A Study on the Flame and Pressure Characteristics of Ultrafine Calcium Carbonate (CaCO3) Powder in Suppressing Gas Explosions
Abstract
1. Introduction
2. Mechanism of CaCO3 Powder in Suppressing Gas Explosions
3. Experimental Equipment and Method
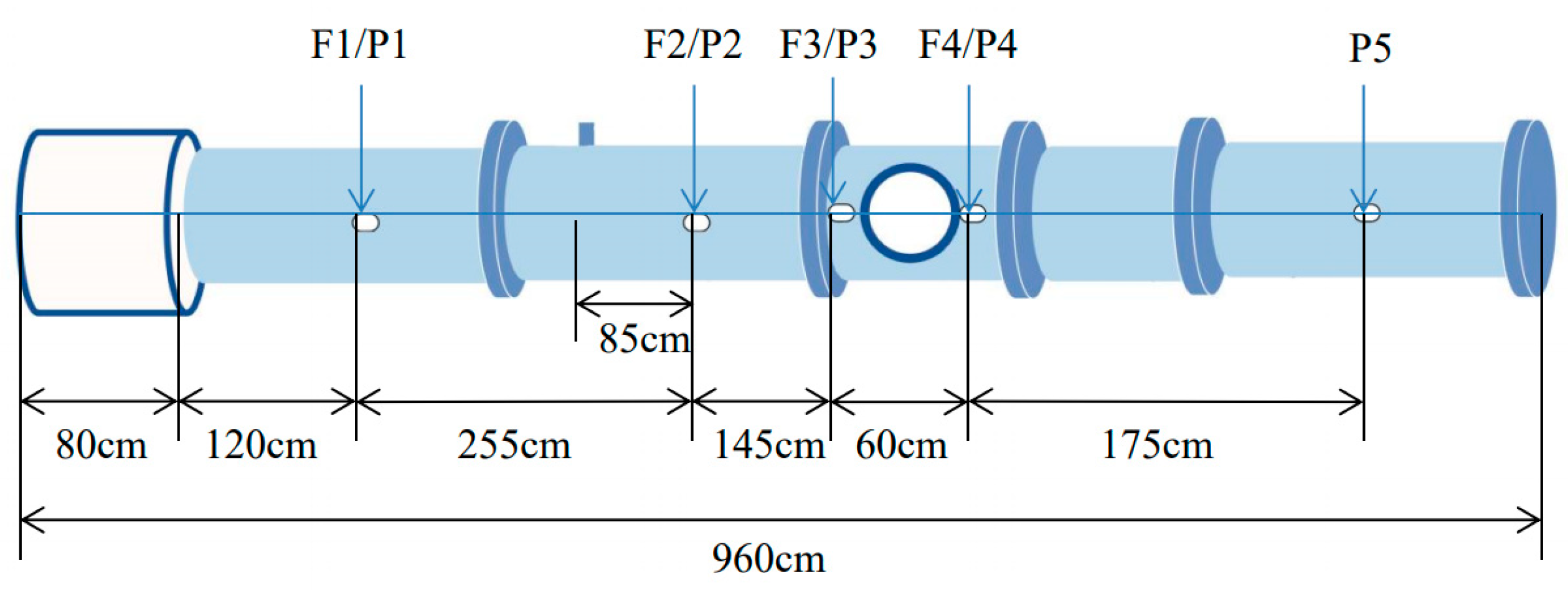
3.1. Experimental System
3.2. Experimental Method
4. Experimental Results
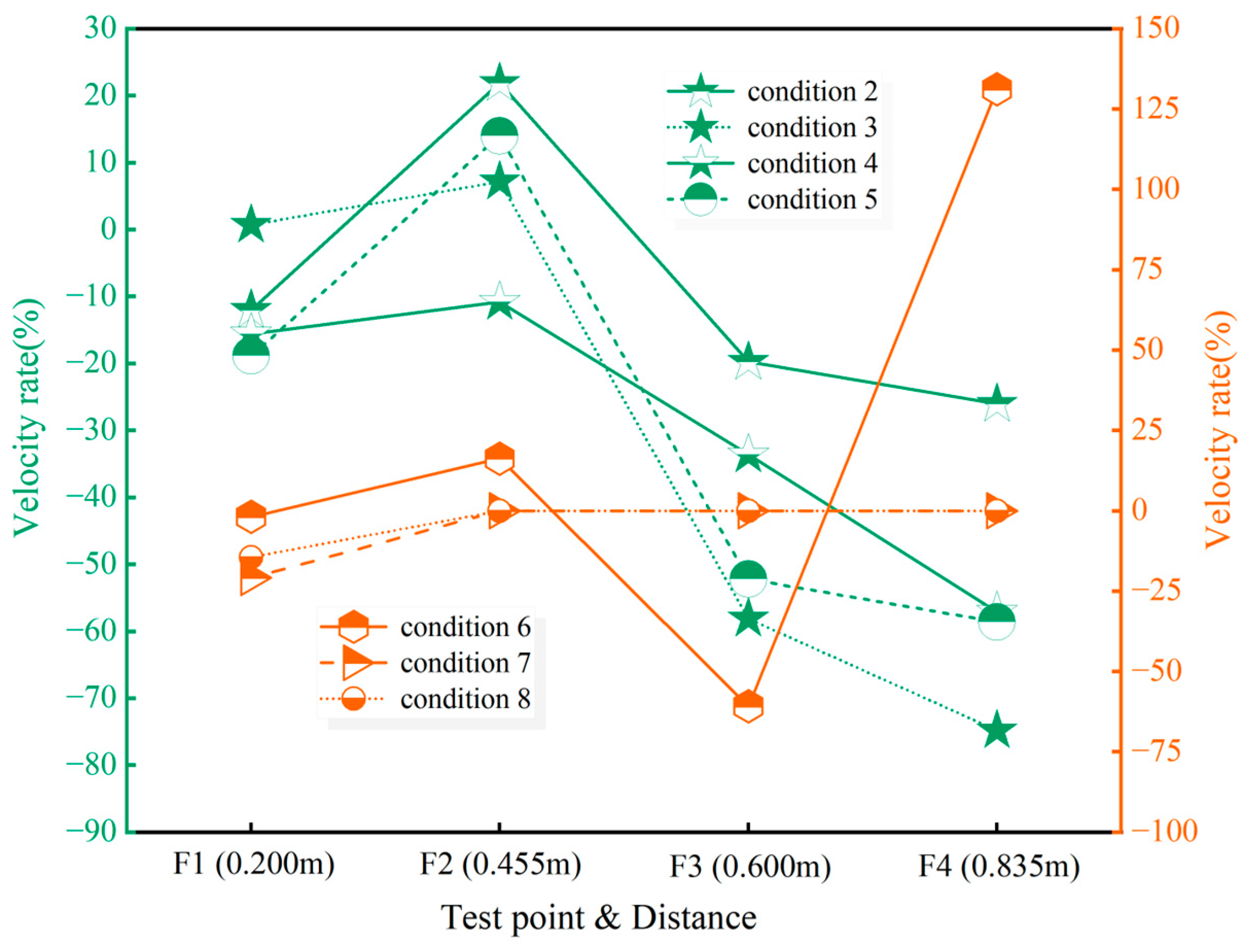
4.1. Flame Propagation Velocity
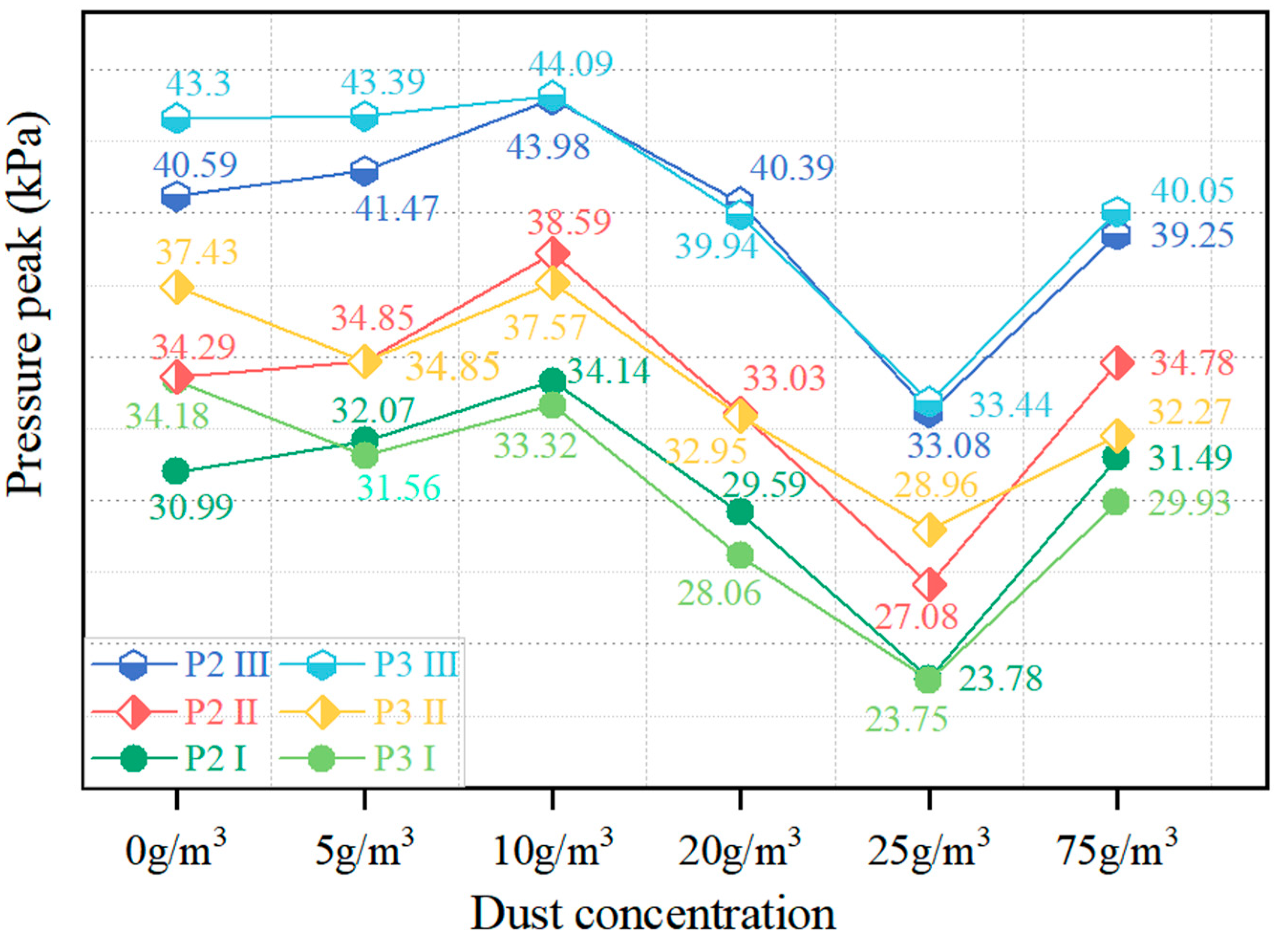
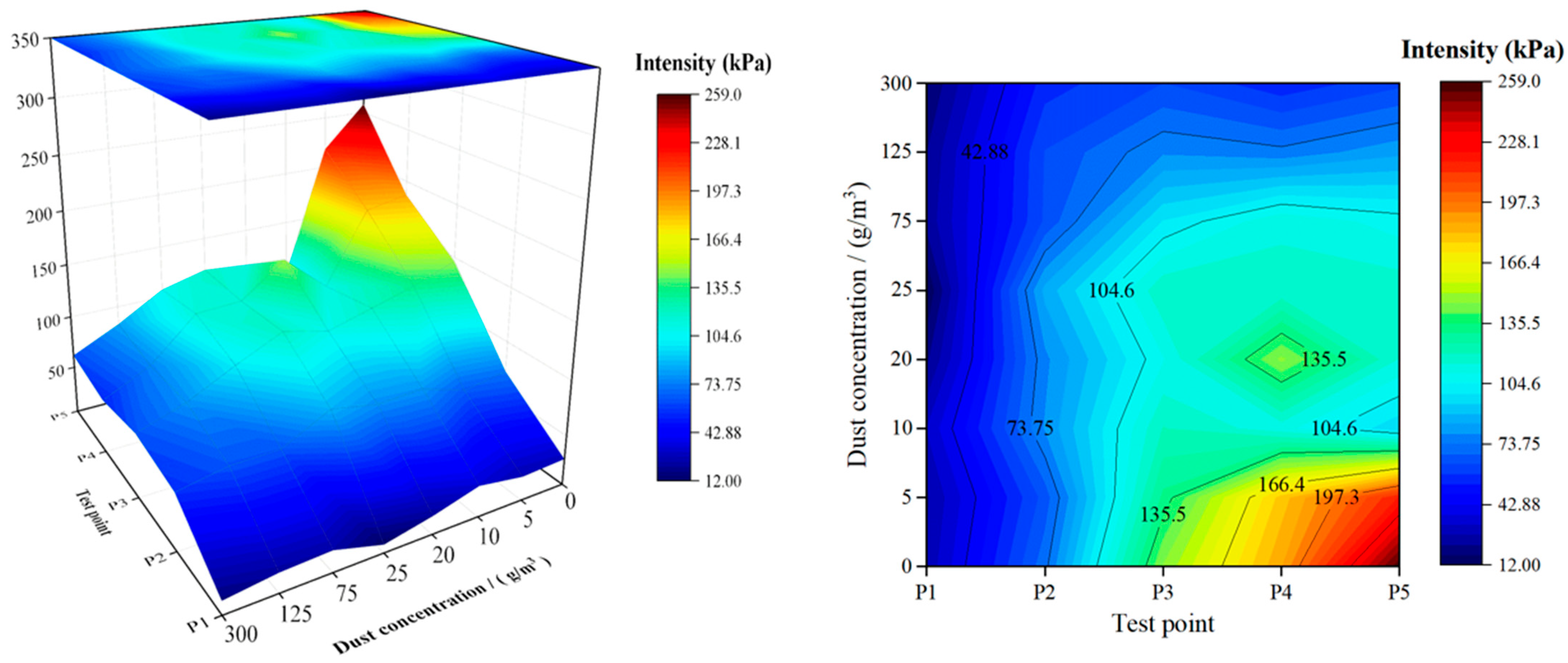
4.2. Explosion Pressure Peak
4.3. Discussion
5. Conclusions
- (1)
- When the concentration of CaCO3 powder is 125 g/m3 and 300 g/m3, the suppression effect on methane explosions is the most significant, with complete flame quenching observed. However, the explosion-induced pressure is not immediately eliminated; it gradually dissipates after repeated collisions of the shockwaves with the duct walls and mutual cancellation between positive and negative pressure zones due to energy loss. When the dust concentration is 20 g/m3, the suppression effect is optimal among the incomplete suppression conditions, with continuous flame suppression observed throughout the entire flame propagation process. Additionally, the first to third pressure waves at test points P2 and P3 are reduced, and the maximum explosion overpressure significantly decreases. When the concentration is 25 g/m3, the powder accelerates flame propagation at F2 (downstream of the injection point), and the resulting maximum overpressure is the highest among all test conditions. Therefore, this concentration is not recommended for industrial applications.
- (2)
- Although increasing the concentration of ultrafine CaCO3 powder can reduce the propagation rate of methane explosion flames to some extent, if the flame propagation is not completely interrupted, both excessively low and high concentrations may participate in chain reactions and temporarily accelerate methane combustion, thereby releasing more heat. As a result, ultrafine CaCO3 may act as a transient catalyst during the explosion process, accelerating flame propagation and further promoting the transmission of the flame and pressure. Therefore, in practical applications, it is recommended that an appropriate concentration of ultrafine CaCO3 is adopted as a suppressant for methane explosions.
- (3)
- The distance between the powder injection point and the test point significantly influences the effectiveness of inert powders in suppressing gas explosions, particularly regarding flame propagation. When the distance between the ignition end and the powder injection point is 3.7 m, and the distance from the injection point to the observation point is 1.7 m, selecting an ultrafine CaCO3 powder concentration of 125 g/m3 can completely suppress the flame and significantly reduce the maximum explosion overpressure. Within 4.55 m from the ignition end, using a concentration of 20 g/m3 is effective in suppressing both the flame and pressure, with a maximum flame suppression rate of 15.58%. Similarly, within 6 m, a concentration of 10 g/m3 can achieve approximately 60% flame suppression, although the initial flame acceleration effect of 75 g/m3 is 2.3 times that of 10 g/m3. Within 6.6 m, 10 g/m3 is preferable for the suppression of the flame speed, with suppression increasing from 58.13% to 74.82%, while 20 g/m3 is optimal when considering both flame and pressure suppression.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Qin, Y.; Lu, N.; Dai, S.; Zhang, Z. Study on the Flame Propagation Characteristics of Multi-point Methane Explosions in Long and Narrow Confined Spaces. Min. Metall. Explor. 2024, 41, 2479–2492. [Google Scholar] [CrossRef]
- Álvarez-Fernández, M.-I.; Prendes-Gero, M.-B.; Pola-Alonso, I.; Conde-Fernández, L.; Luengo-García, J.-C. Determination of the explosion parameters of methane-air mixtures as function of the ignition source and the volume and shape of the explosion chambers. J. Loss Prev. Process Ind. 2022, 80, 104862. [Google Scholar] [CrossRef]
- Shi, Z. Study on the Effect of Initial Pressure on the Methane Deflagration and Detonation Suppression Characteristics of NaHCO3. Master’s Thesis, Henan Polytechnic University, Jiaozuo, China, 2023. [Google Scholar]
- Li, M.; Wang, H.; Wang, D.; Shao, Z.; He, S. Risk assessment of gas explosion in coal mines based on fuzzy AHP and Bayesian network. Process Saf. Environ. Prot. 2020, 135, 207–218. [Google Scholar] [CrossRef]
- Nan, F.; Luo, Z.; Cheng, F.; Xiao, Y.; Su, B.; Li, R.; Wang, T. Study on the instability and suppression mechanism of methane/air deflagration flame by inert gas-halogenated hydrocarbons. Fuel 2024, 374, 132351. [Google Scholar] [CrossRef]
- Liu, R.Z.; Jia, B.S.; Wang, W. Numerical simulation of gas explosion suppression by ultrasonic water mist based on the Cloud, Fog, and Edge Computing. Environ. Technol. Innov. 2021, 21, 101369. [Google Scholar] [CrossRef]
- Li, H.; Zheng, L.; Wang, J.; Wang, X.; Xu, M.; Luo, Q.; Xu, Z. Synergistic inhibition of methane/air explosions by NaHCO3 particles with a bimodal size distribution. Powder Technol. 2024, 439, 119757. [Google Scholar] [CrossRef]
- Yu, M.; Yang, X.; Zheng, K.; Luan, P. Research Progress and Development Trends of Gas Explosion Suppression and Disaster Mitigation Technologies in Chinese Coal Mines. J. China Coal Soc. 2020, 45, 168–188. [Google Scholar]
- Zuo, Q.; Cheng, W.; Tang, J. Current Status and Prospects of Powder Explosion Suppressant Applications in Coal Mines. Coal Technol. 2010, 29, 78–80. [Google Scholar]
- Shi, Z.; Zheng, L.; Zhang, J.; Miao, Y.; Wang, X.; Wang, Y.; Tang, S. Effect of initial pressure on methane/air deflagrations in the presence of NaHCO3 particles. Fuel 2022, 325, 124910. [Google Scholar] [CrossRef]
- Jia, J.; Tian, X. Research Progress and Development Trends of Gas Explosion Suppression. J. Saf. Environ. 2025, 25, 95–107. [Google Scholar]
- Wang, Q.; Wen, H.; Wang, Q.; Sun, J. Inhibiting effect of Al(OH)3 and Mg(OH)2 dust on the explosions of methane-air mixtures in closed vessel. Sci. China Technol. Sci. 2012, 55, 1371–1375. [Google Scholar] [CrossRef]
- Dounia, O.; Vermorel, O.; Poinsot, T. Theoretical analysis and simulation of methane/air flame inhibition by sodium bicarbonate particles. Combust. Flame 2018, 193, 313–326. [Google Scholar] [CrossRef]
- Jia, J.Z.; Tian, X.Y.; Wang, F.X. Study on the effect of KHCO3 particle size and powder spraying pressure on the methane explosion suppression characteristics of pipe networks. ACS Omega 2022, 7, 31974–31982. [Google Scholar]
- Luo, Z.M.; Zhang, J.; Ren, J.Y.; Wang, T.; Kang, K.; Cheng, F.M.; Wang, Y.C. The Role of NH4H2PO4 Powder Thermal Decomposition Products in Gas Explosions. J. China Coal Soc. 2017, 42, 1489–1495. [Google Scholar]
- Liu, R.Z.; Zhang, M.C.; Jia, B.S. Inhibition of Gas Explosion by Nano-SiO2 Powder under the Condition of Obstacles. Integr. Ferroelectr. 2021, 216, 305–321. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhang, Q.; Ren, S.; Wu, J. Experimental investigation on micro-dynamic behavior of gas explosion suppression with SiO2 fine powders. Theor. Appl. Mech. Lett. 2011, 1, 032004. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, H.; Zhou, Y.; Gao, W. Flame Suppression of 100 nm PMMA Dust Explosion by KHCO3 with Different Particle Size. Process Saf. Environ. Prot. 2019, 132, 303–312. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Xu, H.; Piotr, W. Suppression of Gas Explosion Flame Propagation in Large Pipelines by Ultrafine Powder Curtains. J. China Coal Soc. 2017, 42, 1482–1488. [Google Scholar]
- Ding, C.; Wang, X.; Xu, H.; Tang, Q.; Kong, L. Suppression and Enhancement Effects of Sprayed Ultrafine ABC Powder on Gas Explosions. J. China Coal Soc. 2021, 46, 1799–1807. [Google Scholar]
- Omar, D.; Jaravel, T.; Vermorel, O. On the controlling parameters of the thermal decomposition of inhibiting particles: A theoretical and numerical study. Combust. Flame 2022, 240, 111991. [Google Scholar] [CrossRef]
- Li, M.; Xu, J.; Li, Q.; Wang, C.; Wang, B.; Jiang, J. Explosion mitigation of methane/air mixture in combined application of inert gas and ABC dry powders in a closed compartment. Process Saf. Prog. 2020, 39, 12101. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Ding, C.; Wang, X. Hydrophobic nano SiO2 as flow-enhancing additives and flame retardant synergizes with CaCO3 to suppress gas explosion. RSC Adv. 2021, 11, 4672–4681. [Google Scholar] [CrossRef]
- Wen, H.; Wang, Q.H.; Deng, J.; Luo, Z.M. Effect of Ultrafine Al(OH)3 Powder Concentration on Methane Explosion Pressure. J. China Coal Soc. 2009, 34, 1479–1482. [Google Scholar]
- Liu, J.; Meng, X.; Yan, K.; Dai, W.; Wang, Z.; Li, F.; Yang, P.; Liu, Y. Study on the effect and mechanism of Ca(H2PO4)2 and CaCO3 powders on inhibiting the explosion of titanium powder. Powder Technol. 2022, 395, 158–167. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Zhai, F.; Li, J.; Yu, M. Inhibition effect and reaction mechanism of NaHCO3 and NH4H2PO4 on the deflagration of methane/coal dust mixtures. Adv. Powder Technol. 2025, 36, 104866. [Google Scholar] [CrossRef]
- Giammaria, G.; Lefferts, L. Catalytic effect of water on calcium carbonate decomposition. J. CO2 Util. 2019, 33, 341–356. [Google Scholar] [CrossRef]
- Wang, G. Study on the Chemical Kinetic Mechanism of Methane Combustion Based on Shock Tube Experimental Platform. Master’s Thesis, University of Science and Technology of China, Hefei, China, 2008. [Google Scholar]
- Xu, J.; Lu, S.; Lu, H.; Liu, J.; Wang, Z. Numerical Analysis of the Mechanism of Calcium Carbonate Suppression of Methane-Coal Dust Explosions in Pipelines. J. North China Univ. Sci. Technol. 2024, 21, 73–78. [Google Scholar]
- Xu, J.; Lu, S.; Lu, H.; Liu, J.; Xu, Z.; Wang, Z. Experimental Study on the Inhibition of Coal Dust–Methane Explosions in Pipelines by Calcium Carbonate. Blasting. pp. 1–15. Available online: http://kns.cnki.net/kcms/detail/42.1164.tj.20250311.0920.002.html (accessed on 30 April 2025).
- Ajrash, M.J.; Zanganeh, J.; Moghtaderi, B. Deflagration of premixed methane-air in a large scale detonation tube. Process Saf. Environ. Prot. 2017, 109, 374–386. [Google Scholar] [CrossRef]



| Condition Number | Ultrafine CaCO3 Concentration | Condition Number | Ultrafine CaCO3 Concentration |
|---|---|---|---|
| 1 | 0 g/m3 | 2 | 5 g/m3 |
| 3 | 10 g/m3 | 4 | 20 g/m3 |
| 5 | 25 g/m3 | 6 | 75 g/m3 |
| 7 | 125 g/m3 | 8 | 300 gm3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Xu, Z.; Ji, S.; Xu, J.; Hu, Y.; Liu, J.; Wang, Z. A Study on the Flame and Pressure Characteristics of Ultrafine Calcium Carbonate (CaCO3) Powder in Suppressing Gas Explosions. Fire 2025, 8, 203. https://doi.org/10.3390/fire8050203
Li G, Xu Z, Ji S, Xu J, Hu Y, Liu J, Wang Z. A Study on the Flame and Pressure Characteristics of Ultrafine Calcium Carbonate (CaCO3) Powder in Suppressing Gas Explosions. Fire. 2025; 8(5):203. https://doi.org/10.3390/fire8050203
Chicago/Turabian StyleLi, Guiyuan, Zuohui Xu, Sihan Ji, Jingde Xu, Yang Hu, Junhai Liu, and Zhie Wang. 2025. "A Study on the Flame and Pressure Characteristics of Ultrafine Calcium Carbonate (CaCO3) Powder in Suppressing Gas Explosions" Fire 8, no. 5: 203. https://doi.org/10.3390/fire8050203
APA StyleLi, G., Xu, Z., Ji, S., Xu, J., Hu, Y., Liu, J., & Wang, Z. (2025). A Study on the Flame and Pressure Characteristics of Ultrafine Calcium Carbonate (CaCO3) Powder in Suppressing Gas Explosions. Fire, 8(5), 203. https://doi.org/10.3390/fire8050203





