1. Introduction
Peat soils form through the accumulation of partially decomposed organic matter under flooded, anoxic conditions [
1]. Globally, peatlands cover an area of only 4.23 million square kilometers (approximately 2.84% of the world’s land area) [
2]; however, they contain one-third of all soil carbon [
3]. If ignited, peat fires can smolder and burn for months or even years. Greenhouse gas emissions from peat fires account for approximately 15% of anthropogenic emissions [
4].
Indonesia, with its vast areas of peat soils, may see more active fires in the future resulting from the intensification of droughts and decreasing annual precipitation [
5]. The largest, most recent fire in Indonesia was in 2015, which burned approximately 4.6 MHa, releasing 0.89 Gt of carbon dioxide equivalent [
6] and causing USD 28 billion in economic losses [
7]. Peat fires cause smoke pollution, health hazards, economic damage, and other ecological impacts that may extend to neighboring countries [
8].
Various measures have been implemented to manage peat fires, including the development of early fire detection systems [
9], the creation of firebreaks [
10] by digging trenches in the soil, and direct firefighting efforts. Water is the most commonly used firefighting agent and is applied both aerially [
11] and on the ground. In ground-based firefighting, water is either sprayed onto the soil surface or directly injected into the affected area [
12]. The addition of firefighting agents, such as surfactants, to the water used for firefighting has also been explored [
13,
14]. Surfactants reduce the surface tension [
15], enhancing the water’s ability to penetrate combustible materials. For combustion to continue, fuel, oxygen, and heat must be present. If any of these elements are removed, combustion will cease. Water is an effective fire suppressant because it absorbs large amounts of heat as it vaporizes. However, its high surface tension prevents it from penetrating the heat source. The application of aqueous solutions of firefighting agents can help penetrate combusting materials, helping absorb heat and lower the temperature below the combustion threshold. Firefighting agents that enhance surface wetting and penetration may be particularly effective for peat fires because peat soils have hydrophobic surfaces (
Figure 1). However, the widespread use of synthetic surfactants raises concerns about their environmental toxicity.
To mitigate the environmental impact of firefighting agents, soaps have been used as surfactants [
16]. When the soap component of firefighting agents mixes with water, it reacts with calcium ions and other substances, forming soap scum. Because soap scum has minimal toxicity, it does not affect rice germination or growth [
17]. Furthermore, because soap has low toxicity to aquatic organisms [
16], it may have minimal long-term environmental consequences.
Subekti et al. [
18] investigated the use of an optimized formulation using palm-derived soap as a surfactant in firefighting agents that reduced both the volume of water needed and the time required to extinguish fires. Rivai et al. [
19] found that certain additives can improve firefighting efficiency. Rizka et al. [
20] identified the optimal conditions for foam agent production, while Pramuhadi et al. [
21] determined the most effective spraying method.
To evaluate fire suppression performance, Kanyama et al. [
22] investigated the use of soap-based firefighting agents on peat soil that had been burning for 4 h in a 1.5 m × 1.5 m × 0.3 m plot. Subekti et al. [
23] investigated the efficacy of soap-derived firefighting agents on peat soil that had been burning for 3–4 h in a 0.1 m
2 box. However, these studies may have been unable to fully replicate actual peat fire conditions. This is because actual peat fires burn for a long time and spread over a wide area. Although Ramadhan et al. [
24] and Dianti et al. [
25] set fire to peat soil for approximately 20 h, they only evaluated the fire suppression ability of water alone.
Fire-extinguishing experiments on peat fires have been conducted both in the laboratory and the field, but the issue of combustion time has not been adequately addressed. However, while extended burning times have been evaluated at the laboratory scale, very few simulations have been replicated in the field. Peat fires can change combustion stages over time [
26]. Investigating longer burn times are important because a 4 h fire may be insufficient for combustion expansion in peat fires [
27], for which longer burn times have been reported. To reproduce a more realistic peat fire, the combustion time was set to 24 h. In the present study, a long-lasting peat fire was reproduced by setting the combustion time to 24 h and extinguished by spraying either water or a 1 vol.% soap-based firefighting agent solution (SFAS). The efficiency of SFAS in extinguishing the fire was also evaluated by determining the volume of water and length of time required to extinguish the fire. Furthermore, the cost-effectiveness of using SFAS was estimated by evaluating the labor and other costs required for firefighting activities. The characteristics of peatland environments vary by location, which may also impact the fire-extinguishing efficacy. In this study, evaluations were conducted on peat soils from two different locations in Indonesia to compare the effectiveness of soap-based firefighting agents on peat fires in different locations.
2. Materials and Methods
2.1. Preparation of the Soap-Based Firefighting Agent
The two main components of the soap-based agent used in this study were potassium laureate and potassium oleate, which were prepared using lauric acid (Cognis Oleochemicals Co., Ltd., Selangor, Malaysia) and oleic acid (Miyoshi Oil and Fat Co., Ltd., Sumida, Japan). Sodium salt of aspartic acid diacetate (ASDA) was used as the chelating agent, while water, propylene glycol (PG), and methyl pentanediol (MPD) were used as diluents.
Purified water was mixed with 18.3 wt.% ASDA under stirring at 50 °C, followed by the sequential addition of 12.5 wt.% PG, 5.3 wt.% MPD, 3.4 wt.% potassium hydroxide (48 wt.%), 5.7 wt.% lauric acid, and 7.0 wt.% oleic acid to adjust the pH to 10.2. These fatty acid salts exhibited the best performance of foaming and foam stability [
16]. The resulting soap-based firefighting agent was a light-yellow liquid that readily dispersed in water. The viscosity was 21.6 cSt at 20 °C and 302.5 cSt at −10 °C, while the specific gravity was 1.156 at 20 °C.
2.2. Study Area
Firefighting experiments were conducted at two sites in Palangka Raya City, Central Kalimantan, Indonesia. The JR site, was located in the proximity of the University of Palangka Raya, Jekan Raya Sub-District (2°13′23.2″ S, 113°52′56.0″ E), while the KL site, was located in the Kalampangan area of Sabangau Sub-District (2°17′21.6″ S, 114°01′58.4″ E).
2.3. Moisture Content of the Peat Soils
Twenty-five peat soil blocks (0.3 m × 0.3 m × 0.3 m) were collected, packed into 1.5 m × 1.5 m wooden frames, and dried to create peat boxes. Soil moisture was measured at 10 random locations within each peat box using a soil moisture meter (DM-18R, Takemura Electric Works. Ltd., Toshima, Japan; measuring range: 12.1–58.0%; accuracy: ±0.1%) and the values were averaged.
2.4. Water Analysis
The pH and total hardness of the water used in the experiments were measured using a pH meter (LAQUAtwin, Horiba, Ltd., Kyoto, Japan; measuring range: 0–14; accuracy: ±0.1%) and pack test (WAK-TH, Kyoritsu Chemical-Check Lab., Corp., Yokohama, Japan; measuring range: 0–200 ppm), respectively.
2.5. Soil Permeability Test
A soil permeability test was conducted using the water drop penetration time method [
28]. For this method, approximately 60 g of peat soil was collected in a glass Petri dish, and approximately 200 µL water or SFAS diluted to 1 vol.% with groundwater was dripped onto the surface using a syringe (
Figure 2). The time required for the droplets to completely penetrate into the peat soil was recorded.
2.6. Surface Tension Measurements
The surface tension of SFAS diluted to 1 vol.% with purified water and that of purified water were measured using a surface tensiometer (DY-500, Kyowa Interface Science Co., Ltd., Niiza, Japan; measuring range: 0–1000 mN/m; accuracy: ±0.02 mN/m).
2.7. Firefighting Experiments
Firefighting experiments based on the mesoscale experiments described by Kanyama et al. [
22] were conducted on 15–23 September 2023, using 1.5 m × 1.5 m × 0.3 m peat boxes. At each of the JR and KL sites, two plots were prepared for firefighting experiments with water (W1, W2) and another two for firefighting experiments with SFAS (SFAS1, SFAS2). A paraffin igniter and wood were used to burn the peat. Although the ignition method differed from that described by Kanyama et al. [
22], the duration of peat combustion for 24 h was confirmed in a small-scale experiment. Specifically, six paraffin igniters and 1.6 kg of dry acacia wood were placed in the center of each peat box. The igniters were heated using a gas burner for 1 min to ignite the wood (
Figure 3). The fire was allowed to burn for 24 h. Although the soft fiberboard used by Kanyama et al. was unavailable, the peat soil combustion was nevertheless maintained for 24 h, validating the experiment. The temperature distribution on the peat soil surface was captured using a thermal imaging camera (E6xt, FLIR Systems Inc., Wilsonville, OR, USA; measuring range: −20 °C to 550 °C; accuracy: ±2 °C).
The firefighting experiments were initiated 24 h after the start of the fire. Groundwater alone or mixed with soap-based firefighting agents were applied at the recommended concentration of 1 vol.%. The SFAS was prepared using groundwater from each experimental site by consecutively feeding a soap-based firefighting agent and groundwater in that order into a backpack water tank (Jet Shooter EV, Ashimori Industry Co., Ltd., Settsu, Japan). The pH of the SFAS was 10.7. The backpack water tank was used to evenly spray the SFAS or groundwater until the peat surface temperature dropped below 50 °C. Additional firefighting was conducted if smoke was detected within 24 h after the initial efforts, or if the surface temperature exceeded 50 °C, as measured with the thermal imaging camera. The water volume and length of time required for extinguishing the fire were recorded. The extinguishing time was defined as the total work time required from the start of the firefighting activities until the fire was completely extinguished. The amount of water sprayed per unit area was calculated based on the total water used and the burned area. The burned area was measured using an iron ruler and calculated by dividing it into squares, triangles, and other polygons, as required.
3. Results and Discussion
The average temperature and humidity recorded during the experiment were 33.0 °C and 69.7% at the JR site and 32.0 °C and 59.8% at the KL site, respectively. The water content of the peat soil surface in the peat boxes at each site was 32.3% and 40.2%, respectively. The pH and total hardness of the groundwater were 5.0 and 10 ppm at the JR site and 5.3 and 10 ppm at the KL site, respectively. Although soap typically loses its function because of variations in pH and hardness, our experiments confirmed that the soap additive remained effective at approximately pH 5 and maintained a total hardness of up to 100 ppm, indicating no issues with its use in this context [
22].
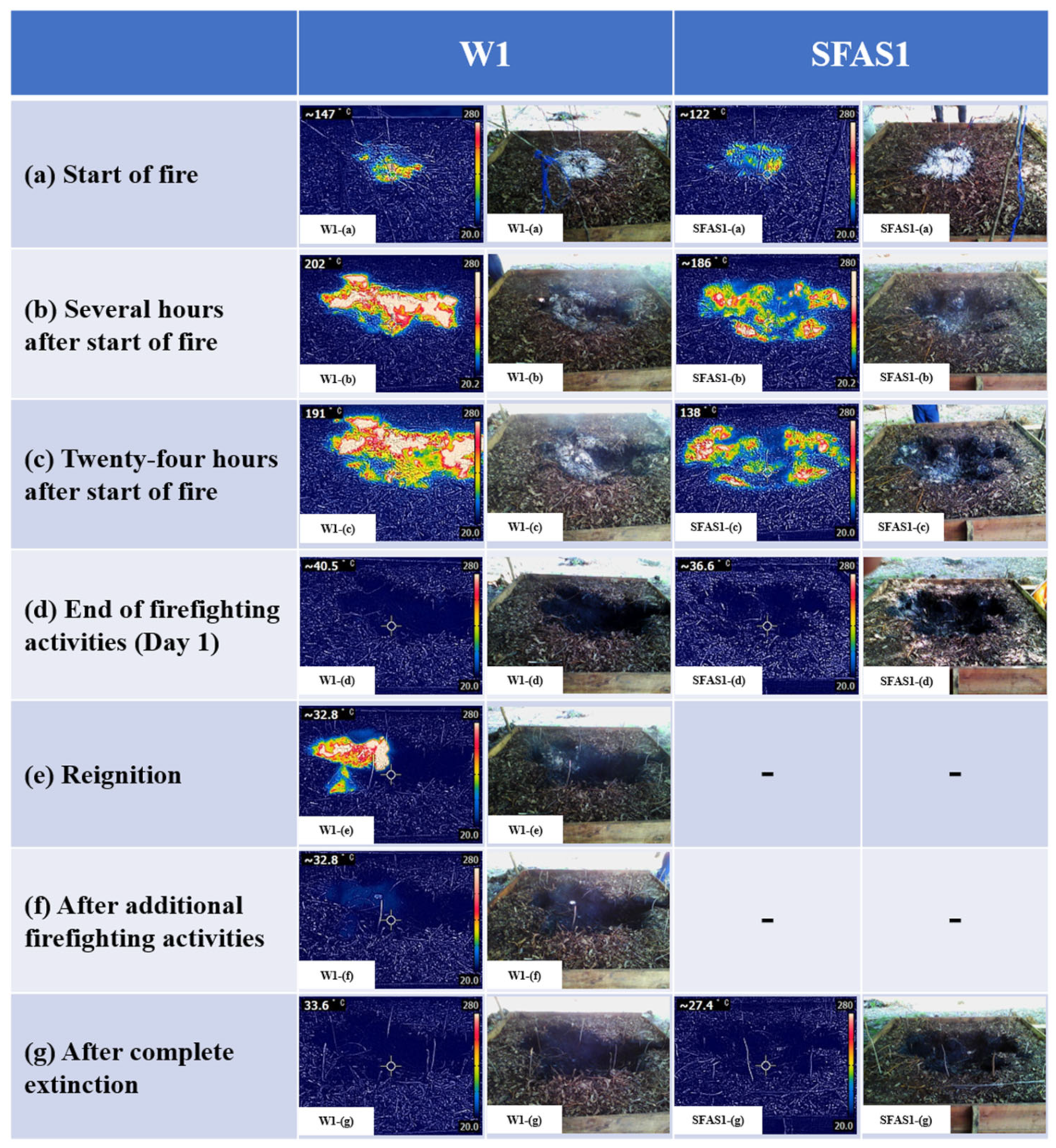
Figure 4 presents thermal and photographic images of one water-treated plot (W1) and one SFAS-treated plot (SFAS1) at the JR site at various stages: start of fire, after several hours, at 24 h, after additional extinguishing, and after complete extinction.
Figure 5 presents images at the KL site. As shown in
Figure 4 and
Figure 5a–c, the combustion area expanded over time. The white regions in
Figure 4 and
Figure 5 indicate temperatures exceeding 280 °C, which is above the 275 °C threshold for peat pyrolysis [
29], thereby confirming the presence of a peat fire. Furthermore, the ground temperatures measured using the thermocouples showed that a 5 cm section of the ground reached approximately 275 °C or higher.
At 24 h after the fire began (
Figure 4 and
Figure 5c), firefighting activities were initiated, and surface temperatures temporarily dropped below 50 °C in all plots during firefighting activities. Two plots at the JR site (W1, SFAS2) and four plots at the KL site were sprayed again when the surface temperatures exceeded 50 °C over time. At the KL site, two water-treated plots (W1, W2) initially had surface temperatures below 50 °C, as shown in
Figure 5W1-(d); however, reignition occurred within 24 h of the initial firefighting activities, and hot spots appeared (
Figure 5W1-(e)); thus, additional firefighting was conducted (
Figure 5W1-(f)). In contrast, no reignition occurred in the SFSA-treated plots at this site, requiring no further firefighting. Furthermore, no reignition occurred within 24 h of the initial firefighting activities for the four plots at the JR site.
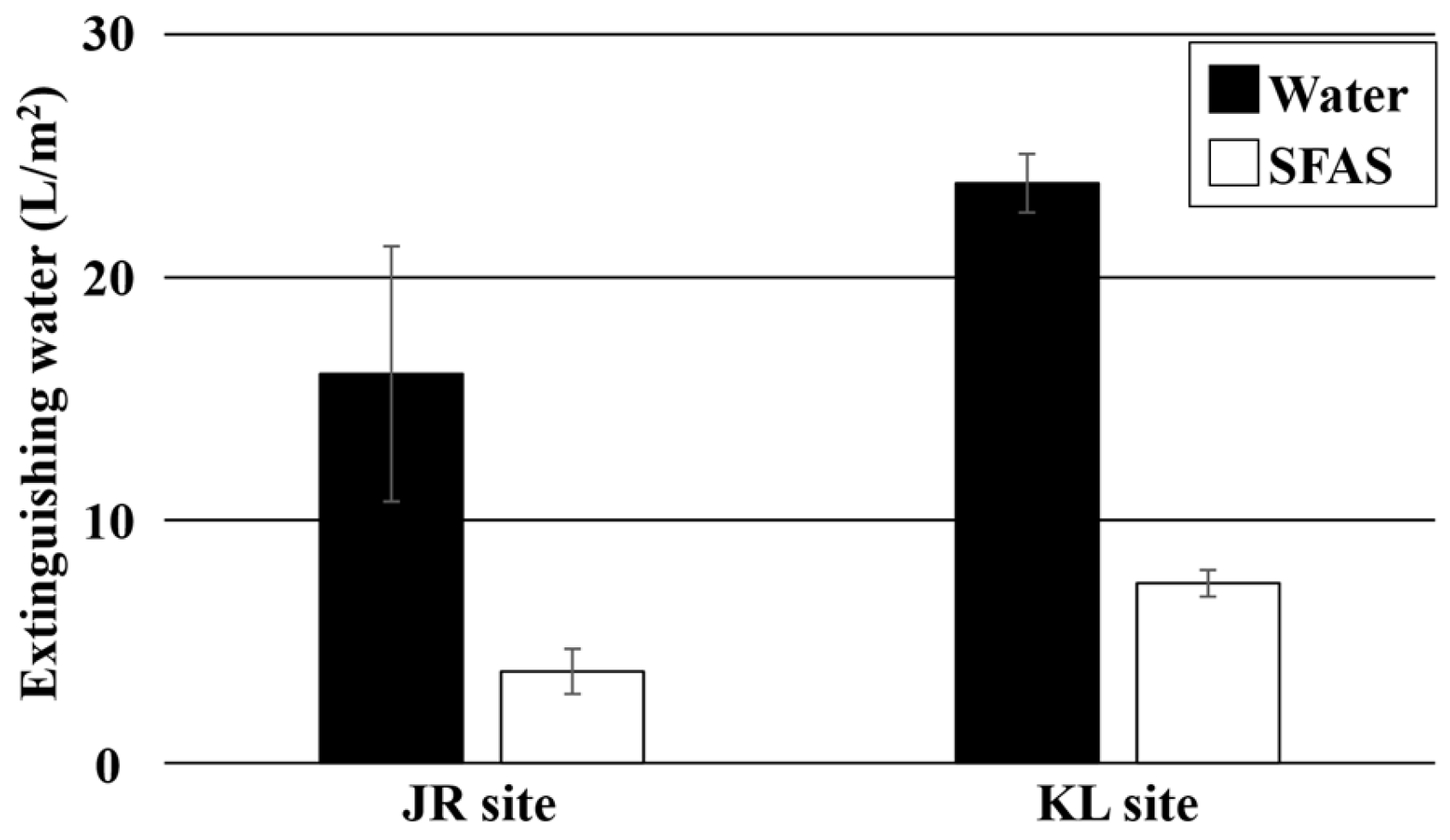
The water-treated plots at the JR site required 17.8 L (W1) and 35.2 L (W2), whereas the SFAS-treated plots required 2.1 L (SFAS1) and 10.6 L (SFAS2). At the KL site, the water-treated plots required 9.2 L (W1) and 15.2 L (W2), whereas the SFAS-treated plots required 1.5 L (SFAS1) and 5.7 L (SFAS2) to extinguish the fire. The amount of water applied per unit area was calculated from the amount of water used and the total burned area in each box. As shown in
Figure 6, the amount of water applied per unit area was 16.0 L/m
2 and 3.8 L/m
2 for the water- and SFAS-treated plots at the JR site, respectively, whereas at the KL site, 23.9 L/m
2 and 7.4 L/m
2 were required for the water- and SFAS-treated plots, respectively. The results were calculated from two averages. The lower water requirement to extinguish an SFAS-treated area than a water-treated plot is similar to the finding from a previous report [
22]. Subekti et al. [
18,
23] also reported a decrease in the amount of water required to extinguish fires when using firefighting agents containing surfactants. Furthermore, SFAS in this study may be effective against long-term burning peat fires.
At both the JR and KL sites, the amount of water required to extinguish the fires in the SFAS-treated plots was approximately one-quarter of that needed for the water-treated plots. This difference was likely due to the high penetrative ability of the SFAS into peat soil. The agent contained a soap component, which acted as a surfactant. It had a surface tension of 31.2 mN/m, which is approximately half that of water (72.2 mN/m). At both sites, the time required for SFAS to penetrate into the peat soil under nonburning conditions was approximately one-sixth of that required for water, indicating that the agent was approximately six times faster than water in penetrating the peat soil. As the surface tension of SFAS was expected to be low even under combustion, it is expected to more efficiently penetrate the peat soil, reaching the combustion point and lowering the sub-surface temperature, thereby exerting a cooling effect through evaporative heat. SFAS can disrupt combustion by absorbing heat and preventing its continuation. As previously mentioned, the use of SFAS reduced the amount of water required to extinguish the peat fires. The difference in the amount of water applied per unit area in the SFAS-treated plots was attributed to the variations in the peat soils in this study. We speculate that the peat soil at the JR site contained more tree roots than those at the KL site, which may have allowed water to penetrate more easily through the root gaps, reducing the amount of water required to extinguish the fire.
Figure 7 shows the time needed to extinguish the fire. Overall, 29 and 11 min were required for the water-treated and SFAS-treated plots at the JR site, respectively, while 21 and 8 min were required for those at the KL site, respectively. More water was needed for the water-treated plots because of the high surface tension of water, which hindered penetration into the peat soil. Conversely, less water was used for the SFAS-treated plots, enabling the fire to be extinguished faster. The use of SFAS reduced the firefighting time, allowing firefighting to be completed more rapidly and efficiently. The use of firefighting agents has been reported to reduce the time required to extinguish fires [
13,
19,
23], but it has been suggested that the composition of the firefighting agents as well as the kind of peat soil may have an effect on the rate of reduction.
In the mesoscale experiment by Kanyama et al. at the same location, 8.1 L/m
2 of water was required to extinguish a 4 h burn [
22], whereas, in this study, 23.9 L/m
2 of water was required for a 24 h burn. We speculate that the reason for the increased water use was greater heat accumulation resulting from the prolonged combustion. Consequently, the amount of water needed to extinguish the 24 h fire was higher.
The extinguishing efficiency of the SFAS was also examined. Considering that in Palangka Raya firefighting activities against peat fires are conducted by a team of 20–30 people or less [
30], a cost comparison was conducted between activities using SFAS and those using water alone, as performed by a 20 person team working 5 h per day at a labor cost of USD 15.8 [
31]. The cost of extinguishing a peat fire using SFAS over an initial burning area of 0.1 ha was lower than that using water alone. The total cost included only labor costs and the cost of the firefighting agent and excluded expenses for fuel, food, and other firefighting-related costs. Furthermore, two advantages associated with the use of SFAS were identified. Because reignition was observed under the treatment with water alone in the present study, this phenomenon can be expected in actual peat fires, indicating the need for additional firefighting activities the next day. Therefore, the first advantage involves avoiding the additional cost for the extra day of additional firefighting. Second, using water alone requires a substantially larger water volume than using SFAS, which may be difficult to obtain during the dry season. Therefore, soap-based firefighting agents are considered a cost-effective option. Furthermore, if the environmental impact of SFAS (e.g., quicker restoration of vegetation) is considered, additional cost benefits can be expected.
The use of SFAS shortens the time needed to extinguish fires, enhances firefighting efficiency, and reduces the burden on firefighters, enabling safer firefighting operations. Furthermore, the ability to quickly extinguish fires can reduce the time spent at a single site, enabling firefighters to manage multiple fire sites. However, the size of the peat fire simulated in this study was small, and its combustion dynamics may thus differ from those of a full-scale real peat fire. Thus, further evaluations need to be conducted on a larger scale. Soap-based firefighting agents may be effective on all types of peat soils, including those found in Indonesia.