Abstract
In recent years, the storage of lithium-ion battery (LIB) containers in general cargo container yards has become an urgent operational requirement for port container terminals. To effectively control the impact range of thermal runaway (TR) incidents in LIB containers and reduce potential economic losses, it is imperative to establish appropriate isolation protocols. This study develops a mathematical–physical model of heat transfer following LIB container TR, incorporating (1) the national regulation limiting stacking height to three layers, (2) the exothermic characteristics of LIB TR, and (3) the fundamental heat transfer theory. Through detailed numerical simulations based on actual engineering scenarios, our analysis demonstrates that when (1) The TR temperature of conventional LIBs remains below 700 °C, (2) the thermal conductivity of goods in adjacent ordinary cargo containers does not exceed 10 W/(m·K). An effective isolation configuration can be achieved by (1) arranging no fewer than four ordinary cargo containers longitudinally and (2) placing no fewer than two ordinary cargo containers transversally. The methodology and conclusions presented in this study provide practical guidance for industrial applications and demonstrate significant engineering value.
1. Introduction
In 2015, the “8·12 Tianjin Port Fire Disaster” heightened concerns about the safety of dangerous goods container yards, leading to explicit requirements for the separate storage of dangerous goods containers from general cargo containers. Currently, most domestic dangerous goods container yards have limited space, making it difficult to accommodate all such containers. This issue is particularly acute for Class 9 dangerous goods containers, where yard space is severely insufficient. To address this operational challenge, the national standard Safety Requirements for Port Operations—Part 3: Dangerous Goods Containers (GB16994.3-2021) [1] was issued. Article 7.7 of this standard permits the storage of Class 9 dangerous goods containers in general cargo container yards, providing a practical solution for port companies.
However, storing Class 9 dangerous goods containers in general cargo yards creates mixed-storage scenarios (referred to as “mixed stacking”). Currently, there are no unified standards for mixed stacking schemes, and safety management lacks relevant experience. This is particularly true for Class 9 LIB containers, which are in high demand for export but pose self-ignition risks. Whether LIB containers can be stored in general cargo yards and how to manage such mixed storage safely have become pressing challenges for port container terminal operators.
LIB container fires have occurred frequently in the port industry. For example, on 3 July 2024, a container fire broke out in a yard in Yantian District, Shenzhen, due to LIB TR, and on 23 September 2024, a LIB container caught fire at the Port of Montreal, Canada. LIB fires do not rely on external ignition sources or oxygen, making them sudden, difficult to detect, and challenging to extinguish. Studying the fire and heat transfer characteristics of LIB containers is crucial for ensuring safe mixed storage with general cargo containers.
To investigate safe storage solutions and effective TR isolation measures for LIB containers in storage yards, this study first examines the hazardous characteristics of such containers and existing safety management protocols in storage facilities. Building on the TR behavior of LIBs, the research further reviews relevant studies covering the following:
- (1)
- TR features across different LIB types;
- (2)
- Fire hazard characteristics;
- (3)
- Fire suppression methods and their effectiveness.
Industry practitioners and research workers such as Rui Yin [2] and Chuanchang Zhang [3,4,5] have qualitatively analyzed the hazards, safety management challenges, and emergency response principles of LIB transportation by water, summarizing existing operational experiences. Doron Aurbach [6] and Stephen J. Harris [7] evaluated the fire hazards of different types of LIB electrolytes, exploring the effects of flame-retardant additives on the combustion performance of LIBs. Current research on LIB fire hazards includes experimental studies [8,9,10] and numerical simulations [11,12,13]. These studies focus on the fire behavior, heat release rate, mass loss, and toxic gas generation of LIBs under electrical, thermal, and mechanical abuse conditions. Yan Cui [14] and Ahmed O. Said [15] evaluated fire suppression methods and their effectiveness for LIB fires, providing theoretical references for firefighting strategies.
LIBs operate through the movement of lithium ions between the positive and negative electrodes. A LIB primarily consists of cathode materials, anode materials, an electrolyte, and a separator. Currently, the cathode materials used in LIBs include LiCoO2, LiFePO4, and ternary materials, among others. Anode materials comprise graphite, lithium titanate (Li4Ti5O12), coke, mesocarbon microbeads (MCMBs), pyrolytic hard carbon, and non-graphitizing carbon. The electrolyte is mainly formulated from organic solvents, lithium salts, and additives, with commercially available electrolytes predominantly using carbonate esters such as dimethyl carbonate (DMC) and diethyl carbonate (DEC). The separator is primarily made of polyolefin microporous membranes, including polyethylene (PE), polypropylene (PP), and their improved composite variants.
The main causes of LIB fire accidents include the following:
- The polyolefin separator may melt at elevated temperatures, altering its dimensions and shape, or even causing rupture, leading to short circuits and ignition [6];
- The organic solvents in the electrolyte have relatively low boiling points. When the battery experiences overheating, overcharging, or over-discharging, it may result in fires or even explosions [16,17];
- LiPF6 in the electrolyte is prone to thermal decomposition at high temperatures. Its thermal chemical reactions with trace amounts of water and organic solvents reduce the thermal stability of the electrolyte [6,18];
- Cathode decomposition: Due to the high-energy P=O bonds in (PO4)3− within lithium iron phosphate crystals, the P-O bonds in the cathode remain robust and resistant to breaking during decomposition reactions, releasing small amounts of oxygen [19];
- Anode–electrolyte reactions: The decomposition of the separator, particularly the solid electrolyte interphase (SEI) layer, releases heat. Without the protection of the separator, the electrolyte comes into direct contact with the lithium-intercalated anode, leading to redox reactions at high temperatures that release significant amounts of heat [20].
TR in LIBs is primarily triggered by three types of abuse: mechanical, electrical, and thermal. Mechanical abuse includes compression, puncture, and collision; electrical abuse involves overcharging, internal short circuits, and over-discharging; and thermal abuse encompasses heat conduction (e.g., from heating plates or rods) and TR in LIBs, which can initiate chain reactions within the battery, leading to the combustion of ejected materials and subsequent ignition of nearby LIBs.
Currently, targeted research on TR in LIB containers and heat transfer within container yards has not been reported. The existing research findings on LIB TR are difficult to apply to container yard scenarios. Thus, targeted research on TR and heat transfer specifically for container yards is needed.
2. Materials and Methods
2.1. Problem Statement
LIB containers fall under Class 9 dangerous goods, “Miscellaneous Dangerous Substances and Articles, Including Environmentally Hazardous Substances.” According to the List of Dangerous Goods (GB12268-2025) [21], LIBs shipped by sea have different UN numbers depending on their form, including UN3480, UN3481, UN3536, UN3171, and UN3091 [22]. The shipping properties of these LIB containers are listed in Table 1.

Table 1.
Shipping properties of LIB containers.
Due to the self-ignition risk of LIBs, storing LIB containers in general cargo yards introduces safety hazards. To mitigate the risk of concentrated LIB fires, these containers must be isolated within the yard. However, determining the optimal isolation measures—such as the maximum stacking height and the minimum number of general cargo containers required for isolation—remains a critical challenge for port operators.
2.2. Methodology
According to engineering practice, general cargo container yards are typically designed for heavy containers with a standard stacking height of up to five tiers. LIB containers, classified as Class 9 dangerous goods, fall under the flammable category. Industry standards generally limit the maximum stacking height for flammable containers to no more than three tiers. Therefore, when LIB containers are stored in mixed stacking with general cargo, their stacking height should not exceed three tiers to facilitate firefighting and emergency response. Specifically, in such mixed-storage yards, the lower two tiers should be reserved for general cargo containers, while LIB containers may occupy the upper three tiers. This arrangement has been proven feasible and acceptable in practical engineering applications.
However, regarding the isolation scheme for LIB containers—including transverse and longitudinal spacing within container rows—there is currently no established industry practice to reference. This necessitates theoretical research and computational analysis to determine appropriate measures.
As outlined above, the isolation of LIB containers within rows involves both transverse and longitudinal spacing, as illustrated in Figure 1 and Figure 2. The primary objectives of isolation are as follows:
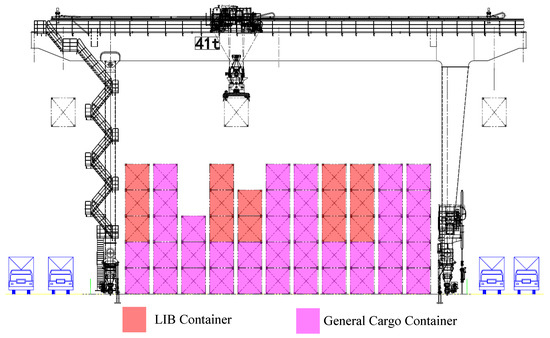
Figure 1.
Cross-sectional schematic diagram of the LIB container isolation scheme.
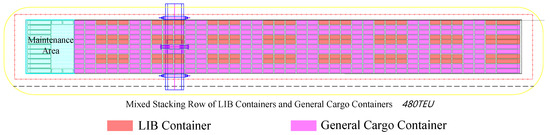
Figure 2.
Schematic plan of LIB container isolation layout.
- To prevent the concentration of large quantities of LIB containers, which could lead to excessive heat release during TR events and ignite adjacent LIB containers;
- To utilize general cargo containers as thermal barriers, confining the impact of TR incidents and preventing domino effects.
To effectively mitigate the consequences of LIB fires, it is essential to quantitatively study the heat release patterns during TR and analyze heat propagation through general cargo containers. This research will provide a theoretical foundation for developing optimized isolation strategies.
2.3. Fire Characteristics of LIBs
Heat transfer analysis [23] reveals that TR in LIB containers induces radiative heating of proximate air volumes, with subsequent temperature stratification in mixed-storage configurations. The heated air then transfers heat to adjacent general cargo containers via convective heat exchange. Subsequently, heat propagates from one side of the general cargo container wall through the cargo to the opposite side via thermal conduction. The opposite side of the container further exchanges heat with the ambient air through convection, heating the air on that side. If the temperature of this air exceeds the minimum threshold required for LIB TR, it may ignite LIB containers on the opposite side.
During the initial stage of TR in a LIB container, it continuously heats both the surrounding air and adjacent ordinary cargo containers used for isolation. As these ordinary containers absorb heat and their temperature rises, they subsequently transfer heat to the air on their opposite side. The general cargo containers then progressively heat the air on their opposite sides. This represents a non-steady-state heating process involving transient convection and conduction. However, over time, as the LIB container continues to radiate heat, temperatures of both the surrounding air and general cargo containers stabilize. Beyond this point, heat transfer enters a steady-state phase with stable convection and conduction processes, under the following assumptions:
- Neglecting thermal conductivity differences between container walls and interior cargo;
- Ignoring variations in thermal conductivity among different types of cargo within general containers.
The temperature distribution curve of heat transfer in the mixed-storage yard during steady-state conditions is shown in Figure 3.
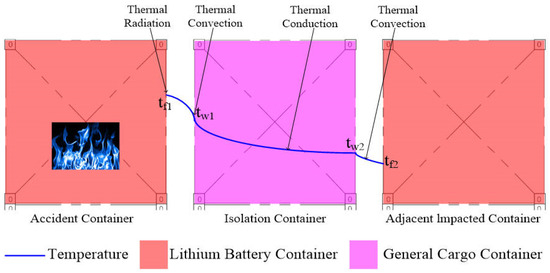
Figure 3.
Thermal propagation diagram during LIB container TR in storage yard.
According to the research by Sun et al. [24], the peak heat release rate (PHRR) during TR varies significantly among different types and capacities of LIBs. Through regression analysis of LIBs with capacities ranging from 10 Wh to 107 Wh, it was found that the PHRR exhibits a linear positive correlation with the 0.6th power of battery capacity, revealing a distinct power-law relationship in TR behavior, as detailed in Figure 4 [24] and Equation (1).
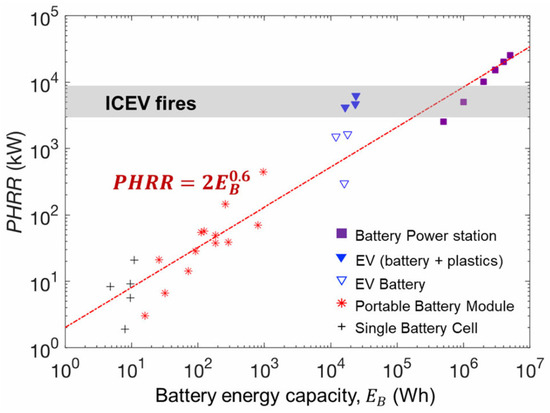
Figure 4.
Relationship between peak heat release rate and battery capacity in LIBs.
In this expression, is the peak heat release rate of the battery (MW), and is the battery capacity (Wh).
This relationship can estimate the peak heat flux released per unit time during TR of LIB containers. The more LIBs a container holds, the higher the state of charge (SOC) of the batteries, and the more densely the containers are stacked, the greater the number of LIBs that may undergo TR—resulting in significantly more heat released.
2.4. Mathematical–Physical Model of Heat Transfer in General Cargo Containers
When a LIB container experiences a TR incident, it emits energy-carrying particles (photons) into the air in the form of electromagnetic waves. The energy carried by these photons constitutes radiant energy. The magnitude of thermal radiation per unit time depends on factors such as the object’s surface temperature, radiating surface area, and surface emissivity, as specifically expressed by Equation (2) [23,25]:
In this expression, Q is radiative power per unit time (W), ε is surface emissivity of the TR LIB container (dimensionless), σ is Stefan–Boltzmann constant (5.669 × 10−8 W/m2·K4), A is radiating surface area (m2), and T is absolute temperature (K).
Generally, the temperature of the thermal radiation-exposed surface (the side facing the incident container) of an ordinary cargo container is significantly higher than that of the opposite side. The container exchanges heat with the surrounding air through convection on both sides, while heat transfers internally from the high-temperature side to the low-temperature side via conduction. This complete heat transfer process comprises two convective stages and one internal conductive stage.
The convective heat transfer between the container walls and surrounding air constitutes forced convection over flat plates. As air flows past the container walls, both laminar and turbulent boundary layers develop at the leading edge. In coastal areas where wind speeds are typically higher and wind direction variable, the laminar boundary layer remains relatively short, making turbulent convection the dominant heat transfer mechanism. Under these conditions, the average heat transfer coefficient at the container wall–air interface can be determined by the following correlation equation:
Given that general cargo containers are constructed from carbon steel—with heat capacity substantially lower than the contained cargo and thermal conductivity significantly higher—the thermal resistance of the container walls themselves can be neglected. Under these conditions, the heat transfer equations can be expressed as Equations (3) and (4) [25]:
In this expression, is the heat transfer rate (W); is the cross-sectional area perpendicular to heat conduction (m2); is the overall heat transfer coefficient (W/m2·K); is high-temperature-side air temperature (K); is the low-temperature-side air temperature (K); is the convective heat transfer coefficients for container wall surfaces (low/high temperature sides) (W/m2·K); is the thickness of the i-th general cargo container in heat conduction direction (m); and is the thermal conductivity of the i-th general cargo container (W/m·K).
In this expression, is the Nusselt number for plate heat transfer; is the Reynolds number for plate heat transfer; is the Prandtl number for plate heat transfer; h is the average heat transfer coefficient of container wall (W/m2·K); L is the windward length of the convective heat transfer wall (m); is the thermal conductivity of air (W/m·K); V is the ambient wind speed (m/s); and is kinematic viscosity of air (m2/s).
To quantitatively investigate the heat release patterns and thermal propagation characteristics during LIB container TR, the coupled solutions of Equations (1)–(6) are required. This enables determination of the following:
- The total heat generation and peak temperatures during TR;
- The resultant air temperature on the opposite side after heat transfer through general cargo container barriers.
The final isolation scheme (i.e., the required number of general cargo containers for effective isolation) can be established by comparing these calculated temperatures against the ignition threshold of LIBs.
3. Results and Discussion
3.1. Numerical Analysis of LIB Container TR
Currently, the most common shipping container configurations are 20-foot and 40-foot units. While 40-foot containers maintain identical width and height dimensions as their 20-foot counterparts, their length doubles that of 20-foot containers. To ensure the universality and generalizability of research findings, this study employs the international-standard 20-foot container (length of 6.100 m outside × width of 2.440 m external × height of 2.590 m external) as the analytical model. Market research indicates that China’s current maritime LIB energy storage containers typically exhibit the following:
- Unit weight range: 35–40 metric tons;
- Battery capacity range: 3.72–5 MWh.
Variations in LIB quantities, individual cell capacities, and states of charge (SOCs) within containers lead to significant differences in total battery capacity. Using Equation (1), the peak heat release rates (PHRRs) for both single and multiple LIB container TR scenarios were calculated, as visually presented in Figure 5 and Figure 6, respectively.
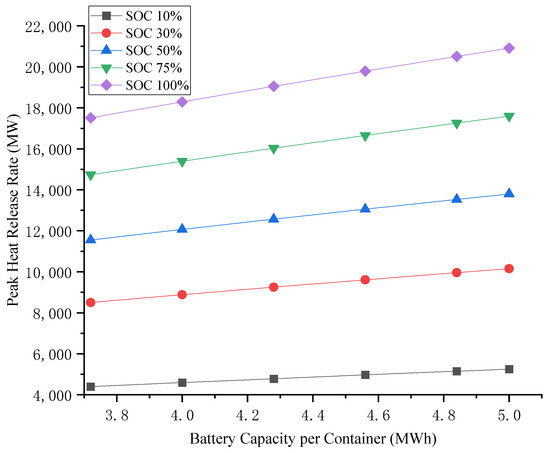
Figure 5.
Heat release rate of a single LIB container during TR.
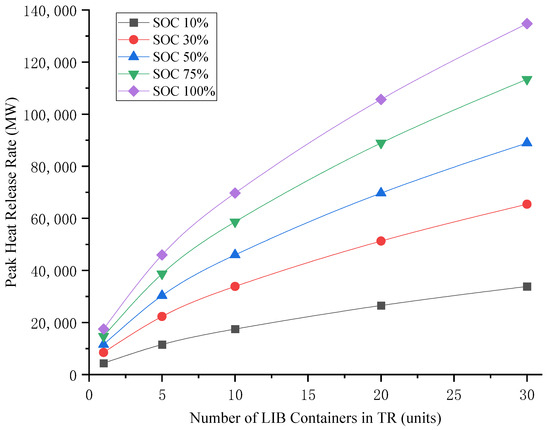
Figure 6.
Heat release rate of multiple LIB containers during TR.
As evidenced in Figure 5, the TR of a single LIB container demonstrates two critical trends:
- State of Charge (SOC) Dependence
- The heat release rate exhibits a strong positive correlation with SOC;
- A SOC increase from 10% to 100% yields a 298% enhancement in heat release rate.
- Capacity Influence
- Larger battery capacities generate higher heat release rates;
- Container energy capacity expands from 3.72 MWh to 5 MWh, enabling a 19.4% higher heat release rate;
- This near-linear relationship remains consistent across operational capacity ranges.
Figure 6 reveals three key findings regarding clustered LIB container storage:
- TR Escalation
- Increased container clustering leads to higher participation in cascade failures;
- Peak heat release rate (PHRR) demonstrates positive correlation with container quantity.
- Diminishing Growth Rate
- The PHRR increase factor decays with container quantity;
- 30-container clusters exhibit 7.7× PHRR versus single-container incidents.
- Energy Release Characteristics
- Total thermal energy output shows weak dependence on stacking density;
- The PHRR-to-quantity relationship follows sublinear progression.
3.2. Numerical Analysis of Heat Transfer in General Cargo Containers
3.2.1. Surface Properties of Conventional Containers
- Surface Properties of Conventional ContainersTraditional containers feature corrugated panel surfaces, which constitute structured surfaces. The surface emissivity (ε) is dependent on the following:
- Corrugation profile geometry;
- Surface area characteristics.
- Current methodologies for calculating emissivity of structured surfaces primarily include the following [26]:
- Empirical formula methods;
- Monte Carlo methods;
- Simulation verification methods.
Materials with poorer thermal conductivity generally exhibit higher surface emissivity. LIB containers utilize non-insulated steel construction (with surface emissivity set at ε = 0.10), demonstrating thermal emission rates comparable to common stainless-steel kettles. The thermal radiation is uniformly emitted from all six surfaces of the container, resulting in a total radiating surface area of 73.6 m2 for a 20-foot LIB container during TR.
3.2.2. TR Process
The TR process of LIBs, induced by external thermal abuse and other factors, can be divided into three distinct phases in Table 2.

Table 2.
TR process.
Thermal radiation from a LIB container undergoing TR heats the surrounding air. This heated air then transfers heat to adjacent ordinary cargo containers via convective heat transfer.
Calculated results of the thermal radiation emitted per unit time from the container walls are shown in Figure 7.
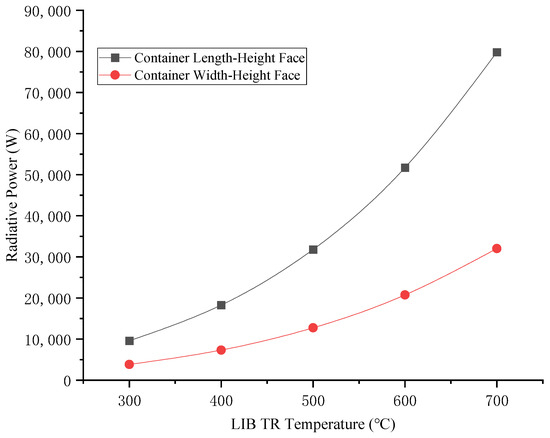
Figure 7.
Thermal radiation after LIB container TR.
Calculated results of the average convective heat transfer coefficient between the heated air and the ordinary cargo containers are shown in Figure 8.
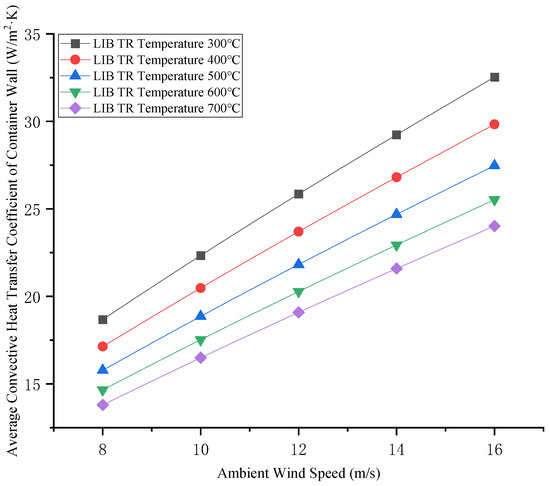
Figure 8.
Average convective heat transfer coefficient between hot air and ordinary cargo container walls.
According to Formulas (2) and (3) and Figure 7, the thermal radiation flux increases proportionally with rising surface temperatures during LIB TR. Due to the larger surface area of the container’s length–height (L-H) plane compared to its width–height (W-H) plane, the L-H face exhibits significantly greater radiative heat emission. As can be seen from Figure 8, the average convective heat transfer coefficient between hot air and general cargo containers is linearly positively correlated with the ambient wind speed, and the wind speed has a significant effect on the average convective heat transfer coefficient. The higher the TR temperature of LIBs, the greater the average convective heat transfer coefficient. When calculating convective heat transfer, the influence of ambient wind speed and TR temperature of LIB cannot be ignored.
After the TR of the LIB container, the heat enters the general cargo container through radiation and convection, and the acceptance rate of the general cargo container is 0.10 according to the emission rate. The thermal conduction of heat in a general cargo container is related to the thermal conductivity of a general cargo container. The thermal conductivity of a general cargo container depends on the filling rate of the container and the type of cargo loaded. The effect of thermal conductivity on the heat transfer coefficient of general cargo containers is shown in Figure 9. The relationship between the ambient wind speed and heat transfer coefficient is shown in Figure 10.
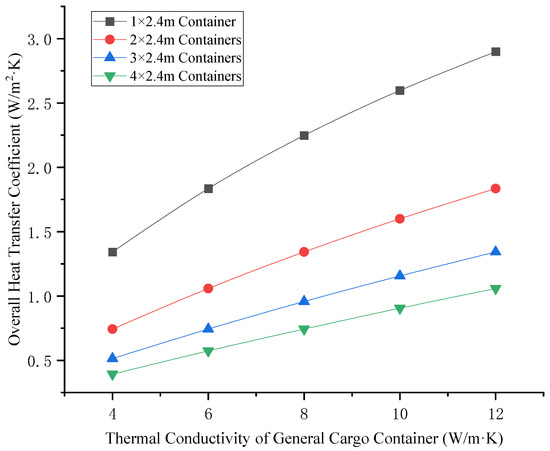
Figure 9.
Relationship between thermal conductivity and heat transfer coefficient of general cargo containers.
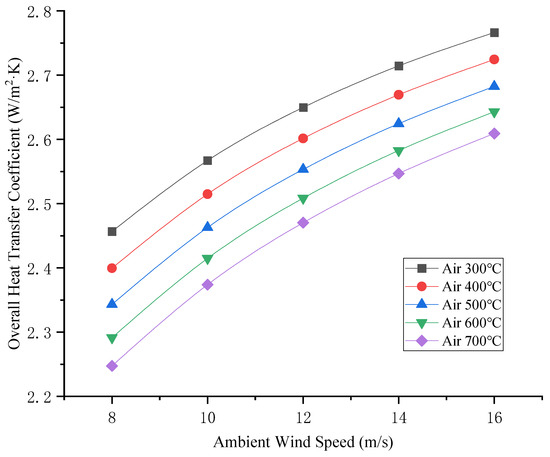
Figure 10.
Relationship between environmental wind speed and heat transfer coefficient.
Once the heat is transferred to the other side of the general cargo container, the general cargo container will be convection-swapped with the cold air on the other side (or the “cold side”) to heat the cold air on the other side. The relationship between the thermal conductivity of a general cargo container and the air temperature on the low temperature side is shown in Figure 11. The relationship between the ambient wind speed and the air temperature on the low temperature side of the general cargo container is shown in Figure 12.
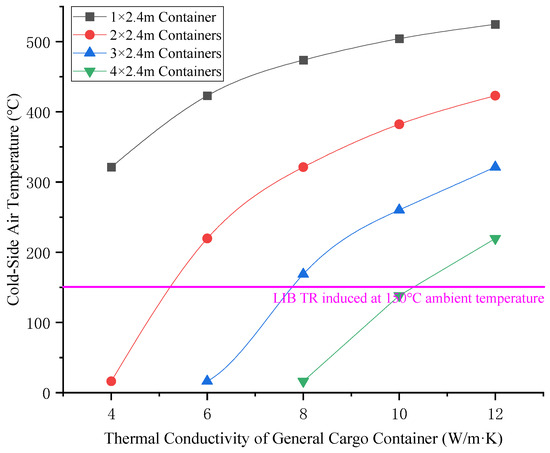
Figure 11.
Relationship between thermal conductivity of general cargo containers and cold-side air temperature.
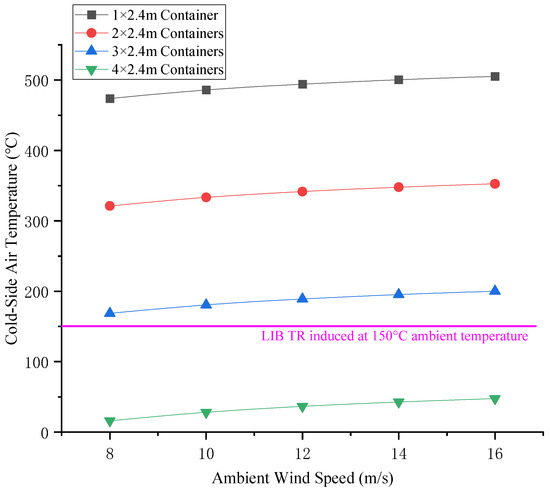
Figure 12.
Relationship between environmental wind speed and cold-side air temperature technical rationale.
As can be seen from Figure 9 and Figure 10 above, the heat transfer coefficient is positively correlated with the thermal conductivity and ambient wind speed of general cargo containers. It is negatively correlated with the width of the general cargo container for isolation (the number of isolation boxes) and the TR temperature of the LIB (the air temperature on the high-temperature side). As can be seen from Figure 11 and Figure 12, the air temperature on the low-temperature side is positively correlated with the thermal conductivity and ambient wind speed of general cargo containers. The variation in ambient wind speed has a relatively minor impact on the air temperature of the low-temperature side. When considering isolation solutions for mixed-stack yards, the influence of ambient wind speed fluctuations may be disregarded.
Further analysis of Figure 9 and Figure 10 can reveal that, in the case that the TR temperature of the traditional LIB does not exceed 700 °C, when the thermal conductivity of the goods in the general cargo container does not exceed 10 W/m·K (such as the goods in the box are clothing, shoes and hats, household goods and other daily necessities, mobile phones, computers, home appliances, and other electronic products), and when the isolation distance of the LIB container is greater than 9.6 m, the air temperature on the low temperature side is lower than 150 °C, which cannot cause the TR of the LIB container on the low temperature side. When the LIB container is isolated in the longitudinal direction of the box for no less than four general cargo containers, and no less than two general cargo containers are arranged in the horizontal direction of the box for isolation, the isolation can be basically guaranteed to be effective and will not cause the domino-effect accident of the LIB container in each concentrated area. When general cargo containers exhibit elevated thermal conductivity (e.g., containing industrial equipment, agricultural machinery, or other high-thermal-conductivity goods), the quantity of buffer containers deployed for isolation must be proportionally augmented.
4. Conclusions
This study investigates the safety issues associated with storing LIB containers prone to TR in ordinary cargo container yards and determines the maximum stacking layers for LIB containers. To explore limited isolation solutions for LIB containers, mathematical and physical models for TR in LIB containers and heat transfer in ordinary cargo containers were established, followed by numerical calculations based on actual engineering conditions. The following conclusions were drawn:
(1) The state of charge (SOC) of the battery significantly influences the heat release rate during TR. To ensure the safe storage of LIB containers, it is advisable to maintain the SOC below 30%. The more LIB containers are stored together, the greater the peak heat release rate in the event of an accident; however, this correlation weakens as the number of containers increases. Therefore, when designing mixed storage solutions, the focus should not be solely on the concentrated quantity of LIB containers, provided the total storage capacity is met.
(2) The ambient wind speed in the storage yard affects both the convective heat transfer between the high-temperature side (hot air) and ordinary cargo containers and the low-temperature side (cold air) and ordinary cargo containers, though the impact on the low-temperature side is negligible. Thus, the influence of wind speed on the low-temperature side can be disregarded when designing isolation solutions. The thermal conductivity of ordinary cargo containers has a significant impact on the heat transfer coefficient. When the thermal conductivity does not exceed 10 W/m·K, arranging no fewer than four ordinary cargo containers for longitudinal isolation and no fewer than two for transverse isolation along the container rows can effectively ensure isolation.
(3) The mathematical and physical models established in this study, along with the quantitative conclusions obtained, can be applied to formulate mixed storage solutions for LIB containers. The research methodology and results can provide guidance for industrial production. Since the thermal conductivity and thermal radiation absorptivity of ordinary cargo containers significantly influence the calculations, and their values depend on the type of goods inside and surrounding environmental conditions, further experimental studies in practical applications are recommended to obtain more precise data for better industrial guidance.
Author Contributions
Conceptualization, Y.G. and C.Z.; methodology, J.D. and C.Z.; software, Y.G. and C.Z.; validation, Y.G., J.D. and C.Z.; formal analysis, J.D.; investigation, Y.G. and C.Z.; resources, J.D.; data curation, Y.G. and C.Z.; writing—original draft preparation, Y.G. and C.Z.; writing—review and editing, J.D.; visualization, Y.G. and C.Z.; supervision, J.D.; project administration, J.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- GB 16994.3-2021; Safety Requirements for Port Operation—Part 3: Dangerous Cargo Container. State Administration for Market Regulation. Standardization Administration of China: Beijing, China, 2021.
- Yin, R.; Du, M.; Shi, F.L.; Cao, Z.X.; Wu, W.Q.; Shi, H.K.; Zheng, Q.G. Risk analysis for marine transport and power applications of lithium ion batteries: A review. Process Saf. Environ. Prot. 2024, 181, 266–293. [Google Scholar] [CrossRef]
- Zhang, C.C.; Sun, H.; Zhang, Y.Y.; Li, G.; Li, S.B.; Chang, J.Y.; Shi, G.Q. Fire accident risk analysis of lithium battery energy storage systems during maritime transportation. Sustainability 2023, 15, 14198. [Google Scholar] [CrossRef]
- Zhao, G.Q.; Xia, L.L.; Sun, Y.Q. Difficulties and countermeasures in marine container transportation of new energy vehicles. World Shipp. 2023, 46, 26–30. [Google Scholar]
- Chen, Y.Y.; Feng, Z.F. Study on maritime supervision of marine export of the “new trio” products. China Marit. Saf. 2024, 8, 25–28. [Google Scholar]
- Doron, A.; Yosef, T.; Boris, M.; Elena, M.; Ella, Z.; Liraz, A.; Joseph, S.G.; Hyeong-Jin, K. Design of electrolyte solutions for Li and Li-ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar]
- Stephen, J.H.; Adam, T.; William, J.P. A combustion chemistry analysis of carbonate solvents used in Li-ion batteries. J. Power Sources 2009, 193, 855–858. [Google Scholar]
- Stephen, W.; Ben, S.; Siddique, K.; Said, A.H. Preventing thermal runaway propagation in lithium ion battery packs using a phase change composite material: An experimental study. J. Power Sources 2017, 340, 51–59. [Google Scholar]
- Khateeb, S.A.; Amiruddin, S.; Farid, M.; Selman, J.R.; Al-Hallaj, S. Thermal management of Li-ion battery with phase change material for electric scooters: Experimental validation. J. Power Sources 2005, 142, 345–353. [Google Scholar]
- Fredrik, L.; Petra, A.; Per, B.; Anders, L.; Bengt-Erik, M. Characteristics of lithium-ion batteries during fire tests. J. Power Sources 2014, 271, 414–420. [Google Scholar]
- Paul, T.C.; Eric, C.D.; Ralph, E.W. Simplified thermal runaway model for assisting the design of a novel safe Li-Ion battery pack. J. Electrochem. Soc. 2022, 169, 040516. [Google Scholar]
- Ben, S.; Stephen, W.; Siddique, K.; Said, A.H. Experimental validation of a 0-D numerical model for phase change thermal management systems in lithium-ion batteries. J. Power Sources 2015, 287, 211–219. [Google Scholar]
- Austin, R.B.; Erik, J.A.; Kevin, C.M.; Ofodike, A.E. Explosion hazards from lithium-ion battery vent gas. J. Power Sources 2020, 446, 227257. [Google Scholar]
- Cui, Y.; Liu, J.H. Research progress of water mist fire extinguishing technology and its application in battery fires. Process Saf. Environ. Prot. 2021, 149, 559–574. [Google Scholar] [CrossRef]
- Ahmed, O.S.; Alex, G.; Yang, P.; Stanislavl, S. Experimental investigation of suppression of 18650 Lithium Ion cell array fires with water mist. Fire Technol. 2022, 58, 523–551. [Google Scholar]
- Andrey, W.G.; David, F.; Julian, W.; Helmar, W.; Christoph, S.; Gisela, F.; Gernot, V.; Alexander, T.; Viktor, H. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar]
- Gnanaraj, J.S.; Zinigrad, E.; Asraf, L.; Gottlieb, H.E.; Sprecher, M.; Aurbach, D.; Schmidt, M. The use of accelerating rate calorimetry (ARC) for the study of the thermal reactions of Li-ion battery electrolyte solutions. J. Power Sources 2003, 119, 794–798. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge. J. Power Sources 2003, 119, 330–337. [Google Scholar] [CrossRef]
- Zaghib, K.; Dubé, J.; Dallaire, A. Enhanced thermal safety and high power performance of carbon-coated LiFePO4 olivine cathode for Li-ion batteries. J. Power Soures 2012, 219, 36–44. [Google Scholar] [CrossRef]
- Liu, X.; Yin, L.; Ren, D.; Wang, L.; Ren, Y.; Xu, W.; Lapidus, S.; Wang, H.; He, X.; Chen, Z.; et al. In situ observation of thermal-driven degradation and safety concerns of lithiated graphite anode. Nat. Commun. 2021, 12, 4235. [Google Scholar] [CrossRef]
- GB 12268-2025; List of Dangerous Goods. State Administration for Market Regulation. Standardization Administration of China: Beijing, China, 2025.
- International Maritime Organization (IMO). International Maritime Dangerous Goods Code (IMDG Code); 2022 edition (Amendment 41-22); IMO Publishing: London, UK, 2022; Available online: https://www.imo.org/en/Publications (accessed on 29 April 2025).
- Holman, J.P. Heat Transfer, 10th ed.; McGraw-Hill Higher Education: Boston, MA, USA, 2010. [Google Scholar]
- Sun, P.; Huang, X.; Bisschop, R.; Niu, H. A review of battery fires in electric vehicles. Fire Technol. 2020, 56, 1361–1410. [Google Scholar] [CrossRef]
- Tao, W.Q. Heat Transfer; Higher Education Press: Beijing, China, 2024; pp. 172–267. [Google Scholar]
- Ji, K.; Guan, Y.; Zhou, X.D.; Li, C.L.; Wang, X.Z.; Xu, J.; Lan, S.F. Design and verification of structured high emissivity radiation surface. Spacecr. Environ. Eng. 2022, 39, 210–213. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).