1. Introduction
Both dust explosion prevention and mitigation of the consequences require measurement of dust explosion parameters. Testing methods defined by standards [
1,
2,
3] require dust cloud testing in explosion chambers of a standard size of 1 m
3 and, alternatively, of 20 L. However, the results are influenced by several processes neglected by the standards, probably due to their small effect in a 1 m
3 chamber. Their effect becomes significant in a smaller 20 L chamber.
Problems with initial conditions and conditions at the time of ignition activation were discussed by Spitzer et al. [
4], who found that dispersion of dust causes compression of gas inside the chamber and a temperature increase to 45 °C at the time of ignition activation, which decreases the measured maximum explosion pressure. A much greater problem is the influence of the pyrotechnical ignitor’s action, known as overdriven ignition. Several authors have evaluated the influence of ignition energy on explosion parameters [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13], minimum explosive concentration [
14,
15,
16], dust inerting concentration [
17,
18] and limiting oxygen concentration [
19]. But only a few authors have been interested in the effect of pyrotechnic mixture combustion on the atmosphere inside the chamber, including tested dust. One of these studies, by Cloney et al. [
20], used CFD finite-volume 1D simulations of the ignitor’s action, examining the effects on dispersed dust. They found that small particles of polyethylene (10–30 µm) at thermal equilibrium have a temperature between 120 and 130 °C.
Taveau et al. [
21] also focused on an explosible system in a 20 L chamber after pyrotechnic ignitor explosion. They hypothesised in their paper that “It is likely that the strong preheating created by the pyrotechnical ignitor(s) affects the dust prior to flame arrival, causing partial reaction and the formation of a more reactive hybrid mixture, consisting of a turbulent flammable gas (or vapour) and dust. We proposed to call this phenomenon an igniter-induced hybrid.” They attributed their high K
st values, which were measured in a 20 L chamber and compared to those measured in a 1 m
3 chamber, to this phenomenon.
These few findings indicate significant pretreatment of the dust cloud in the 20 L chamber at the time of ignition. Such effects are highly limited in the 1 m
3 chamber by its large volume. Consequently, industry uses results measured under different initial conditions, such as higher temperatures and/or hybrid mixtures instead of only a dust cloud, for the design and installation of various safety measures. The aim of this work is to verify Taveau et al.’s [
21] igniter-induced hybrid hypothesis for natural dust, such as lycopodium clavatum, by analysis of gas and solid lycopodium decomposition products after an ignitor’s explosion. Dust clouds were generated in an inert atmosphere. Such a test should show how the dust is influenced by the ignitor’s explosion before the dust flame propagates through the dust cloud. If Taveau’s hypothesis is valid for lycopodium dust, then non-negligible concentrations of gas post-explosion products will be measured.
Analysis of post-explosion products after a dust explosion is not a completely new topic. Most research efforts have focused on the study of coal dust explosion products, specifically in China. Geng et al. [
22] used Fourier transform infrared (FTIR) spectrometry for carboxyl group and aromaticity analysis of an Australian lignite. He et al. [
23] applied FTIR and Raman spectroscopy for functional group analysis in various coals. Li et al. [
24] conducted SEM–EDS analysis of post-explosion solid residues together with TGA/DTG analysis. Their gaseous products were studied by gas chromatography. Lin et al. [
25] investigated the properties of different functional groups and the FTIR structural parameters of a coal sample and its solid residue by FTIR spectra. The use of the same method was described in Lin et al. [
26]. FTIR and gas chromatography were also applied by Nie et al. [
27] in their study on gas and solid residues of coal dust. Qian et al. [
28] employed a gas chromatographic analyser in their coal dust explosion tests. Additionally, a paper originating from the USA can also be found [
29]. Marmo et al. [
30] used X-ray diffraction to highlight the decomposition of sodium carbonate, whereas Li et al. [
31] used the same method for pre- and post-explosion products of coals in a 20 L chamber. Lycopodium dust is studied in the present work and was also analysed by Bidabadi et al. [
32,
33], a paper focused on lycopodium dust flames. They used the assumption that 90% of lycopodium converts into methane gas when it is pyrolyzed and the rest is carbon, according to their calculations. They describe derivative thermogravimetry (DTG) analysis of a thermogravimetry diagram (TG) of lycopodium published by Han et al. [
34]. Mostafavi et al. [
35] published their results obtained from thermogravimetry tests of lycopodium. They analysed the kinetics of reactions of lycopodium particles in a nitrogen environment and also in an oxidising environment by simultaneous thermal analysis (STA) in TG-DTA. Lycopodium elemental composition was also analysed. Spijker [
36] published work focused on the numerical simulation of lycopodium particle behaviour in the flame assuming evaporation and following pyrolysis of the lycopodium oil content. He cited the work of Kern [
37], who reported an oil content of 46% by mass in lycopodium.
2. Materials and Methods
Two types of tests were conducted. A lycopodium clavatum sample was measured in an analytical laboratory during the first phase. Analytical methods such as STA, GC/MS, FTIR and elemental analysis (CHNS) were chosen according to a literature survey for analysis of atmosphere composition after exposure of lycopodium dust as a natural material. The samples were exposed to certain temperatures up to 500 °C and, in some cases, even higher in both air and nitrogen atmospheres. In the second step, the atmospheric composition was measured in a 20 L chamber after dispersions of lycopodium dust in a nitrogen atmosphere and ignition by two 5 kJ Sobbe pyrotechnical ignitors.
To determine the composition of the post-explosion gases, the infrared spectroscopy method (FTIR) was used, by which the concentration of gas mixtures was continuously determined. The Matrix MG2 infrared spectrometer (Bruker OPTIK GmbH, Ettlingen, Germany) and the OPUS GA v5.2.11.20 measurement software were used for the measurements. The flue gases after decomposition/burning were led through a heated sampling line into an FTIR heated gas cell with a stable gas flow through the cell. The concentration of selected gases was determined. The determination of the elemental composition of lycopodium was carried out using method of elemental analysis, which allowed for the determination of the contents of elements C, H, N and S. Thermo Finnigan FLASH EA 1112 Series CHNS/O Analyzer was used for the determination of elemental composition. A furnace designed to determine the ignition and autoignition temperatures of solid materials in a gaseous atmosphere was used (according to the standard ČSN 64 0149:1978 “Determination of flammability of materials” [
38] and ISO 871:2006 “Plastics—Determination of ignition temperature using a hot-air furnace” [
39]) for estimating the behaviour of lycopodium during higher temperatures. The behaviour of lycopodium during gradual heating in a gaseous atmosphere was studied using the method of simultaneous thermal analysis. This thermal analysis technique combines thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). The STA 449 F1 Jupiter instrument was used for the measurements.
The explosion tests were carried out in a 20 L explosion chamber (CA 20 L) by OZM Research according to EN 14034-1,2 [
1,
2] and described in Janovsky et al. [
40]. The chamber is a double-walled spherical-shaped stainless steel (1.4435) vessel with an internal diameter of 336 mm. A rebound nozzle was used for the dispersion of the dust. The dynamic pressure was measured using a pair of PCB-type 112B05 piezoelectric pressure sensors with a sampling rate of 50 kS/s/channel. The automatic procedure was controlled by a Siemens Simatic 1215, connected to the PC interface. The ignition delay time was set to 60 ms according to the requirements of EN 14034-1 [
1].
For the tests, samples of the natural herb lycopodium clavatum and talc dust, a common silicate mineral with the chemical formula Mg
3Si
4O
10(OH)
2, were used. The granulometric distribution was measured by laser diffraction on a Malvern Mastersizer 3000. The moisture content of both samples was measured using a Kern-type DBS60-3 moisture analyser. The results of the measurements are shown in
Table 1.
The chemical composition of lycopodium was determined by elemental analysis showing that the material used consisted of 66.24% of C, 9.24 % of H, 24.40% of O and 0.12% of N. Lycopodium was also tested according to an old, but still valid, Czech standard ČSN 64 0149 [
38] (Setchkin method). The sample spontaneously ignited (no external ignition source) when the temperature of the air flowing around the sample was 390 ± 5 °C.
For standard dust explosion testing, chemical ignitors by Fr. Sobbe GmbH are used all around the world, and therefore, a pair of their 5 kJ chemical ignitors were used. The EN 14034-1,2 standard [
1,
2] defines their pyrotechnic mixture as a mixture of 40% Zr, 30% Ba(NO
3)
2 and 30% BaO
2.
2.1. Lycopodium in Analytical Laboratory
The original lycopodium sample was first characterised using TGA in both air and nitrogen atmospheres to obtain an overview of the response of the dust to heating and differences in the behaviour of the sample in oxidising and inert atmospheres. The sample weights ranged from 12.2 to 14.4 mg. The samples were heated from 20 °C to 600 °C at a rate of 10 °C/min and a gas flow rate of 50 mL/min.
First, the flash point of lycopodium in the testing crucible was determined according to the Czech standard ČSN 64 0149 [
38]. Then, the Setchkin method was slightly modified in the manner of sample insertion. The standard method requires the insertion of a sample in a crucible (cup) into the furnace, but the dust in the crucible does not represent the behaviour of dust particles in dispersion.
To simulate conditions closer to the situation in an explosion chamber, dust was inserted in a Setchkin furnace and warmed to a specific temperature through a small chimney at the top, which led to dust dispersion inside. Measurement accuracy was increased by connecting the FTIR unit to the gas outlet line from the Setchkin furnace. Tests were carried out at temperatures of 200 to 600 °C in air and nitrogen atmospheres. Then, a sample ranging in weight from 0.12 to 0.15 g was slotted into the furnace. CO2, CO, H2O, HBr, HCl, HF, HCN, NO, NO2, SO2, CH4, C2H2, acetaldehyde, acetic acid and NH3 concentrations were monitored in the gas outflow from the furnace every 6 s. The flow rate of air or nitrogen through the FTIR gas cell was 2 L/min. Unfortunately, the FTIR unit cannot detect hydrogen. The same tests were performed on lycopodium, which was dried at 70 °C to a constant weight before testing.
2.2. Lycopodium and Talc in 20 L Chamber
The second phase of measurement was performed in a 20 L chamber made by OZM Research according to EN 14034-1,2 [
1,
2]. Two standard 5 kJ ignitors by Sobbe were ignited in air atmosphere and then in nitrogen atmosphere, where the concentration of oxygen was below 2% by volume. The concentration of remaining oxygen was determined by the ultimate vacuum level of the vacuum pump used and the air remaining in the dust reservoir after insertion of the dust. Samples of the gas atmosphere were taken by a diaphragm pump with a flow rate of 1 L/min and directed to the FTIR instrument for analysis.
The following test series used talc as an inert dust in the air. Sufficient results of the testing of lycopodium dust explosion parameters were produced when lycopodium was tested at concentrations of 125, 250, 500 and 750 g/m3. Therefore, the same concentrations of talc were prepared and exposed to two 5 kJ ignitors by Sobbe. Talc tests were performed in an air atmosphere.
Lycopodium was dispersed into the nitrogen atmosphere in the last series of the test. The oxygen concentration was below 2% by volume. The concentrations of lycopodium exposed to two 5 kJ ignitors by Sobbe were 125, 250, 500 and 750 g/m3. The atmospheric composition after each test was analysed with the FTIR instrument again, and solid residue samples were taken for elemental analysis.
4. Discussion
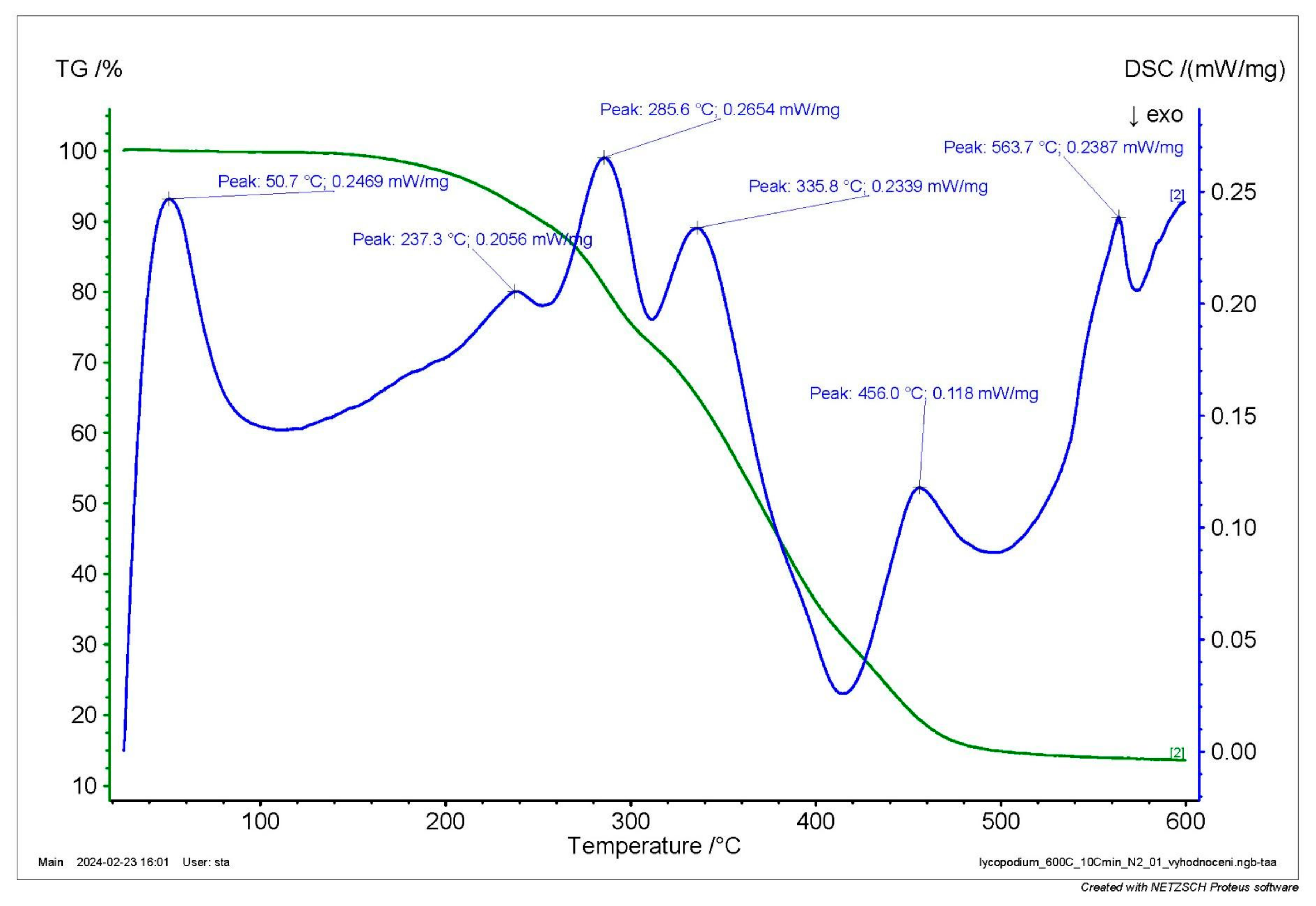
The results of TGA/DSC (
Figure 1) show that the first maximum mass reduction rate occurred in the range of 300 and 340 °C, while the second occurred in the interval between 470 and 500 °C in the atmosphere of the air. However, the DSC curve shows three zones where the rate of energy release increased. In the first zone, a small amount of energy release occurred between 200 and 245 °C (
Figure 1), corresponding to an endo-peak of 237 °C in the nitrogen atmosphere (
Figure 2), followed by a very intensive energy release up to 315 °C. In the nitrogen atmosphere, reacting material (
Figure 2) in this range was characterised by a maximum production rate with a maximum at 286 °C in the endo-curve. Subsequently, the energy release in the air atmosphere (
Figure 1) slowed down to 415 °C with a small peak at 341 °C, corresponding to the endo-peak at 336 °C in the nitrogen atmosphere (
Figure 2). This slowdown was followed by another rapid energy release in air (
Figure 1), reaching the maximum at 477 °C. In the nitrogen atmosphere, the highest rate of flammable material production occurred at 456 °C (
Figure 2). After reaching this temperature, the rate of energy release decreased to the almost zero line at a temperature of 560 °C in the air atmosphere (
Figure 1). The DSC curve correlates well with the TGA curve, with peaks at the same temperatures as the maximum rate of mass reduction in the TGA curves.
Mostafavi et al. [
35] published their TG/DTG lycopodium measurements in air and nitrogen atmospheres. They differentiated two active zones for the measurements under the air atmosphere, with limits of 250 and 424 °C for zone I and 424 and 538 °C for zone II. These ranges fit well with our measurements. Mostafavi et al. [
35] attributed the mass reduction in the first active zone only to the pyrolysis process, while they assumed that the mass reduction in the second active zone was due to both the pyrolysis and combustion of solid particles. The DSC curve measured in the air atmosphere (
Figure 1) shows large energy release in the temperature zone similar to the first active zone by Mostafavi et al. [
35]. It seems that the gas/vapour products of pyrolysis directly oxidise in that zone, increasing the local temperature and intensifying the pyrolysis process. In contrast, Spijker [
36] attributed the mass loss in the range of 177 and 347 °C to oil evaporation. The TGA record (oxygen) in
Figure 1 also shows the decrease in mass in that range. However, the DSC line shows two exo-peaks in that range. It seems that there is not only evaporation of one oil type in that temperature range. This is supported by the three endo-peaks (
Figure 2) in the DSC data for that temperature range. However, the third endo-peak (336 °C) could be a product of cellulose decomposition, which decomposes in the temperature range of 315–390 °C [
35]. Our measurement in a nitrogen atmosphere was also very similar to the measurement taken by Mostafavi et al. [
35], with only a slight temperature shift. But Mostafavi et al. [
35] differentiated only one active zone in the range of 250–450 °C that covered the decomposition of heavier hydrocarbons. The DSC curve in
Figure 2 shows two peaks at 286 °C and 336 °C, respectively. The first one could be a product of heavier oil decomposition, and the second one cellulose decomposition. From the presented findings and Spijker’s [
36] work, Mostafavi et al.’s [
35] statement about two sections of the active zone can be modified as follows:
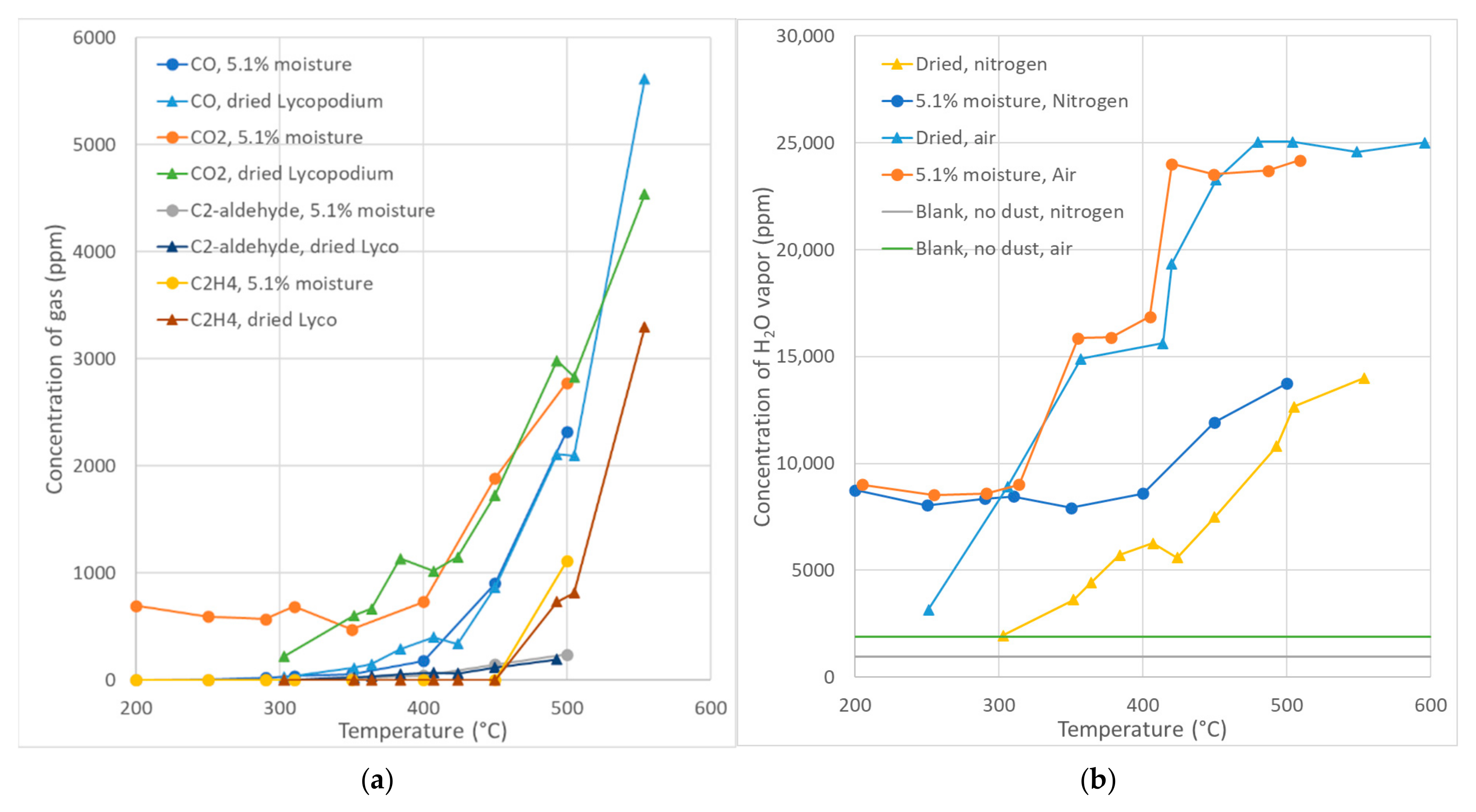
The results of the Setchkin measurements in
Figure 3a show that production of the main reaction products (CO, CO
2 and water) started at temperatures between 250 and 280 °C, which correspond well to the beginning of the first intensive heat production zone with a peak at 315 °C at the DSC curve (
Figure 1, air atmosphere). The concentration of all products increased continuously with increasing furnace temperature. The first sign of hydrocarbon detected appeared at 400 °C with a very small concentration of acetaldehyde. C
2H
4 appeared starting at a temperature of 500 °C. Bidabadi et al. [
32] cited in their article previous works where it was assumed that methane gas was the only product of lycopodium pyrolysis. However, the presented measurements do not support the assumption used for their calculations. Methane was first detected at temperatures above 550 °C in the nitrogen atmosphere. Surprisingly, methane was detected in gas products in tests in the air atmosphere at temperatures at and above 450 °C, where combustion of lycopodium particles was observed. Therefore, we can conclude that the main pyrolysis products in the first active zone (250–424 °C) are CO, CO
2 and water, which corresponds to the primary pyrolysis described by Spijker [
36]. The production of hydrocarbons started in the second active zone (424–538 °C), corresponding to Spijker’s secondary pyrolysis. Note that our experimental setup did not allow for a measurement of the hydrogen concentration.
Regarding the moisture content (
Figure 3b), the moist sample showed a high concentration of water vapour already at a temperature of 200 °C, confirming complete water evaporation at a temperature below 200 °C. The water vapour concentration in the moist sample began to increase above 315 °C in the air atmosphere, corresponding to the DSC peak in
Figure 1. This is in contrast to the dried lycopodium, where the concentration of water vapour started increasing already at a temperature of 250 °C, reaching the same values as the moist lycopodium at about 310 °C. Both temperatures correspond to peaks at 232 °C and 315 °C in the DSC curve in
Figure 1. The reason for this is not clear to the authors, but it seems that the evaporated water vapour from the moist sample inhibited the reactions that produce water in the first heat-producing zone found on the DSC curve (
Figure 1). The curves for the water vapour concentration in the nitrogen atmosphere show the same effect of moisture evaporated, but the nitrogen atmosphere inhibited the reactions producing water until 400 °C in the case of the moist sample, whereas the dried sample showed an increase in the water concentration starting at 300 °C. Nitrogen likely had a similar effect as water vapour on the moist sample in the air atmosphere. However, the moist sample together with nitrogen inhibited water-producing reactions up to 350 °C.
Figure 3 shows good agreement with the DSC data at temperatures between 341 and 425 °C, where heat production decreased. Setchkin measurements show stagnation in water production from reactions within this interval in the air atmosphere. Both samples in the nitrogen atmosphere also did not increase the water concentration within this interval. A very similar effect can be seen in the case of CO
2 production in the nitrogen atmosphere. The release of CO
2 production for the dried sample started at 357 °C and continued up to 420 °C. The moist sample started CO
2 production above 400 °C. The temperature range of 341–425 °C corresponds to the temperature ranges identified by Mostafavi et al. [
35] as the area following the peaks of the first active zone up to the end of the first active zone. The pyrolysis slowed down until the temperature reached the second active zone. The initial production of CO by the dried sample was similar to the production of CO
2. The first hydrocarbon detected was acetaldehyde in small concentrations in the tests with both samples in nitrogen above 350 °C. The ethylene concentration was measured at 500 °C.
The results of the atmosphere composition measured by FTIR after the ignitor’s action in an atmosphere with an oxygen concentration reduced to below 2% by volume show that the main flammable product formed by the ignitor flame was CO, followed by C2H4 and CH4. The sample used was not dried, and therefore, a significant concentration of water vapour was recorded. The almost constant concentration of CO2, regardless of the amount of dust dispersed, confirms the very low oxygen concentration in the atmosphere, and hence, incomplete oxidation reactions. The concentrations of all measured substances increased with increasing dust concentration, which was the expected result, but only the water vapour concentration increased almost linearly. As the dust concentration increased, increases in the flammable product concentrations slowed down, reaching almost constant values at high dust concentrations (500 and 750 g/m3). In contrast, the water vapour concentration increased throughout the whole concentration range of dust used in the tests. The water vapour concentration was mostly due to the evaporation of moisture under temperatures lower than 200 °C according to the results of the Setchkin method, while lycopodium decomposition started at 300 °C. This can be explained by the larger dust mass heated being to 200 °C rather than 300 °C, the temperature necessary for lycopodium decomposition, while water vapor evaporation is not limited in the dust concentration range used.
Taveau et al. [
21] and Kuna et al. [
11] presented pictures of fireball formation after activation of an ignitor. The fireballs shown can easily fill the entire volume of the 20 L chamber. Taveau et al. [
21] stated that the mean temperature inside the 20 L vessel can increase by 350 °C (to a temperature of approximately 640 °C within the entire chamber), with much higher local temperatures, reaching the maximum at the centre of the vessel. They also presented photos of a flame using a fast IR camera, originally published by Scheid et al. [
41], who separated the flame into two temperature zones of 2000–650 °C and 650–200 °C. It is therefore evident that the temperature inside the chamber is stratified, with the maximum at the centre and temperatures near the walls of the chamber close to those before dust dispersion.
Based on the results of the Setchkin method, it is possible to deduce the extent of the ignitor’s fireball effect on a dust cloud based on the concentration of measured gases. The water vapour production region (at a temperature below 200 °C) is larger than the regions for the production of the other products and increases almost linearly with increasing dust concentration. CO production starts above 300 °C, C2H4 is produced at a temperature close to 500 °C, and methane production starts at 550 °C in nitrogen atmosphere. Increasing the lycopodium concentration should increase the production of these substances if the temperature stratification inside the chamber does not change with increasing dust concentration, but this was not observed. The concentration of these three gases did not increase linearly, and the increase slowed down with increasing dust concentration. The increasing dust concentration provided more and more efficient shielding against the dispersion of heat. Therefore, the dust cloud volume with a temperature higher than the gas production limits decreased, but more dust was heated to higher temperatures. The temperature stratification therefore changed; however, the mass of the heated dust increased as its concentration increased. The same process can also be expected in a 1 m3 chamber, but the influenced volume is only one-fiftieth of the chamber volume.
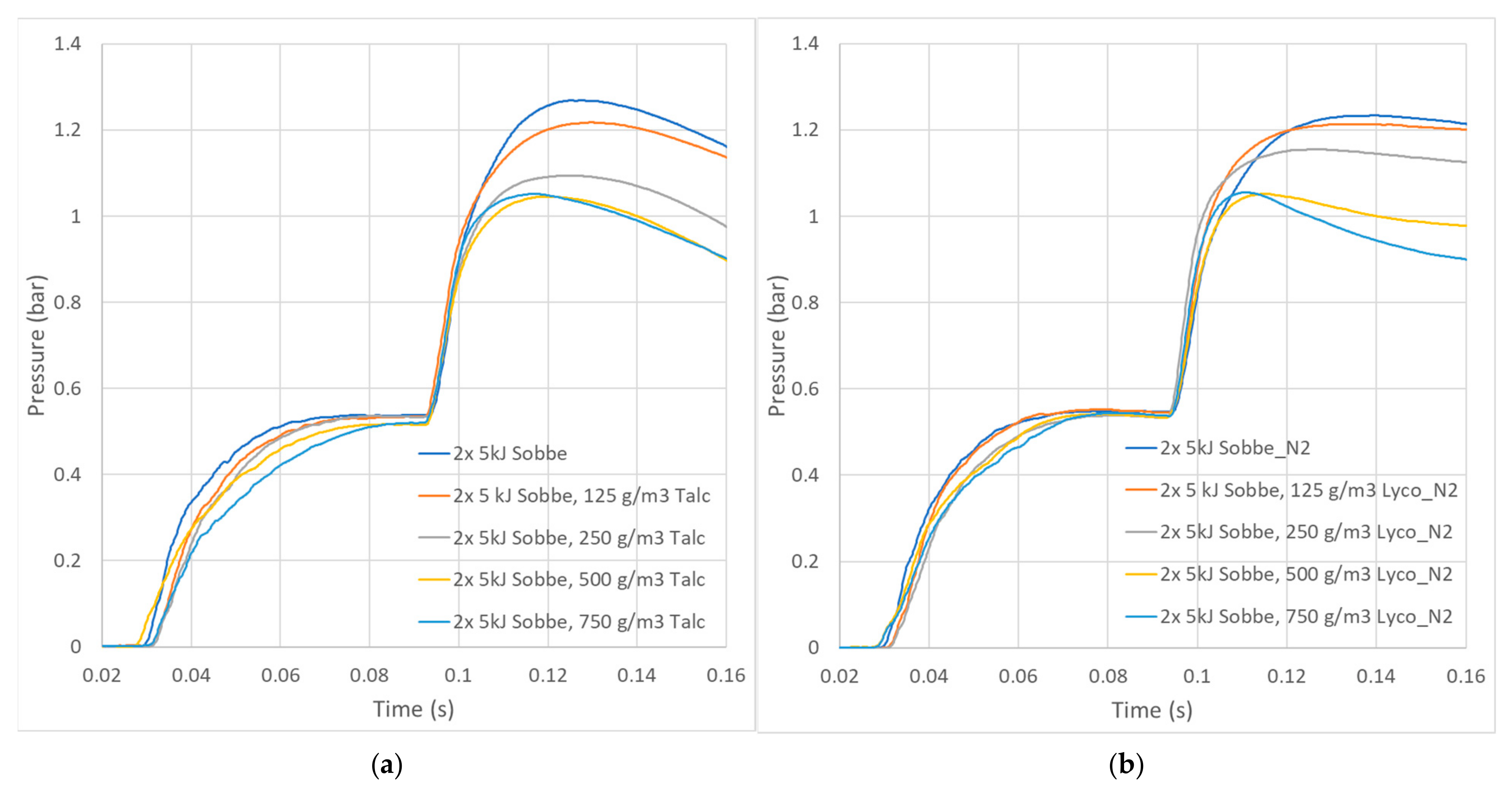
The effect of heat absorption by dust particles is more visible in the pressure data (
Figure 5) recorded during the measurement in the 20 L chamber. The recorded pressure lines show that the dust cloud absorbed the heat and thus behaved as a heat sink (cooler) in the chamber. The maximum overpressures generated decreased with increasing dust concentration from 0.73 bar to 0.53 barg for talc in air and 0.51 barg for lycopodium in nitrogen. The same overpressures were generated with concentrations of 500 and 750 g/m
3 of talc (0.531 and 0.529 barg, respectively) and lycopodium (0.512 and 0.515 barg, respectively). It seems that only a certain maximum portion of the totally released energy could be absorbed by the particles in the chamber. Therefore, the increase in the total mass of the dust does not mean a further decrease in the maximum pressure measured.
Table 3 summarises the results of the pressure measurements in the 20 L sphere.
Table 3 shows that the energy absorbed/lost increased with increasing dust concentration, reaching the maximum at about 26% for both the 500 and 750 g/m
3 concentrations. Similar results were observed in the case of the post-explosion product concentration measurement. This both shows that the total heat absorbed by dust is limited. The reason can be the type of heat energy available and its limiting value. It is known that in various types of fires, heat radiation is immediately available and accounts for about 20–40% of the total heat released, depending on a type of flame [
42]. One could conclude that when the mass of the solid material is larger than the mass absorbing all the radiated heat, then the conditions inside the chamber become more or less constant in terms of the mean temperature and total pre-treated mass of dust. However, this is not the case. The heat capacity of the talc is 0.825 J/g.K [
43], and the heat capacity of the lycopodium is 1.0048 J/g.K, according to Bidabadi [
33].
Table 4 presents the results of the theoretical maximum energy absorbed by dust calculations together with the energy absorbed in the experiments. The theoretical maximum energy absorbed was calculated by multiplying the heat capacity by the mass of the sample and the temperature increase from the initial temperature in the 20 L chamber (25 °C) to the mean temperature.
The results in
Table 4 show that the problem is much more complex and that various factors influence the conditions in the chamber during the explosion. However, it seems that if the dust is inert, then all dust concentrations absorb less energy than the measured energy losses. Therefore, another factor must be causing the energy losses and/or inhibiting the energy production of the burning pyrotechnic mixture in the chamber. If the dust is flammable, then the decomposition of the dust likely complicates the energy situation in the chamber. The same factor as in the case of inert dust is likely involved, but other recently identified, unknown factors make the process complex. A better explanation will require further testing in the future. Another finding is that the explosion of the lower dust concentrations was more influenced by the preheating action of the ignitor than by the higher concentrations. Therefore, a direct comparison of the dust explosion parameters at two dust concentrations is an oversimplification that ignores the different conditions affecting flame spread.
The time to reach maximum pressure, together with the maximum overpressure for talc as an inert system, was almost the same as that of the lycopodium explosion (
Table 3), but in the case of higher concentrations of lycopodium, the time to reach the maximum pressure was shorter. This may have been caused by the complexity of the energy absorption processes, inhibiting the spread of ignitors fireballs, lycopodium decomposition, and other factors. The large differences between the times to reach the maximum pressure and the maximum rate of pressure increase point to two phases of ignitor combustion: the initial fast flame jet from the ignitor’s cup, followed by much slower fireball burning of the released pyrotechnic mixture. This indicates that dust clouds with different preconditioning have different reactivity. All dust clouds are influenced by the initial fast phase, and highly reactive dust that ignites almost immediately can burn faster than the pyrotechnic mixture, resulting in minimal preconditioning. On the other hand, less reactive dusts require a longer time to ignite, and they burn much slower than pyrotechnic mixtures. In this case, the whole dust cloud becomes preconditioned, leading to questionable results. The lycopodium used in the tests can be an example of preconditioning.
At a concentration of 125 g/m
3, lycopodium in the air reached the maximum pressure in 68.9 ms, whereas the same concentration of lycopodium in nitrogen reached the maximum pressure in 43.8 ms. This means that all of the heat released from the ignitors contributed to the maximum pressure/overpressure presented in the test report because standards [
1,
2] disregard the overpressure generated by the igniter when the maximum pressure is higher than 5.5 barg. A similar effect can be seen for the maximum rate of pressure rise. The same concentration of lycopodium in the air reached the maximum rate of pressure rise in 16.9 ms and 43.8 ms in the nitrogen atmosphere. This indicates that a large portion of the pyrotechnic mixture has enough time to burn, changing the initial conditions for dust burning, and exposing the dust to higher temperatures. The situation was even worse at the concentration of 500 g/m
3. Almost the entire mass of the pyrotechnic mixture already burned before the maximum rate of pressure rise was reached. The total concentration of the combustible decomposition products measured for this lycopodium concentration was 1.05% by vol. (
Table 2). It is highly probable that an “igniter-induced hybrid” was present and influenced the burning, at least partially. The concentration of the gas products in the dust cloud will decrease with distance from the centre of the chamber following the temperature stratification described by Taveau et al. [
21].