Neuroprotective and Anti-Microglial Activation Effects of Tocotrienols in Brains of Lipopolysaccharide-Induced Inflammatory Model Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and LPS Treatment
2.2. Experimental Schedule
2.3. Immunohistochemical Staining
2.4. Immunofluorescence Staining
2.5. Western Blotting
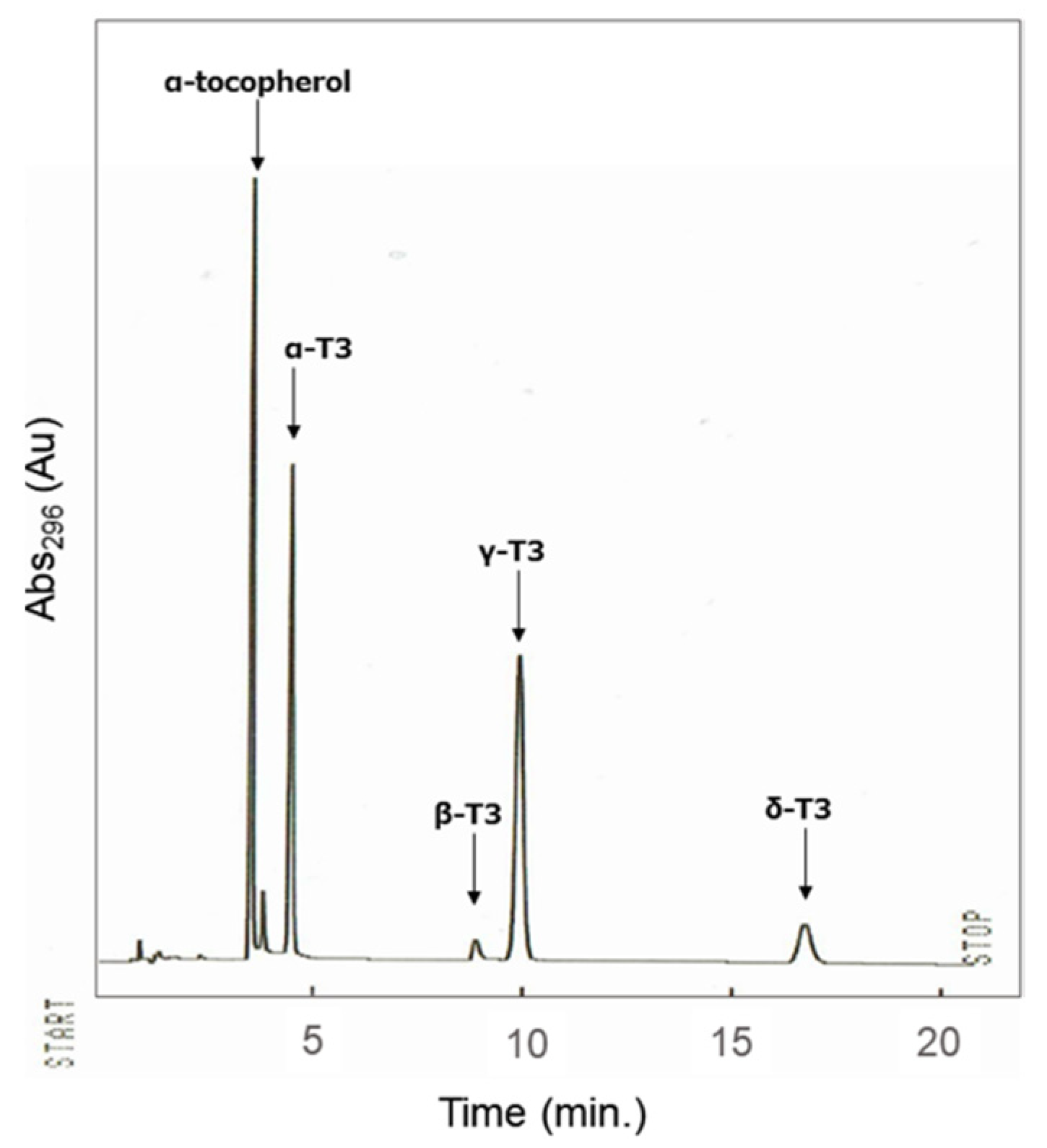
2.6. HPLC Analysis
2.7. Statistical Analysis
3. Results
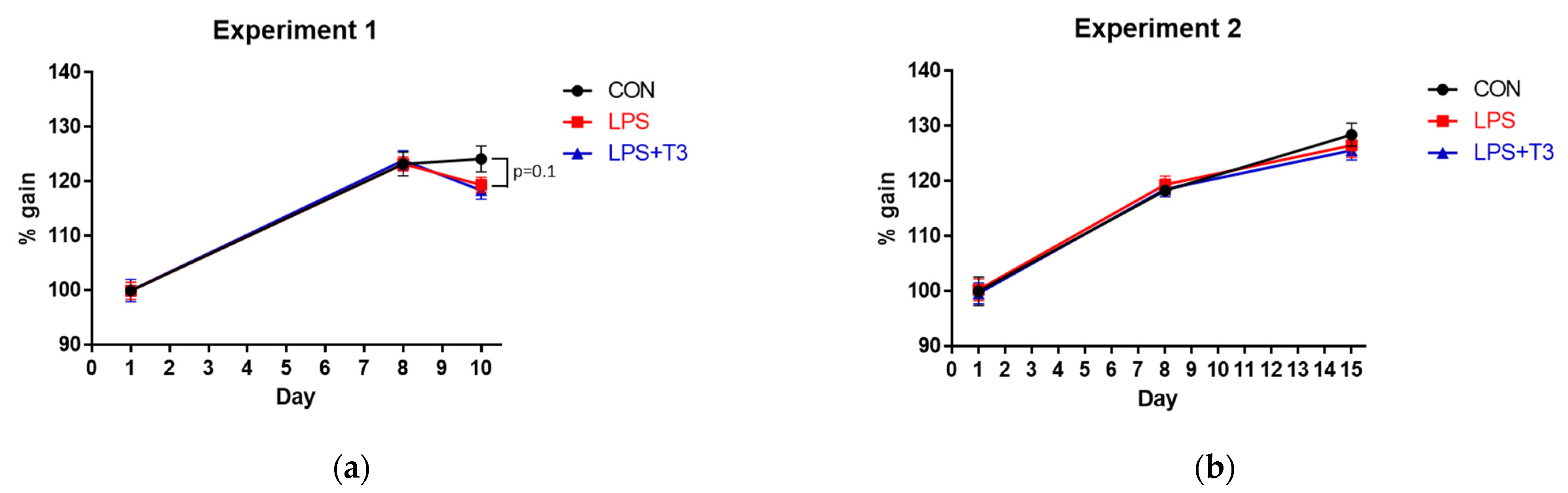
3.1. BW Changes (Experiments 1 and 2)
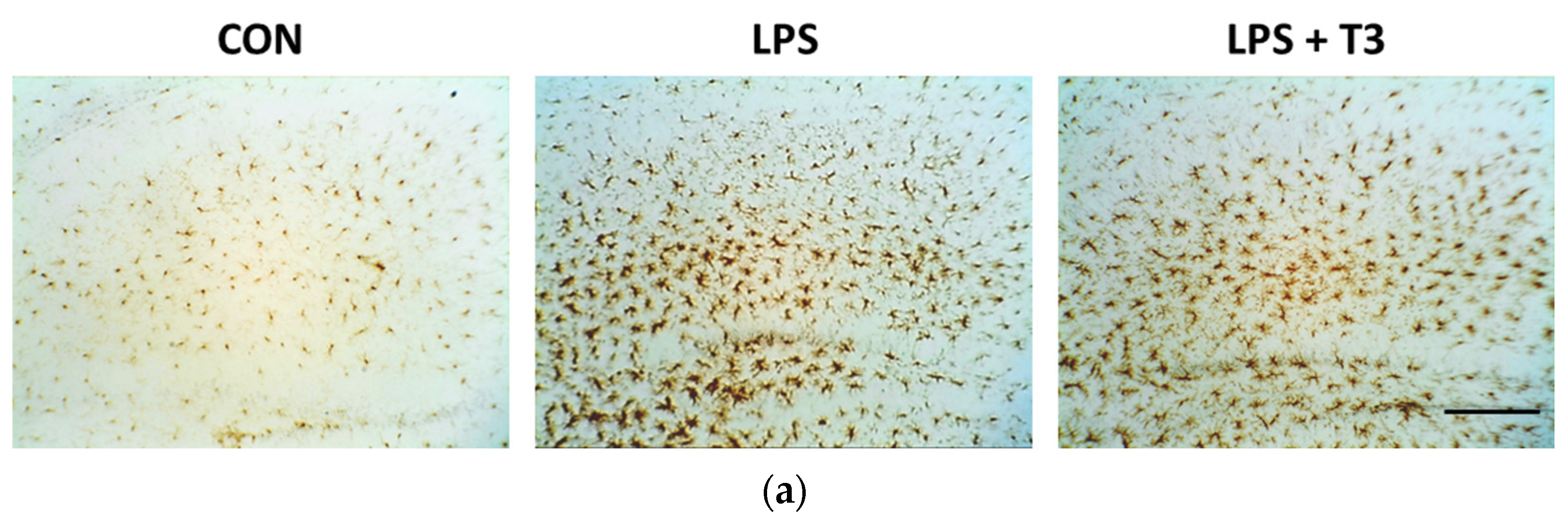
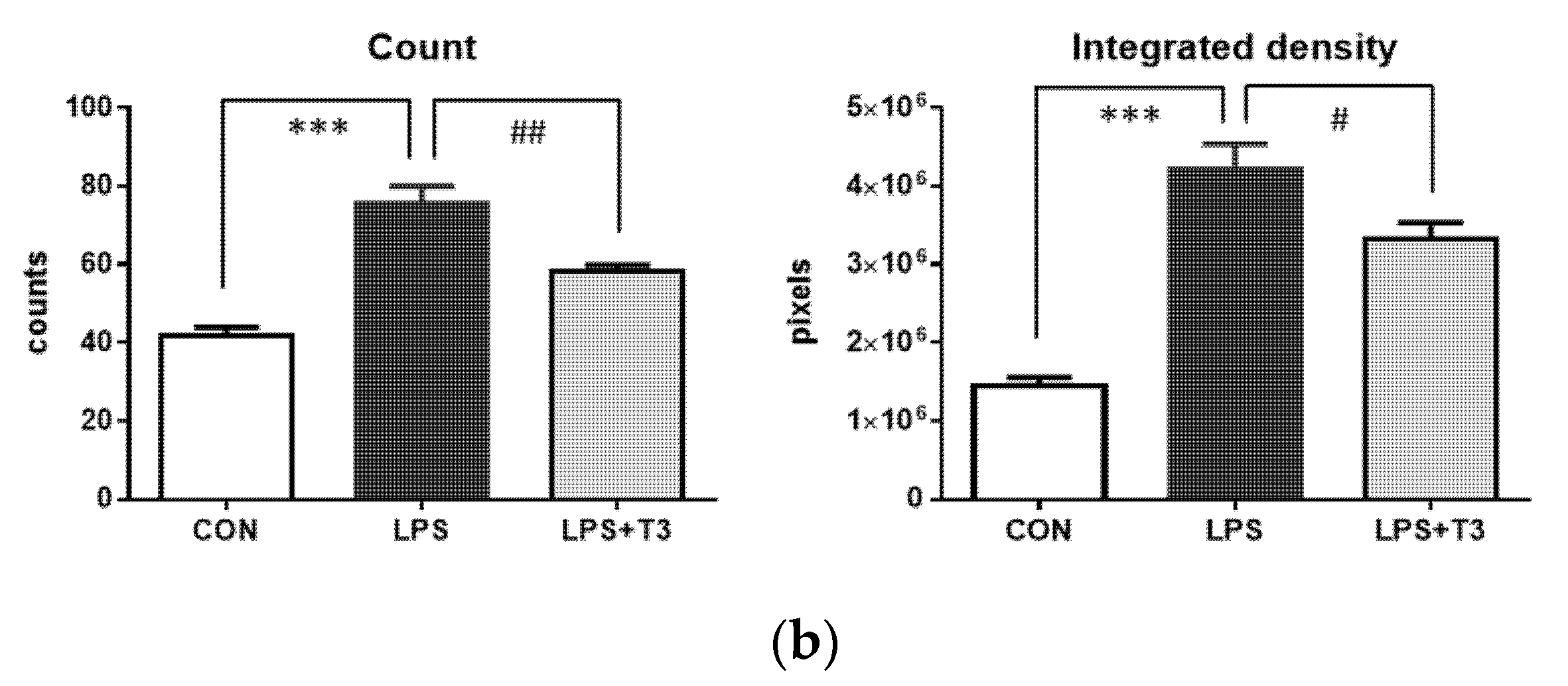
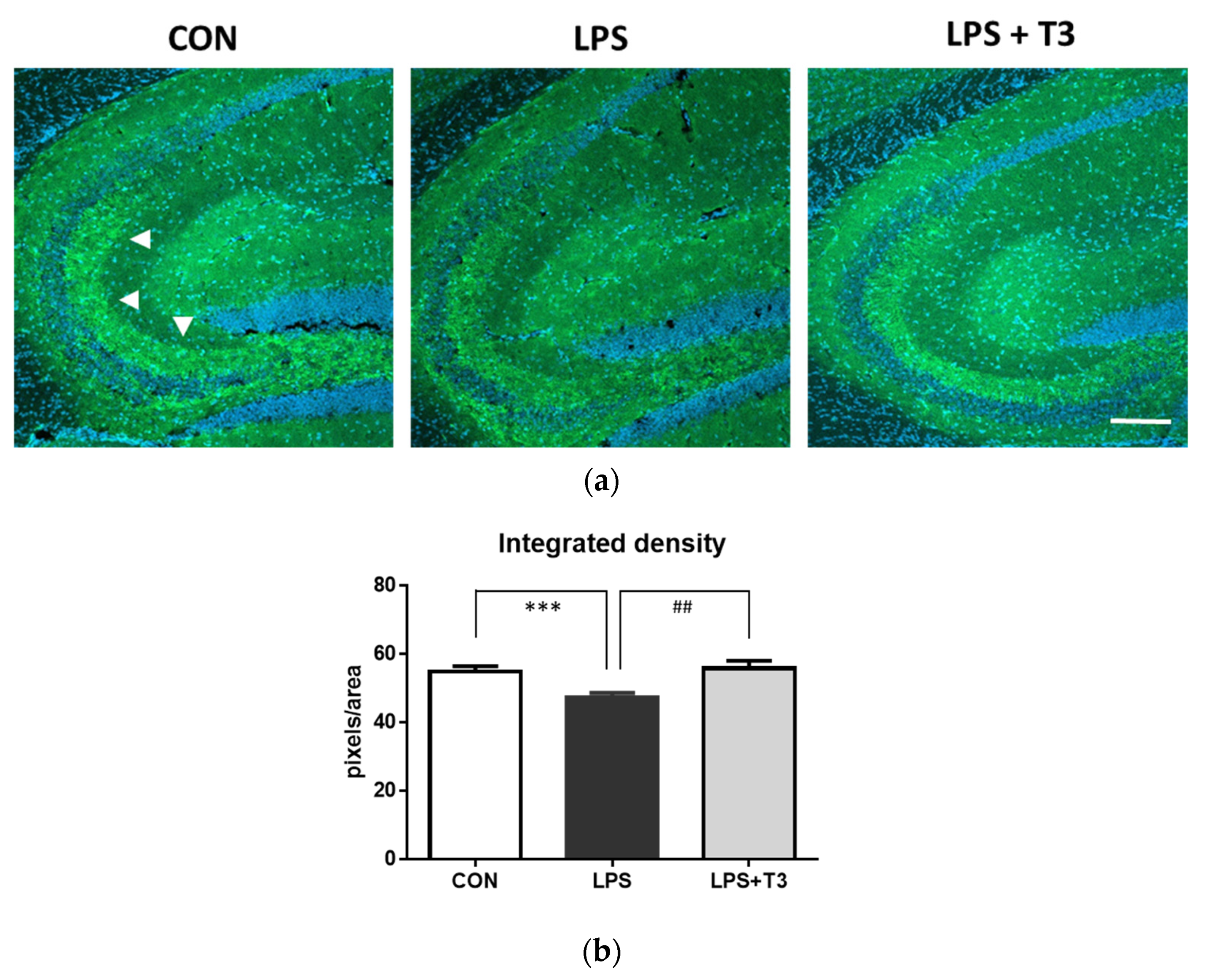
3.2. Suppression of Microglial Activation in the Hippocampus (Experiment 1)
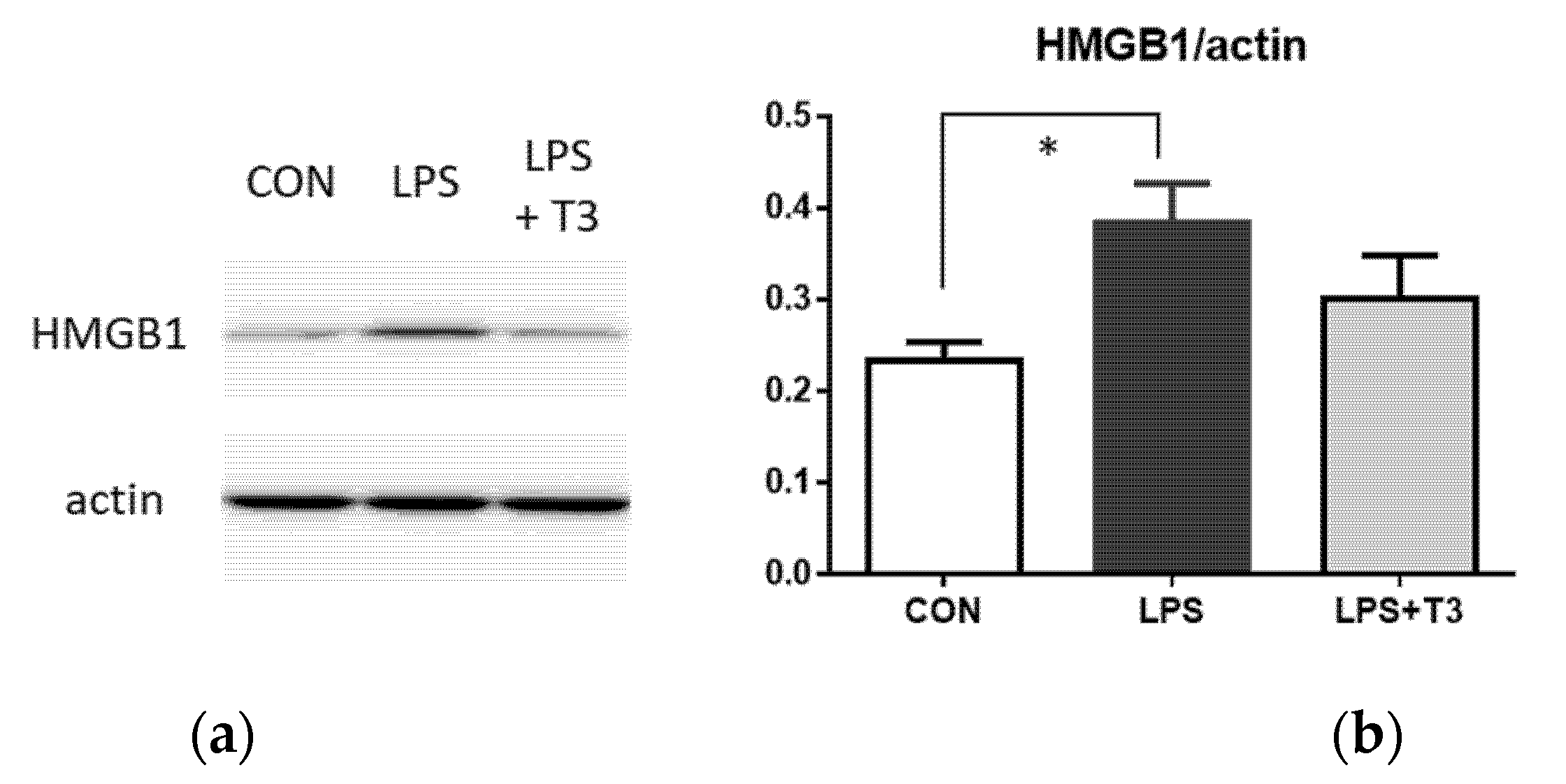
3.3. Western Blot Analysis of Inflammation Markers in the Hippocampus (Experiment 1)
3.4. Suppression of Neuronal Dysfunction in the Hippocampus (Experiment 2)
3.5. HPLC Analysis of the Composition of Tocotrienols
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.J.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noh, H.; Jeon, J.; Seo, H. Systemic injection of LPS induces region-specific neuroinflammation and mitochondrial dysfunction in normal mouse brain. Neurochem. Int. 2014, 69, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Frey, B.N. Disruption in the blood-brain barrier: The missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015, 2015, 708306. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Howe, F.A.; Allen, R.L.; Sofat, N. New insights into the impact of neuro-inflammation in rheumatoid arthritis. Front. Neurosci. 2014, 8, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Toyoda, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Sugawara, K.; Sudo, M.; Nakajima, M.; et al. Auraptene in the peels of Citrus kawachiensis (Kawachi Bankan) ameliorates lipopolysaccharide-induced inflammation in the mouse brain. Evid. Based Complement. Alternat. Med. 2014, 2014, 408503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, S.; Makihata, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Nakajima, M.; Furukawa, Y. Oenothein B suppresses lipopolysaccharide (LPS)-induced inflammation in the mouse brain. Int. J. Mol. Sci. 2013, 14, 9767–9778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haramiishi, R.; Okuyama, S.; Yoshimura, M.; Nakajima, M.; Furukawa, Y.; Ito, H.; Amakura, Y. Identification of the characteristic components in walnut and anti-inflammatory effect of glansreginin A as an indicator for quality evaluation. Biosci. Biotechnol. Biochem. 2020, 84, 187–197. [Google Scholar]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological potential of tocotrienols: A review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, A.A.; Reis, J.C.; Papasian, C.J.; Morrison, D.C.; Qureshi, N. Tocotrienols inhibit lipopolysaccharide-induced pro-inflammatory cytokines in macrophages of female mice. Lipids Health Dis. 2010, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienol: The natural vitamin E to defend the nervous system? Ann. N. Y. Acad. Sci. 2004, 1031, 127–142. [Google Scholar] [CrossRef]
- Tan, S.W.; Abdullah, M.; Ali, D.A.I.; Vidyadaran, S. Palm tocotrienols reduce lipopolysaccharide-stimulated inflammatory responses of microglia. Malays. J. Med. Health Sci. 2016, 12, 1–8. [Google Scholar]
- Chin, K.Y.; Tay, S.S. A review on the relationship between tocotrienol and alzheimer disease. Nutrients 2018, 10, 881. [Google Scholar] [CrossRef] [Green Version]
- Khanna, S.; Roy, S.; Slivka, A.; Craft, T.K.; Chaki, S.; Rink, C.; Notestine, M.A.; DeVries, A.C.; Parinandi, N.L.; Sen, C.K. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke 2005, 36, 2258–2264. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Normal phase high-performance liquid chromatography method for the determination of tocopherols and tocotrienols in cereals. J. Agric. Food Chem. 2003, 51, 3940–3944. [Google Scholar] [CrossRef]
- Shin, T.S.; Godber, J. Improved high-performance liquid chromatography of vitamin E vitamers on normal-phase columns. J. Am. Oil Chem. Soc. 1993, 70, 1289–1291. [Google Scholar] [CrossRef]
- Podda, M.; Weber, C.; Traber, M.G.; Packer, L. Simultaneous determination of tissue tocopherols, tocotrienols, ubiquinols, and ubiquinones. J. Lipid Res. 1996, 37, 893–901. [Google Scholar] [CrossRef]
- Tan, S.W.; Ramasamy, R.; Abdullah, M.; Vidyadaran, S. Inhibitory effects of palm alpha-, gamma- and delta-tocotrienol on lipopolysaccharide-induced nitric oxide production in BV2 microglia. Cell Immunol. 2011, 271, 205–209. [Google Scholar] [CrossRef]
- Wang, H.; Yang, H.; Czura, C.J.; Sama, A.E.; Tracey, K.J. HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 2001, 164, 1768–1773. [Google Scholar] [CrossRef]
- Mu, S.W.; Dang, Y.; Wang, S.S.; Gu, J.J. The role of high mobility group box 1 protein in acute cerebrovascular diseases. Biomed. Rep. 2018, 9, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Othman, I.; Shaikh, M.F. HMGB1-mediated neuroinflammatory responses in brain injuries: Potential mechanisms and therapeutic opportunities. Int. J. Mol. Sci. 2020, 21, 4609. [Google Scholar] [CrossRef]
- Park, H.A.; Mnatsakanyan, N.; Broman, K.; Davis, A.U.; May, J.; Licznerski, P.; Crowe-White, K.M.; Lackey, K.H.; Jonas, E.A. Alpha-Tocotrienol Prevents Oxidative Stress-Mediated Post-Translational Cleavage of Bcl-xL in Primary Hippocampal Neurons. Int. J. Mol. Sci. 2019, 21, 220. [Google Scholar] [CrossRef] [Green Version]
- Serbinova, E.A.; Packer, L. Antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Methods Enzymol. 1994, 234, 354–366. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuyama, S.; Matsuda, M.; Okusako, Y.; Miyauchi, S.; Omasa, T.; Ozawa, A.; Abe, M.; Yaeno, T.; Araki, T.; Sawamoto, A.; et al. Neuroprotective and Anti-Microglial Activation Effects of Tocotrienols in Brains of Lipopolysaccharide-Induced Inflammatory Model Mice. Neuroglia 2021, 2, 89-97. https://doi.org/10.3390/neuroglia2010009
Okuyama S, Matsuda M, Okusako Y, Miyauchi S, Omasa T, Ozawa A, Abe M, Yaeno T, Araki T, Sawamoto A, et al. Neuroprotective and Anti-Microglial Activation Effects of Tocotrienols in Brains of Lipopolysaccharide-Induced Inflammatory Model Mice. Neuroglia. 2021; 2(1):89-97. https://doi.org/10.3390/neuroglia2010009
Chicago/Turabian StyleOkuyama, Satoshi, Masafumi Matsuda, Yuna Okusako, Sanae Miyauchi, Toshiki Omasa, Akiho Ozawa, Masato Abe, Takashi Yaeno, Takuya Araki, Atsushi Sawamoto, and et al. 2021. "Neuroprotective and Anti-Microglial Activation Effects of Tocotrienols in Brains of Lipopolysaccharide-Induced Inflammatory Model Mice" Neuroglia 2, no. 1: 89-97. https://doi.org/10.3390/neuroglia2010009
APA StyleOkuyama, S., Matsuda, M., Okusako, Y., Miyauchi, S., Omasa, T., Ozawa, A., Abe, M., Yaeno, T., Araki, T., Sawamoto, A., Nakajima, M., & Furukawa, Y. (2021). Neuroprotective and Anti-Microglial Activation Effects of Tocotrienols in Brains of Lipopolysaccharide-Induced Inflammatory Model Mice. Neuroglia, 2(1), 89-97. https://doi.org/10.3390/neuroglia2010009





