A Burning Intensity Gradient Modifies Sensitive Soil Properties Depending on Sampled Soil Depth and the Time Since Fire
Abstract
1. Introduction
2. Materials and Methods
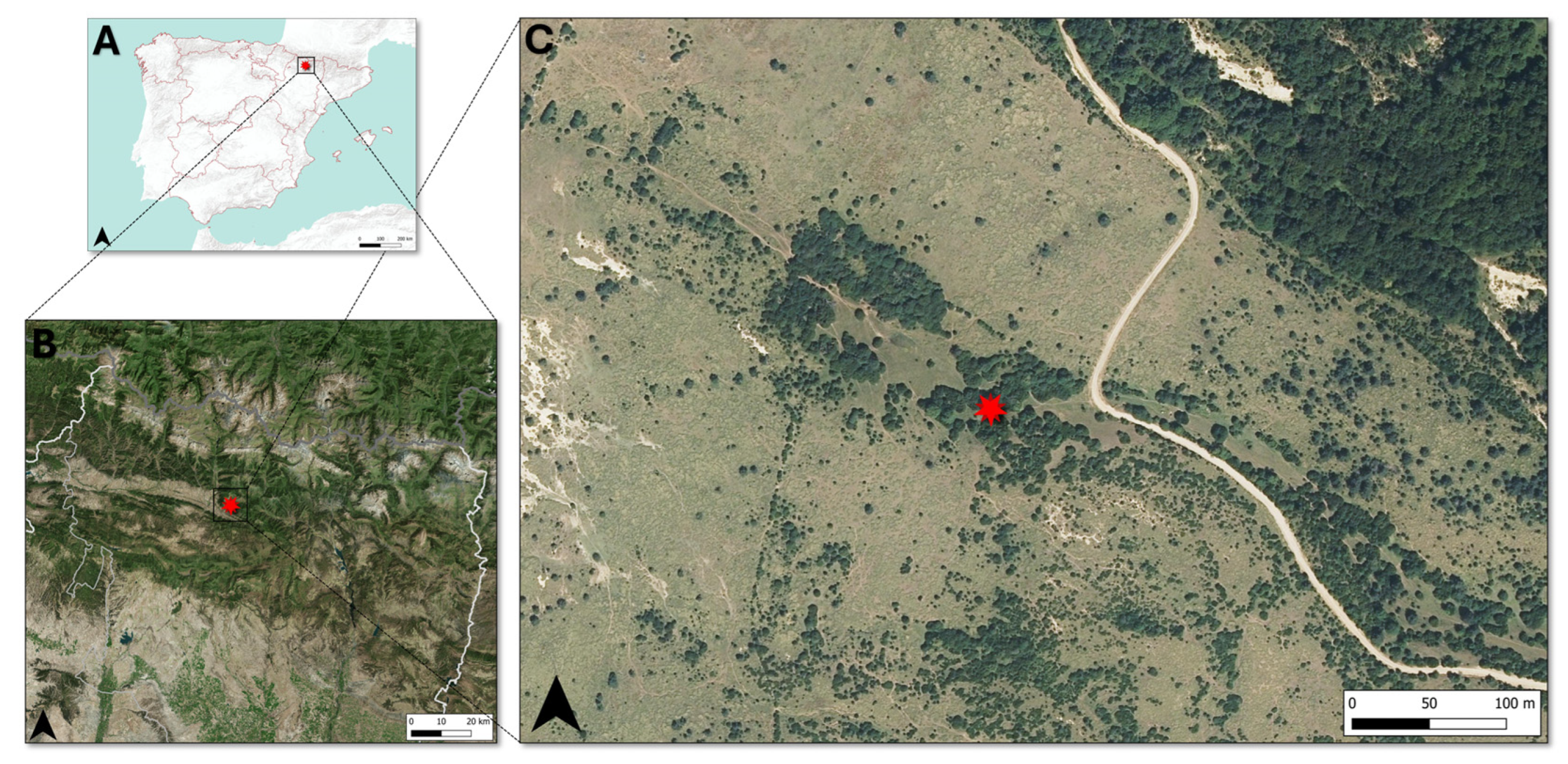
2.1. Sampling Area
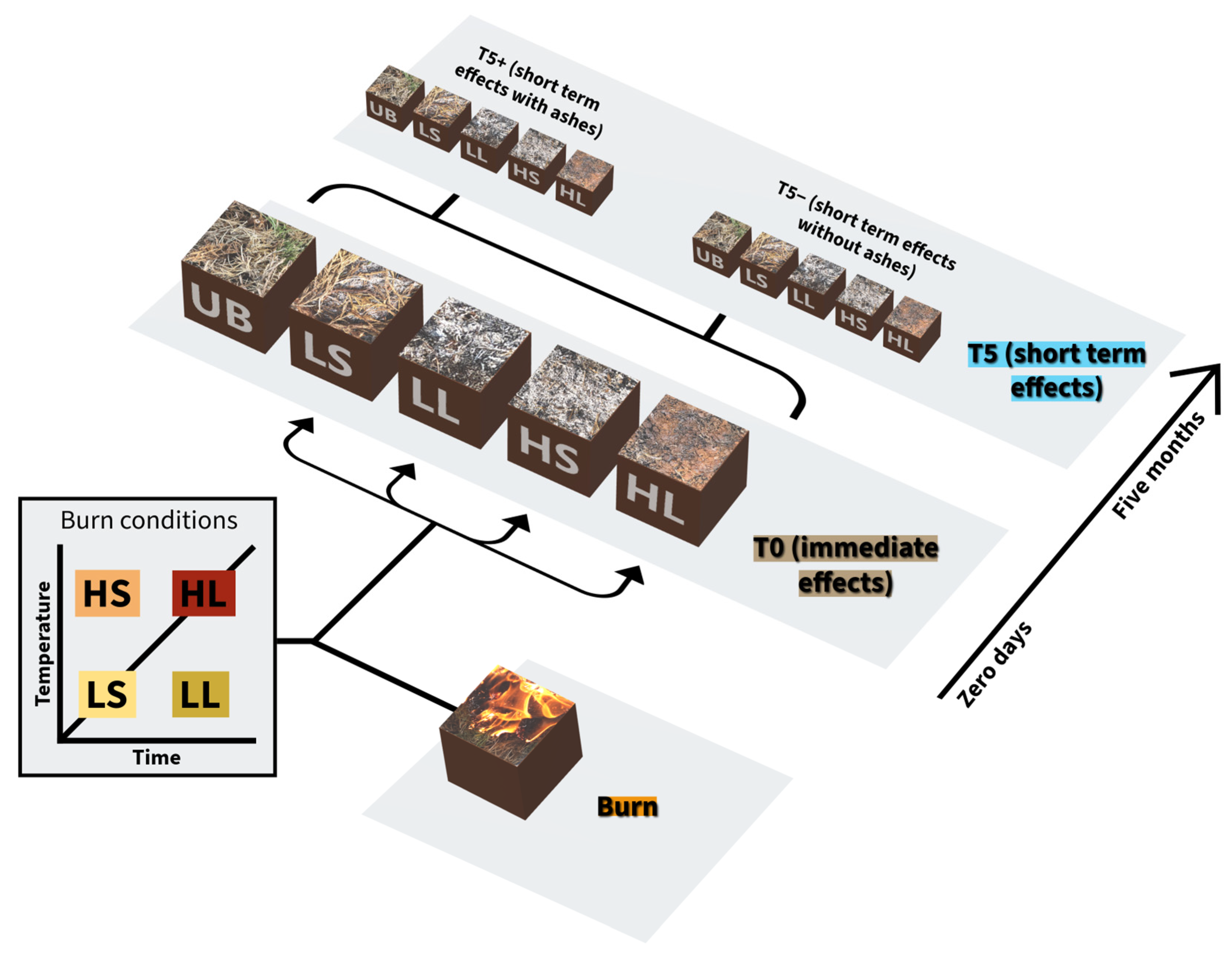
2.2. Experimental Design of Burnings
- ▪
- HL set, high temperature and long duration: high-intensity burning.
- ▪
- HS set, high temperature and short duration: moderate intensity by temperature.
- ▪
- LL set, low temperature and long duration: moderate intensity by duration.
- ▪
- LS set, low temperature and short duration: low-intensity burning.
- ▪
- UB set, unburned or control soil, representative of pre-burn conditions.
2.3. Experimental Burning Execution
| Soil | T Max | Time (min) | CI | ||||
|---|---|---|---|---|---|---|---|
| Depth | Label | (°C) | >50 °C | >100 °C | >300 °C | >500 °C | (°C/min) |
| 0–1 cm | HL | 431 ± 100 | 57.0 ± 18.1 | 33.5 ± 9.2 | 19.8 ± 12.8 | 2.3 ± 4.8 | 13,478 ± 5974 |
| HS | 503 ± 51 | 39.8 ± 18.9 | 27.9 ± 13.7 | 20.6 ± 10.9 | 0.8 ± 1.7 | 11,456 ± 5893 | |
| LL | 254 ± 168 | 24.7 ± 8.8 | 11.8 ± 13.7 | 4.9 ± 10.2 | 3.4 ± 7.3 | 4103 ± 3234 | |
| LS | 305 ± 131 | 22.1 ± 4.7 | 12.4 ± 7.9 | 3.4 ± 5.5 | 0 ± 0 | 3598 ± 2392 | |
| UB | 16 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| 1–3 cm | HL | 76 ± 7 | 57.5 ± 21.3 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 3999 ± 1537 |
| HS | 62 ± 14 | 26.9 ± 24.8 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 2262 ± 1257 | |
| LL | 47 ± 6 | 0.01 ± 0.01 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1041 ± 218 | |
| LS | 43 ± 6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 391 ± 120 | |
| UB | 15 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
2.4. Methods of Analysis of Soil Properties
2.5. Statistical Analysis
3. Results
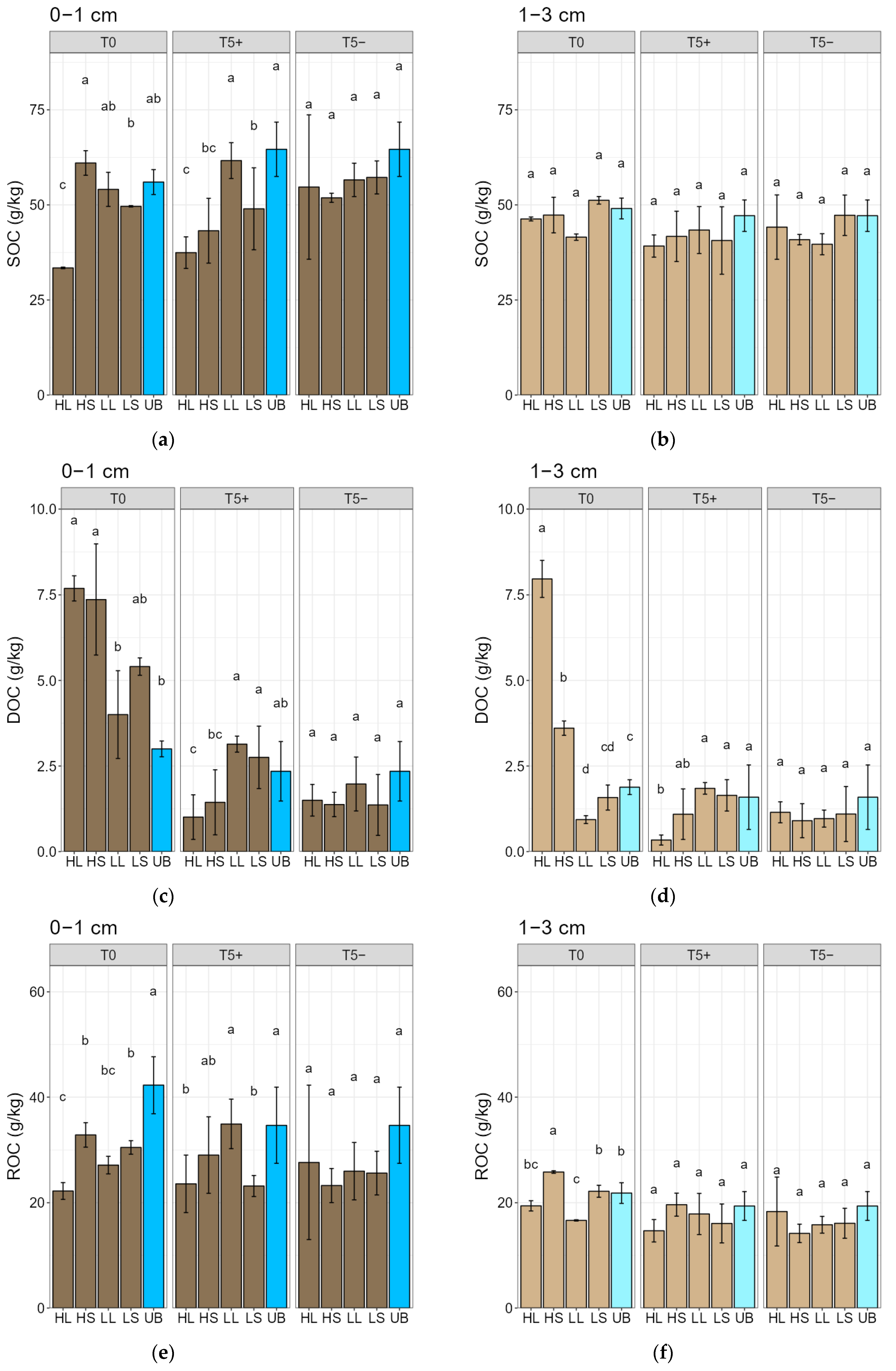
3.1. Fire-Intensity Effects on Quantity and Quality of Soil Organic Carbon Fractions
3.2. Fire-Intensity Effects on Other Chemical Soil Properties: pH and EC
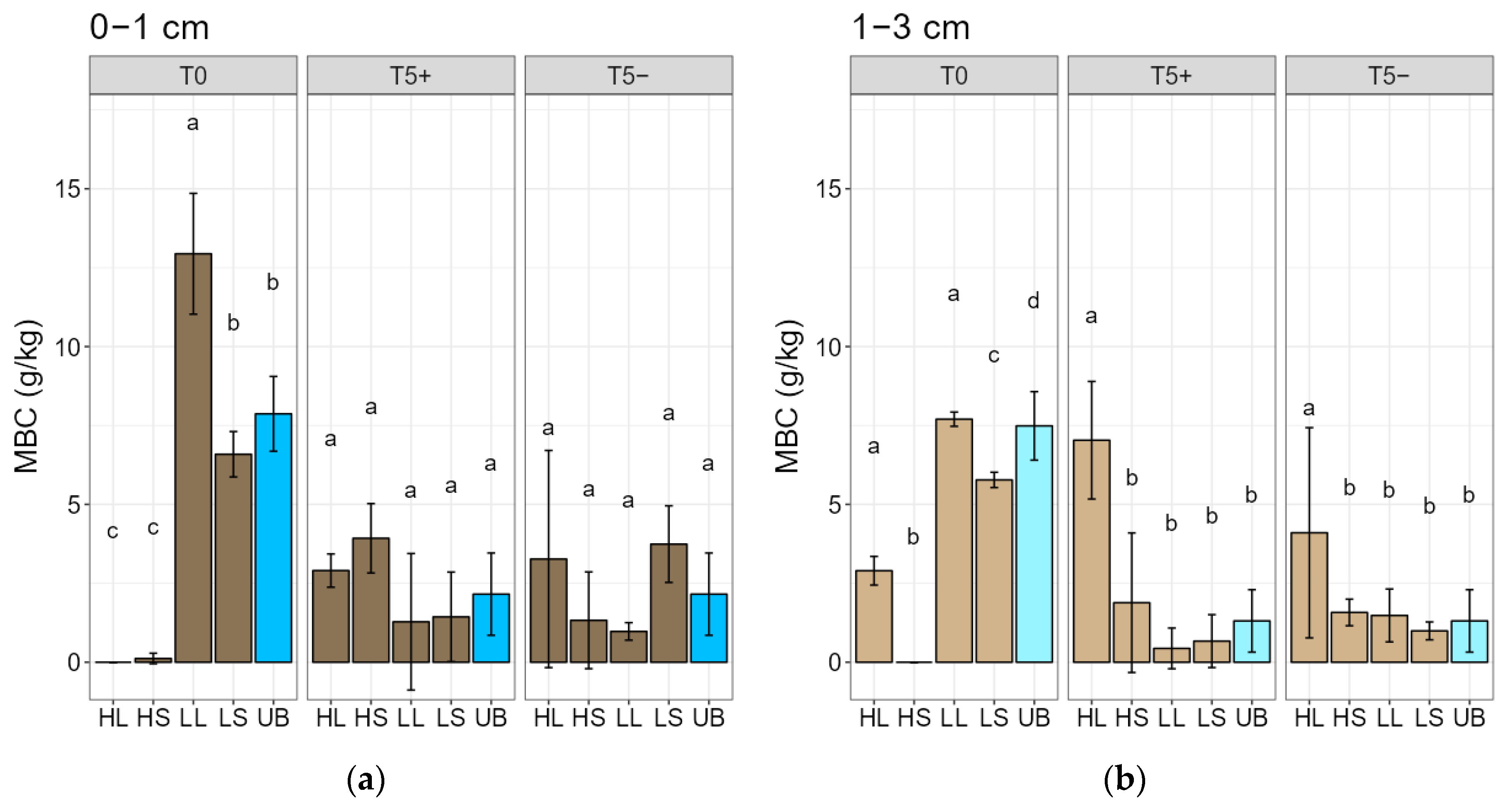
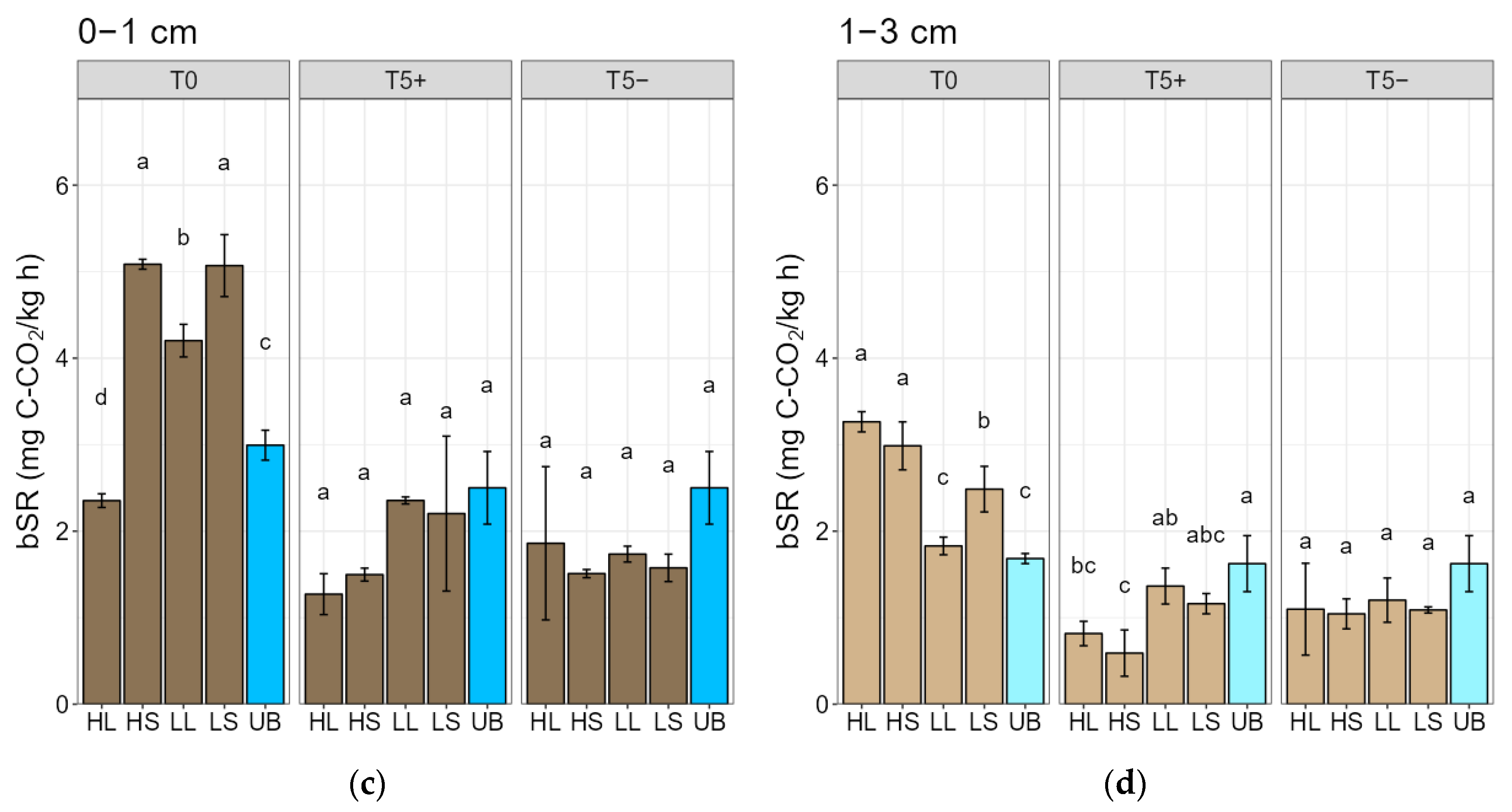
3.3. Fire-Intensity Effects on Soil Microbial Biomass Carbon and Soil Respiration
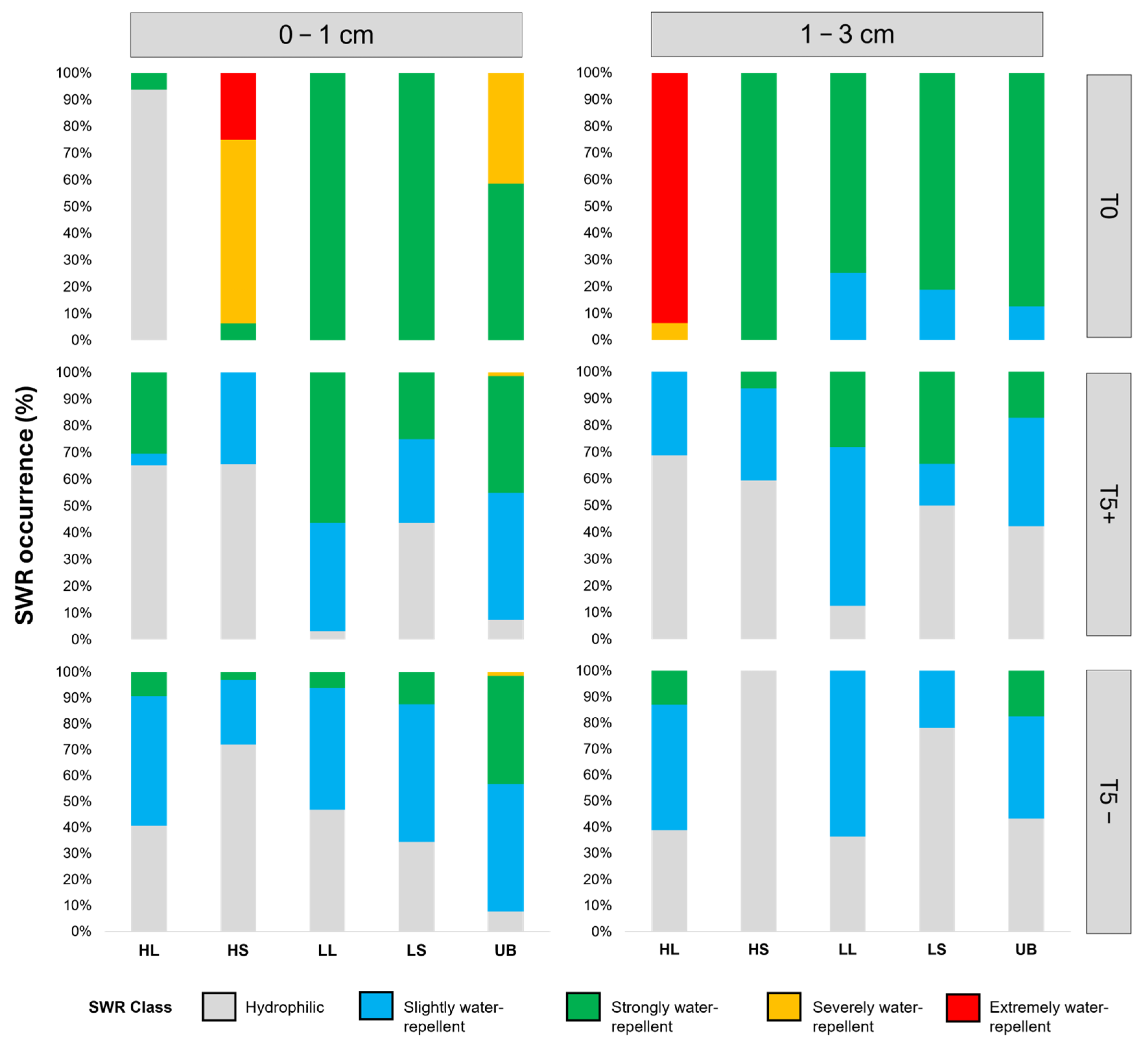
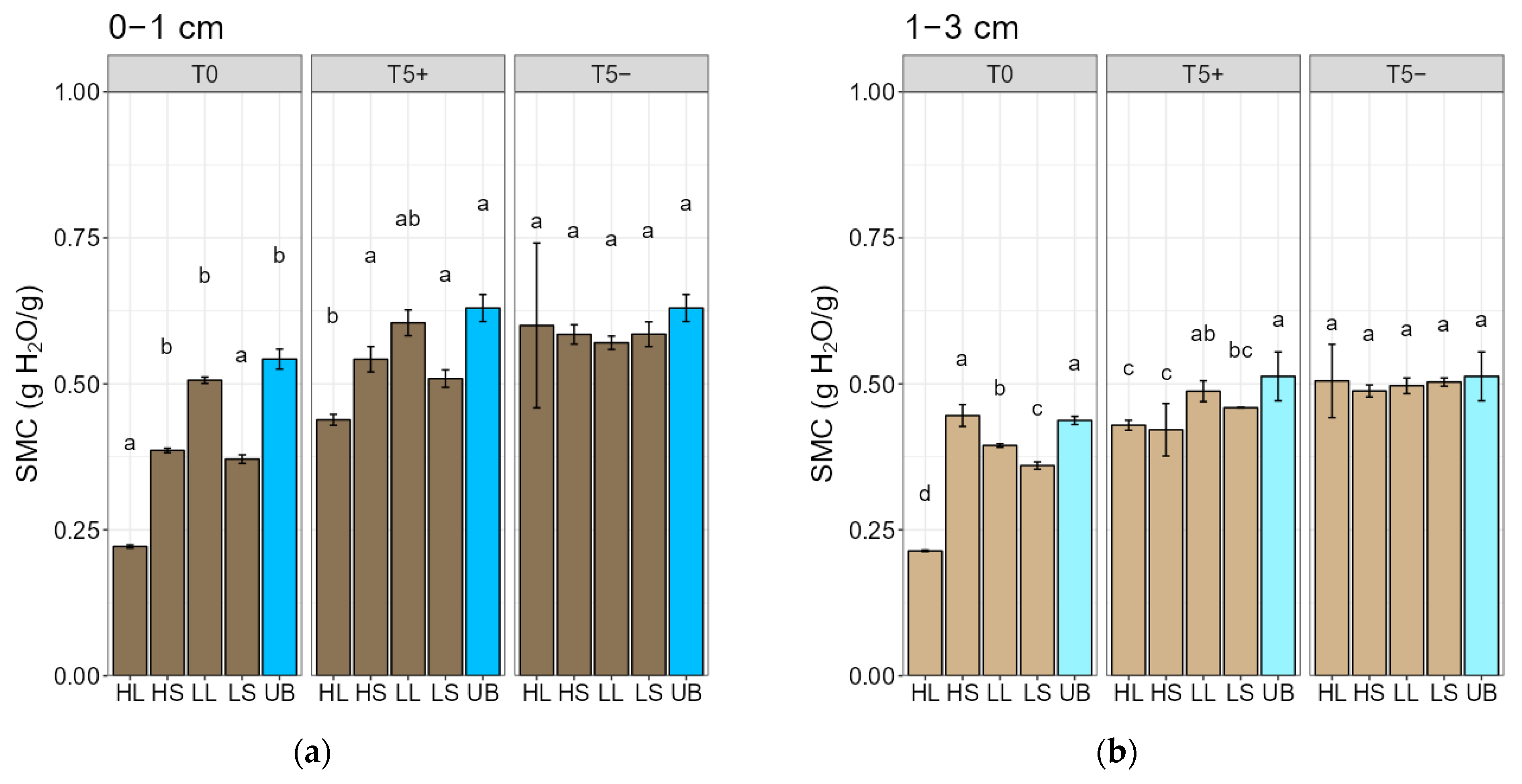
3.4. Fire-Intensity Effects on Physical Soil Properties: Water Repellency and Moisture Content
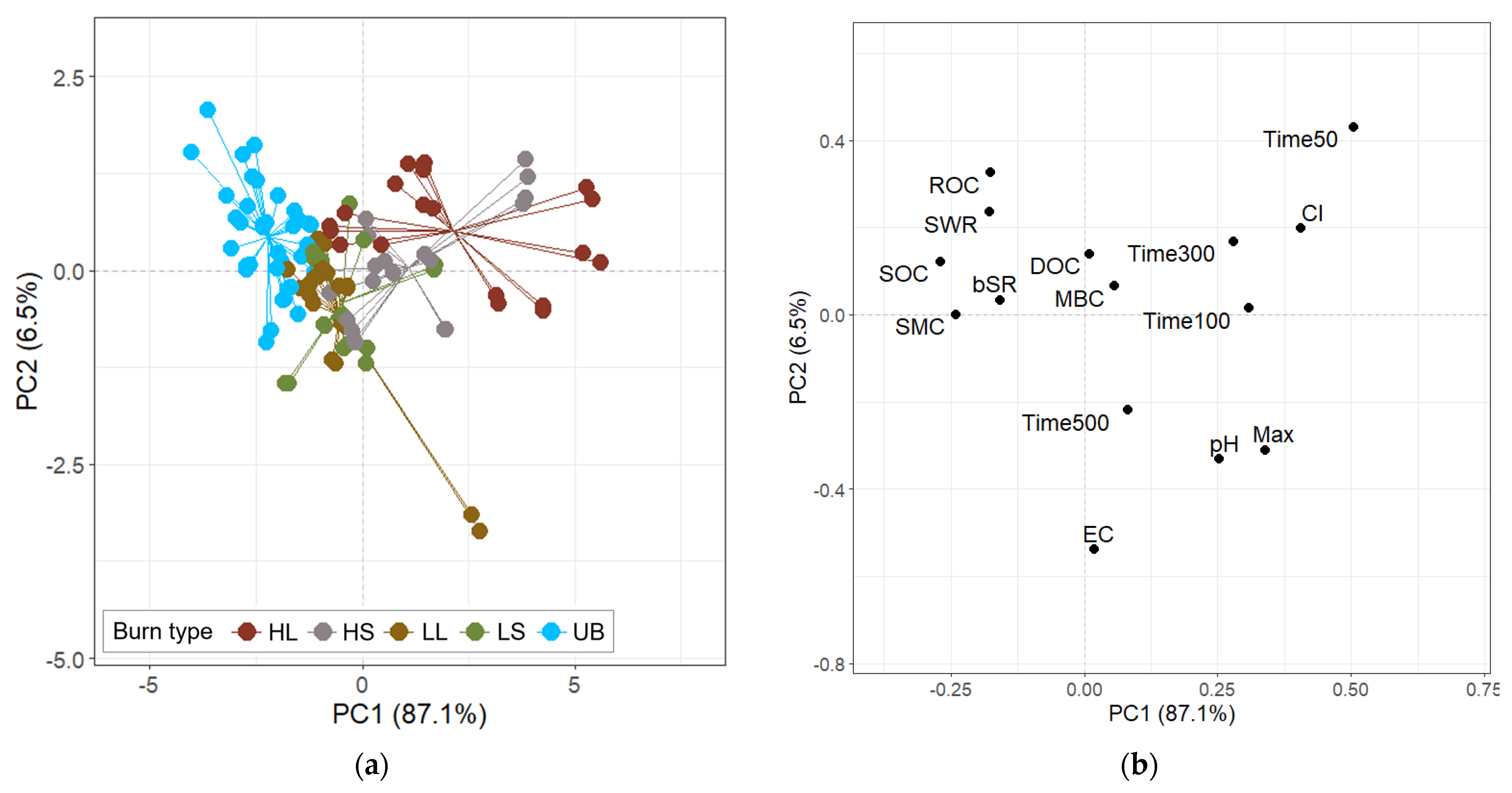
3.5. Relationship Between Soil Properties and Burning Parameters: ASCA
4. Discussion
4.1. Fire-Intensity Effects on Quantity and Quality of Soil Organic Carbon
4.2. Fire-Intensity Effects on Other Chemical Soil Properties: pH and EC
4.3. Fire-Intensity Effects on Soil Microbial Biomass Carbon and Soil Respiration
4.4. Fire-Intensity Effects on Physical Soil Properties: Water Repellency and Moisture Content
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variable | SOC | DOC | ROC | MBC | bSR | SWR | pH | EC |
|---|---|---|---|---|---|---|---|---|
| DOC | 0.16 | |||||||
| ROC | 0.72 ** | 0.30 ** | ||||||
| MBC | 0.04 | −0.06 | 0.16 | |||||
| bSR | 0.48 ** | 0.72 ** | 0.53 ** | 0.20 * | ||||
| SWR | 0.02 | 0.52 ** | −0.02 | −0.04 | 0.30 ** | |||
| pH | 0.17 | 0.02 | 0.18 | −0.01 | 0.13 | 0.04 | ||
| EC | 0.16 | 0.35 ** | 0.24 ** | −0.01 | 0.30 ** | 0.06 | −0.04 | |
| SMC | 0.63 ** | −0.36 ** | 0.48 ** | −0.07 | −0.02 | −0.40 ** | 0.12 | −0.07 |

References
- Montserrat, P.; Villar, L. Ecology and Pastoral Management in the Pyrenees: A Half-Century Perspective. Pirineos 2007, 89–107. [Google Scholar] [CrossRef]
- Clar, E.; Ayuda, M.I. Rural Migration and Agricultural Modernization. An Analysis of Provincial Spain during Its Great Rural Exodus, 1960–1981. Hist. Agrar. 2023, 23, 223–255. [Google Scholar] [CrossRef]
- André, M.F. Depopulation, Land-Use Change and Landscape Transformation in the French Massif Central. Ambio 1998, 27, 351–353. Available online: https://www.jstor.org/stable/4314746 (accessed on 23 September 2024).
- Pinilla, V.; Ayuda, M.-I.; Sáez, L.-A. Rural Depopulation and the Migration Turnaround in Mediterranean Western Europe: A Case Study of Aragon. J. Rural Community Dev. 2008, 3, 1–22. Available online: https://journals.brandonu.ca/jrcd/article/view/91 (accessed on 23 September 2024).
- Perea, P.D. Unravelling the Nutritional Transition in Spain: From Meat Shortages to Excess (1958–1990). Rural Hist. 2024, 255–276. [Google Scholar] [CrossRef]
- Serrano-Zulueta, R.; Gómez-Sal, A.; Pauné, F.; Velado-Alonso, E.; Garzón, J.; del Prado, A.; Herrera, P.M.; Majadas, J.; Pasetti, F.; Prada-Llorente, E.; et al. A Classification of Pastoralism in Spain: Understanding the Past To Address Present Challenges. Nomadic Peoples 2024, 28, 242–274. [Google Scholar] [CrossRef]
- Collantes, F. The Demise of European Mountain Pastoralism: Spain 1500-2000. Nomadic Peoples 2009, 13, 124–145. [Google Scholar] [CrossRef]
- Errea, M.P.; Cortijos-López, M.; Llena, M.; Nadal-Romero, E.; Zabalza-Martínez, J.; Lasanta, T. From the Local Landscape Organization to Land Abandonment: An Analysis of Landscape Changes (1956–2017) in the Aísa Valley (Spanish Pyrenees). Landsc. Ecol. 2023, 38, 3443–3462. [Google Scholar] [CrossRef]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-Use Legacies Rather than Climate Change Are Driving the Recent Upward Shift of the Mountain Tree Line in the Pyrenees. Glob. Ecol. Biogeogr. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Komac, B.; Kefi, S.; Nuche, P.; Escós, J.; Alados, C.L. Modeling Shrub Encroachment in Subalpine Grasslands under Different Environmental and Management Scenarios. J. Environ. Manag. 2013, 121, 160–169. [Google Scholar] [CrossRef]
- Montserrat-Martí, G.; Navarro, T.; Gómez García, D.; Maestro Martínez, M.; Santamaría Pérez, J.J.; Jiménez Jaén, J.J.; Palacio, S. Estudio de la matorralización de pastos en el Parque Nacional de Ordesa-Monte Perdido (PNOMP). In Proyectos de Investigación en Parques Nacionales: 2012–2015; Ed. Ministerio para la Transición Ecológica y el Reto Demográfico: Madrid, Spain, 2021; pp. 247–265. Available online: https://www.researchgate.net/publication/348277616 (accessed on 23 September 2024).
- Komac, B.; Alados, C.; Camarero, J. Influence of Topography on the Colonization of Subalpine Grasslands by the Thorny Cushion Dwarf Echinospartum horridum. Arctic Antarct. Alp. Res. 2011, 43, 601–611. [Google Scholar] [CrossRef]
- Martín-Ramos, P.; Martín-Gil, J.; Gómez-García, D.; Cuchí-Oterino, J.A. On the Physicochemical Characteristics and Applications of an “Undesirable” Pyrenean Thorny Cushion Dwarf: Echinospartum horridum (Vahl) Roth. Plants 2020, 9, 1180. [Google Scholar] [CrossRef]
- Gelabert, P.J.; Rodrigues, M.; de la Riva, J.; Ameztegui, A.; Sebastià, M.T.; Vega-Garcia, C. LandTrendr Smoothed Spectral Profiles Enhance Woody Encroachment Monitoring. Remote Sens. Environ. 2021, 262, 112521. [Google Scholar] [CrossRef]
- Palmer, G.C. Principles and Methods in Landscape Ecology: Towards a Science of Landscape; Springer: Dordrecht, The Netherlands, 2008; Volume 33, ISBN 9781402033285. [Google Scholar]
- Lasanta, T.; Nadal-Romero, E.; Arnáez, J. Managing Abandoned Farmland to Control the Impact of Re-Vegetation on the Environment. The State of the Art in Europe. Environ. Sci. Policy 2015, 52, 99–109. [Google Scholar] [CrossRef]
- Montiel, C.; Kraus, D. Best Practices of Fire Use: Prescribed Burning and Suppression Fire Programmes in Selected Case-Study Regions in Europe; Report 24; European Forest Institute: Joensuu, Finland, 2010; ISBN 978-952-5453-70-6. [Google Scholar]
- Canals, R.M. Landscape in Motion: Revisiting the Role of Key Disturbances in the Preservation of Mountain Ecosystems. Geogr. Res. Lett. 2019, 45, 515–531. [Google Scholar] [CrossRef]
- Bal, M.C.; Pelachs, A.; Perez-Obiol, R.; Julia, R.; Cunill, R. Fire History and Human Activities during the Last 3300cal Yr BP in Spain’s Central Pyrenees: The Case of the Estany de Burg. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 300, 179–190. [Google Scholar] [CrossRef]
- Mora, J.L.; Badía–Villas, D.; Gómez, D. Fire Does Not Transform Shrublands of Echinospartum horridum (Vahl) Rothm. into Grasslands in the Pyrenees: Development of Community Structure and Nutritive Value after Single Prescribed Burns. J. Environ. Manag. 2022, 315, 115125. [Google Scholar] [CrossRef] [PubMed]
- Girona-García, A.; Ortiz-Perpiñá, O.; Badía-Villas, D. Dynamics of Topsoil Carbon Stocks after Prescribed Burning for Pasture Restoration in Shrublands of the Central Pyrenees (NE-Spain). J. Environ. Manag. 2019, 233, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Girona-García, A.; Badía-Villas, D.; Martí-Dalmau, C.; Ortiz-Perpiñá, O.; Mora, J.L.; Armas-Herrera, C.M. Effects of Prescribed Fire for Pasture Management on Soil Organic Matter and Biological Properties: A 1-Year Study Case in the Central Pyrenees. Sci. Total Environ. 2017, 618, 1079–1087. [Google Scholar] [CrossRef]
- Girona-García, A.; Zufiaurre-Galarza, R.; Mora, J.L.; Armas-Herrera, C.; Martí, C.; Ortiz-Perpiñá, O.; Badía-Villas, D. Effects of Prescribed Burning for Pasture Reclamation on Soil Chemical Properties in Subalpine Shrublands of the Central Pyrenees (NE-Spain). Sci. Total Environ. 2018, 644, 583–593. [Google Scholar] [CrossRef]
- Alfaro-Leranoz, A.; Badia-Villas, D.; Martí-Dalmau, C.; Emran, M.; Conte-Domínguez, A.P.; Ortiz-Perpiñá, O. Long-Term Evolution of Shrub Prescribed Burning Effects on Topsoil Organic Matter and Biological Activity in the Central Pyrenees (NE-Spain). Sci. Total Environ. 2023, 888, 163994. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire Effects on Belowground Sustainability: A Review and Synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Certini, G. Effects of Fire on Properties of Forest Soils: A Review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water. In General Technical Report RMRS-GTR-42; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2005; Volume 4, pp. 37–41. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H. Fire Effects on Soils: The Human Dimension. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150171. [Google Scholar] [CrossRef]
- Zavala, L.M.; De Celis, R.; Jordán, A. How Wildfires Affect Soil Properties. A Brief Review. Cuad. Investig. Geográfica 2014, 40, 311–332. [Google Scholar] [CrossRef]
- Badía, D.; Armas, C.; Mora, J.L.; Gómez, D.; Montserrat, G.; Palacios, S. ¿Podemos controlar la expansión del erizón mediante quemas? Lucas Mallada 2017, 19, 69–94. Available online: https://zaguan.unizar.es/record/87645 (accessed on 23 September 2024).
- Girona-García, A.; Ortiz-Perpiñá, O.; Badía-Villas, D.; Martí-Dalmau, C. Effects of Prescribed Burning on Soil Organic C, Aggregate Stability and Water Repellency in a Subalpine Shrubland: Variations among Sieve Fractions and Depths. Catena 2018, 166, 68–77. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A.; Arcenegui, V.; Jordán, A.; Zavala, L.M. Fire Effects on Soil Aggregation: A Review. Earth-Sci. Rev. 2011, 109, 44–60. [Google Scholar] [CrossRef]
- Zheng, W.; Morris, E.K.; Lehmann, A.; Rillig, M.C. Interplay of Soil Water Repellency, Soil Aggregation and Organic Carbon. A Meta-Analysis. Geoderma 2016, 283, 39–47. [Google Scholar] [CrossRef]
- Armas-Herrera, C.M.; Martí-Dalmau, C.; Badía-Villas, D.; Ortiz-Perpiñá, O.; Girona-García, A.; Mora, J.L. Short-Term and Midterm Evolution of Topsoil Organic Matter and Biological Properties after Prescribed Burning for Pasture Recovery (Tella, Central Pyrenees, Spain). Land Degrad. Dev. 2018, 29, 1545–1554. [Google Scholar] [CrossRef]
- Badía-Villas, D.; González-Pérez, J.A.; Aznar, J.M.; Arjona-Gracia, B.; Martí-Dalmau, C. Changes in Water Repellency, Aggregation and Organic Matter of a Mollic Horizon Burned in Laboratory: Soil Depth Affected by Fire. Geoderma 2014, 213, 400–407. [Google Scholar] [CrossRef]
- Knicker, H. How Does Fire Affect the Nature and Stability of Soil Organic Nitrogen and Carbon? A Review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-Term Dynamics of Soil Chemical Properties after a Prescribed Fire in a Mediterranean Forest (Montgrí Massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef]
- Saenger, A.; Cécillon, L.; Poulenard, J.; Bureau, F.; De Daniéli, S.; Gonzalez, J.M.; Brun, J.J. Surveying the Carbon Pools of Mountain Soils: A Comparison of Physical Fractionation and Rock-Eval Pyrolysis. Geoderma 2015, 241–242, 279–288. [Google Scholar] [CrossRef]
- Robichaud, P.R.; Hungerford, R.D. Water Repellency by Laboratory Burning of Four Northern Rocky Mountain Forest Soils. J. Hydrol. 2000, 231–232, 207–219. [Google Scholar] [CrossRef]
- DeBano, L. Water Repellency in Soils: A Historical Overview. J. Hydrol. 2000, 231, 4–32. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Macdonald, L.H. Soil Water Repellency: A Key Factor in Post-Fire Erosion? In Fire Effects on Soils and Restoration Strategies; Cerdà, A., Robichaud, P., Eds.; CRC Press: Boca Raton, FL, USA, 2009; p. 28. ISBN 9780429063596. [Google Scholar]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial Diversity and Soil Functions. Eur. J. Soil Sci. 2003, 68, 12–26. [Google Scholar] [CrossRef]
- Pérez-Valera, E.; Verdú, M.; Navarro-Cano, J.A.; Goberna, M. Soil Microbiome Drives the Recovery of Ecosystem Functions after Fire. Soil Biol. Biochem. 2020, 149, 107948. [Google Scholar] [CrossRef]
- Saccá, M.L.; Caracciolo, A.B.; Di Lenola, M.; Grenni, P. Ecosystem Services Provided By Soil Microorganisms. In Soil Biological Communities and Ecosystem Resilience. Sustainability in Plant and Crop Protection; Lukac, M., Grenni, P., Gamboni, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 9–24. ISBN 978-3-319-63336-7. [Google Scholar]
- Barreiro, A.; Díaz-Raviña, M. Fire Impacts on Soil Microorganisms: Mass, Activity, and Diversity. Curr. Opin. Environ. Sci. Health 2021, 22, 100264. [Google Scholar] [CrossRef]
- Barreiro, A.; Martín, A.; Carballas, T.; Díaz-Raviña, M. Response of Soil Microbial Communities to Fire and Fire-Fighting Chemicals. Sci. Total Environ. 2010, 408, 6172–6178. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Cerdà, A. The Effects of Wildfires on Soils: Synthesis and Conclusions. New Challenges in Research and Management. In Effects of Wildfires on Soils in Spain. The State of the Art as Seen by Spanish Scientists; Càtedra de Divulgació de la Ciència, Universitat de Valencia: Valencia, Spain, 2009; pp. 493–529. ISBN 978-84-370-7653-9. [Google Scholar]
- Choromanska, U.; Deluca, T.H. Microbial Activity and Nitrogen Mineralization in Forest Mineral Soils Following Heating: Evaluation of Post-Fire Effects. Sci. Total Environ. 2002, 34, 263–271. [Google Scholar] [CrossRef]
- Alfaro-Leranoz, A.; Badía-Villas, D.; Martí-Dalmau, C.; Escuer-Arregui, M.; Quintana-Esteras, S. The Effects of Fire Intensity on the Biochemical Properties of a Soil Under Scrub in the Pyrenean Subalpine Stage. Fire 2024, 7, 452. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Massman, W.J. Modeling Soil Heating and Moisture Transport under Extreme Conditions: Forest Fires and Slash Pile Burns. Water Resour. Res. 2012, 48, W10548. [Google Scholar] [CrossRef]
- Pereira, J.S.; Badía, D.; Martí, C.; Mora, J.L.; Donzeli, V.P. Fire Effects on Biochemical Properties of a Semiarid Pine Forest Topsoil at Cm-Scale. Pedobiologia 2023, 96, 150860. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J.; Aznar, J.M.; González-Pérez, J.A.; De la Rosa, J.M.; León, J.; Ibarra, P.; Echeverría, T. Wildfire Effects on Nutrients and Organic Carbon of a Rendzic Phaeozem in NE Spain: Changes at Cm-Scale Topsoil. Catena 2014, 113, 267–275. [Google Scholar] [CrossRef]
- Armas-Herrera, C.M.; Martí, C.; Badía, D.; Ortiz-Perpiñá, O.; Girona-García, A.; Porta, J. Immediate Effects of Prescribed Burning in the Central Pyrenees on the Amount and Stability of Topsoil Organic Matter. Catena 2016, 147, 238–244. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Campbell, C.A.; Paul, E.A.; Rennie, D.A.; Mccallum, K.J. Applicability of the Carbon-Dating Method of Analysis to Soil Humus Studies. Soil Sci. 1967, 104, 217–224. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile, Recalcitrant, and Inert Organic Matter in Mediterranean Forest Soils. Soil Biol. Biochem. 2007, 39, 202–215. [Google Scholar] [CrossRef]
- Anderson, J.P.E. Soil Respiration. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar]
- Wessel, A.T. On Using the Effective Contact Angle and the Water Drop Penetration Time for Classification o f Water Repellency in Dune Soils. Earth Surf. Process. Landf. 1988, 13, 555–561. [Google Scholar] [CrossRef]
- Doerr, S.H. On Standardizing the ‘Water Drop Penetration Time’ and the ‘Molarity of an Ethanol Droplet’ Techniques to Classify Soil Hydrophobicity: A Case Study Using Medium Textured Soils. Earth Surf. Process. Landf. 1998, 23, 663–668. [Google Scholar] [CrossRef]
- Mclean, E.O. Soil PH and Lime Requirement. In Methods of Soil Analysis. Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Thiel, M.; Benaiche, N.; Van, R.; Govaerts, B. Limpca: An R Package for the Linear Modeling of High-dimensional Designed Data Based on ASCA/APCA Family of Methods. J. Chemom. 2023, 37, e3482. [Google Scholar] [CrossRef]
- Alexis, M.A.; Rasse, D.P.; Rumpel, C.; Bardoux, G.; Péchot, N.; Schmalzer, P.; Drake, B.; Mariotti, A. Fire Impact on C and N Losses and Charcoal Production in a Scrub Oak Ecosystem. Biogeochemistry 2007, 82, 201–216. [Google Scholar] [CrossRef]
- Bodí, M.B.; Martin, D.A.; Balfour, V.N.; Santín, C.; Doerr, S.H.; Pereira, P.; Cerdà, A.; Mataix-Solera, J. Wildland Fire Ash: Production, Composition and Eco-Hydro-Geomorphic Effects. Earth-Sci. Rev. 2014, 130, 103–127. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Preston, C.; González-Rodríguez, G. Pyrogenic Organic Matter Production from Wildfires: A Missing Sink in the Global Carbon Cycle. Glob. Change Biol. 2015, 21, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Armas-Herrera, C.M.; Pérez-Lambán, F.; Badía-Villas, D.; Peña-Monné, J.L.; González-Pérez, J.A.; Picazo-Millán, J.V.; Jiménez-Morillo, N.T.; Sampietro-Vattuone, M.M.; Gracia, M.A. Pyrogenic Organic Matter from Palaeo-Fires during the Holocene: A Case Study in a Sequence of Buried Soils at the Central Ebro Basin (NE Spain). J. Environ. Manag. 2019, 241, 558–566. [Google Scholar] [CrossRef]
- Masiello, C.A. New Directions in Black Carbon Organic Geochemistry. Mar. Chem. 2004, 92, 201–213. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The Effect of Fire on Soil Organic Matter - A Review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Knicker, H.; Almendros, G.; González-Vila, F.J.; González-Pérez, J.A.; Polvillo, O. Characteristic Alterations of Quantity and Quality of Soil Organic Matter Caused by Forest Fires in Continental Mediterranean Ecosystems: A Solid-State 13C NMR Study. Eur. J. Soil Sci. 2006, 57, 558–569. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Kane, E.S.; Masiello, C.A.; Ohlson, M.; de la Rosa, J.M.; Preston, C.M.; Dittmar, T. Towards a Global Assessment of Pyrogenic Carbon from Vegetation Fires. Glob. Change Biol. 2016, 22, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, U.; DeLuca, T.H. Prescribed Fire Alters the Impact of Wildfire on Soil Biochemical Properties in a Ponderosa Pine Forest. Soil Sci. Soc. Am. J. 2001, 65, 232–238. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical Composition and Bioavailability of Thermally Altered Pinus Resinosa (Red Pine) Wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Rovira, P.; Romanyà, J.; Duguy, B. Long-Term Effects of Wildfires on the Biochemical Quality of Soil Organic Matter: A Study on Mediterranean Shrublands. Geoderma 2012, 179–180, 9–19. [Google Scholar] [CrossRef]
- Rovira, P.; Duguy, B.; Vallejo, V.R. Black Carbon in Wildfire-Affected Shrubland Mediterranean Soils. J. Plant Nutr. Soil Sci. 2009, 172, 43–52. [Google Scholar] [CrossRef]
- Gartzia, M.; Alados, C.; Pérez-Cabello, F. Assessment of the Effects of Biophysical and Anthropogenic Factors on Woody Plant Encroachment in Dense and Sparse Mountain Grasslands Based on Remote Sensing Data. Prog. Phys. Geogr. 2014, 38, 201–217. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Otal-Laín, I.; Lasanta, T.; Sánchez-Navarrete, P.; Errea, P.; Cammeraat, E. Science of the Total Environment Woody Encroachment and Soil Carbon Stocks in Subalpine Areas in the Central Spanish Pyrenees. Sci. Total Environ. 2018, 636, 727–736. [Google Scholar] [CrossRef]
- Knicker, H.; Müller, P.; Hilscher, A. How Useful Is Chemical Oxidation with Dichromate for the Determination of “Black Carbon” in Fire-Affected Soils? Geoderma 2007, 142, 178–196. [Google Scholar] [CrossRef]
- Certini, G.; Nocentini, C.; Knicker, H.; Arfaioli, P.; Rumpel, C. Wildfire Effects on Soil Organic Matter Quantity and Quality in Two Fire-Prone Mediterranean Pine Forests. Geoderma 2011, 167–168, 148–155. [Google Scholar] [CrossRef]
- Velasco-Molina, M.; Berns, A.E.; Macías, F.; Knicker, H. Biochemically Altered Charcoal Residues as an Important Source of Soil Organic Matter in Subsoils of Fire-Affected Subtropical Regions. Geoderma 2016, 262, 62–70. [Google Scholar] [CrossRef]
- Bird, M.I.; Wynn, J.G.; Saiz, G.; Wurster, C.M.; McBeath, A. The Pyrogenic Carbon Cycle. Annu. Rev. Earth Planet. Sci. 2015, 43, 273–298. [Google Scholar] [CrossRef]
- Hernández, T.; García, C.; Reinhardt, I. Short-Term Effect of Wildfire on the Chemical, Biochemical and Microbiological Properties of Mediterranean Pine Forest Soils. Biol Fertil Soils 1997, 25, 109–116. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Plant Ash and Heat Intensity Effects on Chemical and Physical Properties of Two Contrasting Soils. Arid Land Res. Manag. 2003, 17, 23–41. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Fritze, H. Clear-Cutting and Prescribed Burning in Coniferous Forest: Comparison of Effects on Soil Fungal and Total Microbial Biomass, Respiration Activity and Nitrification. Soil Biol. Biochem. 1995, 27, 101–109. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C. Effect of Simulated Fire on Organic Matter and Selected Microbiological Properties of Two Contrasting Soils. Arid Land Res. Manag. 2003, 17, 55–69. [Google Scholar] [CrossRef]
- Fontúrbel, M.T.; Barreiro, A.; Vega, J.A.; Martín, A.; Jiménez, E.; Carballas, T.; Fernández, C.; Díaz-Raviña, M. Effects of an Experimental Fire and Post-Fire Stabilization Treatments on Soil Microbial Communities. Geoderma 2012, 191, 51–60. [Google Scholar] [CrossRef]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Bååth, E. Soil Microbial Recolonisation after a Fire in a Mediterranean Forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- D’Ascoli, R.; Rutigliano, F.A.; De Pascale, R.A.; Gentile, A.; De Santo, A.V. Functional Diversity of the Microbial Community in Mediterranean Maquis Soils as Affected by Fires. Int. J. Wildl. Fire 2005, 14, 355–363. [Google Scholar] [CrossRef]
- De Marco, A.; Gentile, A.E.; Arena, C.; De Santo, A.V. Organic Matter, Nutrient Content and Biological Activity in Burned and Unburned Soils of a Mediterranean Maquis Area of Southern Italy. Int. J. Wildl. Fire 2005, 14, 365–377. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Walsh, R.P.D. Soil Water Repellency: Its Causes, Characteristics and Hydro-Geomorphological Significance. Earth Sci. Rev. 2000, 51, 33–65. [Google Scholar] [CrossRef]
- Jordán, A.; Zavala, L.M.; Mataix-Solera, J.; Doerr, S.H. Soil Water Repellency: Origin, Assessment and Geomorphological Consequences. Catena 2013, 108, 1–5. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Mataix-Solera, J.; Martin, D.; Oliva, M.; Novara, A. Short-Term Spatio-Temporal Spring Grassland Fire Effects on Soil Colour, Organic Matter and Water Repellency in Lithuania Grassland Fire Effects on Soil Properties. Solid Earth Discuss. 2013, 5, 2119–2154. [Google Scholar] [CrossRef]
- DeBano, L.F.; Mann, L.D.; Hamilton, D.A. Translocation of Hydrophobic Substances into Soil by Burning Organic Litter. Soil Sci. Soc. Am. J. 1970, 34, 130–133. [Google Scholar] [CrossRef]
- DeBano, L.F. The Role of Fire and Soil Heating on Water Repellency in Wildland Environments: A Review. J. Hydrol. 2000, 231, 195–206. [Google Scholar] [CrossRef]
- DeBano, L.F.; Savage, S.M.; Hamilton, D.A. The Transfer of Heat and Hydrophobic Substances during Burning. Soil Sci. Soc. Am. 1976, 40, 779–782. [Google Scholar] [CrossRef]
- Krammes, J.S.; DeBano, L.F. Soil Wettability: A Neglected Factor in Watershed Management. Water Resour. Res. 1965, 1, 283–286. [Google Scholar] [CrossRef]
- Novák, V.; Hlaváčiková, H. Water Repellent Soils. Theory Appl. Transp. Porous Media 2019, 32, 283–291. [Google Scholar] [CrossRef]
- Wallis, M.G.; Horne, D.J. Soil Water Repellency. In Advances in Soil Science; Stewart, B.A., Ed.; Springer: New York, NY, USA, 1992; Volume 20, pp. 91–146. ISBN 978-1-4612-2930-8. [Google Scholar]
- Hubbert, K.R.; Oriol, V. Temporal Fluctuations in Soil Water Repellency Following Wildfire in Chaparral Steeplands, Southern California. Int. J. Wildl. Fire 2005, 14, 439–447. [Google Scholar] [CrossRef]
- Jordán, A.; Gordillo-Rivero, Á.J.; García-Moreno, J.; Zavala, L.M.; Granged, A.J.P.; Gil, J.; Neto-Paixão, H.M. Post-Fire Evolution of Water Repellency and Aggregate Stability in Mediterranean Calcareous Soils: A 6-Year Study. Catena 2014, 118, 115–123. [Google Scholar] [CrossRef]
- Jordán, A.; González, F.A.; Zavala, L.M. Re-Establishment of Soil Water Repellency after Destruction by Intense Burning in a Mediterranean Heathland (SW Spain). Hydrol. Process. 2010, 24, 736–748. [Google Scholar] [CrossRef]
- Macdonald, L.H.; Huffman, E.L. Post-Fire Soil Water Repellency: Persistence and Soil Moisture Thresholds. Soil Sci. Soc. Am. 2004, 68, 1729–1734. [Google Scholar] [CrossRef]
- Hallett, P.D. A Brief Overview of the Causes, Impacts and Amelioration of Soil Water Repellency - A Review. Soil Water Res. 2008, 3, S21–S29. [Google Scholar] [CrossRef]
- Lowe, M.A.; Mathes, F.; Loke, M.H.; McGrath, G.; Murphy, D.V.; Leopold, M. Bacillus Subtilis and Surfactant Amendments for the Breakdown of Soil Water Repellency in a Sandy Soil. Geoderma 2019, 344, 108–118. [Google Scholar] [CrossRef]
- Campbell, G.; Norman, J.M. An Introduction to Environmental Biophysics, 2nd ed.; Springer: New York, NY, USA, 1998; ISBN 0-387-94937-2. [Google Scholar]
- Badía, D.; López-García, S.; Martí, C.; Ortíz-Perpiñá, O.; Girona-García, A.; Casanova-Gascón, J. Burn Effects on Soil Properties Associated to Heat Transfer under Contrasting Moisture Content. Sci. Total Environ. 2017, 601, 1119–1128. [Google Scholar] [CrossRef]


| Coordinates | 42° 30′ 55.0″ N 0° 15′ 47.9″ W |
| Altitude (masl) | 1480 |
| Mean Annual Temperature (°C) | 8.3 |
| Mean Annual Precipitation (mm) | 1015 |
| Aspect | South |
| Mean slope (%) | 5 |
| Echinospartum horridum cover (%) | 75 |
| Aerial biomass (kg/m2) | 9.24 |
| Litter: Oi + Oe (kg/m2) | 1.62 |
| Treatment | Label | Temperature Criteria at 1 cm (°C) | Residence Time of the Temperature Criteria at 1 cm (min) |
|---|---|---|---|
| High temperature, long time | HL | 80 | 24 |
| High temperature, short time | HS | 80 | 12 |
| Low temperature, long time | LL | 50 | 24 |
| Low temperature, short time | LS | 50 | 12 |
| Unburned | UB | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escuer-Arregui, M.; Alfaro-Leranoz, A.; Badía-Villas, D.; Conte-Domínguez, A.P.; Martí-Dalmau, C.; Ortiz-Perpiñá, O. A Burning Intensity Gradient Modifies Sensitive Soil Properties Depending on Sampled Soil Depth and the Time Since Fire. Fire 2025, 8, 351. https://doi.org/10.3390/fire8090351
Escuer-Arregui M, Alfaro-Leranoz A, Badía-Villas D, Conte-Domínguez AP, Martí-Dalmau C, Ortiz-Perpiñá O. A Burning Intensity Gradient Modifies Sensitive Soil Properties Depending on Sampled Soil Depth and the Time Since Fire. Fire. 2025; 8(9):351. https://doi.org/10.3390/fire8090351
Chicago/Turabian StyleEscuer-Arregui, Marta, Andoni Alfaro-Leranoz, David Badía-Villas, Ana P. Conte-Domínguez, Clara Martí-Dalmau, and Oriol Ortiz-Perpiñá. 2025. "A Burning Intensity Gradient Modifies Sensitive Soil Properties Depending on Sampled Soil Depth and the Time Since Fire" Fire 8, no. 9: 351. https://doi.org/10.3390/fire8090351
APA StyleEscuer-Arregui, M., Alfaro-Leranoz, A., Badía-Villas, D., Conte-Domínguez, A. P., Martí-Dalmau, C., & Ortiz-Perpiñá, O. (2025). A Burning Intensity Gradient Modifies Sensitive Soil Properties Depending on Sampled Soil Depth and the Time Since Fire. Fire, 8(9), 351. https://doi.org/10.3390/fire8090351






