Thermophy: A Chebyshev Polynomial-Based Tool for Transport Property Estimation in Multicomponent Gas Systems
Abstract
1. Introduction
2. Theory and Calculations
2.1. Viscosity
2.2. Thermal Conductivity
2.3. Diffusion Coefficient
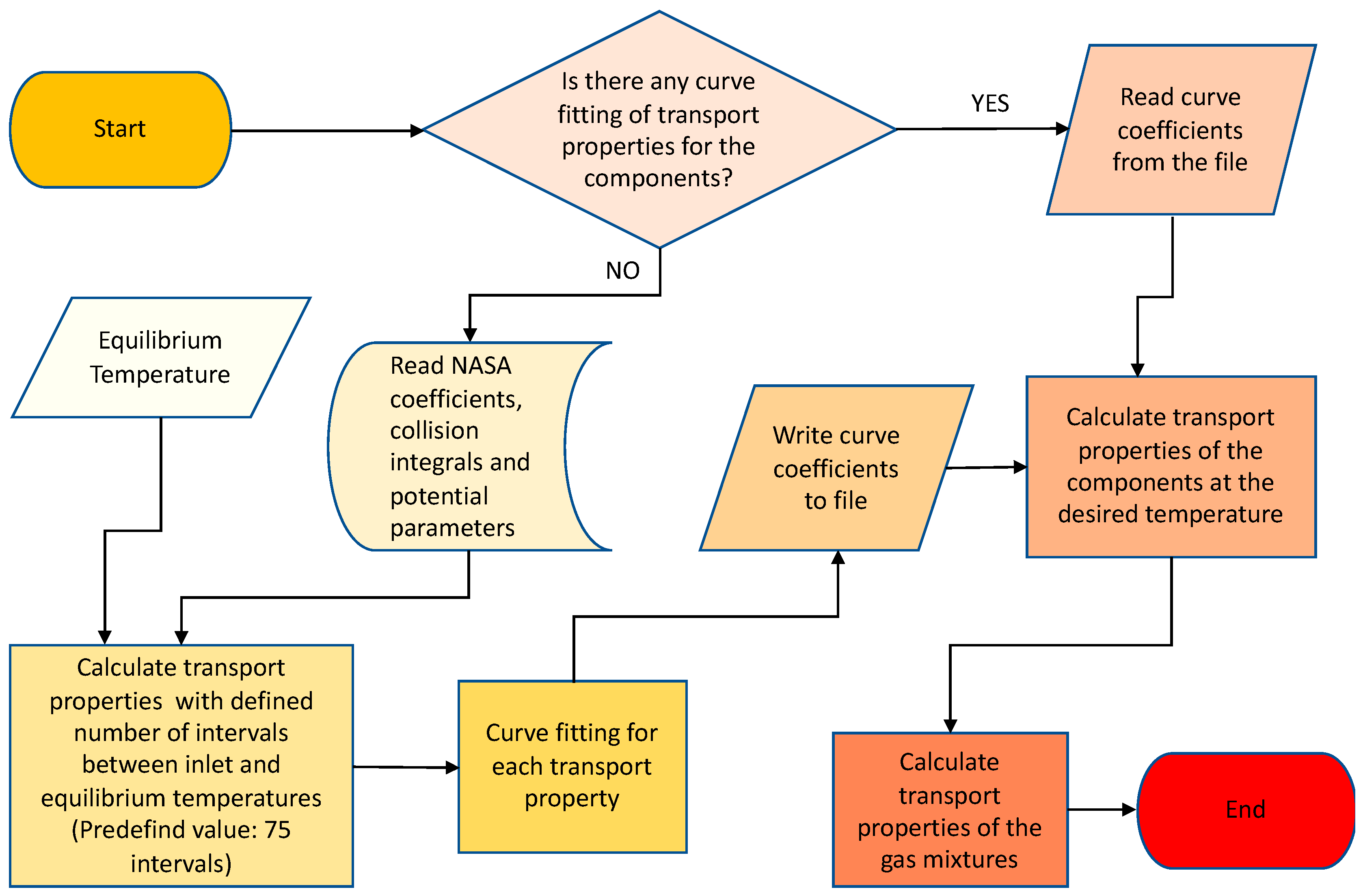
2.4. Program Algorithm
3. Results and Discussion
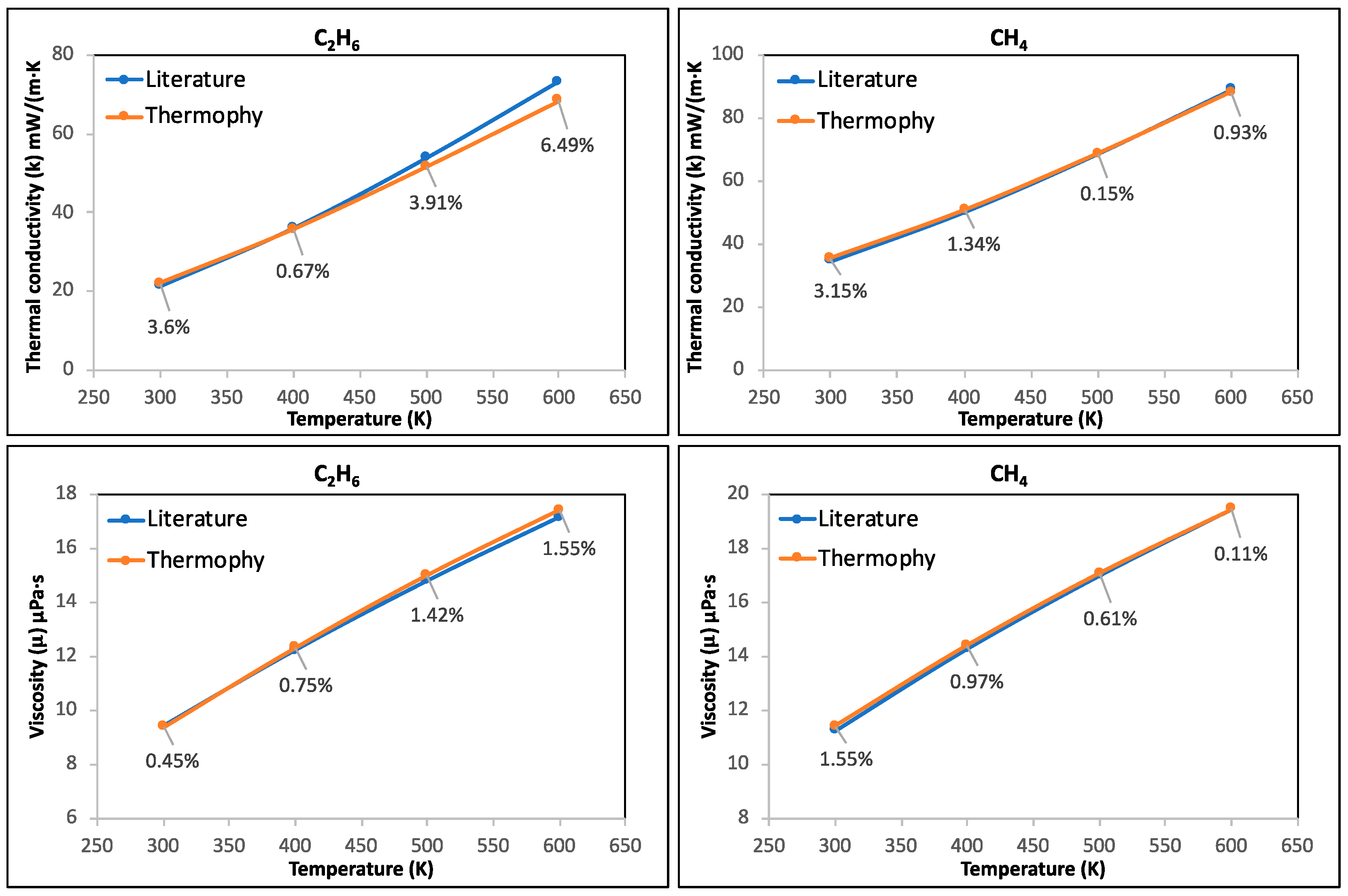
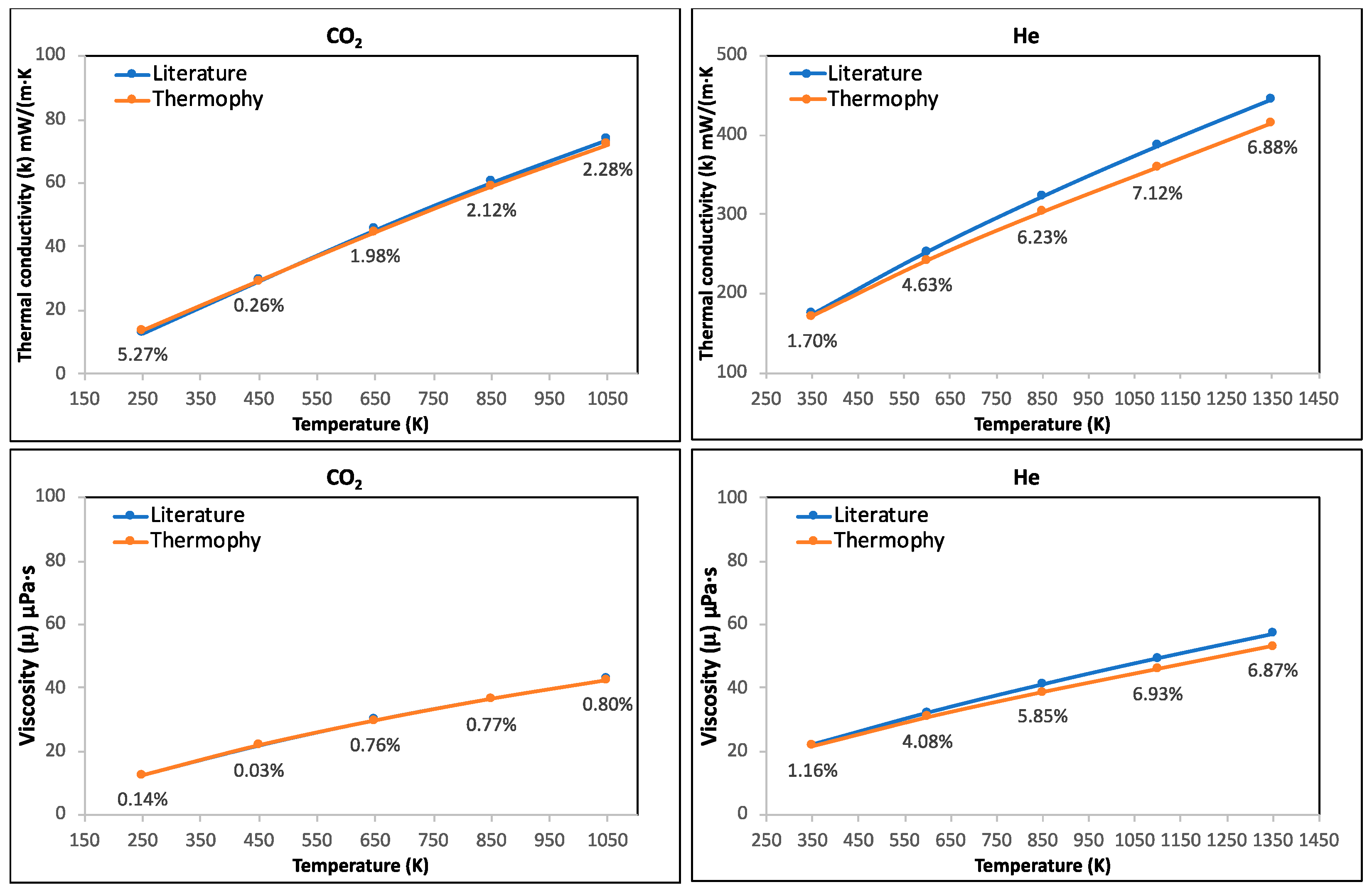
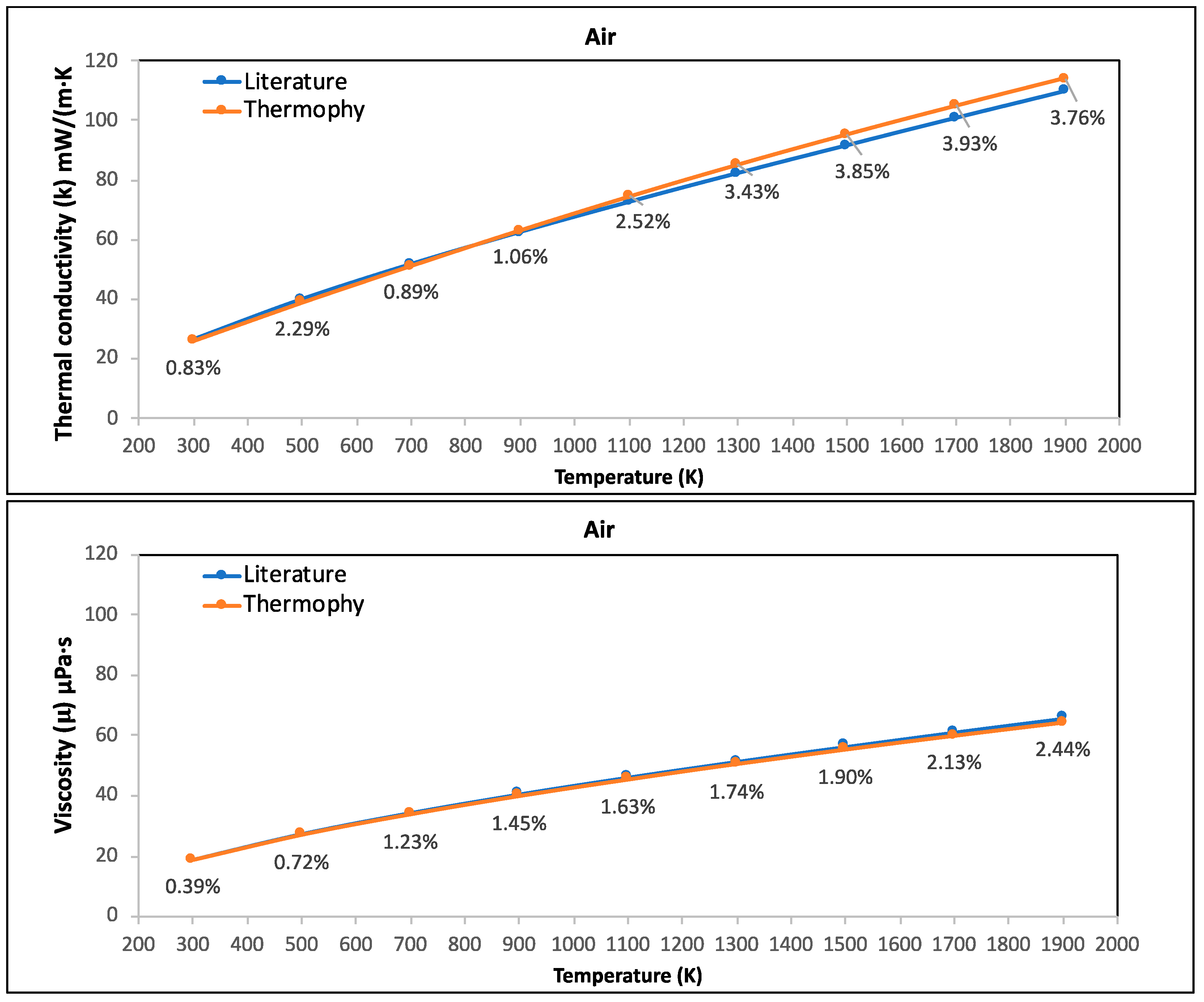
3.1. Case Study 1: Thermal Conductivity and Viscosity of C2H6, CH4, CO2, and He Pure Gases and Air
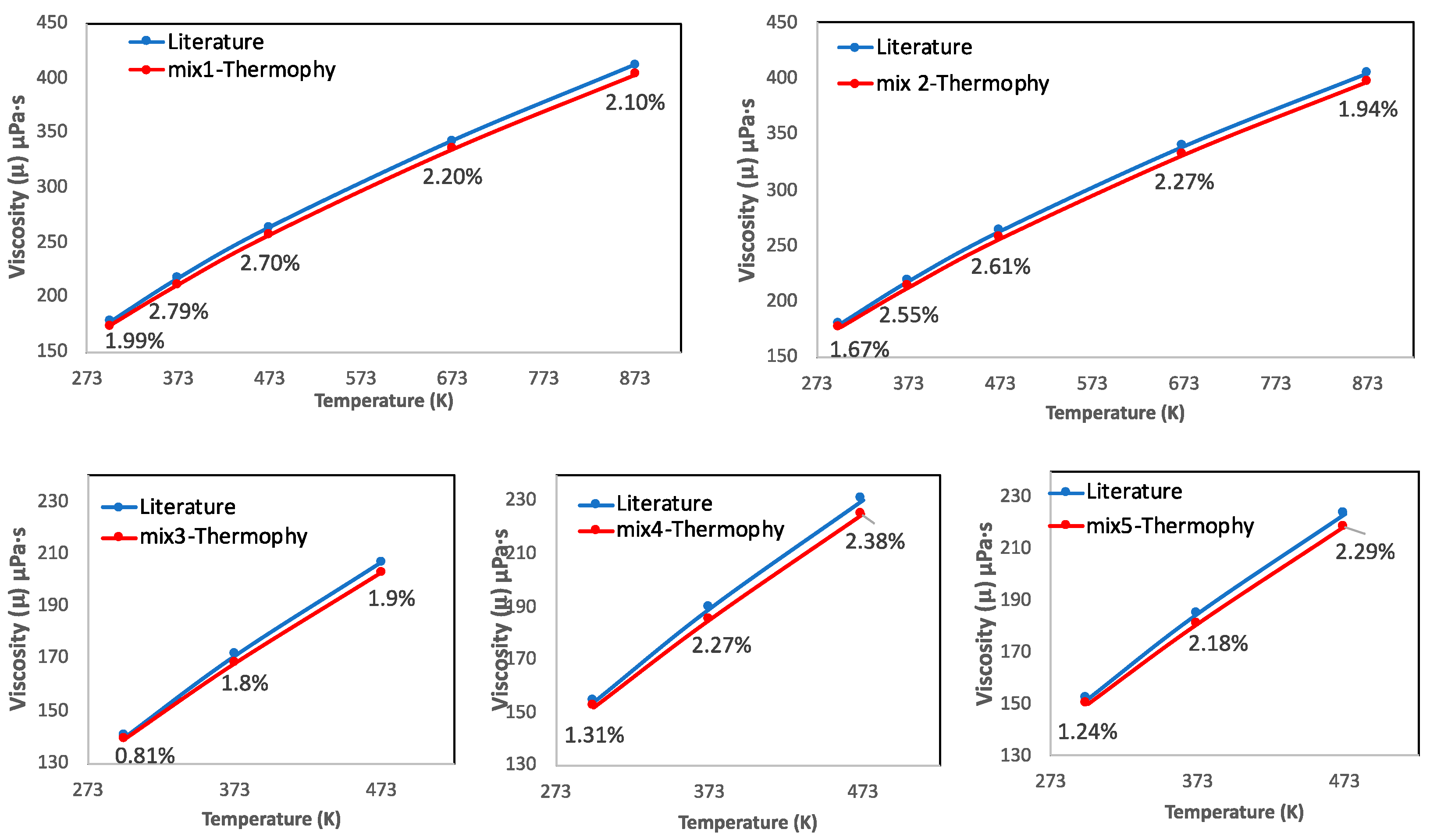
3.2. Case Study 2: Viscosity of N2-Ar-CO2 and N2-CO2-CH4 Ternary Gas Mixtures
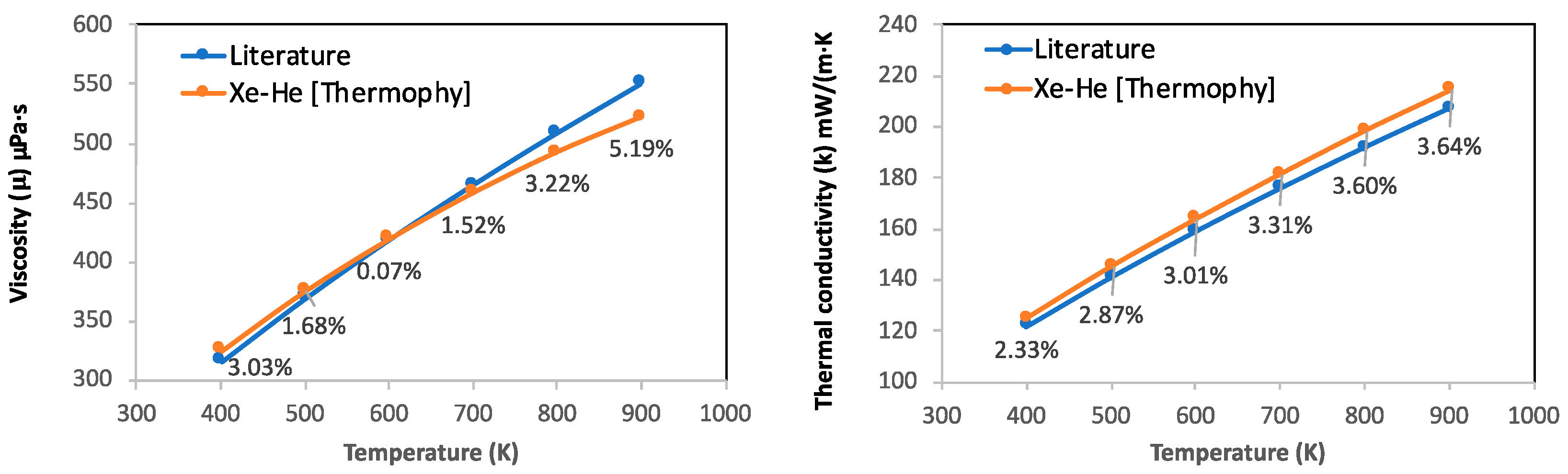
3.3. Case Study 3: Viscosity and Thermal Conductivity of Xe-He Binary Gas Mixture
3.4. Case Study 4: Diffusion Coefficients of Binary Gases
3.5. Error Analysis: Accuracy and Efficiency
4. Conclusions and Future Direction
- Thermophy leverages Chebyshev polynomial fitting to provide numerically stable and accurate estimations of transport properties. Users only need to supply the temperature, number of species, and their mole fractions to perform calculations efficiently.
- Thermophy correctly calculated the thermal conductivity and viscosity of C2H6, CH4, CO2, and He pure gases and air while considering all temperatures, highlighting its potential in calculating the thermophysical properties of other various gases.
- Thermophy computation showed very low deviation from the experimental viscosity data of ternary gas mixtures (overall deviation of mix1, mix2, mix3, mix4, and mix5 are calculated as 0.11%, 0.13%, 0.24%, 0.23%, and 0.22%, respectively, considering each point of temperature) without any processing load during computation.
- Thermal conductivity and viscosity values of the Xe-He binary gas mixture were successfully determined from Thermophy which highlights the potential of using Thermophy in combustion simulations.
- Diffusion coefficients of various binary gas systems were successfully calculated using Thermophy, as confirmed from the literature.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cp | Specific heat at constant pressure, J/mol·K |
| Cυ | Specific heat at constant volume J/mol·K |
| d | Dipole moment |
| Dii | Self diffusion coefficient |
| k0 | Boltzmann constant |
| R | Universal gas constant |
| T | Temperature |
| T* | Reduced temperature |
| W | Molecular weight |
| X | Mole fraction |
| Greek Letters | |
| δ* | Reduced dipole moment |
| ε | Lennard-Jones potential well depth J/molecule |
| λ | Thermal conductivity |
| μ | Viscosity |
| ρ | Density |
| σ | Lennard-Jones collision diameter |
| Ω | Collision integral |
| Subscripts | |
| i | ith species of the mixture |
| j | jth species of the mixture |
| mix | mixture |
| rot | rotational contributions |
| trans | translational contributions |
| vib | vibrational contributions |
| Abbreviation | |
| Ar | Argon |
| C2H4 | Ethylene |
| C2H6 | Ethane |
| C3H8 | Propane |
| CH4 | Methane |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| H2 | Hydrogen |
| H2O | Water |
| He | Helium |
| JANAF | Joint Army–Navy–NASA–Air Force |
| N2 | Nitrogen |
| NASA | National Aeronautics and Space Administration |
| NO2 | Nitrogen dioxide |
| Xe | Xenon |
References
- Jarosinski, J.; Veyssiere, B. Combustion Phenomena: Selected Mechanisms of Flame Formation, Propagation and Extinctions; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Aoki, H.; Miura, T. Combustion Engineering and Thermophysical Properties on Combustion. Netsu Bussei 1995, 9, 30–35. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion with Updates from Active Thermochemical Tables; Argonne National Lab: Argonne, IL, USA, 2005. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, L.; Han, G.; Zhu, T. Numerical Study on the Explosion Reaction Mechanism of Multicomponent Combustible Gas in Coal Mines. Fire 2024, 7, 368. [Google Scholar] [CrossRef]
- Ma, Q.; Guo, Y.; Zhong, M.; You, J.; He, Y.; Chen, J.; Zhang, Z. Theoretical Prediction Model of the Explosion Limits for Multi-Component Gases (Multiple Combustible Gases Mixed with Inert Gases) under Different Temperatures. Fire 2022, 5, 143. [Google Scholar] [CrossRef]
- Williams, F. Combustion Theory: The Fundamental Theory of Chemically Reacting Flow Systems, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; 680p. [Google Scholar]
- Humer, S.; Frassoldati, A.; Granata, S.; Faravelli, T.; Ranzi, E.; Seiser, R.; Seshadri, K. Experimental and kinetic modeling study of combustion of JP-8, its surrogates and reference components in laminar nonpremixed flows. Proc. Combust. Inst. 2007, 31, 393–400. [Google Scholar] [CrossRef]
- Liberman, M. Introduction to Physics and Chemistry of Combustion, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Westbrook, C.K.; Mizobuchi, Y.; Poinsot, T.J.; Smith, P.J.; Warnatz, J. Computational combustion. Proc. Combust. Inst. 2005, 30, 125–157. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. JANAF thermochemical tables. J. Phys. Chem. Ref. Data 1985, 11, 695–940. [Google Scholar] [CrossRef]
- Kee, R.J.; Rupley, F.M.; Miller, J.A. The Chemkin Thermodynamic Data Base; Chemkin Collection: San Diego, CA, USA, 1990. [Google Scholar]
- Zhai, Y.; Li, Y.; Li, H.; McCourt, F.R.W. PENG: A program for transport properties of low-density binary gas mixtures. Comput. Phys. Commun. 2023, 287, 108712. [Google Scholar] [CrossRef]
- Bogdanova, Y.A.; Gubin, S.; Amir, Z.A. Modeling of Thermophysical Properties and Transport Properties of Basic Combustion Products of Organic Substances. Phys. At. Nucl. 2020, 83, 1563–1568. [Google Scholar] [CrossRef]
- Dregalin, A.; Barysheva, O.; Cherenkov, A. Methods for calculating thermophysical properties of gas mixtures. Russ. Aeronaut. (Iz VUZ) 2007, 50, 298–302. [Google Scholar] [CrossRef]
- Copeland, D.A. New Approximate Formulas for Viscosity and Thermal Conductivity of Dilute Gas Mixtures. AIAA J. 2003, 41, 525–537. [Google Scholar] [CrossRef]
- Vesovic, V. Prediction of the Thermal Conductivity of Gas Mixtures at Low Pressures. Int. J. Thermophys. 2001, 22, 801–828. [Google Scholar] [CrossRef]
- Aminian, A. Transport Property Predictions for CH4/H2/CO/CO2/N2/H2O Mixtures Based on Excluded Volume Without Fitting Parameters. Int. J. Thermophys. 2025, 46, 36. [Google Scholar] [CrossRef]
- Kee, R.; Coltrin, M.; Glarborg, P.; Zhu, H. Chemically Reacting Flow: Theory, Modeling, and Simulation; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Goodwin, D.G.; Speth, R.L.; Moffat, H.K.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Zenodo: Geneva, Switzerland, 2018. [Google Scholar]
- Kee, R.; Dixon-lewis, G.; Warnatz, J.; Coltrin, M.; Miller, J. A Fortran Computer Code Package for the Evaluation of Gas-Phase, Multicomponent Transport Properties. SANDIA National Laboratories Report, SAND86-8246: Albuquerque, NM, USA, 1986. [Google Scholar]
- Danciu, B.A.; Frouzakis, C.E. KinetiX: A performance portable code generator for chemical kinetics and transport properties. Comput. Phys. Commun. 2025, 310, 109504. [Google Scholar] [CrossRef]
- Smith, I.M.; Forbes, A.B. Algorithms and software for fitting polynomial functions constrained to pass through the origin. J. Phys. Conf. Ser. 2018, 1065, 212022. [Google Scholar] [CrossRef]
- Montijano, E.; Montijano, J.I.; Sagues, C. Chebyshev Polynomials in Distributed Consensus Applications. IEEE Trans. Signal Process. 2013, 61, 693–706. [Google Scholar] [CrossRef]
- Cai, Q.; Song, L. The Chebyshev interpolation polynomial algorithm error analysis. In Proceedings of the 2012 IEEE International Conference on Information Science and Technology, Wuhan, China, 23–25 March 2012; pp. 745–748. [Google Scholar]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Inst. 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Oh, S.-K. Modified Lennard-Jones Potentials with a Reduced Temperature-Correction Parameter for Calculating Thermodynamic and Transport Properties: Noble Gases and Their Mixtures (He, Ne, Ar, Kr, and Xe). J. Thermodyn. 2013, 2013, 828620. [Google Scholar] [CrossRef]
- Singh, K.; Sood, N.K. Viscosity and thermal conductivity of gas mixtures. Indian J. Pure Appl. Phys. 2003, 41, 121–127. [Google Scholar]
- Design, R. ANSYS Chemkin Theory Manual 17.0 (15151). Reaction Design: San Diego, CA, USA, 2015. [Google Scholar]
- Mian, A.A. Measurement and Prediction of Binary Gaseous Diffusion Coefficients. Ph.D. Thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 1967. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. Properties of Gases and Liquids; McGraw-Hill Education: New York, NY, USA, 2001. [Google Scholar]
- Bird, R.; Stewart, W.; Lightfoot, E. Transport Phenomena, Revised, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Lawson, C.; Hanson, R.; Kincaid, D.; Krogh, F. Basic linear algebra subprograms for FORTRAN usage. ACM Trans. Math. Softw. 1979, 5, 308–323. [Google Scholar] [CrossRef]
- Krogh, F. MATH77/mathc90. Available online: https://www.netlib.org/math (accessed on 1 January 2020).
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.; Hanson, R.; Song, S.; Gardiner, W., Jr. GRI-MECH 3.0. sn. Available online: http://combustion.berkeley.edu/gri-mech (accessed on 25 May 2021).
- Green, D.W. Perry’s Chemical Engineers’ Handbook; Mcgraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Kestin, J.; Ro, S.T. The viscosity of nine binary and two ternary mixtures of gases at low density. Berichte Bunsenges. Für Phys. Chem. 1974, 78, 20–24. [Google Scholar] [CrossRef]
- Johnson, P.K. A Method for Calculating Viscosity and Thermal Conductivity of a Helium-Xenon Gas Mixture; BiblioGov: Asheville, NC, USA, 2006. [Google Scholar]
- Hashim, E.; Al-Shorachi, H. Prediction of diffusion coefficients for binary gas system. Pet. Sci. Technol. 2007, 25, 983–989. [Google Scholar] [CrossRef]
- Langenberg, S.; Carstens, T.; Hupperich, D.; Schweighoefer, S.; Schurath, U. Determination of binary gas-phase diffusion coefficients of unstable and adsorbing atmospheric trace gases at low temperature–arrested flow and twin tube method. Atmos. Chem. Phys. 2020, 20, 3669–3682. [Google Scholar] [CrossRef]
- Zangi, P.; Rausch, M.H.; Fröba, A.P. Binary Diffusion Coefficients for Gas Mixtures of Propane with Methane and Carbon Dioxide Measured in a Loschmidt Cell Combined with Holographic Interferometry. Int. J. Thermophys. 2019, 40, 18. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Curtiss, C.F.; Bird, R.B. The Molecular Theory of Gases and Liquids; John Wiley & Sons: Hoboken, NJ, USA, 1964. [Google Scholar]
- Loianno, V. On the measurement of the mutual diffusivity of binary gas mixtures with FTIR Spectroscopy. Chem. Eng. Sci. 2023, 270, 118546. [Google Scholar] [CrossRef]
- Neufeld, P.D.; Janzen, A.R.; Aziz, R.A. Empirical Equations to Calculate 16 of the Transport Collision Integrals Ω(l, s)* for the Lennard-Jones (12–6) Potential. J. Chem. Phys. 1972, 57, 1100–1102. [Google Scholar] [CrossRef]
- Bell, I.H. Efficient Approximation with Space Filling Quadtrees: Application to Phase Equilibria in Binary Mixtures. Ind. Eng. Chem. Res. 2024, 63, 13895–13903. [Google Scholar] [CrossRef] [PubMed]
| Binary Gas System | Literature | Temperature (K) | Experimental DAB (cm2/s) | Thermophy DAB (cm2/s) | Deviation (%) |
|---|---|---|---|---|---|
| Ar-CH4 | [39] | 298 | 0.205 | 0.214 | 4.39 |
| Ar-H2 | [39] | 295 | 0.840 | 0.790 | 5.95 |
| Ar-He | [39] | 276 | 0.655 | 0.654 | 0.15 |
| Ar-Xe | [39] | 378 | 0.180 | 0.173 | 3.89 |
| CO2-He | [39] | 298 | 0.620 | 0.610 | 1.61 |
| CO2-He | [39] | 498 | 1.433 | 1.446 | 0.91 |
| CO-N2 | [39] | 373 | 0.322 | 0.302 | 6.21 |
| H2-N2 | [39] | 294 | 0.773 | 0.751 | 2.85 |
| H2-N2 | [39] | 573 | 2.449 | 2.317 | 5.39 |
| He-H2O | [39] | 352 | 1.136 | 1.176 | 3.52 |
| He-N2 | [39] | 298 | 0.696 | 0.709 | 1.87 |
| CH4-He | [40] | 273.15 | 0.618 | 0.596 | 3.56 |
| C2H4-He | [40] | 273.15 | 0.497 | 0.498 | 0.20 |
| NO2-N2 | [40] | 273.15 | 0.145 | 0.146 | 0.69 |
| C3H8-CH4 | [41] | 293.29 | 0.118 | 0.119 | 1.42 |
| C3H8-CH4 | [41] | 313.59 | 0.138 | 0.136 | 1.92 |
| CO2-CH4 | [43] | 298.45 | 0.173 | 0.167 | 3.47 |
| Ternary Gas Mixtures | Molar Compositions | Overall Deviation (%) Compared to Thermophy | Overall Deviation (%) Compared to Cantera |
|---|---|---|---|
| Mixture 1 | 0.11 | 3.54 | |
| Mixture 2 | 0.13 | 1.29 | |
| Mixture 3 | 0.24 | 0.23 | |
| Mixture 4 | 0.23 | 0.88 | |
| Mixture 5 | 0.22 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydın, N.Ö.; Kopaç, M. Thermophy: A Chebyshev Polynomial-Based Tool for Transport Property Estimation in Multicomponent Gas Systems. Fire 2025, 8, 372. https://doi.org/10.3390/fire8090372
Aydın NÖ, Kopaç M. Thermophy: A Chebyshev Polynomial-Based Tool for Transport Property Estimation in Multicomponent Gas Systems. Fire. 2025; 8(9):372. https://doi.org/10.3390/fire8090372
Chicago/Turabian StyleAydın, Nuri Özgür, and Mehmet Kopaç. 2025. "Thermophy: A Chebyshev Polynomial-Based Tool for Transport Property Estimation in Multicomponent Gas Systems" Fire 8, no. 9: 372. https://doi.org/10.3390/fire8090372
APA StyleAydın, N. Ö., & Kopaç, M. (2025). Thermophy: A Chebyshev Polynomial-Based Tool for Transport Property Estimation in Multicomponent Gas Systems. Fire, 8(9), 372. https://doi.org/10.3390/fire8090372






