Pan et al. [40,41,42]. Notably, it was Rose Johnstone who coined the term exosomes [43]. A major breakthrough occurred when it was found that parenteral EVexos contained both messenger ribonucleic acid (mRNA) and microRNA (miRNA) and that they could be translated into proteins by target cells [44,45]. The interest and fascination with exosomes have expanded exponentially in the past few years because it has been found that these exosomes not only signal via an autocrine, paracrine (intercellular communication) but also an endocrine mechanism to signal distant organs and cells (inter-organ communication) from the parental cells generating exosomes [46]. Furthermore, it has been found that exosomes contain multiple biologically versatile cargoes including various proteins, fats (fatty acids, cholesterol, sphingomyelins, and ceramides), and nucleic acids including coding and long-non-coding RNA (incRNAs), mRNA, and miRNAs that are capable of signaling adjacent and distant cells and organs [46,47].
Stressed cells (especially those with endoplasmic reticulum stress) as in obesity and T2DM are capable of producing greater numbers of exosomes as occur in the obese PVAT adipocytes with hypertrophy and crown-like structures (CLS) of macrophages with or without rupture that may be capable of producing more exosomes than quiescent homeostatic non-stressed adipocytes in healthy lean models [48]. This concept is especially important given that WAT (SAT and VAT) may be the largest secretory, endocrine organ in the human body [49].
Notably, many publications regarding AT derived-exosomes are related to their protective roles such as adipocyte-derived mesenchymal stem cells with anti-inflammatory and regenerative potential. However, we are only recently beginning to learn of the potential negative and damaging roles that obese adipocytes (VAT and PVAT) are playing in multiple diseases such as occurs in obesity, MetS, IR, LR, and T2DM [50,51,52]. This overview will primarily focus on small EVexos that are generated in PVAT adipocytes (PVAT-dEVexos) and macrophages (PVATMΦ-dEVexos) as observed with TEM in the obese, IR, LR, female, diabetic, db/db preclinical rodent models. Extracellular vesicles including exosomes, microvesicles, and apoptotic bodies are identified in the VAT-PVAT of obese, prediabetic, and manifest diabetic models such as occurs in the db/db preclinical models (Figure 3).
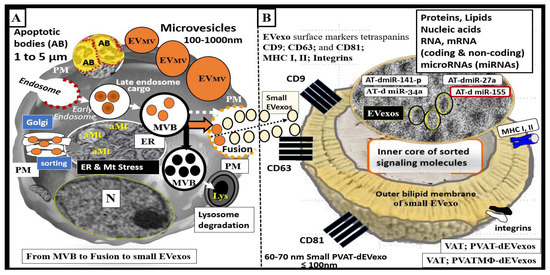
Figure 3.
Extracellular vesicles (EV): Exosomes, microvesicles, and apoptotic bodies as found in the obese and diabetic preclinical models. (A) illustrates a representative cell with early and late endosomes and multivesicular body formation and fusion with the plasma membrane (pm) and secretion of exosomes along with the alternative pathway for lysosomal degradation of MVB contents. (B) illustrates a cross-section view of a small exosome. Exosomes are derived from late endosome-multivesicular bodies (MVB), which fuse with the plasma membrane and are secreted into the extracellular space for paracrine intercellular and/or endocrine inter-organ communication. They are dependent on endosomal sorting complexes required for transport (ESCRT), cluster of differentiation tetraspanins (CD-9, CD-63, CD-81), ceramides, and various stimuli for proper secretion. In contrast, microvesicles are a heterogeneous population of membrane vesicles produced by membrane budding, and apoptotic bodies (AB) are liberated via membrane blebbing during the late stages of apoptotic cellular death. Exosomes are considered to be small when they are less than 100 nm as depicted in panel B. Note the cropped image of uniform and multiple small exosomes from ruptured adipocytes in the obese, diabetic db/db model that are approximately 60–70 nm in diameter. Exosomes have an outer bilipid membrane (colored yellow) and surround an inner core that contains various proteins, lipids, nucleic acids, coding messenger RNAs (mRNA) coding and long-non-coding RNA (incRNAs), microRNAs (miRNAs), which are small single-stranded non-coding molecules (19–23 nucleotides) that function in RNA silencing and/or post-transcriptional regulation of gene expression in recipient cells. miRNAs accomplish this via cleavage, destabilization, and less efficient translation of mRNAs into proteins [53,54]. EV exosomes such as perivascular adipose tissue-derived EV exosomes (PVAT-dEVexos) may include various miRNAs including miR-155 (promoting proinflammatory macrophage (MΦ) M1 polarization), miR-34a (inhibiting MΦ M2 polarization), miR-27a (promoting insulin resistance (IR) in skeletal muscle), miR-141-3p (promoting IR in hepatocytes) [55]. Importantly, small EVexos are able to be transferred horizontally to adjacent cells (paracrine signaling) or to distant cells and organs (endocrine–inter-organ signaling). aMt = aberrant mitochondria; AT = adipose tissue; ER = endoplasmic reticulum; M1 = classically activated macrophage polarization-proinflammatory; MHC = major histocompatibility complex; Mt = mitochondria; PVAT-dEVexo = perivascular adipose tissue-derived small extracellular vesicle exosomes; PVATMΦ-dEVexo = perivascular adipose tissue macrophage-derived small extracellular vesicle exosomes.
2. PVAT Adipokines, Peripheral Cytokines/Chemokines (pCC), Adipocyte PVAT-dEVexos and Macrophages PVATMΦ-dEVexos
The PVAT synthesizes and secretes a variety of proinflammatory and anti-inflammatory factors, including the peripheral adipokines leptin (proinflammatory-upregulated), adiponectin (anti-inflammatory-downregulated), resistin (proinflammatory), and visfatin (proinflammatory), and omentin (anti-inflammatory-downregulated) in obesity. Furthermore, the PVAT synthesizes and secretes proinflammatory cytokines such as TNF-α, IL-6, and chemokines including monocyte chemoattractant protein-1 (MCP-1) also termed CXCL2, in obesity [56,57].
The normal healthy SAT and VAT depots are known to consist of multiple cells in a bed of loose connective tissue. These cells consist of the parenchymal unilocular (WAT) or multilocular (BAT) adipocytes and the stromal-vascular fraction, which includes endothelial cells, vascular smooth muscles cells and pericytes, unmyelinated neurons, resident innate immune cells consisting of macrophage(s) (MΦ), mast cell(s) (MC), fibrocytes, pre-adipocytes, and adipocyte mesenchymal stem cells [10,11,12,13]. However, in obesity, there are multiple remodeling changes that may be observed such as the obese models and obese, diabetic db/db models. In these models, the VAT becomes inflamed and more so in the VAT as compared to the SAT. Importantly, in the transdifferentiated PVAT (Figure 1) the unilocular VAT becomes inflamed along with the other multiple VAT depots. These depots form what are classically known as crown-like structures (CLS) with MΦs forming the crowns over the adjacent engorged, ruptured, dying unilocular adipocytes that are frequently adherent to the plasma membrane of these adipocytes. The most frequent innate inflammatory cell is the MΦ and the second most frequent is the MC, which acts as first responder cells [49,58]. Notably, the adaptive immune cell lymphocytes were only rarely noted in the db/db models by TEM studies (Figure 4) [13,20,58].
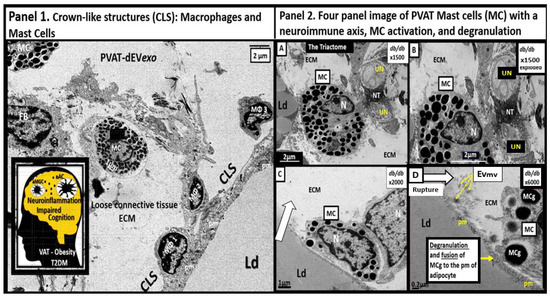
Figure 4.
Crown-like structures (CLS) with activated macrophages (MΦ), a neuroimmune axis (triactome), and activated Mast Cells (MC) with degranulation. Panel 1 depicts three activated and attached MΦs to the plasma membrane (pm) of a hypertrophic adipocyte within the Perivascular adipose tissue (PVAT) of the descending aorta. Furthermore, note a MC and a fibroblast (FB). Insert depicts neuroinflammation within the brain and impaired cognition due to obesity, type 2 diabetes mellitus (T2DM), and PVAT metainflammation. Panel 2 with (A–D). (A,B) depict unmyelinated neurons innervating a MC that is also in contact with an adipocyte creating the triactome (neuro–immune–adipose interaction). (C) depicts the homing of a MC to the pm of the unilocular adipocyte that is undergoing rupture (open arrow). (D) depicts the actual fusion of the MC and its active degranulation of its mast cell granule (MCg) to fuse with the pm of the adjacent unilocular adipocyte. Importantly, note the liberation of extracellular vesicle microvesicles (EVmv) (yellow arrows) measuring ~100–120 nm and larger than small EVexosomes (<100 nm). These modified images are provided with permission by CC 4.0 [20]. ECM = extracellular matrix; Ld = lipid droplet; N = nucleus; NT = neurotransmitters; PVAT-dEVexos = perivascular adipose tissue-derived extracellular small exosomes; UN = unmyelinated neuron.
Notably, the PVAT in the db/db model is known to be richly innervated with unmyelinated neurons that are capable of interacting with the resident immune cells (MΦs and MCs) to form a neuro-immune-adipose axis or triactome of the descending thoracic aorta [20,21,22]. The aortic PVAT contained numerous MCs that were innervated by unmyelinated neurons and interestingly, there was observed degranulation of the MCs in these regions that could contribute to the neuroimmunomodulation and remodeling of the PVAT (Figure 4) [59]. MC degranulation contributes to a proinflammatory environment via MC secretion of numerous preformed cytokines and chemokines within the MC granules and contributes the formation of CLS due to activation of resident MΦs as previously depicted (Figure 4).
The continued hypertrophic expansion and hyperplasia in the PVAT (in combination with multiple other VAT depots) combine to contribute to damaging ectopic lipid deposition in multiple tissues [60] and certainly may outstrip their vascular supply of the vasa vasorum capillaries in the PVAT and result in hypoxic, dying, and dead adipocytes that trigger CLS (Figure 4). As the constantly engorging PVAT adipocytes expand with triglyceride storage, their lipid droplet expands with eventual plasma membrane thinning, loss of integrity, and rupture. These adipocyte ruptures allow their contents (multiple toxic lipids including toxic free fatty acids, oxidized lipids, cholesterol-oxidized cholesterol, sphingomyelins, ceramides, and PVAT-dEVexos) to be extruded,.
PVAT-derived adipokines and pCC are known to signal adjacent cells via a paracrine mechanism but can also signal distant organs such as the CNS via an endocrine, inter-organ mechanism. Likewise, adipocyte PVAT-dEVexos and PVATMΦ-dEVexos are also capable of adjacent paracrine cellular and long distant endocrine signaling such as the CNS [31,61,62]. Importantly, PVAT-dEVexos and PVATMΦ-dEVexos are extruded in the descending thoracic aorta of the PVAT and are capable of entering the systemic circulation from the PVAT via the vasa vasorum capillary beds within these depots to signal the brain (Figure 5 and Figure 6).
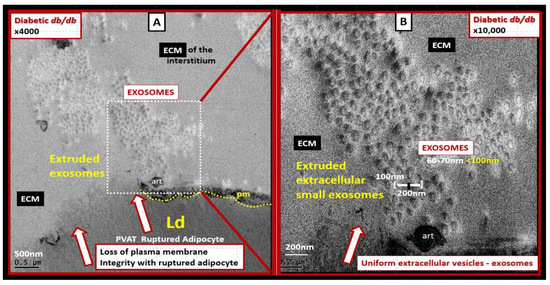
Figure 5.
Ruptured adipocyte in the perivascular adipose tissue (PVAT) of the descending aorta in the obese, diabetic db/db model at 20 weeks of age. (A) depicts a ruptured adipocyte in the PVAT (open red arrows) and note the dashed boxed-in region depicting uniform small extracellular vesicles within the interstitial extracellular matrix (ECM). Magnification ×4000; scale bar = 500 nm. (B) depicts a higher magnification of the dashed boxed-in region in panel A in order to increase the clarity and measurement of the small extracellular vesicle exosomes. Upon careful measurements, these ECM vesicles measured approximately 60–70 nm and are therefore considered to be adipocyte PVAT-derived extracellular vesicles small exosome(s) (PVAT-dEVexos). Magnification ×10,000; scale bar = 200 nm. Notably, these findings of PVAT adipocyte rupture and extracellular vesicle extrusions were not observed in control or db/db models treated for 10-weeks with the antidiabetic sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin. These modified images (highly magnified) are provided by CC 4.0 [20]. art = artifact; Ld = lipid droplet; pm = plasma membrane.
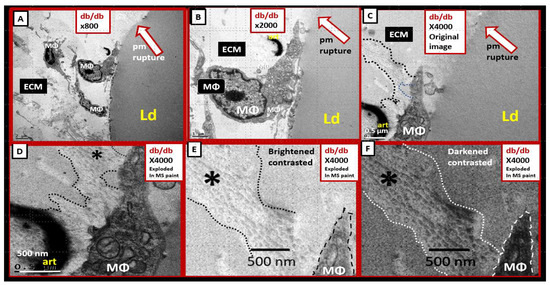
Figure 6.
Perivascular adipose tissue macrophage-derived extracellular vesicle small exosomes (PVATMΦ-dEVexos) in the descending thoracic of the obese, diabetic db/db models. (A–C) depict the increasing magnification (×800–×4000) of the PVAT macrophage that is adherent to the adipocyte plasma membrane (pm). (D–F) depict the progressive exploded images of panel C in Microsoft paint with intact scale bars. Note how the protrusions from the PVAT macrophage depict definite multiple small extracellular vesicles (sEVs) (outlined with dashed lines and labeled with asterisks). (E) is brightened and contrasted and (F) is darkened and contrasted to better illustrate these sEVs that are definitely less than 100 nm (~ 60–70 nm and similar to adipocyte derived EVexos) in diameter allowing them to be considered PVATMΦ-derived small exosomes (PVATMΦ-dEVexo). Similar to Figure 5, these images of PVATMΦ-dEVexos were not observed in control models or db/db treated for 10-weeks with the antidiabetic sodium-glucose transporter 2 (SGLT2) inhibitor empagliflozin [20]. These modified images (highly magnified) are provided by CC 4. [20]. Magnifications and scale bars are identified in each panel.
Mechanisms That Help Explain Why Peripherally Derived VAT and PVAT-dEVexos Signal the CNS
Based on these TEM observational findings in the PVAT of the descending thoracic aorta of the obese, IR, LR, female diabetic db/db models (Figure 5 and Figure 6) and the knowledge that EVexosomes may not only result in intercellular paracrine communication but also endocrine inter-organ and long-distance communication a working model was inspired [51,52]. Indeed, Figure 5 and Figure 6 were the inspiration for the presented concept of neuroglia activation by peripherally derived PVAT EVexosomes. PVAT-dEVexos of the VAT and PVAT depots could signal the CNS via an endocrine inter-organ signaling mechanism to activate neuroglia [51,52]. Additionally, the exosomes released from these two cells (ruptured adipocytes and the CLS activated macrophages) would be capable of being absorbed by the local vasa vasorum capillaries within the PVAT of the aorta and the regional capillaries of VAT. Subsequently, these adipocyte PVAT-dEVexos and PVATMΦ-dEVexos could enter the systemic circulation to enter and pass the blood–brain barrier (BBB) and the choroid plexus blood-cerebrospinal fluid barrier (BCSFB) interfaces. Further, once these small EVexos are delivered to the brain they could signal the component cells of the neurovascular unit endothelial cells, pericytes, astrocytes, and the adjacent ramified microglia to result in neuroglial activation with neuroinflammation, CNS remodeling and impaired cognition. Importantly, it is known from previous TEM studies that the neurovascular unit and neuroglia become highly remodeled in the 20-week-old obese, diabetic preclinical db/db models [32,33,34,36]. The neurovascular unit (NVU), BBB, and brain endothelial cell(s) (BECs) become activated and remodel their tight and adherens junctions (TJ/AJ) to become attenuated and/or lost with increased permeability. The ramified microglia cell(s) (rMGCs) undergo polarization to become activated proinflammatory M1-like microglia cell(s) (aMGCs). Astrocytes become reactive (rACs) and retract from the NVU with NVU uncoupling and regional ischemia, retract from neuronal dendritic synapses and instigate reactive astrogliosis. Oligodendrocytes remodel with nuclear chromatin condensation, and dysmyelination develops with myelin splitting, separation, and ballooning with subsequent axonal collapse. These multiple neuroglia activation remodeling changes are associated with impaired cognition in the obese, diabetic db/db models [32,33,34,36]. The following illustrations depict these proposed events (endocrine, inter-organ long distance signaling) of small EVexos being delivered from the PVAT to the brain via the systemic circulation that enter the CNS via the BBB and BCSFB interfaces (Figure 7).
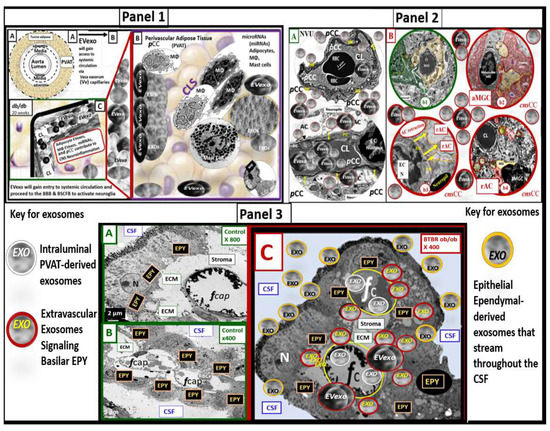
Figure 7.
Illustration demonstrating how the perivascular-derived small extracellular vesicle exosomes (PVAT-dEVexos) and PVAT macrophage-derived extracellular vesicles exosomes (PVATMΦ-dEVexos) signals the brain via the endocrine, inter-organ, and long-distance signaling by the systemic circulation. Panel 1 depicts PVAT-dEVexos that are synthesized and secreted by the activated, stressed, and ruptured hypertrophic adipocytes and the accumulated activated crown-like structure (CLS) macrophages to enter the systemic circulation to enter the brain (images (A–C)). Panel 2 depicts the systemically derived PVAT-dEVexos and PVATMΦ-dEVexos entering the neurovascular unit blood–brain barrier (BBB) (image (A)) and activating the microglia and the resulting reactive AC (image (B)). Panel 3 depicts the PVAT-dEVexos readily entering the blood-CSF barrier (BCSFB) via its fenestrated capillaries (images (A,B)) to activate the choroid plexus basilar ependymal cells (EPY), which in turn result in the synthesis and secretion of EPY-derived EVexos that traverse and stream across the CSF to enter the epithelial ependymal lining cells of the lateral ventricles of the CSF that then enter the CNS interstitium in the subventricular zone to undergo bulk flow throughout the CNS interstitium and result in microglia activation and the subsequent formation of reactive astrocyte formation (image (C)). Note the color-coded key describing the different exosomes. Please see the text for further descriptions of these processes. Of course, the exosomes depicted are not to scale in order to emphasize their importance. The modified cropped transmission electron microscopic images were provided with permission by CC 4.0 [20,32,33,34,36]. AC = astrocyte; aMGC = activated MGC; aMt = aberrant mitochondria; CL = capillary lumen; cnsCC = central nervous system cytokines/chemokines; CSF = cerebrospinal fluid; EC = endothelial cell(s); ECM = extracellular matrix; EPY = ependymal cells of the choroid plexus; EVexos = extracellular vesicle exosomes; fCap = fenestrated capillary; microRNA = micro ribosomal nucleic acid; MΦ = macrophage; NVU = neurovascular unit; pCC = peripheral cytokines/chemokines; rAC = reactive astrocyte; WBC = white blood cell.
These PVAT-dEVexos and PVATMΦ-dEVexos will enter the PVAT capillaries along with the peripheral PVAT adipokines, pCC, and enter the systemic circulation in an endocrine mechanism to eventually traverse the BBB and BCSFB of the CNS and will synergistically result in the activation of the CNS neuroglia. Importantly, Morales-Prieta et al., have demonstrated that small EVs pass the BBB and induce neuroglia activation [63]. Once MGC and AC neuroglia are activated and in a reactive state, they will result in a chronic and ongoing vicious cycle wherein reactive neuroglia promote more reactive neuroglia and contribute to chronic neuroinflammation via increased cnsCC. This chronic neuroinflammation and CNS remodeling could certainly result in clinically impaired cognition (Figure 7) [62,63].
Peripheral inflammation including the descending aorta PVAT and its PVAT-dEVexos and PVATMΦ-dEVexos, along with adipokines, and pCC provide endocrine, inter-organ long distance communication between the periphery and the CNS at the NVU BBB [62] and also the BCSFB [64]. In an elegant study by Balusu et al., they were able to demonstrate that soluble EVexos were capable of activating choroid plexus epithelial ependymal cells to induce ependymal sEVexos to be secreted into the CSF and deliver their miRNA cargo to the brain via the cerebrospinal fluid (Figure 7 [64]. Furthermore, Li JJ et al., were able to demonstrate that the microglia were activated primarily around the lateral and third ventricles following LPS infusions to simulate peripheral inflammation and activation of neuroglia cells (Figure 7 Panel 3) [65]. As the peripheral PVAT-dEVexos and PVATMΦ-EVexos enter the choroid plexus capillaries via the CNS choroidal arterial supply they will readily cross the fenestrated capillaries that supply the CSF epithelial ependymal cells (EPY) to result in epithelial ependymal cell activation to produce small EVexos and EPY derived cytokines and chemokines and liberate them and their cargo into the cerebrospinal fluid. In turn, these EPY-dEVexos will stream throughout the CSF to enter the EPY CSF lining ventricular cells to enter the subventricular zone interstitium. Upon this entry into the CNS interstitium, the EPY-derived EVexos (EPY-dEVexos) will be capable of diffusing via interstitial fluid bulk flow throughout the CNS interstitium to activate neuroglia with aMGCs and subsequent rACs that increase CNS CC (cnsCC) to result in neuroglia activation, CNS remodeling, neurodegeneration, and impaired cognition. Additionally, these peripherally derived exosomes will also readily enter the circumventricular organs with their fenestrated capillaries.
3. MicroRNA’s Role in Ongoing PVAT Inflammation Remodeling, and the Development of Peripheral Systemic Insulin Resistance
The inflammatory trigger in obesity is metabolic (nutrient excess and decreased physical activity) and the visceral adipocytes are the cells that becomes the triggering cells for PVAT metainflammation via macrophage CLSs. This metainflammation appears to be self-sustaining in the DIO Western and the obese, diabetic db/db models, which results in the interruption of metabolic homeostasis [66]. The hypertrophic adipocytes undergo increased lipolysis with the liberation of increased toxic unesterified free fatty acid(s) (FFA) and this is likely to be one of the early triggering factors for inflammation [60,67] along with the paracrine intercellular communication of exosome miR-155 (promoting M1 MΦ polarization) [56] and miR-34a (inhibiting M2 MΦ polarization) (Figure 3) [56,68]. Thus, VAT-PVAT adipocytes continue to instigate chronic ongoing metainflammation with the polarization of macrophages and the formation of CLS. Additionally, the excess liberation of unesterified toxic FFA and cholesterol into the systemic circulation from the VAT and PVAT depots provide the mechanism, in which ectopic lipids deposit in cardiac and skeletal muscle, liver, and pancreatic beta-cells to create the lipotoxicity in these ectopic sites that is associated with the development of systemic insulin resistance [69,70,71,72].
Zvikowski et al. have recently outlined multiple common adipocyte (PVAT-dEVexos) and macrophage (PVATMΦ-dEVexo) miRNAs and their respective roles in miRNA paracrine intercellular signaling and endocrine inter-organ signaling and their contribution to peripheral insulin resistance (Table 1) [68,73,74,75,76,77,78,79].

Table 1.
PVAT-dEVexos and PVATMΦ-dEVexos microRNAs (miRNAs) contribution to peripheral insulin resistance (IR) via intercellular and inter-organ communication with liver and skeletal muscle [68,73,74,75,76,77,78,79]. M1 = classically activated macrophage polarization-proinflammatory; MΦ = macrophage; PVAT = perivascular adipose tissue; PVAT-dEVexos = perivascular adipose tissue-derived extracellular vesicles exosomes; PVATMΦ-dEVexos = perivascular adipose tissue macrophage-derived extracellular vesicles exosomes.
Additionally, Huang et al. have shared that there are an additional seven other miRNAs that are capable of inducing peripheral systemic IR in regard to examining new insights into the role of miRNAs in the MetS [80]. These include miR-143 (inhibits insulin induced Akt activation and induces IR); miR-145 (inhibits phosphorylation if IRS-1 and Akt); miR-190 -190b (can decrease the level of IGF-1); miR-21 (can inhibit TGF-B1/Smad3 pathway); miR-93, miR-106b (inhibits glucose transporter 4 (GLUT4)); miR-222; miR-30d (inhibits PI3K) [80].
There is considerable crosstalk (intercellular paracrine communication) between obese PVAT adipocytes and macrophages such that the thinned-walled, engorged, and rupture-prone PVAT adipocytes are capable of signaling regional macrophages via exosomes with their cargo of miR-155 to polarize MΦs to a M1 phenotype and in turn, these M1 MΦs can signal PVAT adipocytes via exosomes with their cargo of miR-155 and miR-130b to result in PVAT adipocyte insulin resistance [81,82,83]. Notably, in both prediabetes and overt T2DM it has been recently found that proinflammatory miR-222 is increased and anti-inflammatory miR-126-3p and miR-146a are decreased [84]. This increased proinflammatory miR-222 was also found to be associated with increased TNFa, Il-6, and IR and decreased anti-inflammatory miR-126-3p and miR-146a were negatively correlated with TNFa, Il-6 and IR. Further, Zeinali et al., suggested that this miRNA profile could potentially contribute to the pathogenesis of T2DM [84].
4. Adipocyte (PVAT-dEVexos), Macrophage (PVATMΦ-dEVexos), and MicroRNAs Contribution to Neuroglia Activation, Neuroinflammation, Remodeling, and Impaired Cognition
T2DM is a progressive metabolic disease that has adverse functional and structural remodeling effects within the CNS (Figure 2) [14,20,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39]. Both learning and memory are known to be impaired in the obese, IR, LR, and diabetic db/db models as early as 17 weeks of age [85]. Additionally, Yermakov et al. have been able to demonstrate impaired cognitive flexibility in the db/db models at 24 weeks of age [86].
T2DM doubles the risk of dementia in older individuals [87] and even if these T2DM individuals do not have dementia their cognitive impairment is of major importance [88,89,90,91]. Notably, decreased long-term memory [3,4,92,93], cognitive flexibility [86,94,95], and working memory may be impaired [96]. Importantly, these impairments in cognitive function can interfere with the necessary self-care provided by these individuals for the proper control of their T2DM [86,97,98]. Depression in T2DM is also known to commonly occur and interferes with executive function, which will compound the problem with the necessary self-care that is required of these individuals [98]. Therefore, the obese, IR, LR, and diabetic db/db preclinical models are thought to provide a reliable model for studying impaired cognition and the associated neuroinflammation, and CNS remodeling that develops around 17–20 weeks of age and older [85,86].
EVexos are capable of transferring proteins and fats as well as genetic information horizontally via RNA, mRNA and microRNAs [99] and are increasingly recognized as mediators of BBB and BCSFB intercellular communication in the CNS [63,100]. Notably, current evidence demonstrates that small and soluble EVs are also involved in the regulation of inflammation-neuroinflammation and oxidative stress, which are capable of affecting a multitude of human pathologies [101].
Currently, it is now recognized that peripheral inflammatory stimuli such as the PVAT adipocyte PVAT-dEVexos, macrophage PVATMΦ-dEVexos, and their microRNAs are capable of activating CNS microglia and in turn these activated MGCs (aMGCs) are capable of rendering the ACs to polarize to a reactive phenotype (rACs) that are capable of promoting pathologic astrogliosis, which contributes to chronic neuroinflammation and impaired cognition [65,102,103,104,105].
The uptake of peripherally derived PVAT adipocyte (PVAT-dEVexos) and macrophage (PVATMΦ-dEVexos) is via the neurovascular unit at the BBB interface via BEC transcytosis, paracellular junctions, and fenestrations of the choroid plexus capillaries at the BCSFB interface [106,107,108,109]. Further, Shetgaonkar et al. [108] and Rufino-Ramos et al. [110] have shared that one of the most important core features of exosomes is their ability to pass the CNS barrier (BBB and BCSFB) interfaces. The following figure illustrates how PVAT-dEVexos are capable of entering and passing through the NVU BBB and the choroid plexus BCSFB to enter the cerebral spinal fluid of the ventricles (Figure 8) [108,110].
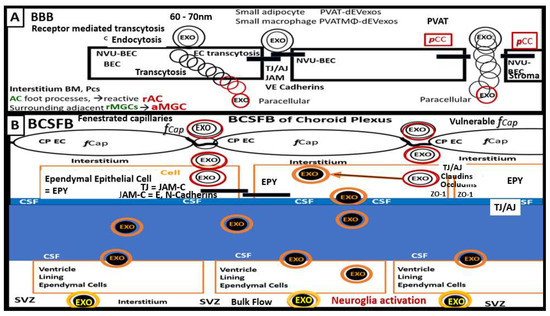
Figure 8.
Illustration of the PVAT-derived exosomes (PVAT-dEVexos) passing the blood–brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB). (A) depicts the adipocyte perivascular adipose tissue-derived extracellular exosomes (PVAT-dEVexos) and the perivascular adipose tissue macrophage-derived extracellular exosomes (PVATMΦ-dEVexos) passing the BBB neurovascular unit (NVU) brain endothelial cell(s) (BEC) via receptor mediated endocytosis and transcytosis and the paracellular tight/adherens junctions (TJ/AJ). Importantly, the passage of exosomes via the paracellular junctional regions is enhanced in the presence of peripheral cytokine/chemokines (pCC) that will be elevated via the macrophage inflammation from the PVAT (outlined in red) and may contribute to the impairment of paracellular junctions. (B) demonstrates the passage of PVAT-dEVexos and PVATMΦ-dEVexos (black center outlined in orange) via the more vulnerable fenestrated capillaries of the choroid plexus (CP) endothelial cell(s) (EC) into the interstitial stromal regions to enter the basilar ependymal cells (EPY) of the BCSFB. Here, the exosomes may result in the activation of the epithelial ependymal cells (EPY)-derived (blackened exosomes lined by orange color) (EPY-dEVexos) that will be secreted into the cerebrospinal fluid (CSF) and pass through the CSF via a streaming effect to be eventually taken up by CSF ventricle lining EPY cells to be delivered to the interstitial fluid space of the subventricular zones (SVZ) (blackened center with golden outline) and be capable of activating the neuroglia microglia cells which activated the astrocytes to form reactive astrocytes with resulting remodeling astrogliosis and the activation of central nervous system cytokines and chemokines with ongoing neuroinflammation as they flow through the interstitial fluid space via bulk flow. AC = astrocyte(s); aMGC = activated microglia cell(s); BM = basement membrane; EXO = exosome(s); JAM-C = junctional adherens molecule-C; JAM-C = E or N Cadherens; rAC = reactive astrocytes; rMGC = reactive microglia cell(s) or aMGCs; TJ/AJ = tight and adherens junctions; ZO-1= zona occludens-1.
Even though the small PVAT exosomes are now known to pass the CNS barriers the exact mechanism of interaction is not completely understood; however, both the BEC transcellular routes and the paracellular routes are thought to be involved at both the BBB and BCSFB [108,110]. Additionally, the BCSF has fenestrated capillaries within the choroid plexus and this more vulnerable route of exosome passage has been previously discussed [64].
PVAT derived miRNA EVexos are thought to be proinflammatory and result in neuroglia activation of MGCs. Specifically, miR-155 seems to be a constant proinflammatory miRNA and is delivered via EVexos to the CNS and is capable of passing the BBB and BCSFB interfaces to affect the recipient neuroglia. MiR-155 is thought to promote inflammation in recipient CNS cells (MGCs and ACs) through its effect of downregulating Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase 1 (SHIP1) and suppressor of cytokine signaling 1 (SOCS1), which are associated with increased nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor (p65) and multiple downstream cytokines such as cnsCC, which include TNFα, IL-1β, IL-6, and MCP-1 [105,111]. Once these exosomes have passed the BBB interface and reside within the CNS interstitium they will be able to signal and activate CNS MGCs that will, in turn, result in the formation of rACs and the promotion of pathologic astrogliosis in addition to promoting ongoing neuroinflammation [112,113]. In regard to the importance of miR-155 and neuroinflammation, Gaudet et al., have created an miR-155 (-5p and -3p) knock out model and were able to demonstrate in female mice models that these models were resistant to weight gain when fed a high-fat diet. Their findings suggested that by deleting miR-155 (5p and -3p) there was increased adipogenesis, improved insulin sensitivity, and improved energy uncoupling machinery by increasing UCP-1 in adipose depots, along with the limitation of inflammation in WAT [114].
Once these exosomes have passed the choroid plexus endothelial cells they will be taken up at the basal side of the epithelial choroid plexus ependymal cells and result in activation of these ependymal cells to produce secondary epithelial ependymal exosomes (EPY-EVexos) to be secreted into the CSF where they can then interact and traverse the other lining ependymal cells of the CSF ventricles and enter the CNS interstitium and pass throughout the interstitial fluid space by bulk flow to activate the neuroglia MGCs and result in reactive ACs [64,107].
PVAT derived miRNA EVexos are thought to be proinflammatory and result in neuroglia activation of MGCs. Specifically, miR-155 seems to be a constant proinflammatory miRNA and is delivered via EVexos to the CNS and are capable of passing the BBB and BCSFB interfaces to affect the recipient neuroglia. miR-155 is thought to promote inflammation in recipient CNS cells through its effect of downregulating Src homology 2 (SH2) domain containing inositol polyphosphate 5-phosphatase 1 (SHIP1) and suppressor of cytokine signaling 1 (SOCS1), which are associated with increased nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) transcription factor (p65) and multiple downstream cnsCC, such as TNFα, IL-1β, IL-6, MCP-1 [111,115].
The development of aMGCs and rACs may result in NVU uncoupling. This loss of NVU coupling with decreased cerebral blood flow results in regional CNS ischemia. This ischemic injury will result in a compensatory response to injury wound healing mechanism to result in pathologic astrogliosis and ensuing impaired cognition. These remodeling changes due to neuroglia activation may be summarized in the following schematic illustration of the possible sequence of events that result in neuroglia activation, neuroinflammation, CNS remodeling and impaired cognition (Figure 9) [20,42,63,66,67,116,117].
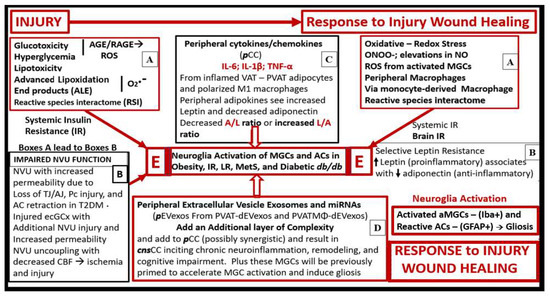
Figure 9.
Possible sequence of events leading to neuroglia activation, neuroinflammation, brain remodeling, and impaired cognition due to descending thoracic aorta perivascular tissue (PVAT)-derived adipocyte and macrophage extracellular vesicle exosomes (EVexos) (A–E). This schematic illustration suggests that an injury and response to injury wound healing mechanism is present in the central nervous system (CNS) [62]. Further, this injury would be induced by perivascular adipose tissue (PVAT) inflammation and dysregulation of peripheral adipokines (increased proinflammatory leptin and decreased anti-inflammatory adiponectin), and peripheral cytokines and chemokines (pCC). Increase pCC would activate the CNS ramified microglia cells (rMGCs) to undergo polarization to an M1-like ameboid activated (aMGC) proinflammatory phenotype, while the astrocytes (ACs) would become reactive, proinflammatory phenotypes (rAC) and result in pathological astrogliosis. Furthermore, neuroglia activation would result in an ongoing chronic neuroinflammation with increased central nervous system cytokines/chemokines (cnsCC) production associated with cellular and brain remodeling in a response to injury mechanism with ensuing impaired cognition. Additionally, rAC detachment, retraction, and separation from the NVU will result in NVU uncoupling and decreased regional cerebral blood flow (CBF) and ischemia, which would also result in injury and a response to injury wound healing mechanism. Note that boxes A–D all lead to the final outcome of neuroglia activation in the central box E. While the possible sequence of events has been denoted in sequential lettering (A–E), it is likely that each of these injuries and response to injuries is occurring concurrently rather than occurring in a hard and fast sequence of (A–E). A = adiponectin; AGE/RAGE = advanced glycation end products and its receptor; CBF = cerebral blood flow; ecGCx = endothelial cell glycocalyx; GFAP = glial fibrillary acidic protein; iba1 = ionized calcium binding adaptor molecule1; IL- 1β = interleukin-1 beta; IL- 6 = interleukin- 6; IR = insulin receptor; L = leptin; LR = leptin resistance; miRNA(s) = micro ribonucleic acid(s); NO = nitric oxide; NVU = neurovascular unit; O2.- = superoxide; ONOO- = peroxinitrite; Pc = pericyte; ROS = reactive oxygen species; T2DM = type 2 diabetes mellitus; TJ/AJ = tight and adherens junctions; TNFα = tumor necrosis alpha.
Importantly, previously published experimental findings from rodents in association with genome wide association studies suggest that there may be a direct association between neuroinflammation and the development and progression of neurodegeneration [65,118,119,120,121,122,123]. Notably, neuroinflammation and the development and progression of neurodegeneration in humans have been documented and further, the aMGCs and rACs have a bidirectional influence on one another [124,125].
Bidirectional Crosstalk between Microglia, Astrocytes, and Neuronal Synapses
Astrocytes are the largest and most numerous glial cell type in the CNS and play a major role in orchestrating CNS homeostasis [126,127]. Importantly, they are the connecting cells within the brain and upon activation, reactive ACs (rACs) are capable of bidirectional signaling with MGCs to result in the activation/polarization of MGCs to the classical M1-like aMGC. It has been found that reactive astrocytes lose their supportive role of maintaining homeostasis and gain toxic and pathologic functions in the progression of neurodegenerative diseases [113,126]. Importantly, both injury and disease including obesity and T2DM result in an evolutionarily conserved program, which plays an important role in the protection and the repair due to a response to injury CNS wound healing mechanism [20,37,38,62,128] with induction of both aMGCs and rACs. Further, rACs are induced by aMGCs and these two cells undergo bidirectional signaling [113,126]. Astrocytes and their foot processes (ACfp) maintain ionic and neurotransmitter homeostasis, refine synaptic connections, and provide neuronal metabolic substrates. Furthermore, AC diversity is even more pronounced in human individuals as compared to rodent models [40,127]. Notably, ACs may be divided into protoplasmic ACs in cortical grey matter regions where their foot processes interact with the NVU and synapses, which provide a cradling effect for the tripartite synapse (including the pre-and postsynaptic neuron terminals, AC cradle, and the perineuronal nets). Additionally, ACs contribute to cerebral blood flow and synapse signaling and maintenance [40,41,44,45,129,130,131], whereas fibrous ACs are present exclusively in the white matter and interact with myelinated neurons [42,130].
ACs are key cells important for the formation of neural circuity from the early guidance in their formation to maintenance in fully developed individuals [40,126,127,129,130]. In regard to the use of the term reactive AC and reactive astrogliosis readers are strongly urged to read the recent paper regarding AC nomenclature, definitions, and future directions [131].
Microglia monitor synaptic elements and various networks and importantly include the NVU. They are known to respond to dyshomeostasis by inducing or removing synaptic elements and by modulating neuronal activity. Additionally, MGCs also participate in neuromodulation, synaptic plasticity, learning and memory formation and even pruning of synapses when necessary [130]. Therefore, if MGCs are activated they may lose their important neuromodulation capabilities and contribute to impaired cognition known to occur in db/db models.
MGCs and ACs respond to neuronal injury with various programs including the synthesis and secretion of cnsCC and are capable of undergoing proliferation, morphological alterations, mediator production, and engulfment of cells and subcellular elements as well as bidirectional signaling. These changes represent the CNS tissue response to injury and response to injury CNS wound healing. Once these CNS MGCs are activated via pCC and PVAT-dEVexos and PVATMΦ-dEVexo and the AC phenotype and function are altered to become rACs they are capable of exchanging various miRNA containing EVexos bidirectionally [130].
Oligodendrocytes and oligodendrocyte precursor cells are capable of synthesizing and elaborating myelin sheaths, which protect and nourish myelinated neuronal segments [34]. However, in obese diabetic db/db models the ACfp detachment and remodeling are associated with significant aberrant myelinated neuronal remodeling [32,34].
Neuroglia (MGCs and ACs) play an extremely important and key role in the bidirectional crosstalk or signaling between one another and utilize cnsCCs, EVexos, and microvesicles when the pCC initiate or instigate chronic neuroglia activation from the PVAT-dEVexos and PVATMΦ-dEVexos. Indeed, aMGC and rAC interactions may determine the eventual phenotype that develops due to the chronic pCC delivered to the CNS. These two neuroglia cells (MGCs and ACs) express cnsCC, EVexosomes, and microvesicles during chronic neuroglia activation and neuroinflammation; thus, create a vicious cycle in obesity and diabetes as occurs in the preclinical db/db mouse models (Figure 10) [129,132,133].
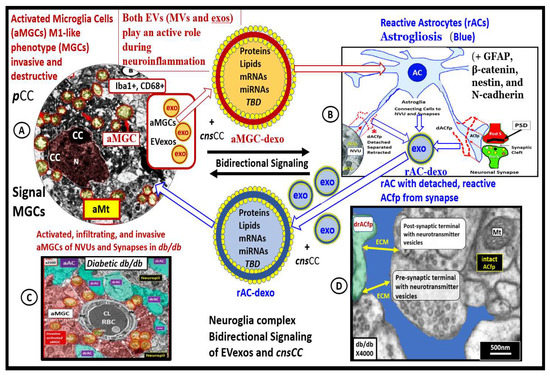
Figure 10.
Peripheral extracellular exosomes (EVexos) that include adipocyte PVAT-dEVexos, macrophage PVATMΦ-EVexos, and peripheral cytokines/chemokines (pCC) are capable of activating central nervous system (CNS) microglia (aMGCs) with microgliosis and bidirectional signaling with astrocytes. In turn these aMGCs undergo bidirectional crosstalk signaling with astrocytes (AC) that result in reactive astrocytes (rACs) and astrogliosis via cnsEVexos and cnsCC. Subsequently, these rACs are capable of signaling MGCs into becoming aMGCs and reinforce this vicious cycling resulting in neuroinflammation, CNS remodeling, and impaired cognition. Representative transmission electron microscopic images of aMGC (A), rAC (B), encroaching aMGC of neurovascular unit (C), and detached rAC from neuronal synapse (D). These modified transmission electron microscopic images are provided with permission by CC 4.0 [32,33,36]. AC-dEVexo = astrocyte derived exosomes; aMGC = activated microglia cell; aMt = aberrant mitochondria; CC = chromatin condensation; cnsCC = central nervous cytokines/chemokines; CD68 = cluster of differentiation 68; dAFfp = detached astrocyte foot process(es); EV = extracellular vesicles; exos = exosomes; GFAP = glial fibrillary acidic protein; iba1 = ionized calcium-binding adapter molecule 1; MGC(s) = microglia cells; MΦ = macrophage; mRNA = messenger ribonucleic acid; miRNA = micro ribonucleic acid; MVs = microvesicles; N = nucleus; PVAT = perivascular adipose tissue; PostS = post synaptic neuron; PreS = presynaptic neuron; PSD = postsynaptic density; RBC = red blood cell; TBD = to be determined.
Importantly, the EVexos derived from both adipocytes and macrophages also have the capability to carry and deliver EVexo containing mitochondrial deoxyribonucleic acid (MtDNA), which is known to contribute to peripheral insulin resistance in obese T2DM human individuals [134]. The capability of adipocyte (PVAT-dEVexos) and macrophage (PVATMΦ-dEVexos) to be delivered via EVexos via the endocrine inter-organ signaling to the brain, allows one to ponder the possibility that these peripherally derived EVexos could contribute to not only to peripheral IR as discussed by Yuzefovych et al., but also to central BIR [134]. However, to date, this hypothesis has not been tested.
5. Conclusions
During the past decade, EVexos have emerged as a novel class of signaling molecules that are known to mediate autocrine, intercellular (paracrine) and inter-organ (endocrine) communication. These EVexos are released by various cell types including the VAT and specifically PVAT adipocytes and macrophages in the descending thoracic aorta of the obese, insulin and leptin-resistant, diabetic db/db preclinical models that can be widely distributed to other cells and organs via an endocrine, inter-organ communication including the brain (Figure 7 and Figure 8). These EVexos are protected by a bilayer lipid membrane and therefore do not readily undergo degradation and digestion by phagocytic cells or degradative enzymes [135]. Their contents including proteins, lipids and nucleic acids (mRNA, coding and long-non-coding RNA (incRNAs), and microRNA, which allow these EVexos from the PVAT to signal neuroglia within the CNS. Once signaled by PVAT-dEVexos these recipient cells such as the microglia and astrocytes undergo activation, remodeling and result in neuroinflammation, CNS remodeling, neurodegeneration, neuronal synaptic dysfunction, and impaired cognition. Because of these basic and fundamental features, EVexos are well suited to serve as versatile carriers and transporters of their cargo, which allow for the transmission of signals from parental cells such as the PVAT adipocytes and macrophages to the recipient neuroglia cells in the CNS. A better understanding of the roles that EVexos play in the crosstalk between multiple tissues and organs (inter-organ communication) will allow for a new perspective in order to better understand not only the pathogenesis of obesity and T2DM but also diabetic end-organ disease including the activation of the CNS neuroglia, neuroinflammation, remodeling, and impaired cognition [63,65,136,137].
The TEM images, illustrations, and the discussions presented in this overview of the obese, diabetic db/db preclinical models paralleled many of the similar findings by Giordano et al., where they additionally were able to demonstrate (NRL3P) inflammasome activation of caspase-1 induced adipocyte death by pyroptosis and demonstrate the electron-dense staining of the thinned and prone-to-rupture plasma membrane with calcium deposition (by calcium-specific positive von Kossa staining) [138]. Because of their diversity of active molecules and biological signaling information (proteins, lipids, and especially genetic material such as mRNA, incRNAs, and miRNAs) these EVexos contribute to inter-organ cellular signaling in the CNS. Cerebral NVU ECs, Pcs, perivascular ACs, MGCs, and neurons, which are all a part of the NVU have been shown to release and take up exosomes in a bidirectional manner [139]. Importantly, EVexos are able to traverse the BBB and BCSFBs, which allow for communication between peripheral cells of the descending aortic PVAT and the CNS cells with multicellular crosstalk [105,106,107,108,109,138]. Notably, it has been determined that T2DM is associated with impaired cognition in humans [140,141] and in obese, diabetic db/db preclinical models [142,143]. Further, this impaired cognition is closely related to hyperglycemia, oxidative stress, and neuroinflammation due to neuroglia activation (aMGCs and rACs), that result in CNS remodeling [14,20,32,33,34,36,37,38].
This overview has attempted to create an integrated approach utilizing the findings of PVAT engorged unilocular rupture-prone adipocyte PVAT-dEVexos (Figure 5 and Figure 7) and PVAT CLS macrophages PVATMΦ-dEVexos (Figure 6) and how they might be responsible for the development of neuroglia activation, remodeling, and impaired cognition in obese, IR, LR, and diabetic db/db models. This presented concept has integrated the production of adipocyte (PVAT-dEVexos) and macrophage (PVATMΦ-dEVexos) that are formed in the PVAT of the obese, diabetic db/db descending thoracic aorta from the transdifferentiated multilocular BAT in controls to the engorged and rupture-prone unilocular WAT in the db/db models.
Throughout this overview, author has been able to add additional knowledge to the above by discussing the integration of peripheral inflammation with increased pCC, identify peripheral obese adipocyte PVAT-dEVexos (Figure 6) and macrophage PVATMΦ-dEVexos (Figure 7) and PVAT remodeling in the descending thoracic aorta of the obese, diabetic db/db models (Section 2). Furthermore, to elucidate how these mechanisms allow for the PVAT-dEVexos and PVATMΦ-dEVexos and their miRNAs to promote peripheral IR and possibly brain insulin resistance (Section 3). Discuss how the PVAT-dEVexos and PVATMΦ-dEVexos and their miRNAs enter and cross the CNS BBB interface to enter the CNS interstitium, the BCSFB interfaces to enter the CSF at the choroid plexus and then enter the CNS interstitium via CSF lining ependymal cells to activate MGCs (neuroinflammation) and result in reactive ACs (CNS remodeling astrogliosis) and contribute to impaired cognition (Section 4) and bidirectional crosstalk between microglia, astrocytes, and neuronal synapses (Section Bidirectional Crosstalk between Microglia, Astrocytes, and Neuronal Synapses).
This overview has discussed the recurring importance of VAT (utilizing the PVAT depot as a surrogate for VAT) as a source for metainflammation due to the formation of CLS of activated MΦs in this depot to generate pCC and EVexos from not only MΦs but also PVAT remodeled adipocytes due to obesity. Further, PVAT-dEVexos and PVATMΦ-dEVexos have been demonstrated to be capable of both paracrine and endocrine (inter-organ signaling) to the CNS. The ongoing VAT-PVAT chronic metainflammation is thought to drive peripheral-systemic IR. It is now known that obesity is one of the main causes of peripheral systemic insulin/IGF-1 resistance that correlates strongly with brain insulin resistance [144,145,146,147,148,149]. Notably, Spielman et al., have put forth the concept that inflammation and insulin/IGF-1 resistance may be the link between obesity, neuroinflammation, and neurodegeneration [145].
Exosome-derived miRNAs may now be considered to play an important and central role in obesity, MetS, IR, and T2DM by allowing peripherally derived miRNAs to signal the CNS via an endocrine inter-organ mechanism and result in the activation of neuroglia including the microglia and astrocyte populations (cnsCC) along with the pCC mechanisms [150]. Not only are MGCs and ACs capable of regulating homeostasis but also theseneuroglia play an important and emerging role of instigating CNS remodeling and impaired cognition in obesity and diabetes in human individuals and the obese, diabetic preclinical db/db mouse models when they are activated and reactive. At this point in time, many of the EVexos are not completely understood and their miRNAs have not been fully studied; however, in due time we will begin to understand not only their physiologic and protective roles but also their damaging and disease-supporting mechanisms. To confirm the author’s thoughts, Gaudet et al. (2018), have recently stated the following: “the miRNA-neuroinflammation field is in its infancy” [103]. Furthermore, we are now beginning to understand some of the roles that EVexos are playing in the development of the end-organ remodeling complications of diabetes and the various end-organ involvement of the diabetic-opathies such as neuropathy, retinopathy, nephropathy, accelerated atherosclerosis–atheroscleropathy, cardiomyopathy, and diabetic encephalopathy–cognopathy that is manifest by impaired cognition due to neuroglia activation, chronic neuroinflammation, CNS remodeling with microgliosis and reactive astrogliosis as presented in this overview [151].
Furthermore, many authors sometimes single out just one or two miRNAs to examine their roles; however, we may come to understand that the exosome miRNA profiles may be equally important, since some miRNAs are upregulated and concurrently some are downregulated, to create a specific profile of miRNAs to better understand their roles in obesity, MetS, and T2DM with neuroglia activation, neuroinflammation, and impaired cognition. For example, Kim et al., have recently provided heat maps of individuals with obesity and diabetes that reveal definite profiles, which consist of multiple miRNAs that are different from those without obesity or diabetes [152]. These findings strongly suggest that instead of just looking at one or two specific miRNAs that we may need to examine multiple miRNAs to better define the miRNA profiles that will emerge as biomarkers or therapeutic profiles as we continue to learn more about these unique miRNA profiles in obesity and T2DM and examine and compare both those miRNAs that are upregulated along with those that are down-regulated. Even though it is important to single out just one miRNA and determine whether or not it is upregulated or downregulated in a given situation, it may be even more important to study the various miRNA profiles in obesity and T2DM that is associated with neuroglia activation, neuroinflammation, and impaired cognition [152].
With novel findings come new knowledge and it will be exciting to continue to observe the growth in the field of obesity and T2DM exosomes and their microRNAs and how this knowledge not only adds a new layer of complexity but also allows for our continued growth and understanding of these two complex multifactorial and polygenic diseases in the development of neuroglia activation, neuroinflammation, remodeling, and neurodegeneration with impaired cognition.
Funding
This research received no external funding.
Institutional Review Board Statement
The tissues provided for the representative electron microscopic images utilized in this manuscript were all approved in advance by the University of Missouri Institutional Animal Care and Use Committee, and animals were cared for in accordance with National Institutes of Health guidelines and by the Institutional Animal Care and Use Committees at the Harry S Truman Memorial Veterans’ Hospital and the University of Missouri, Columbia, MO, USA and conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data and materials will be provided upon reasonable request.
Acknowledgments
Author would like to acknowledge DeAna Grant Research Specialist & Interim Director of the Electron Microscopy Core Facility at the NextGen Precision Health Research Center, University of Missouri, Columbia, Missouri. Furthermore, author would like to acknowledge Shelly Erickson Research Assistant Professor at the University of Washington and a Research Biologist at the VA Medical Center-Seattle, Washington, for providing the lanthanum perfused control models in this review.
Conflicts of Interest
Author declares no conflict of interest.
References
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA 2012, 307, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.M.; Carroll, M.D.; Kuczmarski, R.J.; Johnson, C.L. Overweight and obesity in the United States: Prevalence and trends, 1960–1994. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Prevalence of overweight and obesity among adults with diagnosed diabetes–United States, 1988–1994 and 1999–2002. MMWR Morb Mortal Wkly Rep. 2004, 53,1066–1068. Prevalence of overweight and obesity among adults with diagnosed diabetes--United States, 1988–1994 and 1999–2002. MMWR Morb. Mortal Wkly. Rep. 2004, 53, 1066–1068. [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults: Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2002, 285, 2486–2497. [Google Scholar] [CrossRef]
- Grundy, S.M.; Brewer, H.B., Jr.; Cleeman, J.I.; Smith, S.C., Jr.; Lenfant, C.; American Heart Association; National Heart, Lung, and Blood Institute. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004, 109, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Flint, A.J.; Hu, F.B.; Glynn, R.J.; Caspard, H.; Manson, J.E.; Willett, W.C.; Rimm, E.B. Excess weight and the risk of incident coronary heart disease among men and women. Obesity 2010, 18, 377–383. [Google Scholar] [CrossRef]
- Lee, C.M.; Huxley, R.R.; Wildman, R.P.; Woodward, M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than bmi: A meta-analysis. J. Clin. Epidemiol. 2008, 61, 646–653. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ: Morphological perspectives of adipose tissues. Proc. Nutr. Soc. 2001, 60, 319–328. [Google Scholar] [CrossRef]
- Cinti, S. The adipose organ. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 915. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. The adipose organ at a glance. Dis. Model Mech. 2012, 5, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. The Mighty Mitochondria Are Unifying Organelles and Metabolic Hubs in Multiple Organs of Obesity, Insulin Resistance, Metabolic Syndrome, and Type 2 Diabetes: An Observational Ultrastructure Study. Int. J. Mol. Sci. 2022, 23, 4820. [Google Scholar] [CrossRef]
- Bjørndal, B.; Burri, L.; Staalesen, V.; Skorve, J.; Berge, R.K. Different Adipose Depots: Their Role in the Development of Metabolic Syndrome and Mitochondrial Response to Hypolipidemic Agents. J. Obes. 2011, 2011, 490650. [Google Scholar] [CrossRef]
- Wajchenberg, B.L. Subcutaneous and visceral adipose tissue: Their relation to the metabolic syndrome. Endocr. Rev. 2000, 21, 697–738. [Google Scholar] [CrossRef]
- Chowdhury, B.; Sjöström, L.; Alpsten, M.; Kostanty, J.; Kvist, H.; Löfgren, R. A multicompartment body composition technique based on computerized tomography. Int. J. Obes. Relat. Metab. Disord. 1994, 18, 219–234. [Google Scholar] [PubMed]
- Hoffstedt, J.; Arner, P.; Hellers, G.; Lönnqvist, F. Variation in adrenergic regulation of lipolysis between omental and subcutaneous adipocytes from obese and non-obese men. J. Lipid Res. 1997, 38, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.K.; Chen, M.; Clements, R.H.; Abrams, G.A.; Aprahamian, C.J.; Harmon, C.M. Human mesenteric adipose tissue plays unique role versus subcutaneous and omental fat in obesity related diabetes. Cell Physiol. Biochem. 2008, 22, 531–538. [Google Scholar] [CrossRef]
- Hayden, M.R. Empagliflozin Ameliorates Tunica Adiposa Expansion and Vascular Stiffening of the Descending Aorta in Female db/db Mice. Adipobiology 2020, 10, 1. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Beltowsky, J.; Ghenev, P.I.; Fiore, M.; Panayotov, P.; Rančič, G.; Aloe, L. Adipoparacrinology-vascular periadventitial adipose tissue (tunica adiposa) as an example. Cell Biol. Int. 2012, 36, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G.N.; Fiore, M.; Ghenev, P.I.; Beltowski, J.; Ranćić, G.; Tunçel, N.; Aloe, L. Triactome: Neuro-immune-adipose interactions. Implication in vascular biology. Front. Immunol. 2014, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S. Adipocyte differentiation and transdifferentiation: Plasticity of the adipose organ. J. Endocrinol Invest. 2002, 25, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.; Jenkins, N.T.; Vieira-Potter, V.J.; Laughlin, M.H. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R543–R552. [Google Scholar] [CrossRef]
- Hayden, M.R.; Joginpally, T.; Salam, M.; Sowers, J.R. Childhood and adolescent obesity in cardiorenal metabolic syndrome and Type 2 diabetes: A clinical vignette and ultrastructure study. Diabetes Manag. 2011, 1, 601–614. [Google Scholar] [CrossRef]
- Hayden, M.R.; Sowers, J.R. Childhood-Adolescent Obesity in the Cardiorenal Syndrome: Lessons from Animal Models. Cardiorenal Med. 2011, 1, 75–86. [Google Scholar] [CrossRef]
- Aroor, A.R.; Sowers, J.R.; Bender, S.B.; Hayden, M.R.; Nistala, R.; DeMarco, V.G.; Hayden, M.R.; Johnson, M.S.; Salam, M.; Whaley-Connell, A.; et al. Dipeptidylpeptidase Inhibition Is Associated with Improvement in Blood Pressure and Diastolic Function in Insulin-Resistant Male Zucker Obese Rats. Endocrinology 2013, 154, 2501–2513. [Google Scholar] [CrossRef]
- Hayden, M.R.; Banks, W.A.; Shah, G.N.; Gu, Z.; Sowers, J.R. Cardiorenal metabolic syndrome and diabetic cognopathy. Cardiorenal Med. 2013, 3, 265–282. [Google Scholar] [CrossRef]
- Salameh, T.S.; Shah, G.N.; Price, T.O.; Hayden, M.R.; Banks, W.A. Blood-Brain Barrier Disruption and Neurovascular Unit Dysfunction in Diabetic Mice: Protection with the Mitochondrial Carbonic Anhydrase Inhibitor Topiramate. J. Pharm. Exp. Ther. 2016, 359, 452–459. [Google Scholar] [CrossRef]
- Habibi, J.; Aroor, A.R.; Sowers, J.R.; Jia, G.; Hayden, M.R.; DeMarco, V.G.; Barron, B.; Mayoux, E.; Rector, R.S.; Whaley-Connell, A.; et al. Sodium glucose transporter 2 (SGLT2) inhibition with empagliflozin improves cardiac diastolic function in a female rodent model of diabetes. Cardiovasc. Diabetol. 2017, 16, 9. [Google Scholar] [CrossRef]
- Aroor, A.R.; Habibi, J.; Kandikattu, H.K.; Hayden, M.R.; Garro-Kacher, M.; Bender, S.B.; Hayden, M.R.; Whaley-Connell, A.; Bender, S.B.; Klein, T.; et al. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc. Diabetol. 2017, 16, 61. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of The Neurovascular Unit in The Female Diabetic db/db Model—Part I: Astrocyte. Neuroglia. Neuroglia 2018, 1, 220–244. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of The Neurovascular Unit in in the Female Diabetic db/db Model–Part II: Microglia and Mitochondria. Neuroglia 2018, 1, 311–326. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; Demarco, V.G. Ultrastructural Remodeling of the Neurovascular Unit in the Female Diabetic db/db Model—Part III: Oligodendrocyte and Myelin. Neuroglia 2018, 1, 351–364. [Google Scholar] [CrossRef]
- Aroor, A.R.; Das, N.A.; Carpenter, A.J.; Habibi, J.; Jia, G.; Ramirez-Perez, F.I.; Martinez-Lemus, L.; Manrique-Acevedo, C.M.; Hayden, M.R.; Duta, C.; et al. Glycemic control by the SGLT2 inhibitor empagliflozin decreases aortic stiffness, renal resistivity index and kidney injury. Cardiovasc. Diabetol. 2018, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Hypothesis: Astrocyte Foot Processes Detachment from the Neurovascular Unit in Female Diabetic Mice May Impair Modulation of Information Processing-Six Degrees of Separation. Brain Sci. 2019, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Type 2 Diabetes Mellitus Increases the Risk of Late-Onset Alzheimer’s Disease: Ultrastructural Remodeling of the Neurovascular Unit and Diabetic Gliopathy. Brain Sci. 2019, 9, 262. [Google Scholar] [CrossRef]
- Hayden, M.R.; Grant, D.G.; Aroor, A.R.; DeMarco, V.G. Empagliflozin Ameliorates Type 2 Diabetes-Induced Ultrastructural Remodeling of the Neurovascular Unit and Neuroglia in the Female db/db Mouse. Brain Sci. 2019, 9, 57. [Google Scholar] [CrossRef]
- Hayden, M.R.; Banks, W.A. Deficient Leptin Cellular Signaling Plays a Key Role in Brain Ultrastructural Remodeling in Obesity and Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 5427. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Endocytosis and intracellular processing of transferrin and colloidal gold-transferrin in rat reticulocytes: Demonstration of a pathway for receptor shedding. Eur. J. Cell Biol. 1984, 35, 256–263. [Google Scholar]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-derived microvesicles: Important and underappreciated mediators of cell-to-cell communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Xu, A. Adipose Extracellular Vesicles in Intercellular and Inter-Organ Crosstalk in Metabolic Health and Diseases. Front. Immunol. 2021, 12, 608680. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Kanemoto, S.; Nitani, R.; Murakami, T.; Kaneko, M.; Asada, R.; Matsuhisa, K.; Saito, A.; Imaizumi, K. Multivesicular body formation enhancement and exosome release during endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2016, 480, 166–172. [Google Scholar] [CrossRef]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Gao, X.; Salomon, C.; Freeman, D.J. Extracellular Vesicles from Adipose Tissue-A Potential Role in Obesity and Type 2 Diabetes? Front. Endocrinol. 2017, 8, 202. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, C.; Wei, M.; Yang, G.; Yuan, L. Multifaceted Roles of Adipose Tissue-Derived Exosomes in Physiological and Pathological Conditions. Front. Physiol. 2021, 12, 669429. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Fabian, M.R.; Sonenberg, N.; Filipowicz, W. Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 2010, 79, 351–379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tan, C. miRNAs in Adipocyte-Derived Extracellular Vesicles: Multiple Roles in Development of Obesity-Associated Disease. Front. Mol. Biosci. 2020, 7, 171. [Google Scholar] [CrossRef]
- Fantuzzi, G. Adipose tissue, adipokines, and inflammation. J. Allergy Clin. Immunol. 2005, 115, 911–919. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Nat Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Altintas, M.M.; Azad, A.; Nayer, B.; Contreras, G.; Zaias, J.; Faul, C.; Jochen Reiser, J.; Ali Nayer, A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011, 52, 480–488. [Google Scholar] [CrossRef]
- Zelechowska, P.; Agier, J.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Mast cells participate in chronic low-grade inflammation within adipose tissue. Obes. Rev. 2018, 19, 686–697. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Gurung, S.; Perocheau, D.; Touramanidou, L.; Baruteau, J. The exosome journey: From biogenesis to uptake and intracellular signalling. Cell Commun Signal 2021, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.R. Hypothesis: Neuroglia Activation Due to Increased Peripheral and CNS Proinflammatory Cytokines/Chemokines with Neuroinflammation May Result in Long COVID. Neuroglia 2021, 2, 7–35. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Murrieta-Coxca, J.M.; Stojiljkovic, M.; Diezel, C.; Streicher, P.E.; Henao-Restrepo, J.A.; Röstel, F.; Lindner, J.; Witte , O.W.; Weis, S.; et al. Small Extracellular Vesicles from Peripheral Blood of Aged Mice Pass the Blood-Brain Barrier and Induce Glial Cell Activation. Cells 2022, 11, 625. [Google Scholar] [CrossRef]
- Balusu, S.; Van Wonterghem, E.; De Rycke, R.; Raemdonck, K.; Stremersch, S.; Gevaert, K.; Brkic, M.; Demeestere, D.; Vanhooren, V.; Hendrix, A.; et al. Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol. Med. 2016, 8, 1162–1183. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Kumar, S.; Xinjun Wang, X.; Yue, J.; Liao, F.F. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflammation 2018, 15, 8. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Wueest, S.; Rapold, R.A.; Rytka, J.M.; Schoenle, E.J.; Konrad, D. Basal lipolysis, not the degree of insulin resistance, differentiates large from small isolated adipocytes in high-fat fed mice. Diabetologia 2009, 52, 541–546. [Google Scholar] [CrossRef]
- Pan, Y.; Hui, X.; Hoo, R.L.C.; Ye, D.; Chan, C.Y.C.; Feng, T.; Wang, Y.; Lam, K.S.L.; Xu, A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J. Clin. Investig. 2019, 129, 834–849. [Google Scholar] [CrossRef]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Rutkowski, J.M.; Stern, J.H.; Scherer, P.E. The cell biology of fat expansion. J. Cell Biol. 2015, 208, 501–512. [Google Scholar] [CrossRef]
- Slawik, M.; Vidal-Puig, A.J. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res. Rev. 2006, 5, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Haczeyni, F.; Bell-Anderson, K.S.; Farrell, G.C. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes. Rev. 2018, 19, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Żbikowski, A.; Błachnio-Zabielska, A.; Galli, M.; Zabielski, P. Adipose-Derived Exosomes as Possible Players in the Development of Insulin Resistance. Int. J. Mol. Sci. 2021, 22, 7427. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal miR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.C.; Cheng, P.; Shao, H.G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358. [Google Scholar] [CrossRef]
- Dang, S.Y.; Leng, Y.; Wang, Z.X.; Xiao, X.; Zhang, X.; Wen, T.; Gong, H.Z.; Hong, A.; Ma, Y. Exosomal transfer of obesity adipose tissue for decreased miR-141–3p mediate insulin resistance of hepatocytes. Int. J. Biol. Sci. 2019, 15, 351–368. [Google Scholar] [CrossRef]
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 171, 372–384.e12. [Google Scholar] [CrossRef]
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517. [Google Scholar] [CrossRef]
- Li, D.; Song, H.; Shuo, L.; Wang, L.; Xie, P.; Li, W.; Liu, J.; Tong, Y.; Zhang, C.Y.; Jiang, X.; et al. Gonadal white adipose tissue-derived exosomal MiR-222 promotes obesity-associated insulin resistance. Aging 2020, 12, 22719–22743. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, Y.; Xv, W.; Qian, G.; Li, C.; Zou, H.; Li, Y. A New Insight into the Roles of MiRNAs in Metabolic Syndrome. Biomed. Res. Int. 2018, 2018, 7372636. [Google Scholar] [CrossRef]
- Khalyfa, A.; Kheirandish-Gozal, L.; Gozal, D. Exosome and Macrophage Crosstalk in Sleep-Disordered Breathing-Induced Metabolic Dysfunction. Int. J. Mol. Sci. 2018, 19, 3383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shi, L.; Mei, H.; Zhang, J.; Zhu, Y.; Han, X.; Zhu, D. Inflamed macrophage microvesicles induce insulin resistance in human adipocytes. Nutr. Metab. 2015, 12, 21. [Google Scholar] [CrossRef] [PubMed]
- Tryggestad, J.B.; Teague, A.M.; Sparling, D.P.; Jiang, S.; Chernausek, S.D. Macrophage-Derived microRNA-155 Increases in Obesity and Influences Adipocyte Metabolism by Targeting Peroxisome Proliferator-Activated Receptor Gamma. Obesity 2019, 27, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, F.; Aghaei Zarch, S.M.; Jahan-Mihan, A.; Kalantar, S.M.; Vahidi Mehrjardi, M.Y.; Fallahzadeh, H.; Hosseinzadeh, M.; Rahmanian, M.; Mozaffari-Khosravi, H. Circulating microRNA-122, microRNA-126–3p and microRNA-146aare associated with inflammation in patients with pre-diabetes and type 2 diabetes mellitus: A case control study. PLoS ONE. 2021, 16, e0251697. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zheng, Y.; Zhao, L.; Chen, M.; Bai, G.; Hu, Y.; Hu, W.; Yan, Z.; Gao, H. Cognitive decline in type 2 diabetic db/db mice may be associated with brain region-specific metabolic disorders. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017, 1863, 266–273. [Google Scholar] [CrossRef]
- Yermakov, L.M.; Griggs, R.B.; Drouet, D.E.; Sugimoto, C.; Williams, M.T.; Vorhees, C.V.; Susuki, K. Impairment of cognitive flexibility in type 2 diabetic db/db mice. Behav. Brain Res. 2019, 371, 111978. [Google Scholar] [CrossRef]
- Stoeckel, L.E.; Arvanitakis, Z.; Gandy, S.; Small, D.; Kahn, C.R.; Pascual-Leone, A.; Pawlyk, A.; Sherwin, R.; Smith, P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Research 2016, 5, 353. [Google Scholar] [CrossRef]
- Biessels, G.J.; Strachan, M.W.J.; Visseren, F.L.J.; Kappelle, L.J.; Whitmer, R.A. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: Towards targeted interventions. Lancet Diabetes Endocrinol. 2014, 2, 246–255. [Google Scholar] [CrossRef]
- Kodl, C.T.; Seaquist, E.R. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008, 29, 94–511. [Google Scholar] [CrossRef]
- Cukierman, T.; Gerstein, H.C.; Williamson, J.D. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia 2005, 48, 2460–2469. [Google Scholar] [CrossRef]
- McCrimmon, R.J.; Ryan, C.M.; Frier, B.M. Diabetes and cognitive dysfunction. Lancet 2012, 379, 2291–2299. [Google Scholar] [CrossRef]
- Palta, P.; Schneider, A.L.C.; Biessels, G.J.; Touradji, P.; Hill-Briggs, F. Magnitude of cognitive dysfunction in adults with type 2 diabetes: A meta-analysis of six cognitive domains and the most frequently reported neuropsychological tests within domains. J. Int. Neuropsychol. Soc. 2014, 20, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.M.; Dziobek, I.; Sweat, V.; Tirsi, A.; Rogers, K.; Bruehl, H.; Tsui, W.; Richardson, S.; Javier, E.; Convit, A. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia 2007, 50, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Thabit, H.; Tun, T.K.; McDermott, J.; Sreenan, S. Executive Function and Diabetes Mellitus A Stone Left Unturned? Curr. Diabetes Rev. 2012, 8, 109–115. [Google Scholar] [CrossRef]
- Sadanand, S.; Balachandar, R.; Bharath, S. Memory and executive functions in persons with type 2 diabetes: A meta-analysis. Diabetes Metab. Res. Rev. 2016, 32, 132–142. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Z.; Zhang, J.; Xu, K.; Zhang, S.; Wei, D.; Zhang, Z. Altered brain activation patterns under different working memory loads in patients with type 2 diabetes. Diabetes Care 2014, 37, 3157–3163. [Google Scholar] [CrossRef]
- Feil, D.G.; Zhu, C.W.; Sultzer, D.L. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J. Behav. Med. 2012, 35, 190–199. [Google Scholar] [CrossRef]
- Black, S.; Kraemer, K.; Shah, A.; Simpson, G.; Scogin, F.; Smith, A. Diabetes, Depression, and Cognition: A Recursive Cycle of Cognitive Dysfunction and Glycemic Dysregulation. Curr. Diab. Rep. 2018, 18, 118. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Sheridan, G.K. Extracellular vesicles and intercellular communication in the central nervous system. FEBS Lett. 2021, 595, 1391–1410. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Cho, W.C.; Yee, B.K.; Kwan, Y.W.; Tai, W.C. Exosomes in Inflammation and Inflammatory Disease. Proteomics. 2019, 19, e1800149. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflammation 2015, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist 2018, 24, 221–245. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.G.; Ferreira, S.T. Inflammation, Defective Insulin Signaling, and Mitochondrial Dysfunction as Common Molecular Denominators Connecting Type 2 Diabetes to Alzheimer Disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Aloi, M.S.; Garden, G.A. MicroRNAs mediating CNS inflammation: Small regulators with powerful potential. Brain Behav Immun. 2016, 52, 1–8. [Google Scholar] [CrossRef]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Jing, Z. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Krämer-Albers, E.M. Extracellular Vesicles at CNS barriers: Mode of action. Curr. Opin. Neurobiol. 2022, 75, 102569. [Google Scholar] [CrossRef]
- Shetgaonkar, G.G.; Marques, S.M.; DCruz, C.E.M.; Vibhavari, R.J.A.; Kumar, L.; Shirodkar, R.K. Exosomes as cell-derivative carriers in the diagnosis and treatment of central nervous system diseases. Drug Deliv. Transl. Res. 2022, 12, 1047–1079. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Carmona, V.; Perfeito, R.; Nobre, R.J.; Pereira de Almeida, L. Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. J. Control. Release 2017, 262, 247–258. [Google Scholar] [CrossRef]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, A.D.; Fonken, L.K.; Gushchina, L.V.; Aubrecht, T.G.; Maurya, S.K.; Periasamy, M.; Nelson, R.J.; Popovich, P.G. miR-155 Deletion in Female Mice Prevents Diet-Induced Obesity. Sci. Rep. 2016, 6, 22862. [Google Scholar] [CrossRef] [PubMed]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Alexaki, V.I. The Impact of Obesity on Microglial Function: Immune, Metabolic and Endocrine Perspectives. Cells 2021, 10, 1584. [Google Scholar] [CrossRef]
- Ramesh, G.; MacLean, A.G.; Philip, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediat. Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef]
- Boillee, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef]
- Yamanaka, K.; Chun, S.J.; Boillee, S.; Fujimori-Tonou, N.; Yamashita, H.; Gutmann, D.H.; Takahashi, R.; Misawa, H.; Cleveland, D.W. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nat. Neurosci. 2008, 11, 251–253. [Google Scholar] [CrossRef]
- Boillee, S.; Vande Velde, C.; Cleveland, D.W. ALS: A disease of motor neurons and their nonneuronal neighbors. Neuron 2006, 52, 39–59. [Google Scholar] [CrossRef]
- Lobsiger, C.S.; Cleveland, D.W. Glial cells as intrinsic components of non-cellautonomous neurodegenerative disease. Nat. Neurosci. 2007, 10, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Walter, J. The triggering receptor expressed on myeloid cells 2: A molecular link of neuroinflammation and neurodegenerative diseases. J. Biol. Chem. 2016, 291, 4334–4341. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E. TREM2 and risk of Alzheimer’s disease—Friend or foe? N. Engl. J. Med. 2015, 372, 2564–2565. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 42, 1–12. [Google Scholar] [CrossRef]
- Di Filippo, M.; Chiasserinia, D.; Tozzia, A.; Picconib, B.; Calabresi, P. Mitochondria and the Link Between Neuroinflammation and Neurodegeneration. J. Alzheimer’s Dis. 2010, 20, S369–S379. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Bush, N.A.; Nedergaard, M.; Butt, A. The Special Case of Human Astrocytes. Neuroglia 2018, 1, 21–29. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Physiology of Astroglia. Physiol. Rev. 2018, 98, 239–389. [Google Scholar] [CrossRef]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Nedergaard, M. Astroglial cradle in the life of the synapse. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130595. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Carandini, T.; Colombo, F.; Finardi, A.; Casella, G.; Garzetti, L.; Verderio, C.; Furlan, R. Microvesicles: What is the role in multiple sclerosis? Front. Neurol. 2015, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Matias, D.; Balça-Silva, J.; da Graça, G.C.; Wanjiru, C.M.; Macharia, L.W.; Nascimento, C.P.; Roque, N.R.; Coelho-Aguiar, J.M.; Pereira, C.M.; Dos Santos, M.F.; et al. Microglia/Astrocytes–Glioblastoma Crosstalk: Crucial Molecular Mechanisms and Microenvironmental Factors. Front. Cell. Neurosci. 2018, 12, 235. [Google Scholar] [CrossRef] [PubMed]
- Yuzefovych, L.V.; Pastukh, V.M.; Ruchko, M.V.; Simmons, J.D.; Richards, W.O.; Rachek, L.I. Plasma mitochondrial DNA is elevated in obese type 2 diabetes mellitus patients and correlates positively with insulin resistance. PLoS ONE 2019, 14, e0222278. [Google Scholar] [CrossRef]
- Dang, X.T.T.; Kavishka, J.M.; Zhang, D.X.; Pirisinu, M.; Le, M.T.N. Extracellular Vesicles as an Efficient and Versatile System for Drug Delivery. Cells 2020, 9, 2191. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Tian, Y.; Huang, W.; Tong, N.; Fu, X. Integrative biology of extracellular vesicles in diabetes mellitus and diabetic complications. Theranostics 2022, 12, 1342–1372. [Google Scholar] [CrossRef]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef]
- Zagrean, A.M.; Hermann, D.M.; Opris, I.; Zagrean, L.; Popa-Wagner, A. Multicellular Crosstalk Between Exosomes and the Neurovascular Unit After Cerebral Ischemia. Therapeutic Implications. Front. Neurosci. 2018, 12, 811. [Google Scholar] [CrossRef]
- Mollon, J.; Curran, J.E.; Mathias, S.R.; Knowles, E.E.; Carlisle, P.; Fox, P.T.; Olvera, R.L.; Göring, H.H.; Rodrigue, A.; Almasy, L. Neurocognitive impairment in type 2 diabetes: Evidence for shared genetic aetiology. Diabetologia 2020, 63, 977–986. [Google Scholar] [CrossRef]
- Sharma, G.; Parihar, A.; Talaiya, T.; Dubey, K.; Porwal, B.; Parihar, M.S. Cognitive impairments in type 2 diabetes, risk factors and preventive strategies. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 20190105. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Sharma, A.N.; Elased, K.M.; Guest, P.C.; Rahmoune, H.; Bahn, S. Diabetic db/db mice exhibit central nervous system and peripheral molecular alterations as seen in neurological disorders. Trans. Psychiatry 2013, 3, e263. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, Z.; Qian, X.; Liu, X.; Chen, S.; Tang, H. Multi-Omics Characterization of Type 2 Diabetes Mellitus-Induced Cognitive Impairment in the db/db Mouse Model. Molecules 2022, 27, 1904. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Koibuchi, N.; Hasegawa, Y.; Sueta, D.; Toyama, K.; Uekawa, K.; Ma, M.; Nakagawa, T.; Kusaka, H.; Kim-Mitsuyama, S. Glycemic control with empagliflozin, a novel selective SGLT2 inhibitor, ameliorates cardiovascular injury and cognitive dysfunction in obese and type 2 diabetic mice. Cardiovasc. Diabetol. 2014, 13, 148. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J. Neuroimmunol. 2014, 273, 8–21. [Google Scholar] [CrossRef]
- Kern, P.A.; Ranganathan, S.; Li, C.; Wood, L.; Ranganathan, G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E745–E751. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Farb, M.G.; Mott, M.M.; Hess, D.T.; Carmine, B.; Fiscale, A.; Joseph, L.; Apovian, C.M.; Gokce, N. Relation of depot-specific adipose inflammation to insulin resistance in human obesity. Nutr. Diabetes 2012, 2, e30. [Google Scholar] [CrossRef]
- De la Monte, S.M.; Wands, J.R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J. Alzheimers Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef]
- De la Monte, S.M. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer’s disease. Curr. Alzheimer Res. 2012, 9, 35–66. [Google Scholar] [CrossRef]
- Zhong, H.; Ma, M.; Liang, T.; Guo, L. Role of MicroRNAs in Obesity-Induced Metabolic Disorder and Immune Response. J. Immunol. Res. 2018, 2018, 2835761. [Google Scholar] [CrossRef]
- Chang, W.; Wang, J. Exosomes and Their Noncoding RNA Cargo Are Emerging as New Modulators for Diabetes Mellitus. Cells 2019, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Bae, Y.U.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Han, D.C.; Byun, D.W.; Kim, S.H.; Park, H.K.; et al. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res. Care 2020, 8, e001403. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).