The Effect of Optogenetically Activating Glia on Neuronal Function
Abstract
1. Introduction
2. Results
2.1. Larval and Adult Behavior
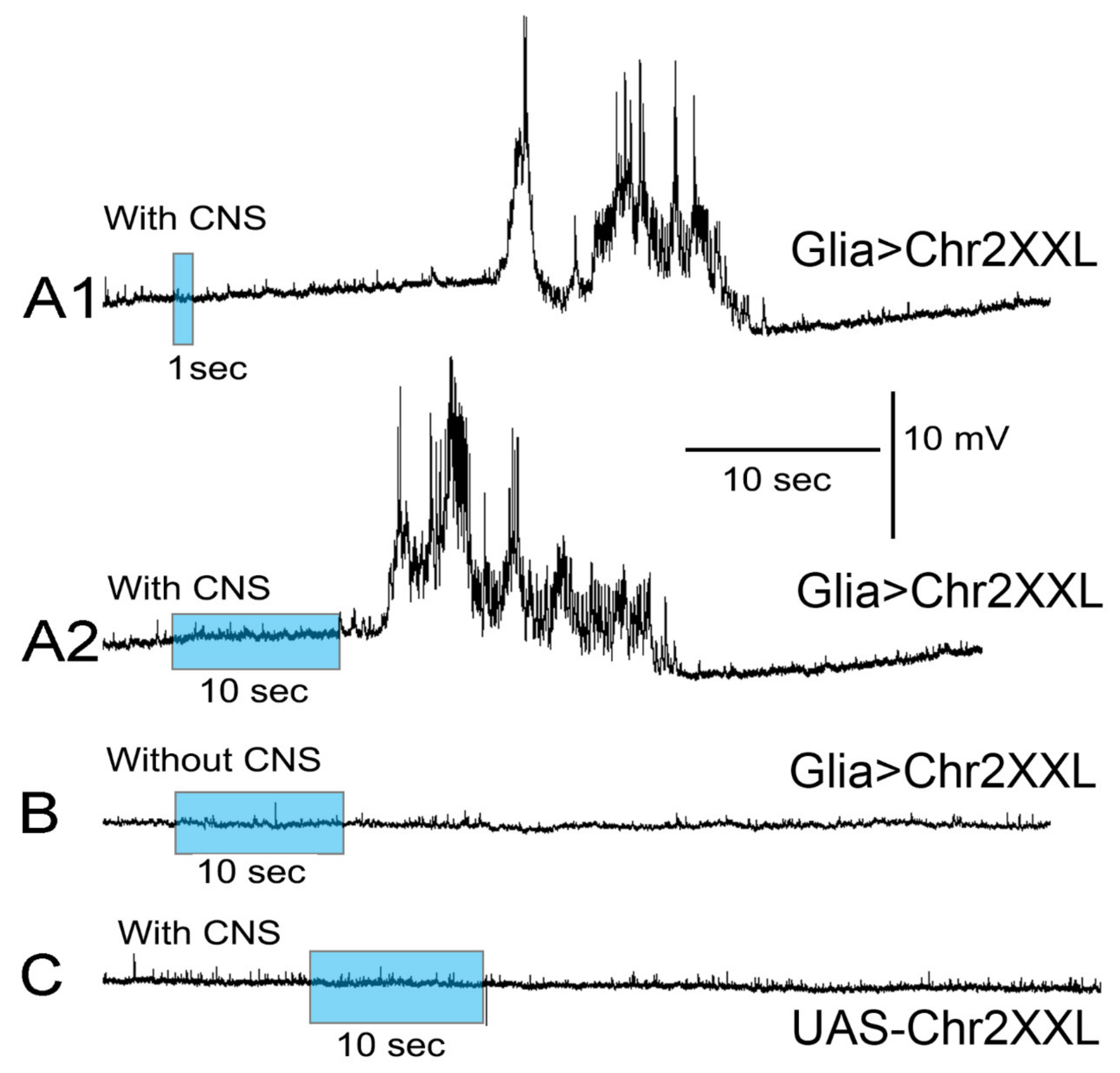
2.2. Activity of Motor Neurons with Intact CNS
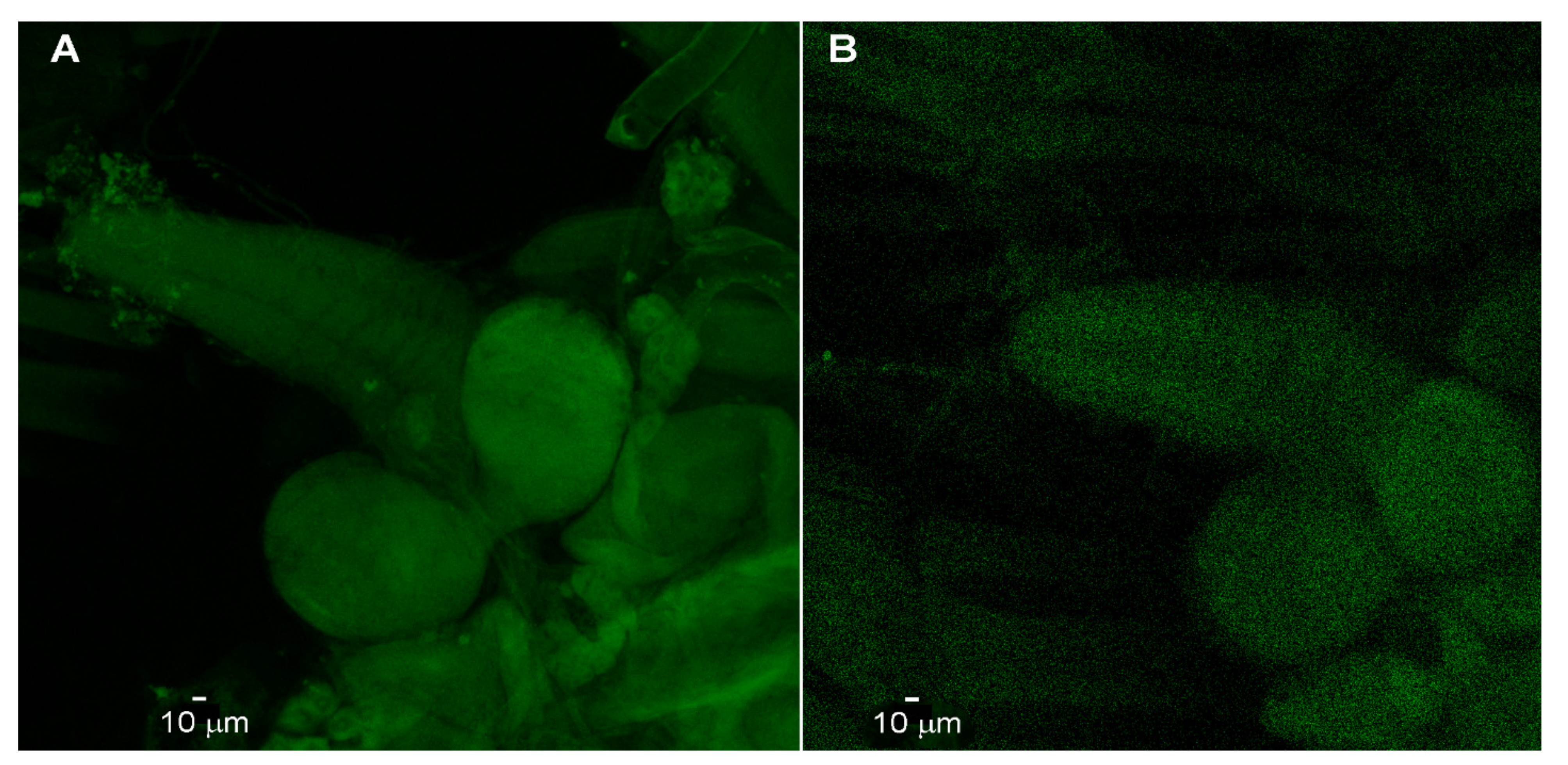
2.3. Antibody Staining for Channel Rhodopsin
3. Discussion
4. Methods and Materials
4.1. Fly Stock Maintenance
4.2. Light Stimulation
4.3. Electrophysiology
4.4. Larval and Adult Behavior
4.5. Immunocytochemistry
4.6. Statistical Analysis
4.7. Study Area
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sancho, L.; Contreras, M.; Allen, N.J. Glia as sculptors of synaptic plasticity. Neurosci. Res. 2021, 167, 17–29. [Google Scholar] [CrossRef]
- Einheber, S.; Bhat, M.; Salzer, J. Disrupted axo-glial junctions result in accumulation of abnormal mitochondria at nodes of Ranvier. Neuron Glia Biol. 2006, 2, 165–174. [Google Scholar] [CrossRef][Green Version]
- Baylor, D.A.; Nicholls, J.G. Changes in extracellular potassium concentration produced by neuronal activity in the central nervous system of the leech. J. Physiol. 1969, 203, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, B.S.; Robitaille, R.; Charlton, M.P. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron 1992, 8, 1069–1077. [Google Scholar] [CrossRef]
- Todd, K.J.; Darabid, H.; Robitaille, R. Perisynaptic glia discriminate patterns of motor nerve activity and influence plasticity at the neuromuscular junction. J. Neurosci. 2010, 30, 11870–11882. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, R. Purinergic receptors and their activation by endogenous purines at perisynaptic glial cells of the frog neuromuscular junction. J. Neurosci. 1995, 15, 7121–7131. [Google Scholar] [CrossRef] [PubMed]
- Todd, K.J.; Robitaille, R. Purinergic modulation of synaptic signalling at the neuromuscular junction. Pflugers Arch. 2006, 452, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S.; Robitaille, R. Glial cells and neurotransmission: An inclusive view of synaptic function. Neuron 2003, 40, 389–400. [Google Scholar] [CrossRef]
- Barik, A.; Li, L.; Sathyamurthy, A.; Xiong, W.C.; Mei, L. Schwann cells in neuromuscular junction formation and maintenance. J. Neurosci. 2016, 36, 9770–9781. [Google Scholar] [CrossRef]
- Benraiss, A.; Wang, S.; Herrlinger, S.; Li, X.; Chandler-Militello, D.; Mauceri, J.; Burm, H.B.; Toner, M.; Osipovitch, M.; Xu, Q.J.; et al. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat. Commun. 2016, 7, 11758. [Google Scholar] [CrossRef]
- Onur, T.S.; Laitman, A.; Zhao, H.; Keyho, R.; Kim, H.; Wang, J.; Mair, M.; Wang, H.; Li, L.; Perez, A.; et al. Downregulation of glial genes involved in synaptic function mitigates Huntington’s disease pathogenesis. eLife 2021, 10, e64564. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.A.; Mariani, J.N.; Madsen, P.M. Glial progenitor cell-based repair of the dysmyelinated brain: Progression to the clinic. Semin. Cell Dev. Biol. 2021, 116, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Song, B.; Lee, I.S. Drosophila Glia: Models for Human Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 4859. [Google Scholar] [CrossRef]
- Ballinger-Boone, C.; Anyagaligbo, O.; Bernard, J.; Bierbower, S.M.; Dupont-Versteegden, E.E.; Ghoweri, A.; Greenhalgh, A.; Harrison, D.; Istas, O.; McNabb, M.; et al. The effects of bacterial endotoxin (LPS) on cardiac and synaptic function in various animal models: Larval Drosophila, crayfish, crab, and rodent. Int. J. Zool. Res. 2020, 16, 33–62. [Google Scholar] [CrossRef]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef]
- Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 2015, 18, 1213–1225. [Google Scholar] [CrossRef]
- Han, X.; Boyden, E.S. Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS ONE 2007, 2, e299. [Google Scholar] [CrossRef]
- Sasaki, T.; Beppu, K.; Tanaka, K.F.; Fukazawa, Y.; Shigemoto, R.; Matsui, K. Application of an optogenetic byway for perturbing neuronal activity via glial photostimulation. Proc. Natl. Acad. Sci. USA 2012, 109, 20720–20725. [Google Scholar] [CrossRef]
- Higgins, J.; Hermanns, C.; Malloy, C.; Cooper, R.L. Considerations in repetitive activation of light sensitive ion channels for long term studies: Channel rhodopsin in the Drosophila model. Neurosci. Res. 2017, 125, 1–10. [Google Scholar] [CrossRef]
- Akasaka, T.; Ocorr, K. Drug discovery through functional screening in the Drosophila heart. Methods Mol. Biol. 2009, 577, 235–249. [Google Scholar] [CrossRef]
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 years of Drosophila research and its impact on vertebrate neuroscience: A history lesson for the future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef] [PubMed]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef]
- MacNamee, S.E.; Liu, K.E.; Gerhard, S.; Tran, C.T.; Fetter, R.D.; Cardona, A.; Tolbert, L.P.; Oland, L.A. Astrocytic glutamate transport regulates a Drosophila CNS synapse that lacks astrocyte ensheathment. J. Comp. Neurol. 2016, 524, 1979–1998. [Google Scholar] [CrossRef]
- Ito, K.; Urban, J.; Technau, G.M. Distribution, classification, and development of Drosophila glial cells in the late embryonic and early larval ventral nerve cord. Roux’s Arch. Dev. Biol. 1995, 204, 284–307. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.M.; Rickert, C.; Altenhein, B.; Technau, G.M. Postembryonic development of the midline glia in the CNS of Drosophila: Proliferation, programmed cell death, and endocrine regulation. Mech. Dev. 2008, 125, 542–557. [Google Scholar] [CrossRef]
- Awasaki, T.; Lai, S.-L.; Ito, K.; Lee, T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J. Neurosci. 2008, 28, 13742–13753. [Google Scholar] [CrossRef]
- Muthukumar, A.K.; Stork, T.; Freeman, M.R. Activity-dependent regulation of astrocyte GAT levels during synaptogenesis. Nat. Neurosci. 2014, 17, 1340–1350. [Google Scholar] [CrossRef]
- Peco, E.; Davla, S.; Camp, D.; Stacey, S.M.; Landgraf, M.; van Meyel, D.J. Drosophila astrocytes cover specific territories of the CNS neuropil and are instructed to differentiate by Prospero, a key effector of Notch. Development 2016, 143, 1170–1181. [Google Scholar] [CrossRef]
- Ng, F.S.; Tangredi, M.M.; Jackson, F.R. Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr. Biol. 2011, 21, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef]
- Agulhon, C.; Fiacco, T.A.; McCarthy, K.D. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 2010, 327, 1250–1254. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, B.; Yan, W.; Lei, Z.; Zhao, X.; Zhang, K.; Guo, A. Astrocyte-like glial cells physiologically regulate olfactory processing through the modification of ORN PN synaptic strength in Drosophila. Eur. J. Neurosci. 2014, 40, 2744–2754. [Google Scholar] [CrossRef]
- Xiang, Y.; Yuan, Q.; Vogt, N.; Looger, L.L.; Jan, L.Y.; Jan, Y.N. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature 2010, 468, 921–926. [Google Scholar] [CrossRef]
- Arlow, R.L.; Foutz, T.J.; McIntyre, C.C. Theoretical principles underlying optical stimulation of myelinated axons expressing channelrhodopsin-2. Neuroscience 2013, 248, 541–551. [Google Scholar] [CrossRef]
- Chamorro, E.; Bonnin-Arias, C.; Pérez-Carrasco, M.J.; Muñoz de Luna, J.; Vázquez, D.; Sánchez-Ramos, C. Effects of light-emitting diode radiations on human retinal pigment epithelial cells in vitro. Photochem. Photobiol. 2013, 89, 468–473. [Google Scholar] [CrossRef]
- Lewis, E.B. A new standard food medium. Drosoph. Inf. Ser. 1960, 34, 117–118. [Google Scholar]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.; Sifers, J.; Yocom, E.; Blümich, S.L.E.; Potter, R.; Nadolski, J.; Harrison, D.A.; Cooper, R.L. Acute and chronic effects of inhibiting dTOR by rapamycin on development, behavior, and physiology in Drosophila. Biol. Open 2019, 8, bio046508. [Google Scholar] [CrossRef] [PubMed]
- Govorunova, E.G.; Sineshchekov, O.A.; Janz, R.; Liu, X.; Spudich, J.L. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 2015, 349, 647–650. [Google Scholar] [CrossRef] [PubMed]
- Mauss, A.S.; Busch, C.; Borst, A. Optogenetic neuronal silencing in Drosophila during visual processing. Sci. Rep. 2017, 7, 13823. [Google Scholar] [CrossRef]
- Zhao, S.; Cunha, C.; Zhang, F.; Liu, Q.; Gloss, B.; Deisseroth, K.; Augustine, G.J.; Feng, G. Improved expression of halorhodopsin for light-induced silencing of neuronal activity. Brain Cell Biol. 2008, 36, 141–154. [Google Scholar] [CrossRef]
- Stewart, B.A.; Atwood, H.L.; Renger, J.J.; Wang, J.; Wu, C.F. Improved stability of Drosophila larval neuromuscular preparation in haemolymph-like physiological solutions. J. Comp. Physiol. A 1994, 175, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.J.; Nicholls, J.G.; Stent, G.S. Neurobiology of the Leech; Cold Spring Harbor Laboratory: New York, NY, USA, 1981; p. 254. [Google Scholar]
- DeCastro, C.; Titlow, J.; Majeed, Z.R.; Cooper, R.L. Analysis of various physiological salines for heart rate, CNS function, and synaptic transmission at neuromuscular junctions in Drosophila melanogaster larvae. J. Comp. Physiol. A 2014, 200, 83–92. [Google Scholar] [CrossRef] [PubMed]
- DeCastro, N.; Cooper, R.L. Impedance measures and a mounting technique for drosophila: Larval movements, heart rate, imaging, and electrophysiology. Meth. Protoc. 2020, 3, 12. [Google Scholar] [CrossRef]
- Majeed, Z.R.; Abdeljaber, E.; Soveland, R.; Cornwell, K.; Bankemper, A.; Koch, F.; Cooper, R.L. Modulatory action by the serotonergic system: Behavior and neurophysiology in Drosophila melanogaster. Neural Plast. 2016, 2016, 7291438. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pankau, C.; McCubbin, S.; Cooper, R.L. The Effect of Optogenetically Activating Glia on Neuronal Function. Neuroglia 2021, 2, 57-67. https://doi.org/10.3390/neuroglia2010007
Pankau C, McCubbin S, Cooper RL. The Effect of Optogenetically Activating Glia on Neuronal Function. Neuroglia. 2021; 2(1):57-67. https://doi.org/10.3390/neuroglia2010007
Chicago/Turabian StylePankau, Cecilia, Shelby McCubbin, and Robin L. Cooper. 2021. "The Effect of Optogenetically Activating Glia on Neuronal Function" Neuroglia 2, no. 1: 57-67. https://doi.org/10.3390/neuroglia2010007
APA StylePankau, C., McCubbin, S., & Cooper, R. L. (2021). The Effect of Optogenetically Activating Glia on Neuronal Function. Neuroglia, 2(1), 57-67. https://doi.org/10.3390/neuroglia2010007






