Halogen-Free Flame Retardant Impact on Rigid Polyisocyanurate Foam Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PIR Foams and Characterisation of Foaming Parameters
2.3. Characterisation of PIR Foams
3. Results and Discussion
3.1. Rigid PIR Foam Foaming Parameters
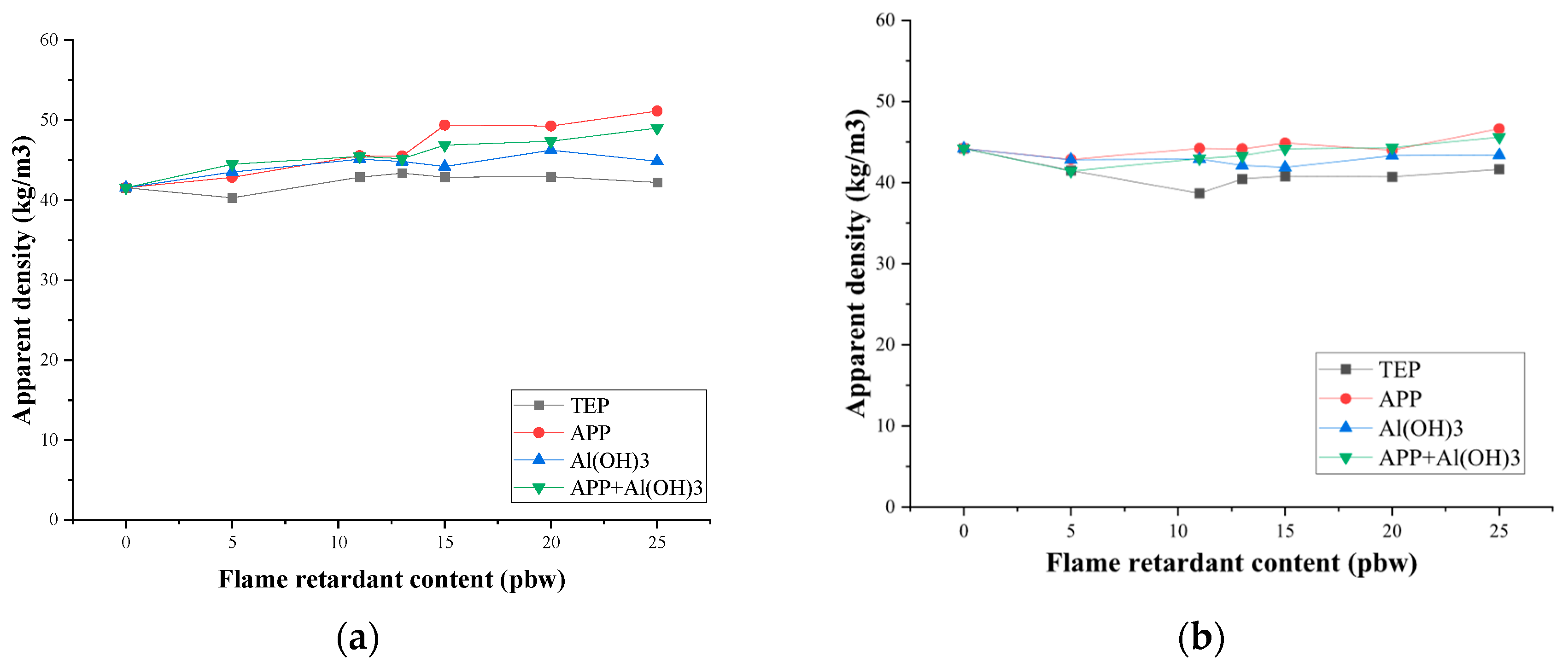
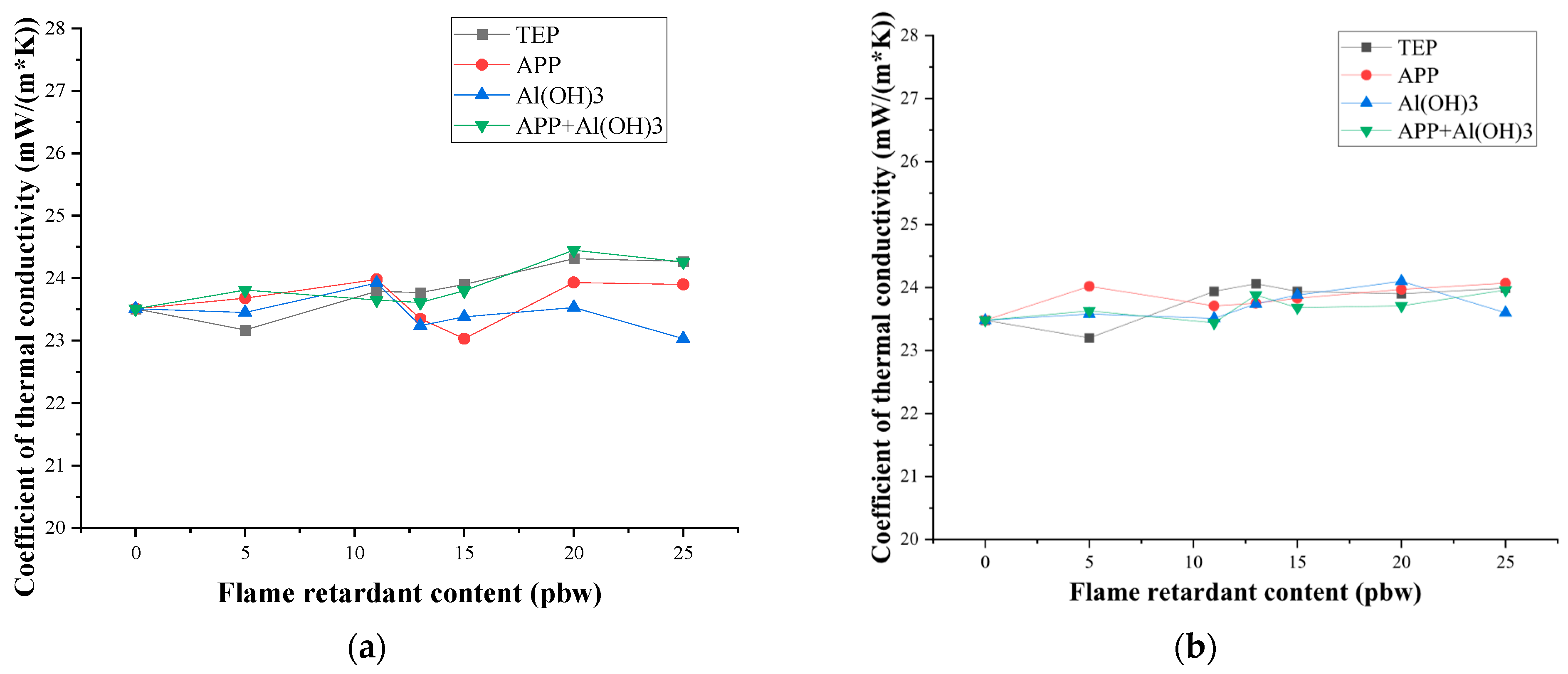
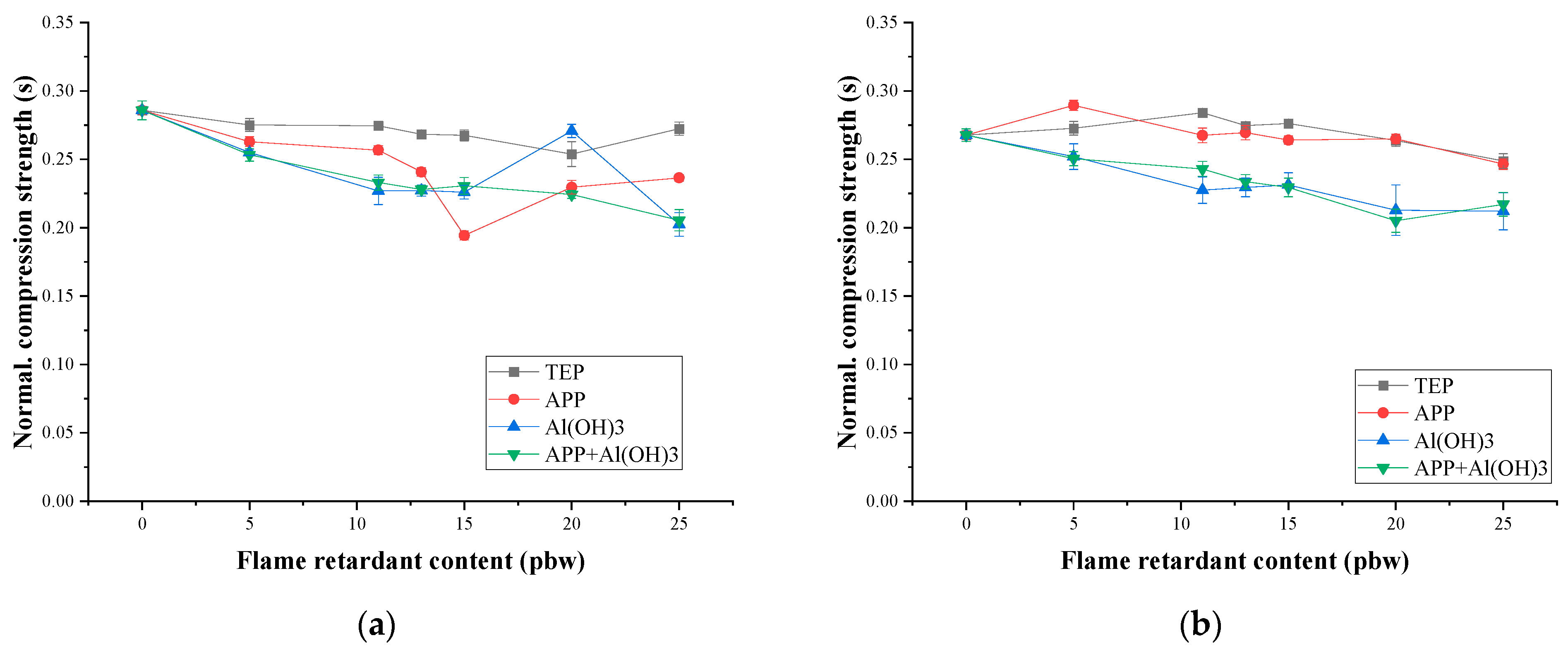
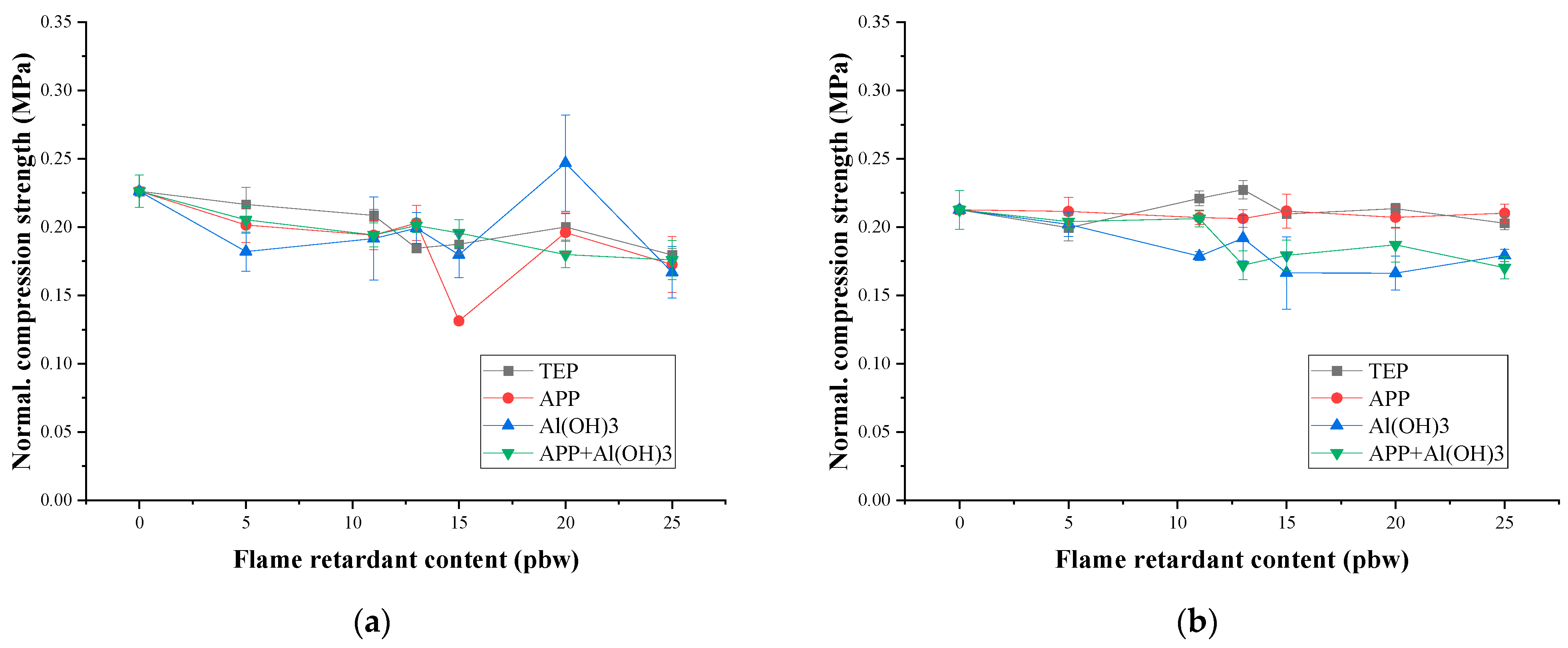
3.2. Mechanical Properties and Thermal Conductivity
- σnormal—normalised compressive strength, MPa;
- σ—compressive strength, MPa;
- ρ—density, kg/m3.
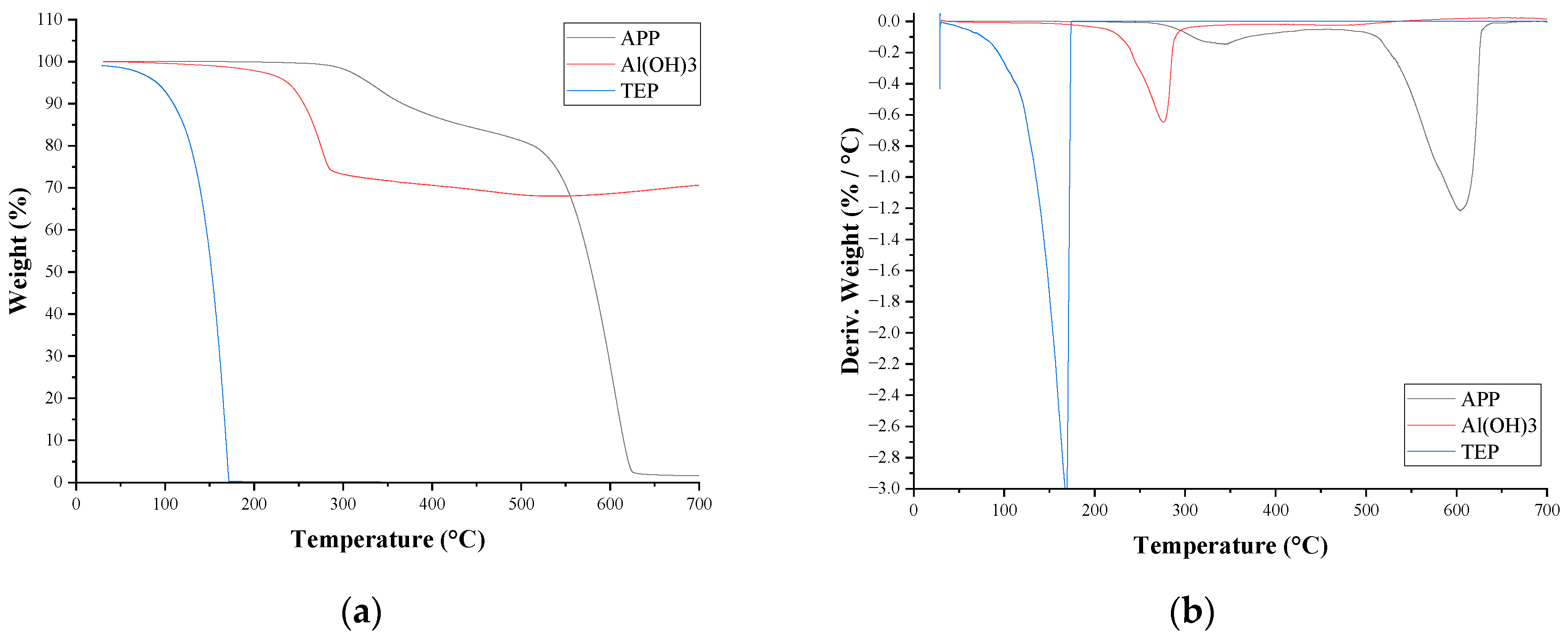
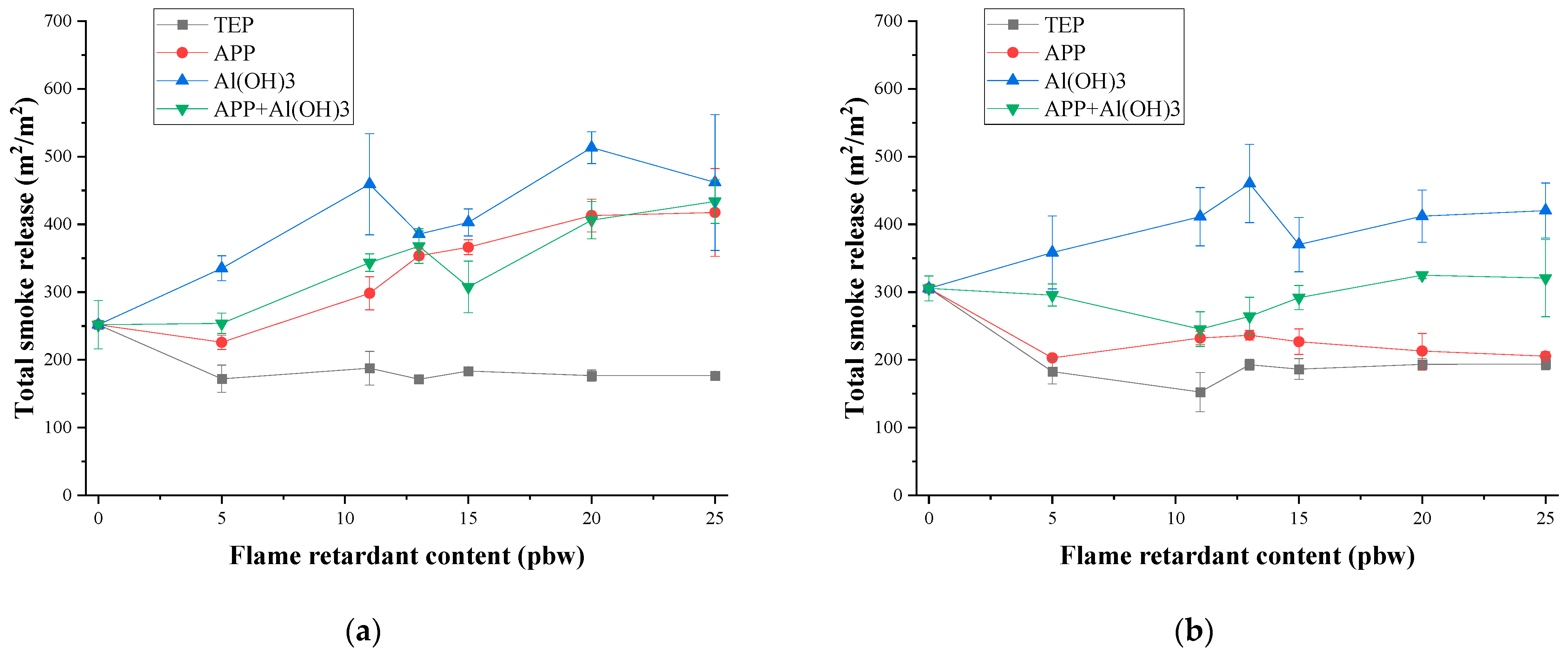
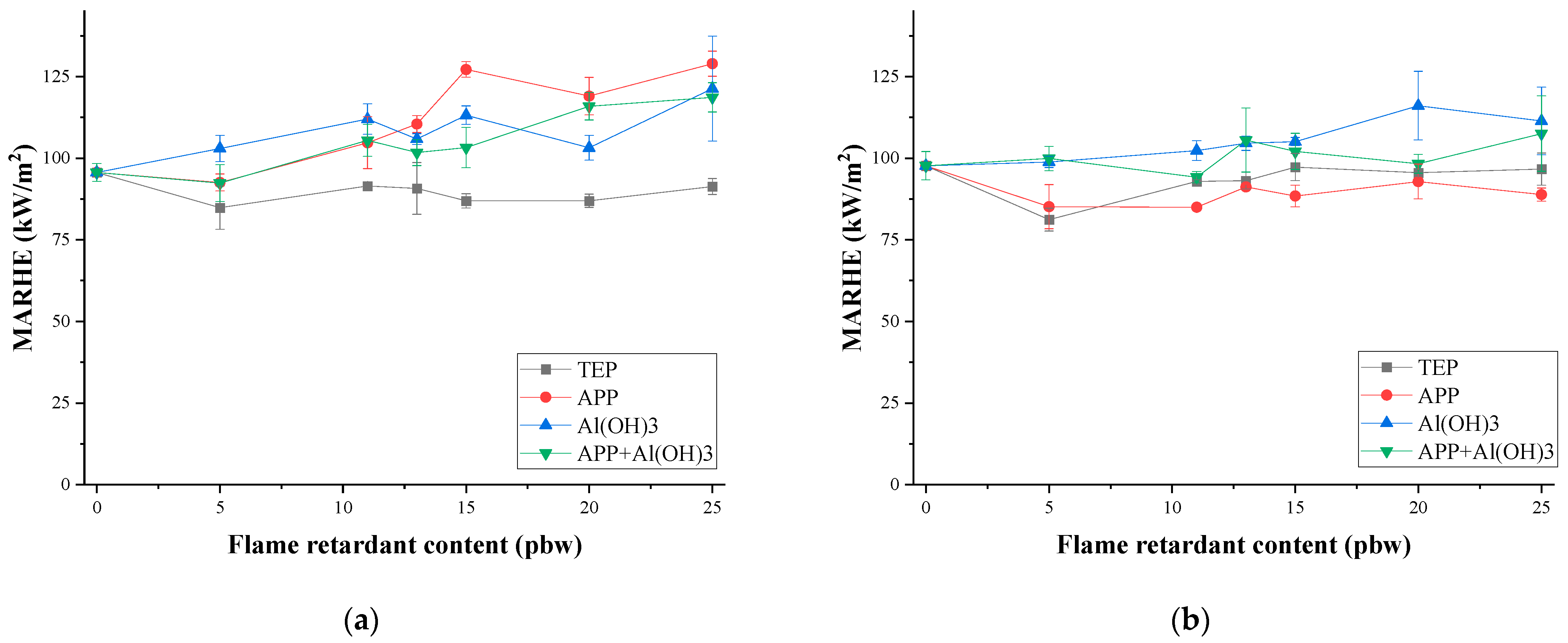
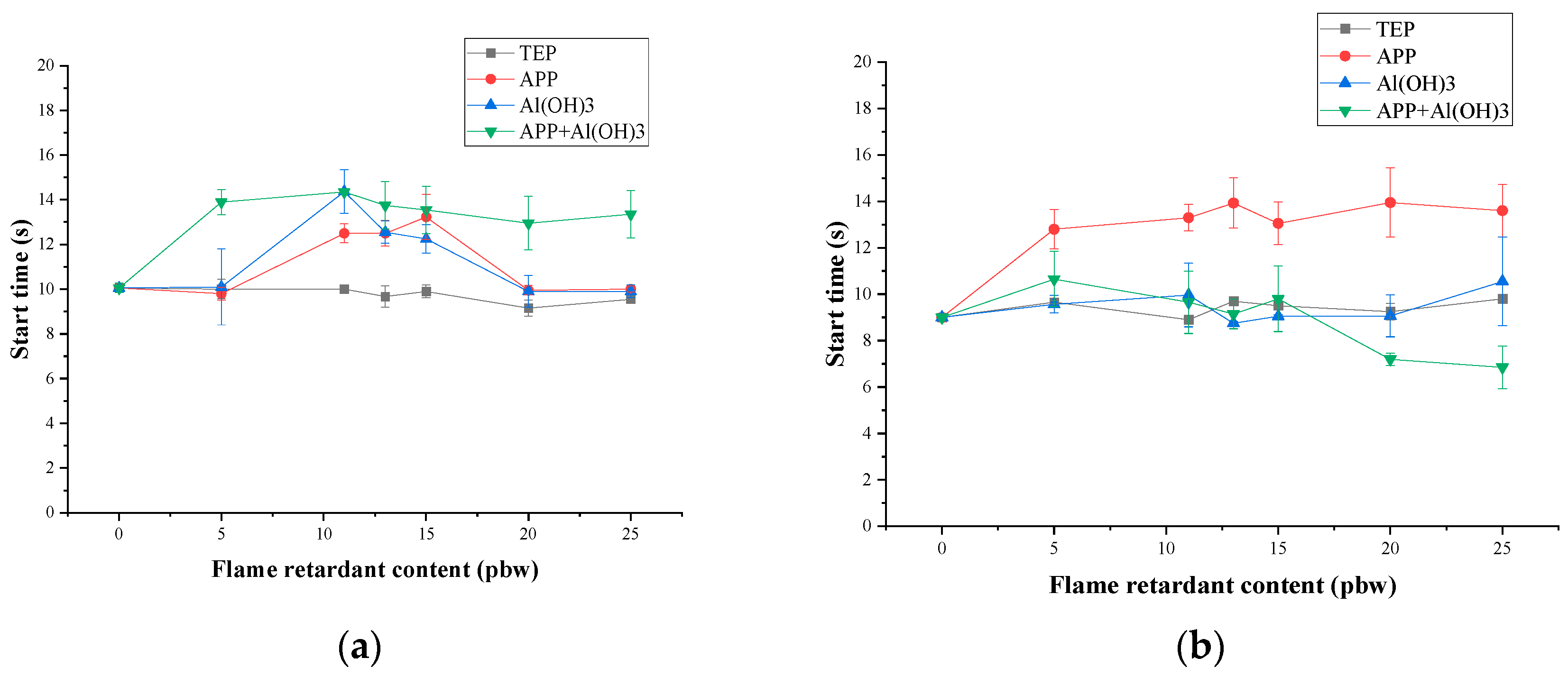
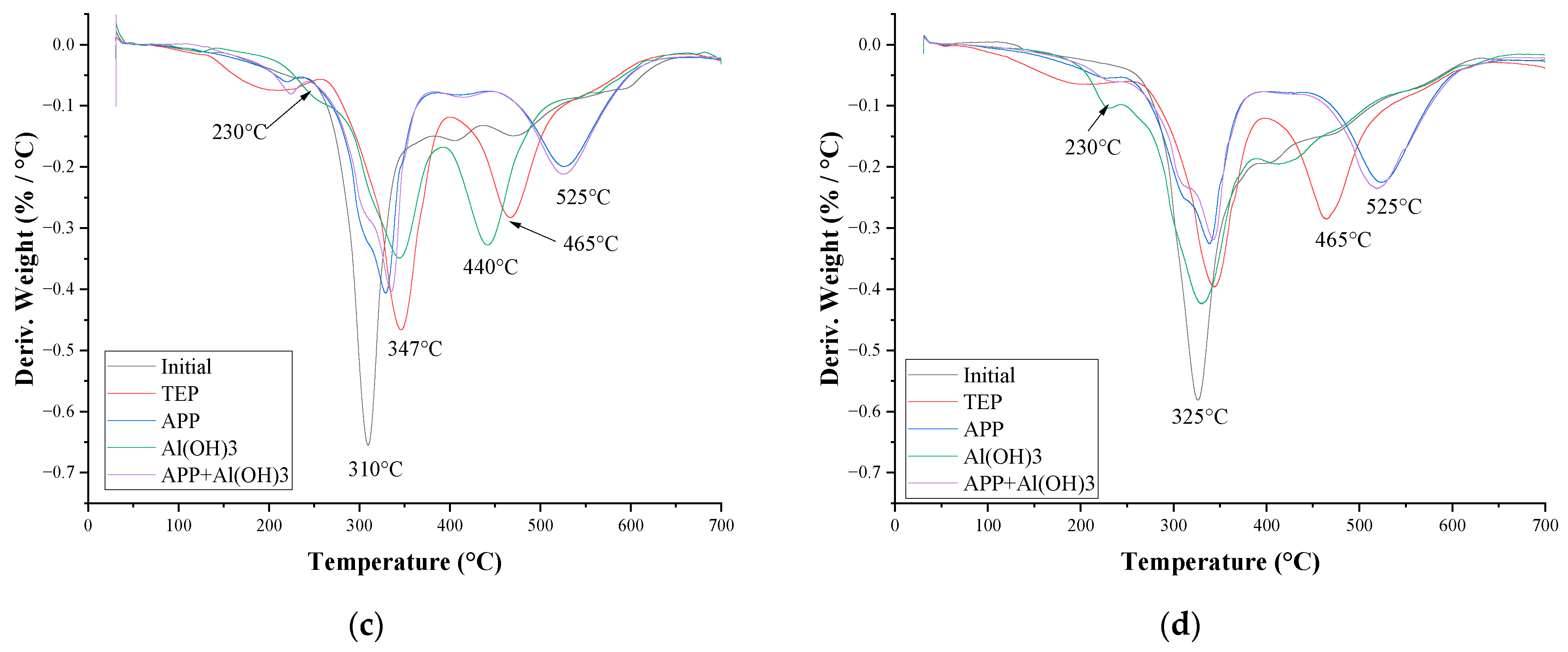
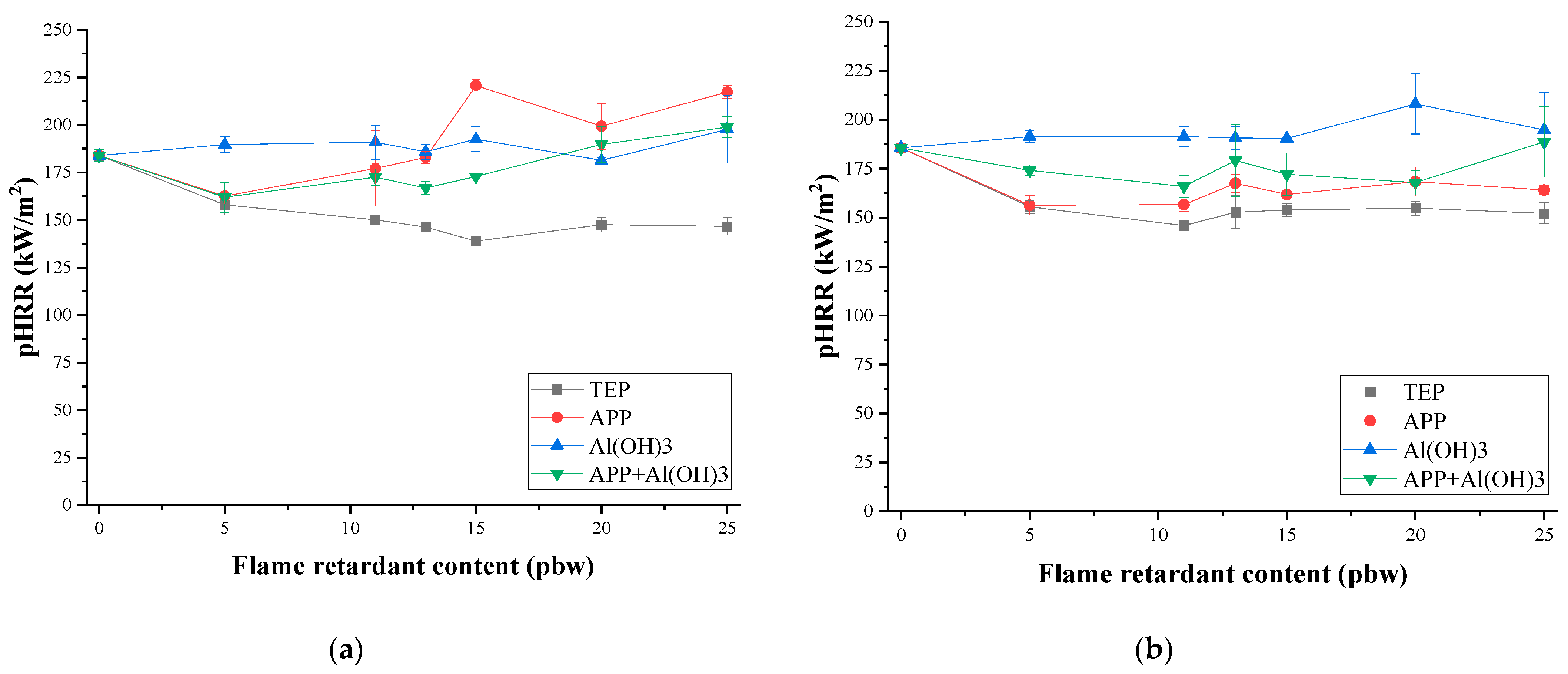
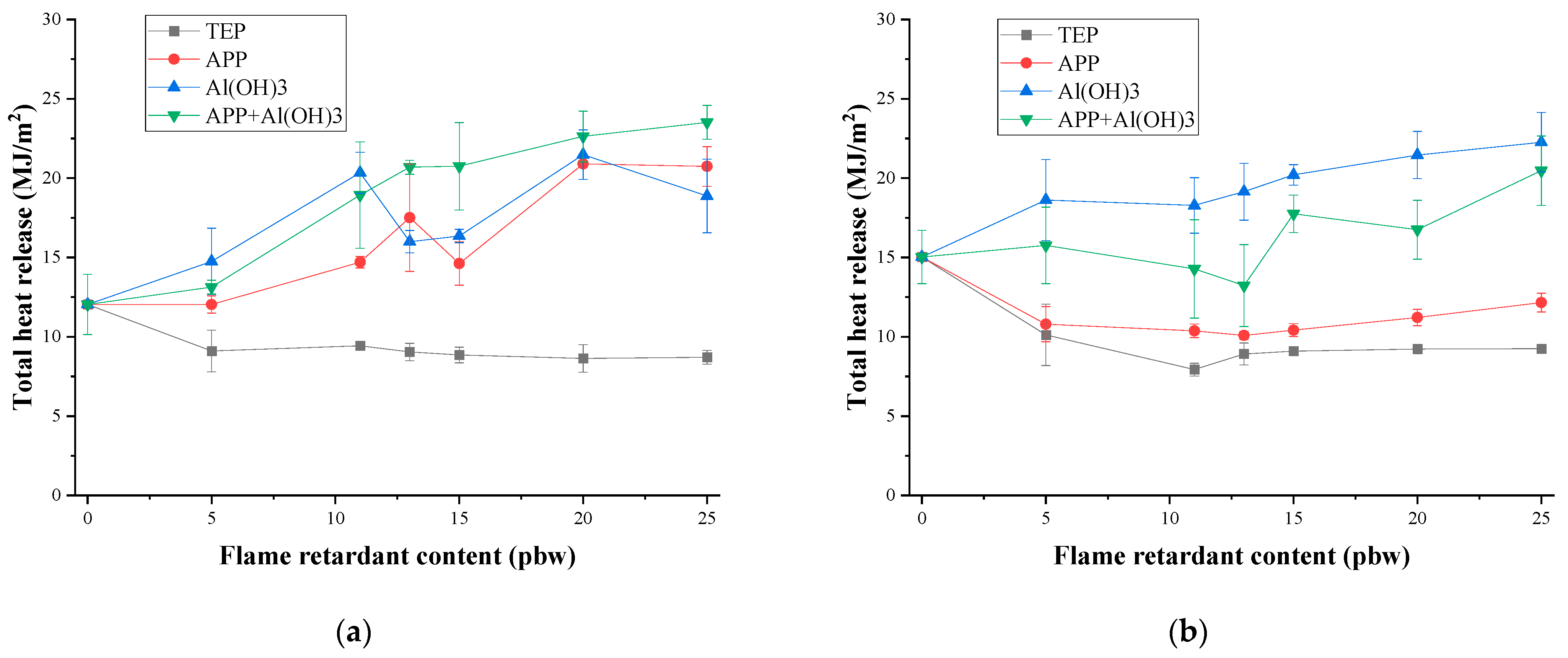
3.3. Thermal Stability and Fire Behaviour
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PIR | polyisocyanurate |
| PUR | polyurethane |
| TEP | triethyl phosphate |
| APP | ammonium polyphosphate |
| TCPP | tris(chloropropyl)phosphate |
| pMDI | polymeric 4,4-methylene diphenyl isocyanate |
| ATC | advanced test container |
| LOI | limited oxygen index |
| TGA | thermogravimetric analysis |
| DTG | differential thermogravimetric analysis |
| pHRR | peak heat release rate |
Appendix A
References
- Sun, Y.; Liu, L.Y.; Sverko, E.; Li, Y.F.; Li, H.L.; Huo, C.Y.; Ma, W.L.; Song, W.; Zhang, Z.F. Organophosphate flame retardants in college dormitory dust of northern Chinese cities: Occurrence, human exposure and risk assessment. Sci. Total Environ. 2019, 665, 731–738. [Google Scholar] [CrossRef]
- Wei, G.L.; Li, D.Q.; Zhuo, M.N.; Liao, Y.S.; Xie, Z.Y.; Guo, T.L.; Li, J.J.; Zhang, S.Y.; Liang, Z.Q. Organophosphorus flame retardants and plasticizers: Sources, occurrence, toxicity and human exposure. Environ. Pollut. 2015, 196, 29–46. [Google Scholar] [CrossRef]
- Ali, N.; Ali, L.; Mehdi, T.; Dirtu, A.C.; Al-Shammari, F.; Neels, H.; Covaci, A. Levels and profiles of organochlorines and flame retardants in car and house dust from Kuwait and Pakistan: Implication for human exposure via dust ingestion. Environ. Int. 2013, 55, 62–70. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Shi, Z. Organophosphorus flame retardants in breast milk from Beijing, China: Occurrence, nursing infant’s exposure and risk assessment. Sci. Total Environ. 2021, 771, 145404. [Google Scholar] [CrossRef] [PubMed]
- Chupeau, Z.; Mercier, F.; Rouxel, E.; Le Bot, B.; Chauvet, G.; Siméon, T.; Bonvallot, N.; Zaros, C.; Chevrier, C.; Glorennec, P. Pre- and post-natal exposure of children to organophosphate flame retardants: A nationwide survey in France. Environ. Int. 2022, 168, 107435. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, H.; Raffy, G.; Le Bot, B.; Bossanne, E.; Rawas, C.; Cardin, P.; Desauziers, V. Kinetic analysis of TCPP emission from fireproofed upholstered furniture under realistic indoor conditions. Build. Environ. 2025, 267, 112286. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Li, M.; Du, M.; Li, X.; Li, Y. A review of a class of emerging contaminants: The classification, distribution, intensity of consumption, synthesis routes, environmental effects and expectation of pollution abatement to organophosphate flame retardants (opfrs). Int. J. Mol. Sci. 2019, 20, 2874. [Google Scholar] [CrossRef]
- European Commission. Restrictions Roadmap Under the Chemicals Strategy for Sustainability. Ongoing MDR and IVDR Guidance Plan. 2022. Available online: https://ec.europa.eu/docsroom/documents/49734 (accessed on 31 August 2025).
- Duquesne, S.; Le Bras, M.; Bourbigot, S.; Delobel, R.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T.; Vezin, H. Mechanism of fire retardancy of polyurethanes using ammonium polyphosphate. J. Appl. Polym. Sci. 2001, 82, 3262–3274. [Google Scholar] [CrossRef]
- Xiao, F.; Guo, R.; Wang, J. Flame retardant and its influence on the performance of asphalt—A review. Constr. Build. Mater. 2019, 212, 841–861. [Google Scholar] [CrossRef]
- Hu, S.; Chen, F.; Li, J.G.; Shen, Q.; Huang, Z.X.; Zhang, L.M. The ceramifying process and mechanical properties of silicone rubber/ammonium polyphosphate/aluminium hydroxide/mica composites. Polym. Degrad. Stab. 2016, 126, 196–203. [Google Scholar] [CrossRef]
- Abosnina, A.E.; Mohamad, Z.B.; Majid, R.A. Thermal Stability and Flammability Properties of Filled RigidPolyurethane/Aluminium Hydroxide Composite Foam. Chem. Eng. Trans. 2014, 108, 61–66. [Google Scholar] [CrossRef]
- ISO 845:2006; Cellular Plastics and Rubbers. Determination of Apparent Density. ISO: Geneva, Switzerland, 2006.
- ISO 8301:1991; Thermal Insulation—Determination of Steady-State Thermal Resistance and Related Properties—Heat Flow Meter Apparatus. ISO: Geneva, Switzerland, 1991.
- ISO 844:2021; Rigid Cellular Plastics—Determination of Compression Properties. ISO: Geneva, Switzerland, 2021.
- ISO 4589-2; Plastics—Determination of Burning Behaviour by Oxygen Index. Part 2 Ambient Temperature Test. ISO: Geneva, Switzerland, 2017.
- ISO 11925-2:2020; Reaction to the Fire Tests—Ignitability of Products Subjected to Direct Impingement of Flame. Part 2: Single Flame Source Test. ISO: Geneva, Switzerland, 2020.
- ISO/TR 5660-1:2015; Reaction to Fire Tests—Heat Release, Smoke Production and Mass loss RATE. Part 1 Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement). ISO: Geneva, Switzerland, 2015.
- Borreguero, A.M.; Rodríguez, J.F.; Valverde, J.L.; Peijs, T.; Carmona, M. Characterization of rigid polyurethane foams containing microencapsulted phase change materials: Microcapsules type effect. J. Appl. Polym. Sci. 2013, 128, 582–590. [Google Scholar] [CrossRef]
- Vevere, L.; Sture-Skela, B.; Yakushin, V.; Němeček, P.; Beneš, H.; Cabulis, U. Phase-Change Materials as Cryo-Shock Absorbers in Rigid Polyurethane Cryogenic Insulation Foams. Polymers 2025, 17, 729. [Google Scholar] [CrossRef]
- Kuranska, M.; Prociak, A.; Cabulis, U.; Kirpluks, M.; Ryszkowska, J.; Auguscik, M. Innovative porus polyurethane—Polyisocyanurate foams based on rapessed oil and modified with expandable graphite. Ind. Crops Prod. 2017, 95, 316–323. [Google Scholar] [CrossRef]
- Hetimy, S.; Megahed, N.; Eleinen, O.A.; Elgheznawy, D. Exploring the potential of sheep wool as an eco-friendly insulation material: A comprehensive review and analytical ranking. Sustain. Mater. Technol. 2024, 39, e00812. [Google Scholar] [CrossRef]
- Hawkins, M.C.; O’Toole, B.; Jackovich, D. Cell morphology and mechanical properties of rigid polyurethane foam. J. Cell. Plast. 2005, 41, 267–285. [Google Scholar] [CrossRef]
- Hosseinpourpia, R.; Adamopoulos, S.; Parsland, C. Utilization of different tall oils for improving the water resistance of cellulosic fibers. J. Appl. Polym. Sci. 2019, 136, 47303. [Google Scholar] [CrossRef]
- Honeywell Blowing Agents. Solstice Liquid Blowing Agent Technical Information. Available online: https://ess.honeywell.com/content/dam/advancedmaterials/en/documents/document-lists/blowing-agents/technical/TDS-Solstice-LBA-Feb2022.pdf (accessed on 31 August 2025).
- Andersons, J.; Grūbe, R.; Vēvere, L.; Cābulis, P.; Kirpluks, M. Anisotropic thermal expansion of bio-based rigid low-density closed-cell polyurethane foams. J. Mater. Res. Technol. 2022, 16, 1517–1525. [Google Scholar] [CrossRef]
- Modesti, M.; Lorenzetti, A. Halogen-free flame retardants for polymeric foams. Polym. Degrad. Stab. 2002, 78, 167–173. [Google Scholar] [CrossRef]
- Asimakopoulou, E.; Zhang, J.; Mckee, M.; Wieczorek, K.; Krawczyk, A.; Andolfo, M.; Kozlecki, T.; Scatto, M.; Sisani, M.; Bastianini, M.; et al. Effect of layered double hydroxide, expanded graphite and ammonium polyphosphate additives on thermal stability and fire performance of polyisocyanurate insulation foam. Thermochim. Acta 2020, 693, 178724. [Google Scholar] [CrossRef]
- Lazo, M.; Puga, I.; Macías, M.A.; Barragán, A.; Manzano, P.; Rivas, A.; Rigail-Cedeño, A. Mechanical and thermal properties of polyisocyanurate rigid foams reinforced with agricultural waste. Case Stud. Chem. Environ. Eng. 2023, 8, 100392. [Google Scholar] [CrossRef]
- Reinerte, S.; Kirpluks, M.; Cabulis, U. Thermal degradation of highly crosslinked rigid PU-PIR foams based on high functionality tall oil polyol. Polym. Degrad. Stab. 2019, 167, 50–57. [Google Scholar] [CrossRef]
- Bruhn, I.H.; Craig, J.L.; Hinge, M.; Richard, T.; Hansen, I.; Laura, J.; Hinge, M.; Richard, T. Ammonium polyphosphates: Correlating structure to application. Eur. Polym. J. 2024, 223, 113644. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Liu, G. Synthesis of ammonium polyphosphate with crystalline form v (APP-V) from melamine polyphosphate (MPP). Polym. Degrad. Stab. 2012, 97, 2567–2570. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]
- Kurańska, M.; Prociak, A.; Kirpluks, M.; Cabulis, U. Polyurethane-polyisocyanurate foams modified with hydroxyl derivatives of rapeseed oil. Ind. Crops Prod. 2015, 74, 849–857. [Google Scholar] [CrossRef]
- Hu, X.M.; Wang, D.M. Enhanced fire behavior of rigid polyurethane foam by intumescent flame retardants. J. Appl. Polym. Sci. 2013, 129, 238–246. [Google Scholar] [CrossRef]
- Duval, A.; Sarazin, J.; de Haas, C.; Sarbu, A.; Bourbigot, S.; Avérous, L. Influence of the chemical structure of polyester polyols on the properties and fire resistance of polyisocyanurate foams. Eur. Polym. J. 2024, 210, 112938. [Google Scholar] [CrossRef]
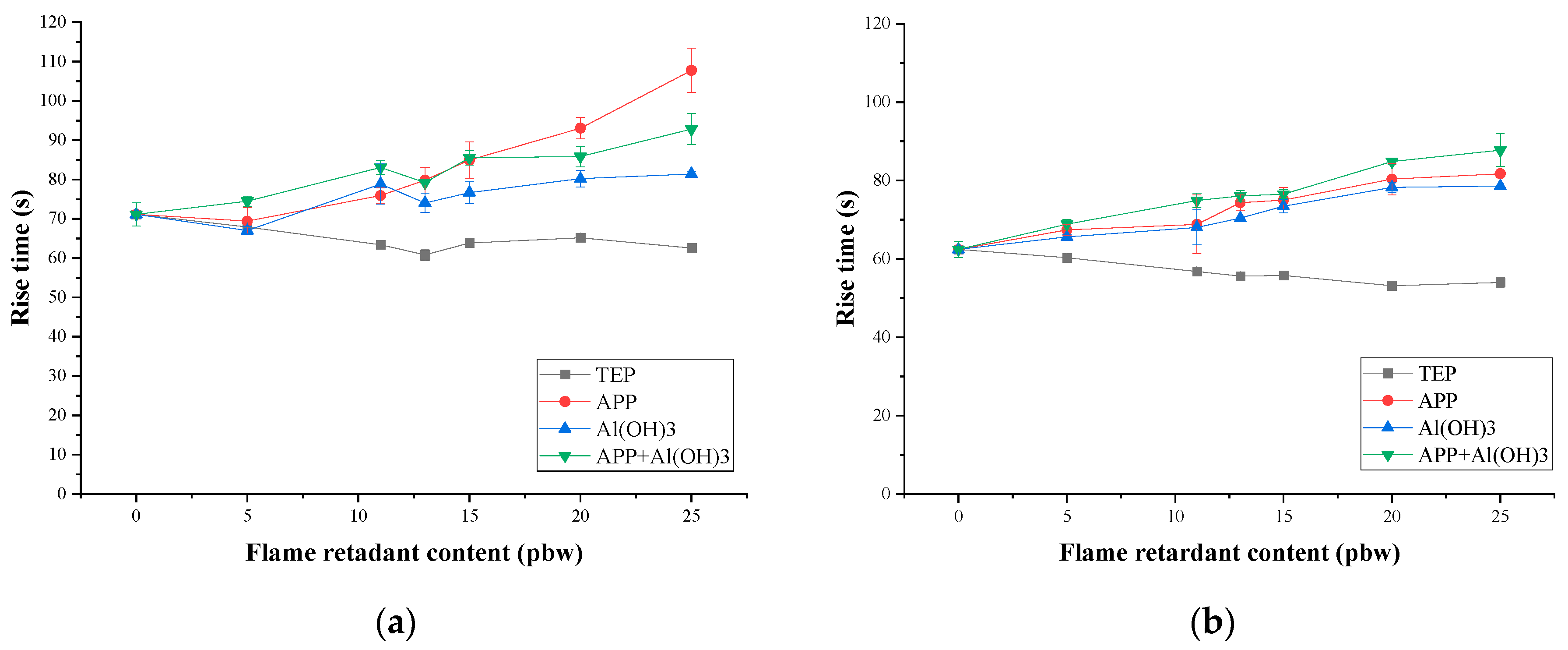
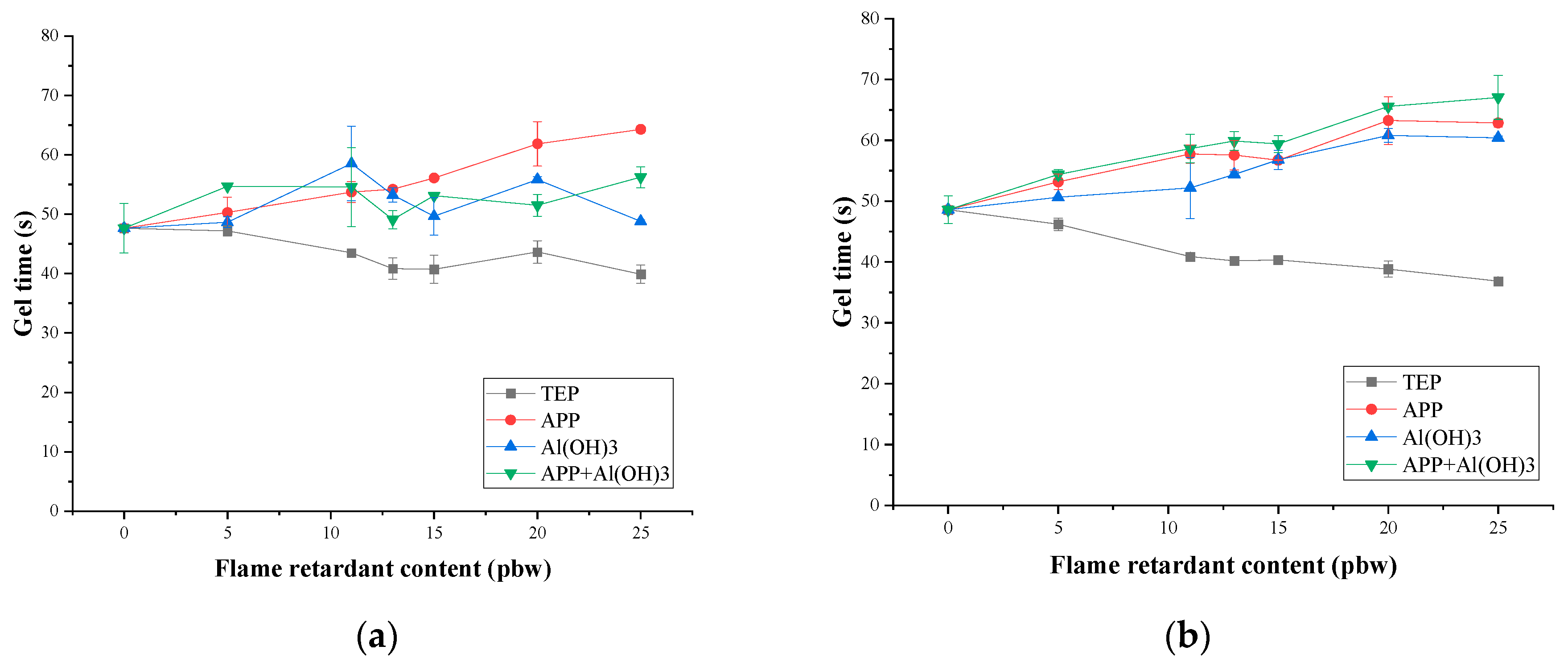
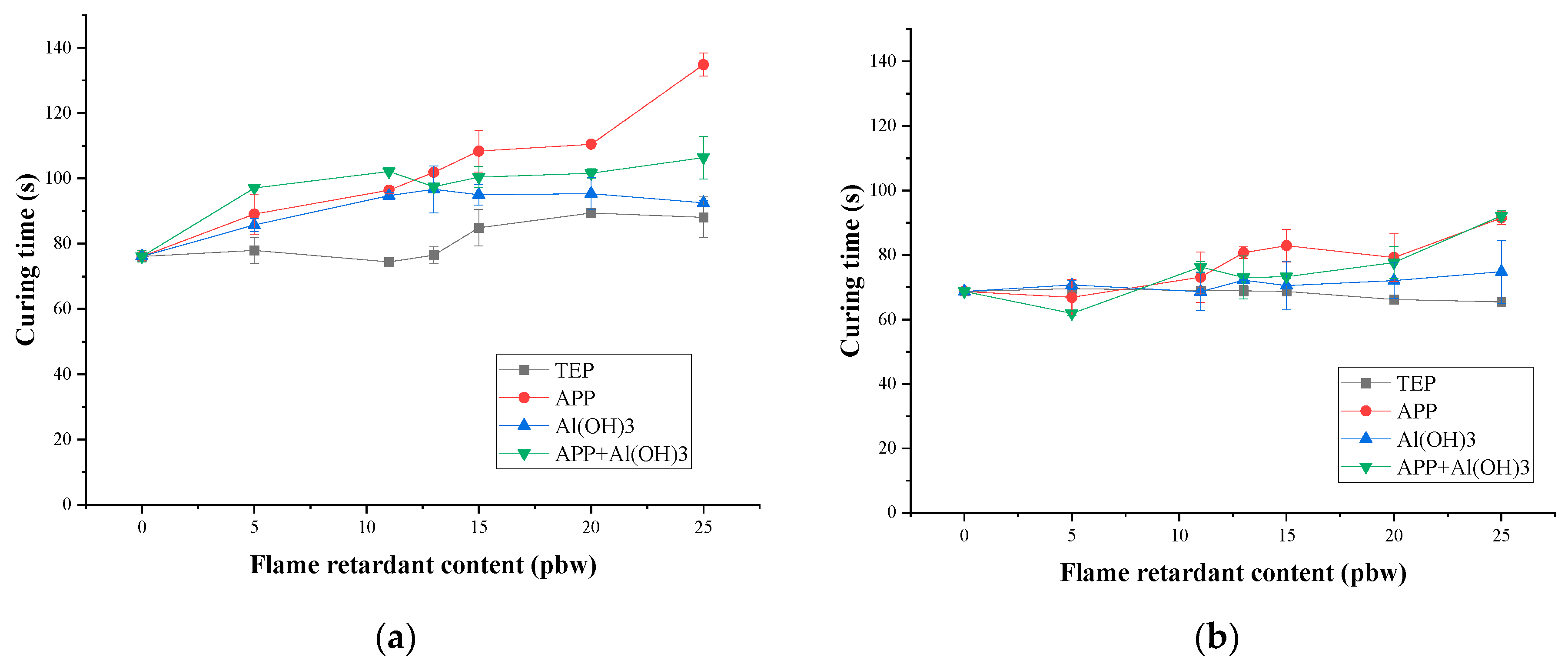
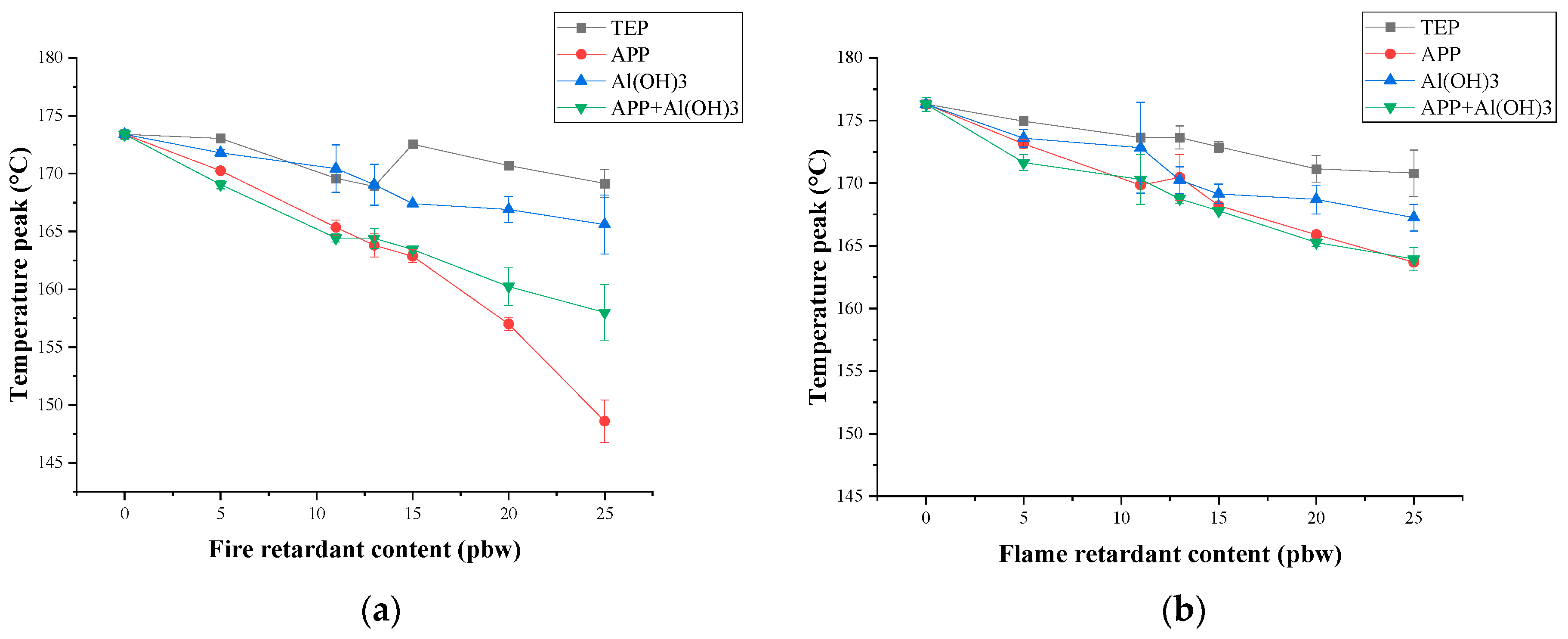

| TEP Content, pbw | Length of Damaged Area for PIR Foams Containing TEP, cm | Oxygen Index | ||||
|---|---|---|---|---|---|---|
| TEP | APP + Al(OH)3 | |||||
| Isocyanate Index 335 | Isocyanate Index 400 | Isocyanate Index 335 | Isocyanate Index 400 | Isocyanate Index 335 | Isocyanate Index 400 | |
| 0 | 12.8 | 9.4 | 22.4 | 22.7 | 22.4 | 22.7 |
| 5 | 10.0 | 8.0 | 24.2 | 24.3 | 23.9 | 24.0 |
| 11 | 7.8 | 8.0 | 25.7 | 25.7 | 24.8 | 25.1 |
| 13 | 8.9 | 8.3 | 25.1 | 26.3 | 25.1 | 24.6 |
| 15 | 9.3 | 8.0 | 26.0 | 27.2 | 25.5 | 25.7 |
| 20 | 7.6 | 7.3 | 27.5 | 27.7 | 25.8 | 26.3 |
| 25 | 8.1 | 28.6 | 28.3 | 26.3 | 27.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vevere, L.; Sture-Skela, B.; Dhalivala, V.; Bariss, U.; Pasters, U.; Kurma, N.; Cabulis, U.; Kirpluks, M. Halogen-Free Flame Retardant Impact on Rigid Polyisocyanurate Foam Properties. Fire 2025, 8, 360. https://doi.org/10.3390/fire8090360
Vevere L, Sture-Skela B, Dhalivala V, Bariss U, Pasters U, Kurma N, Cabulis U, Kirpluks M. Halogen-Free Flame Retardant Impact on Rigid Polyisocyanurate Foam Properties. Fire. 2025; 8(9):360. https://doi.org/10.3390/fire8090360
Chicago/Turabian StyleVevere, Laima, Beatrise Sture-Skela, Vanesa Dhalivala, Uldis Bariss, Uldis Pasters, Nikolajs Kurma, Ugis Cabulis, and Mikelis Kirpluks. 2025. "Halogen-Free Flame Retardant Impact on Rigid Polyisocyanurate Foam Properties" Fire 8, no. 9: 360. https://doi.org/10.3390/fire8090360
APA StyleVevere, L., Sture-Skela, B., Dhalivala, V., Bariss, U., Pasters, U., Kurma, N., Cabulis, U., & Kirpluks, M. (2025). Halogen-Free Flame Retardant Impact on Rigid Polyisocyanurate Foam Properties. Fire, 8(9), 360. https://doi.org/10.3390/fire8090360







