Sustainable Synthesis of Hydro Magnesite Fire Retardants Using Seawater: Characterization, Yield Modeling and Process Optimization
Abstract
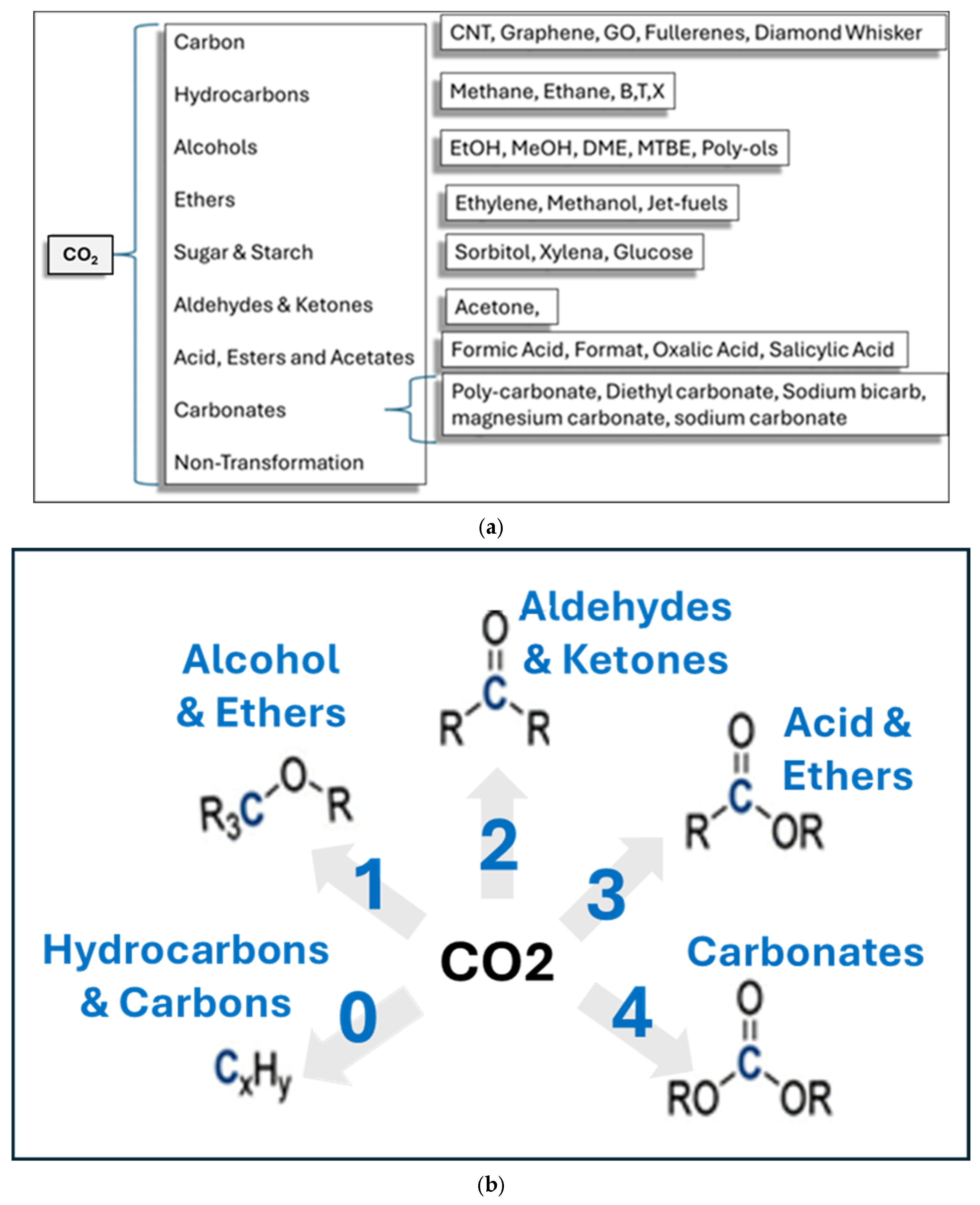
1. Introduction
2. State of the Art
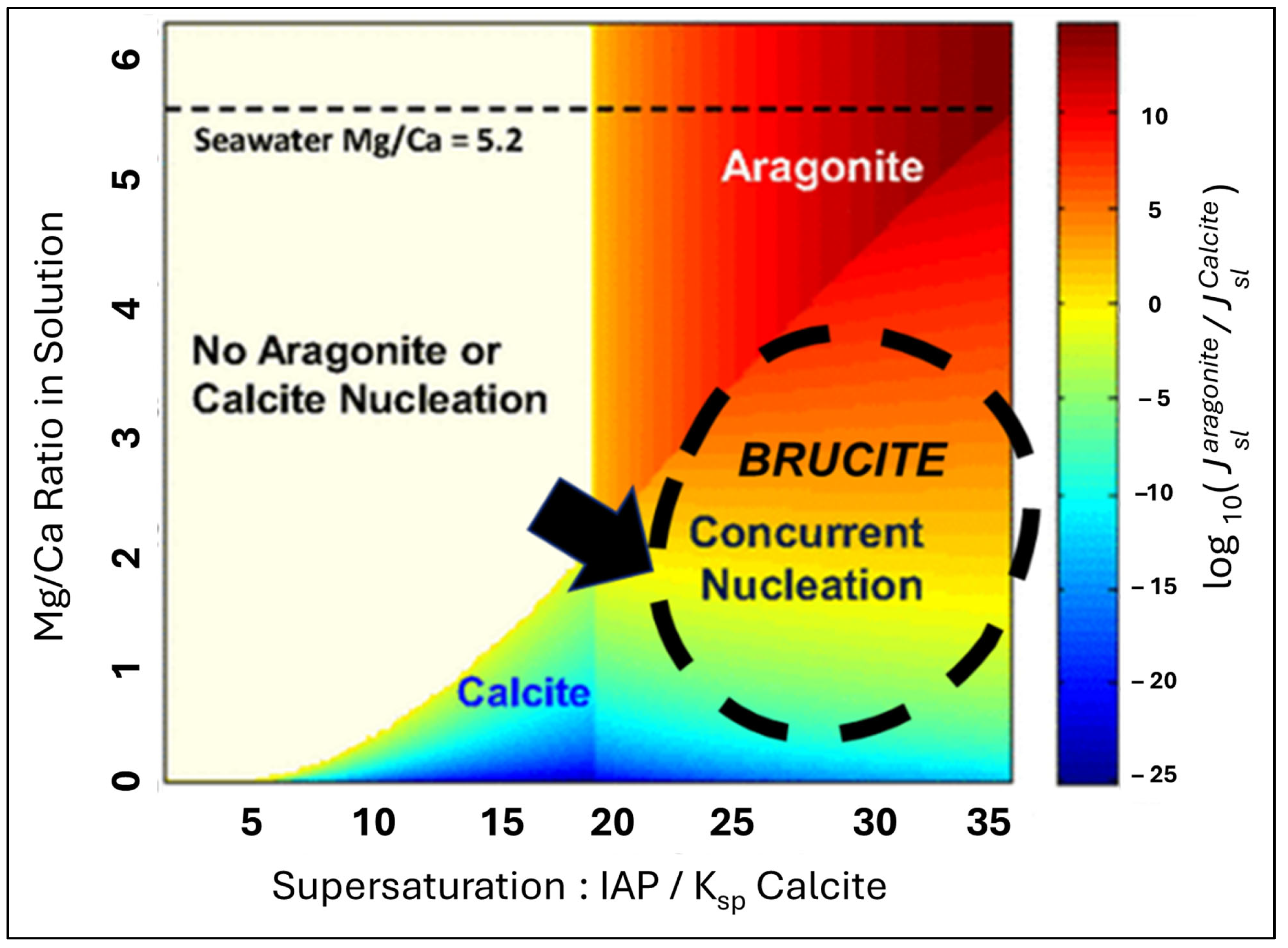
3. Theory
4. Method
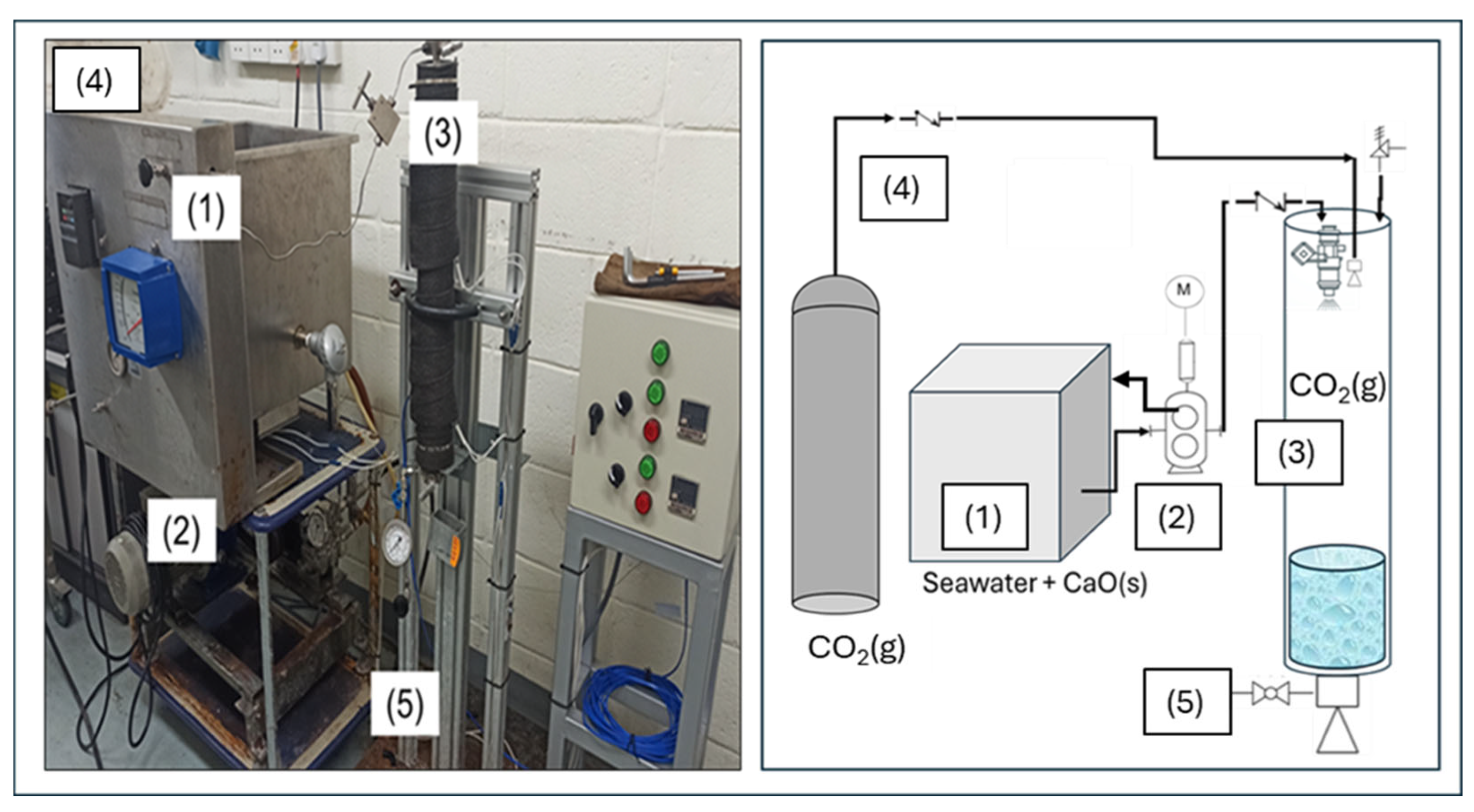
4.1. Experimental Setup and Procedure Using Lab Scale Glass Reactor
4.2. Feedstock Preparation
4.3. Experimental Setup and Procedure Using Continuous Reactor
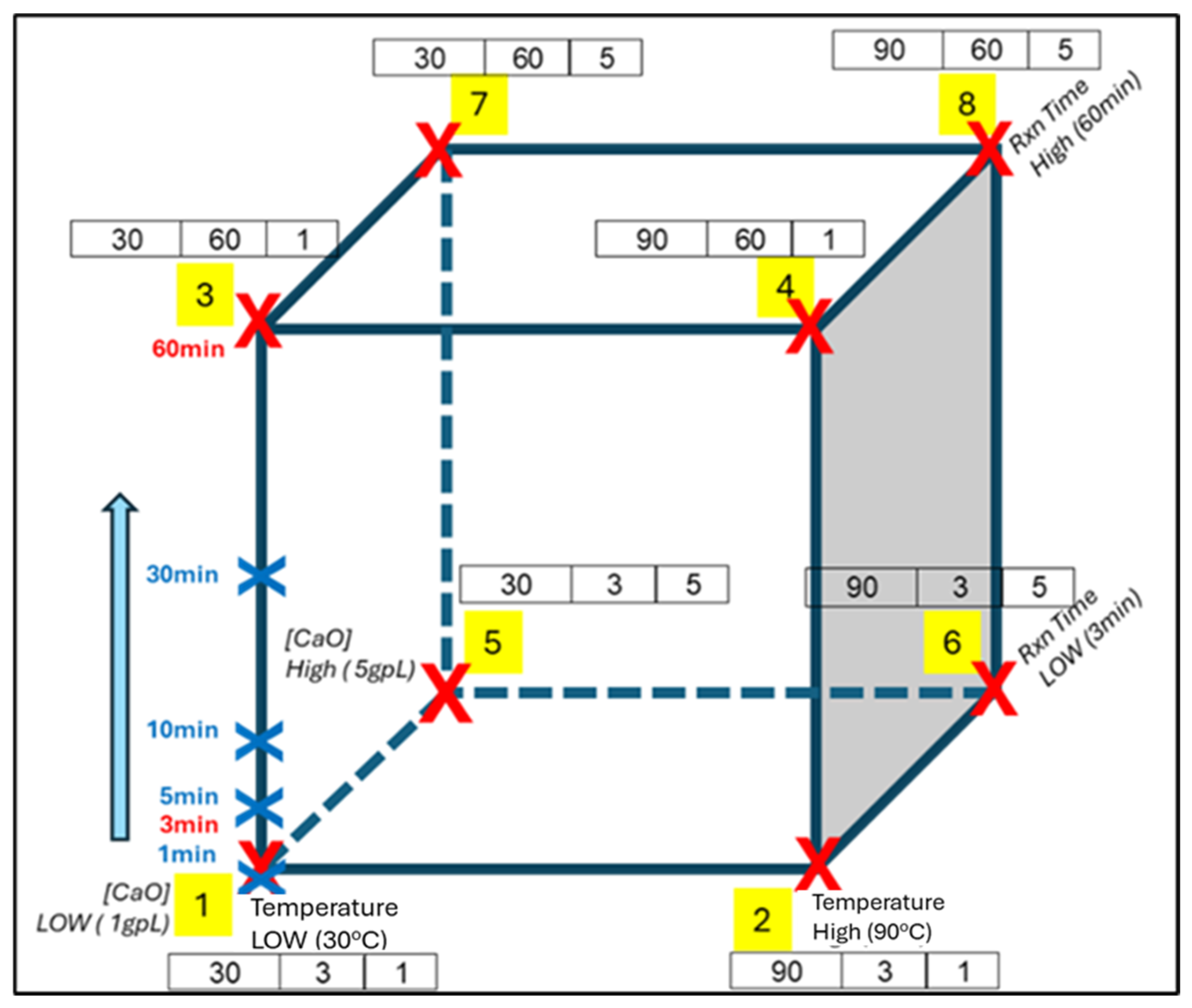
5. Design of Experiment
5.1. Design of Experiment
α_1 × A + α_2 × B + α_3 × C + α_4 × AB + α_5 × BC + α_c × AC + α_7 × ABC
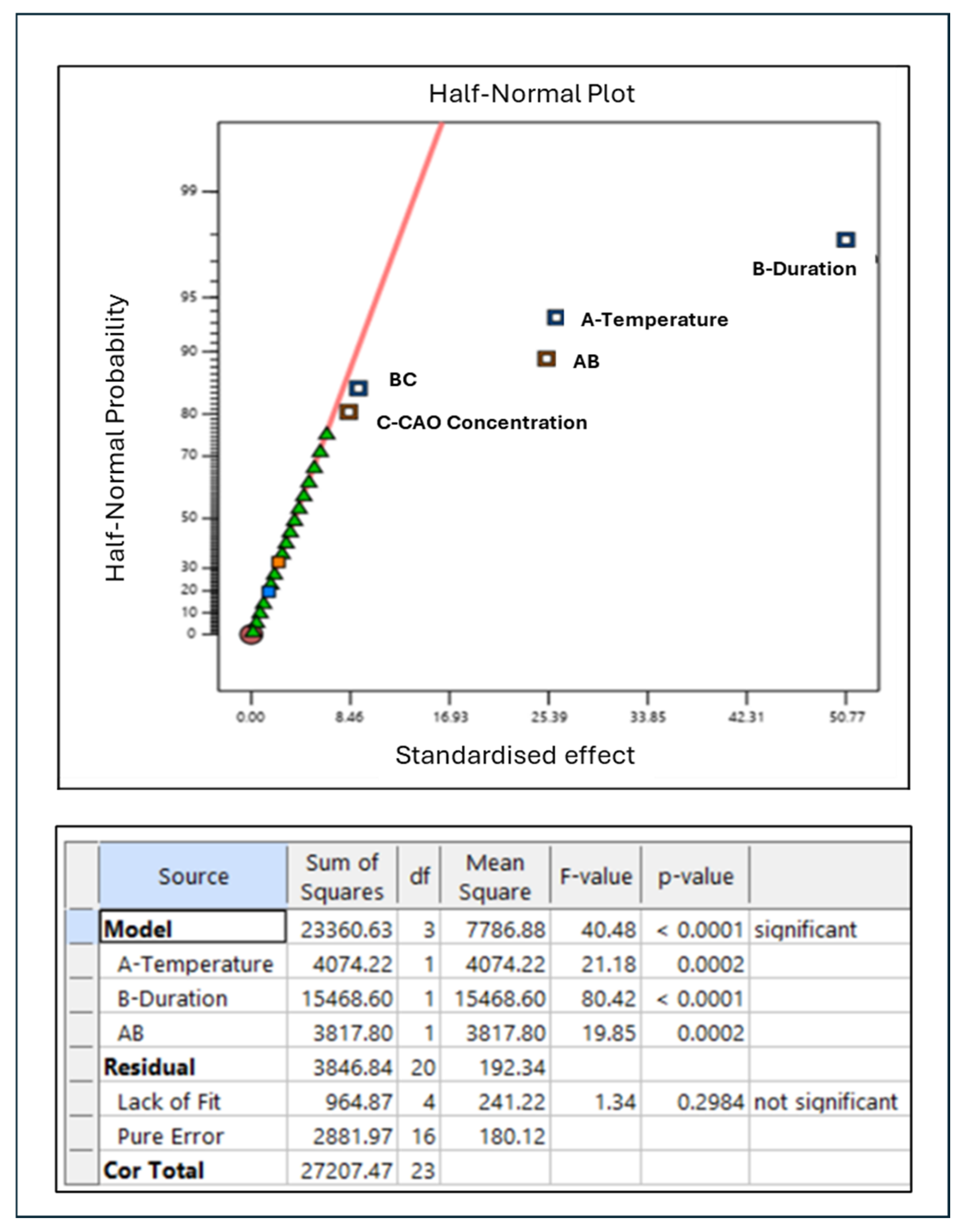
5.2. Results
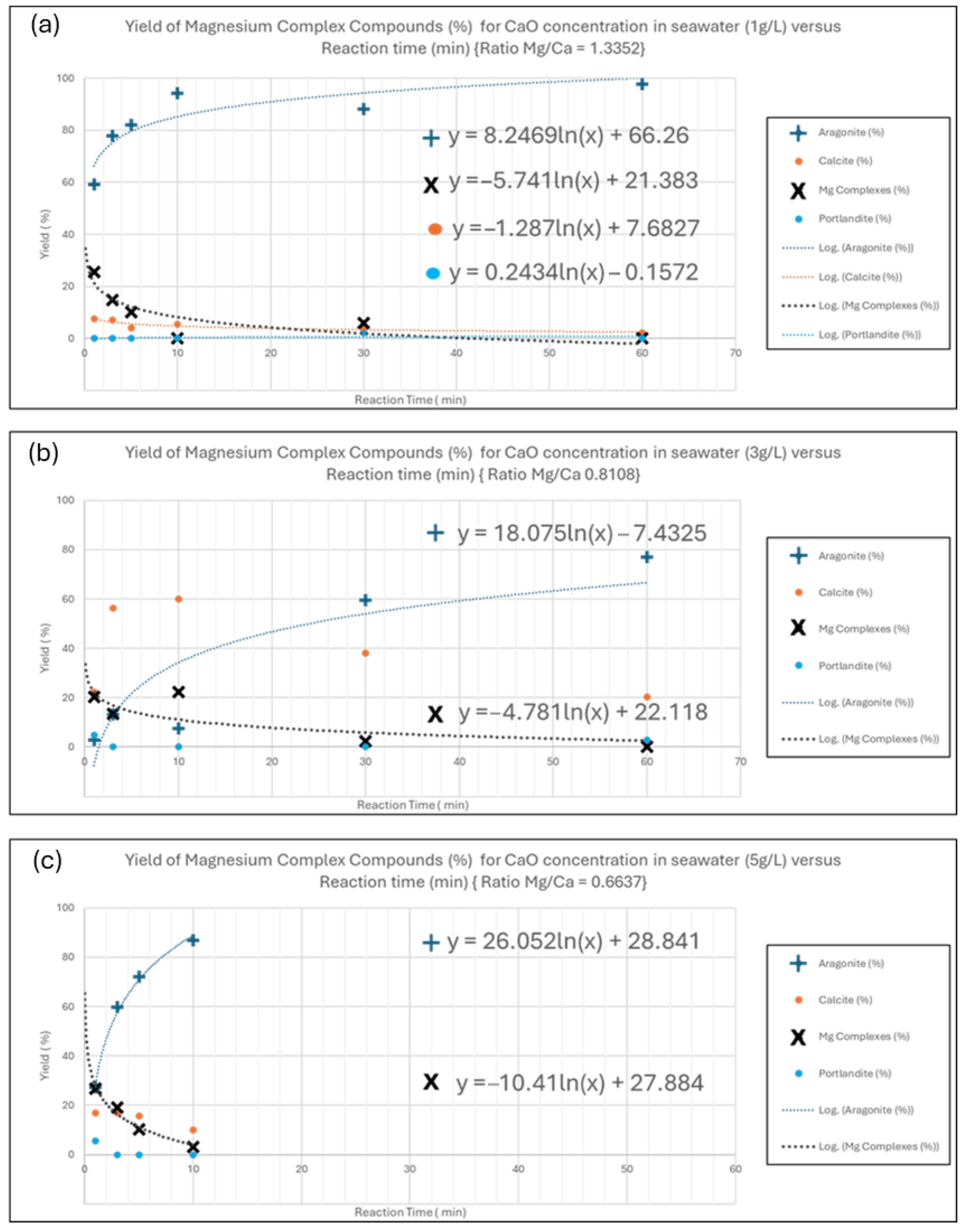
5.3. Investigation of Main Factors for Magnesium Complex Compounds Yield
5.4. Optimization of Aggregate Hydro Magnesite, Brucite, and Huntite Formation in Seawater Media
6. Physico-Chemical Characteristics of Magnesium-Based Compounds Synthesized
6.1. FESEM Imaging
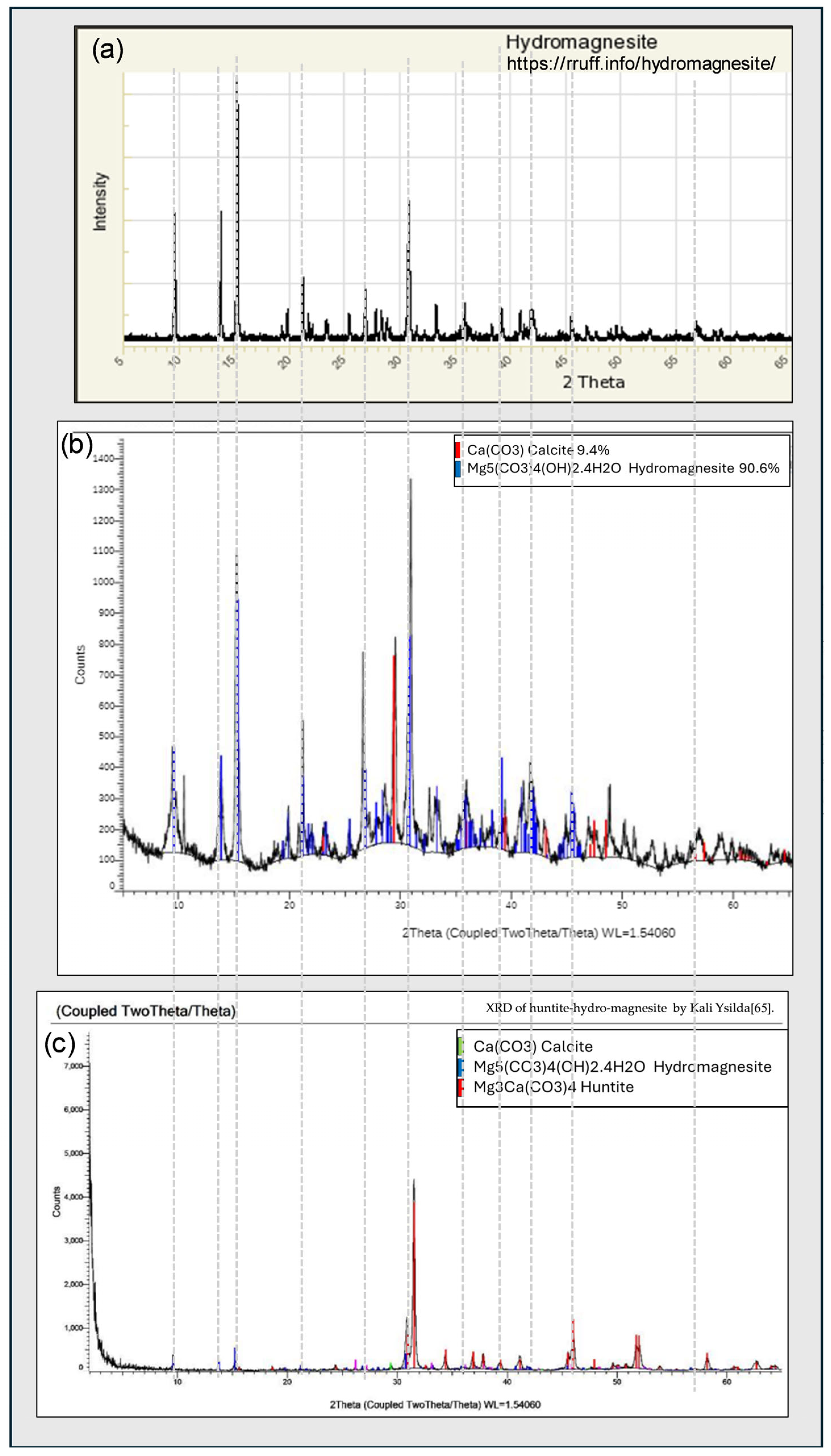
6.2. X-Ray Diffractogram XRD Analysis
6.3. Anion Analysis
6.4. Thermal Gravimetric Analysis TGA
7. Fire Testing
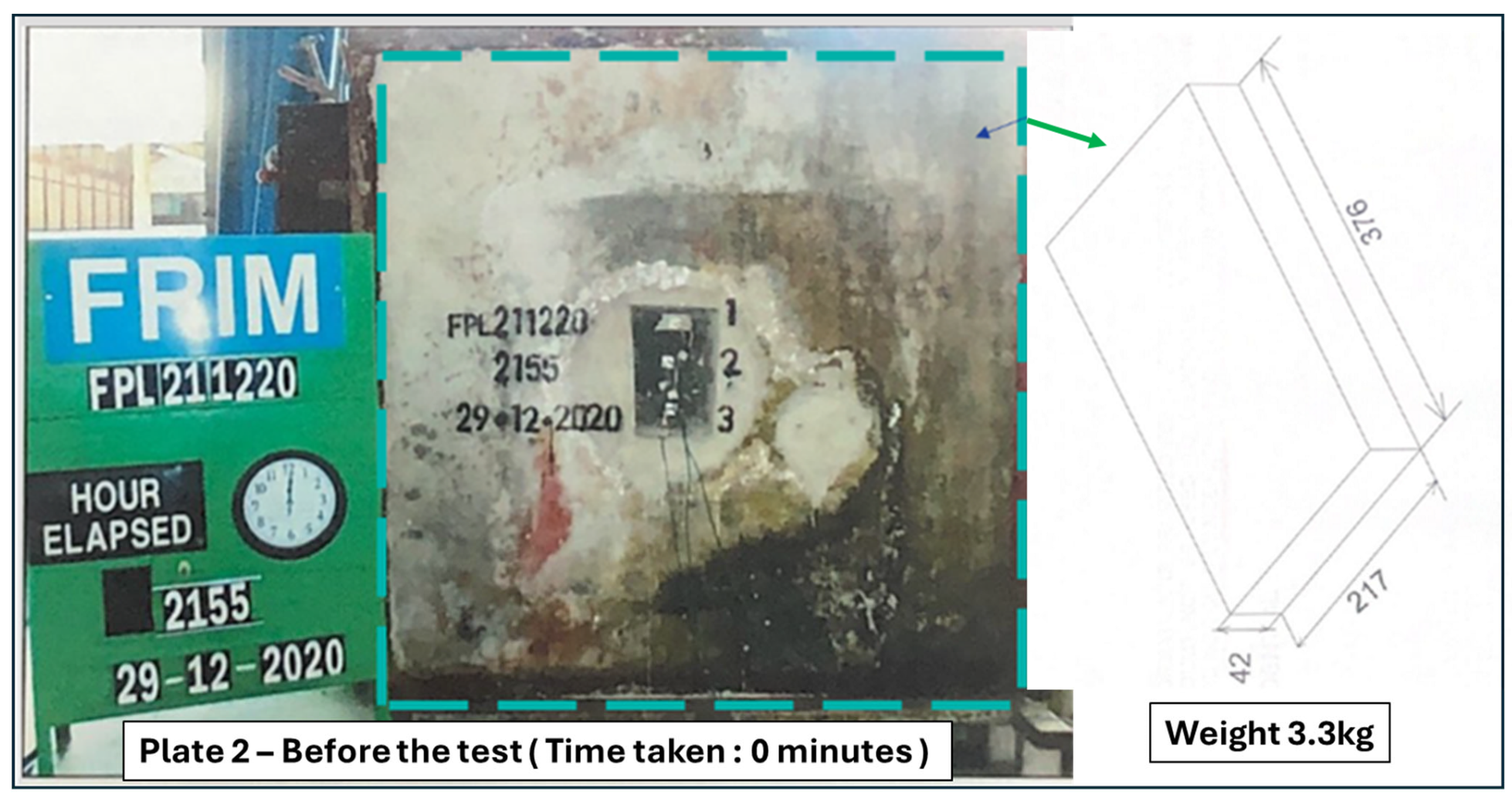
7.1. Test Methods and Installation Setup
- Integrity—A failure of the test construction to maintain integrity shall be deemed to have occurred when collapse of sustained flaming for more than 10 s on the unexposed face, or one of the following occurs, such as a through gap between 6 and 25 mm exists or develops in the specimen.
- Insulation—Failure shall be deemed to have occurred when one of the following occurs: the mean temperature of the unexposed surface temperature increases by more than 140 °C above its initial values, and the temperature recorded at any position on the unexposed face is in excess of 180 °C above the initial mean unexposed temperature, or collapse of the mechanical structure of the fire-retardant block.
7.2. BS 476-22:1987—Fire Tests on Building Materials and Structures Results
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Energy Agency. Net Zero by 2050. A Roadmap for the Global Energy Sector; Report; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Ma, J.; Li, L.; Wang, H.; Du, Y.; Ma, J.; Zhang, X.; Wang, Z. Carbon Capture and Storage: History and the Road Ahead. Engineering 2022, 14, 33–43. [Google Scholar] [CrossRef]
- Wang, M.; Oko, E. Special issue on carbon capture in the context of carbon capture, utilization and storage (CCUS). Int. J. Coal Sci. Technol. 2017, 4, 1–4. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Hanson, E.; Nwakile, C.; Hammed, V.O. Carbon capture, utilization, and storage (CCUS) technologies: Evaluating the effectiveness of advanced CCUS solutions for reducing CO2 emissions. Results Surf. Interfaces 2025, 18, 100381. [Google Scholar] [CrossRef]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef]
- Chang, S.-F.; Chiu, H.-H.; Jao, H.-S.; Shang, J.; Lin, Y.-J.; Yu, B.-Y. Comprehensive evaluation of various CO2 capture technologies through rigorous simulation: Economic, equipment footprint, and environmental analysis. Carbon Capture Sci. Technol. 2025, 14, 100342. [Google Scholar] [CrossRef]
- Global Cement, Concrete Association. Concrete Future—The GCCA 2050 Cement and Concrete Industry Roadmap for Net Zero Concrete; Global Cement and Concrete Association: London, UK, 2021; pp. 1–48. [Google Scholar]
- LeClerc, H.O.; Erythropel, H.C.; Backhaus, A.; Lee, D.S.; Judd, D.R.; Paulsen, M.M.; Ishii, M.; Long, A.; Ratjen, L.; Bertho, G.G.; et al. The CO2 Tree: The Potential for Carbon Dioxide Utilization Pathways. ACS Sustain. Chem. Eng. 2025, 13, 5–29. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Angelini, A. The changing paradigm in CO2 utilization. J. CO2 Util. 2013, 3–4, 65–73. [Google Scholar] [CrossRef]
- Aresta, M.; Quaranta, E.; Tommasi, I.; Giannoccaro, P.; Ciccarese, A. Interaction of CO2 with C–C Multiple Bonds: Reactions with Olefins, Cumulated and Conjugated Dienes, and Alkynes. In Reaction Mechanisms in Carbon Dioxide Conversion; Springer: Berlin/Heidelberg, Germany, 2016; pp. 143–182. [Google Scholar] [CrossRef]
- Danieli, S.; Neto, J.S.A.; Soares, E.G.; Oliveira, T.F.; Brito, B.L.; Kirchheim, A.P. Shaping a sustainable path: Exploring opportunities and challenges in carbon capture and utilization in cement and concrete industry. CEMENT 2025, 19, 100135. [Google Scholar] [CrossRef]
- Carbon Upcycling Technologies. Carbon Upcycling Technologies. 2023. Available online: https://carbonupcycling.com/ (accessed on 28 January 2023).
- Sinha, A. A Mechanochemical Process to Produce Exfoliated Nanoparticles. WO/2019/012474, 17 January 2019. [Google Scholar]
- Sinha, A. A Mechanically Carboxylate Fly Ash, Methods of Its Production and Uses Thereof. WO/2021/087605, 14 May 2021. [Google Scholar]
- Sinha, A. Compositions Comprising a Mechanochemically Carboxylated Mineral Filler and a Cement and/or Asphalt Binder. WO/2021/087606, 4 September 2024. [Google Scholar]
- Billing, M. Carbon Storage Startup Paebbl Raises €8m Seed Round; Sifted: London, UK, 2023. [Google Scholar]
- Paebbl. Paebbl 2024. Available online: https://paebbl.com/ (accessed on 3 January 2024).
- Hills, C.D.; Carey, P.J. Production of Secondary Aggregates. WO/2007/096671A1, 9 July 2019. [Google Scholar]
- Carey, P. KentCarbon8 Systems Limited Improved Production of Aggregates, 2017. European Patent Application EP 4 279 465 A2, 22 November 2023. [Google Scholar]
- Constantz, B.R.; Schneider, J.; Bewernitz, M.; Camire, C. Carbon Sequestration Methods and Systems, and Compositions Produced Thereby. US 2020/0370001A1, 15 July 2015. [Google Scholar]
- Constantz, B.R.; Bewernitz, M.A.; Camire, C.L.; Seung-Hee, K.; Schneider, J. Continuous Carbon Sequestration Material Production Methods and Systems for Practicing the Same. WO 2016/057709A2, 8 September 2020. [Google Scholar]
- Constantz, B.R.; Bewernitz, M.A. Carbon Sequestration Methods and Systems. US10197747B2, 5 February 2019. [Google Scholar]
- Constantz, B.; Youngs, A.; O’Neil, J.; Farsad, K.; Patterson, J.; Stagnaro, J.; Thatcher, R.; Camire, C. Rocks and Aggregate, and Methods of Making and Using the Same. US20100247410A1, 29 March 2011. [Google Scholar]
- Constantz, B.R.; Ryan, C.; Clodic, L. Hydraulic Cements Comprising Carbonate Compound Compositions. US7906028B2, 14 October 2014. [Google Scholar]
- Neustark. Neustark 2023. Available online: https://www.neustark.com/en/ (accessed on 7 October 2023).
- Orbix. Carbstone 2022. Available online: https://www.orbix.be/en/technologies/carbonation (accessed on 8 October 2022).
- Vito. Carbstone 2022. Available online: https://vito.be/en/carbstone (accessed on 8 October 2022).
- Quaghebeur, M.; Nielsen, P.; Laenen, B.; Nguyen, E.; van Mechelen, D. Carbstone: Sustainable valorisation technology for fine grained steel slags and CO2. Refract. Worldforum 2010, 2, 75–79. [Google Scholar]
- Vito. First Footpath Constructed with Carbstone Clinkers 2020. Available online: https://vito.be/en/news/first-footpath-constructed-carbstone-clinkers (accessed on 8 October 2022).
- Shao, Y.; Mahoutian, M.; Ghouleh, Z. Carbonate-Bonded Construction Products from Steel-Making Residues and Method for Making the Same. WO 2015/139121 A1, 30 October 2018. [Google Scholar]
- CarbiCrete. Lower Your Carbon Footprint 2021:2. Available online: https://carbicrete.com/wp-content/uploads/2021/03/carbicrete-datasheet-3-en.pdf (accessed on 7 October 2023).
- CarbonBuilt. CarbonBuilt 2022. Available online: https://www.carbonbuilt.com/ (accessed on 4 October 2022).
- Carbon Leadership Forum. CarbonBuilt: The Age of Incrementalism Must Come to a Close 2023. Available online: https://carbonleadershipforum.org/carbonbuilt/ (accessed on 9 June 2023).
- Kuppler, J.P.; Atakan, V.; Smith, K.; Hu, X. Curing Systems for Materials That Consume Carbon Dioxde and Method of Use Thereof. US9.221027B2, 29 December 2015. [Google Scholar]
- Solidia. The Science Behind Solidia 2019:1–2. Available online: https://assets.ctfassets.net/jv4d7wct8mc0/5DwEAeEYqsFAYA9UC53EF7/4f8b7566221a8d9cb38f970867003226/Solidia_Science_Backgrounder_11.21.19__5_.pdf (accessed on 7 October 2023).
- Meyer, V.; De Cristofaro, N.; Bryant, J.; Sahu, S. Solidia cement an example of carbon capture and utilization. Key Eng. Mater. 2018, 761, 197–203. [Google Scholar] [CrossRef]
- Gilliam, R.; Krugh, K.; Fortera; Hanson, L. Low-CO2 Cement Inspired by Nature. Global Cement 2021. Available online: https://www.globalcement.com/magazine/articles/1230-fortera-low-CO2-cement-inspired-by-nature (accessed on 9 June 2023).
- CarbonCure. CarbonCure 2023. Available online: https://www.carboncure.com/ (accessed on 6 September 2022).
- Toure, B.; Cuesta, J.-M.L.; Gaudon, P.; Benhassaine, A.; Crespy, A. Fire resistance and mechanical properties of a huntite/hydromagnesite/antimony trioxide/decabromodiphenyl oxide filled PP-PE copolymer. Polym. Degrad. Stab. 1996, 53, 371–379. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Influence of flame-retardant magnesium hydroxide on the mechanical properties of high density polyethylene composites. J. Reinf. Plast. Compos. 2017, 36, 1802–1816. [Google Scholar] [CrossRef]
- Hornsby, P.R. Application of Magnesium Hydroxide as a Fire Retardant and Smoke-Suppressing Additive for Polymers. Fire Mater. 1994, 18, 269–276. [Google Scholar] [CrossRef]
- Ciullo, P.A. (Ed.) 1. Inorganic compounds—Industrial applications—Handbooks. manuals, etc. I. In Industrial Minerals and Their Uses: A Handbook and Formulary; Includes index; William Andrew: Norwich, NY, USA; ISBN 0-8155-1408-5.
- Hornsby, P.R.; Watson, C.L. A Study of the Mechanism of Flame Retardance and Smoke Suppression in Polymers Filled with Magnesium Hydroxide. Polym. Degrad. Stab. 1990, 30, 73–87. [Google Scholar] [CrossRef]
- Hollingberya, L.A.; Hul, T.R. The Thermal Decomposition of Huntite and Hydromagnesite—A Review. Thermochim. Acta 2010, 509, 1–11. [Google Scholar] [CrossRef]
- Jamsaz, A.; Goharshadi, E.K. Graphene-based flame-retardant polyurethane: A critical review. Polym. Bull. 2022, 80, 11633–11669. [Google Scholar] [CrossRef]
- Available online: https://www.futuremarketinsights.com/reports/flame-retardant-market (accessed on 7 October 2023).
- Kim, D.; Kim, M.-J. Study on Carbon Dioxide Storage through Mineral Carbonation using Sea Water and Paper Sludge Ash. J. Korean Soc. Mar. Environ. Energy 2016, 19, 18–24. [Google Scholar] [CrossRef]
- Ho, H.-J.; Iizuka, A. Mineral carbonation using seawater for CO2 sequestration and utilization: A review. Sep. Purif. Technol. 2023, 307, 122855. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jensen, O.E.; Cliffe, K.A.; Maroto-Valer, M.M. A Model of Carbon Dioxide Dissolution and Mineral Carbonation Kinetics. Proc. Math. Phys. Eng. Sci. 2010, 466, 1265–1290. Available online: https://www.jstor.org/stable/25661496 (accessed on 10 August 2025). [CrossRef]
- Sun, W.; Jayaraman, S.; Chen, W.; Persson, K.A.; Ceder, G. Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl. Acad. Sci. USA 2015, 112, 3199–3204. [Google Scholar] [CrossRef]
- Bischoff, J.L. Kinetics of calcite nucleation: Magnesium ion inhibition and ionic strength catalysis. J. Geophys. Res. 1968, 73, 3315–3322. [Google Scholar] [CrossRef]
- Bischoff, J.L.; Fyfe, W.S. Catalysis, inhibition, and the calcite-aragonite problem; [Part] 1, The aragonite-calcite transformation. Am. J. Sci. 1968, 266, 65–79. [Google Scholar] [CrossRef]
- Berner, R.A. The Role of Magnesium in the Crystal Growth of Calcite and Aragonite from Sea Water. Geochim. Cosmochim. Acta 1975, 39, 489–494. [Google Scholar] [CrossRef]
- Astilleros, J.M.; Fernández-Díaz, L.; Putnis, A. The role of magnesium in the growth of calcite: An AFM study. Chem. Geol. 2010, 271, 52–58. [Google Scholar] [CrossRef]
- De Choudens-Sánchez, V.; González, L.A. Calcite and aragonite precipitation under controlled instantaneous supersaturation: Elucidating the role of CaCO3 saturation state and Mg/Ca ratio on calcium carbonate polymorphism. J. Sediment. Res. 2009, 79, 363–376. [Google Scholar] [CrossRef]
- Meldrum, F.C. Calcium carbonate in biomineralization and biomimetic chemistry. Int. Mater. Rev. 2003, 48, 187–224. [Google Scholar] [CrossRef]
- Available online: https://g1.redseafish.com/red-sea-salts/red-sea-salt (accessed on 7 October 2023).
- Santoro De Vico, F.; Bonilla-Correa, S.; Pelayo-Punzano, G.; Elert, K.; Rodríguez-Navarro, C.; Ruiz-Agudo, E. Additive impact on early-stage magnesium carbonate mineralisation. Geochem. Perspect. Lett. 2024, 32, 46–51. [Google Scholar] [CrossRef]
- Santos, H.S.S.; Nguyen, H.; Venâncio, F.Q.; Ramteke, D.; Zevenhoven, R.; Kinnunen, P. Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage. Inorg. Chem. Front. 2023, 10, 2507–2546. [Google Scholar] [CrossRef]
- Boyd, V.; Yoon, H.; Zhang, C.; Oostrom, M.; Hess, N.; Fouke, B.; Valocchi, A.J.; Werth, C.J. Influence of Mg2+ on CaCO3 precipitation during subsurface reactive transport in a homogeneous silicon-etched pore network. Geochim. Cosmochim. Acta 2014, 135, 321–335. [Google Scholar] [CrossRef]
- Available online: https://handbookofmineralogy.org/pdfs/hydromagnesite.pdf (accessed on 7 October 2023).
- Available online: https://rruff.info/hydromagnesite/display=default (accessed on 7 October 2023).
- Akao, M.; Iwai, S. The hydrogen bonding of hydromagnesite. Acta Cryst. B 1977, 33, 1273–1275. [Google Scholar] [CrossRef]
- Ysilda, K. Huntite-Hydromagnesite, Brucite, Dawnosite application as Fire Retardant Fillers. Bachelor’s Thesis, University of Athens, Athens, Greece, 3 December 2021. [Google Scholar]
- Rothon, R.N.; Hornsby, P.R. Flame retardant effects of magnesium hydroxide. Polym. Degrad. Stab. 1996, 54, 383–385. [Google Scholar] [CrossRef]
- Haurie, L.; Fernández, A.I.; Velasco, J.I.; Chimenos, J.M.; Cuesta, J.L.; Espiell, F. Thermal stability and flame retardancy of LDPE/EVA blends filled with synthetic hydromagnesite/aluminium hydroxide/montmorillonite and magnesium hydroxide/aluminium hydroxide/montmorillonite mixtures. Polym. Degrad. Stab. 2007, 92, 1082–1087. [Google Scholar] [CrossRef]
- Inglethorpe, S.D.J.; Stamatakis, M.G. Thermal decomposition of natural mixtures of hydromagnesite and huntite from Kozani, Northern Greece. Miner. Wealth 2003, 7–18. [Google Scholar]



| Standard Run | Randomized Run | Factor A | Factor C | Factor B | Response Y |
|---|---|---|---|---|---|
| Temperature | [CaO] | Reaction Time | Hydro Magnesite/Brucite Yield | ||
| °C | g/L | minutes | % | ||
| 1 | 11 | 90 | 60 | 1 | Not detected |
| 2 | 2 | 30 | 3 | 1 | 70.3 |
| 3 | 4 | 90 | 3 | 1 | 14.9 |
| 4 | 14 | 30 | 3 | 5 | 85.0 |
| 5 | 20 | 30 | 60 | 5 | Not detected |
| 6 | 19 | 30 | 60 | 5 | Not detected |
| 7 | 9 | 30 | 60 | 1 | 1.0 |
| 8 | 5 | 90 | 3 | 1 | 14.9 |
| 9 | 18 | 90 | 3 | 5 | 15.0 |
| 10 | 6 | 90 | 3 | 1 | 14.9 |
| 11 | 24 | 90 | 60 | 5 | Not detected |
| 12 | 15 | 30 | 3 | 5 | 83.0 |
| 13 | 8 | 30 | 60 | 1 | 2.0 |
| 14 | 16 | 90 | 3 | 5 | 13.6 |
| 15 | 3 | 30 | 3 | 1 | 70.1 |
| 16 | 7 | 30 | 60 | 1 | 2.0 |
| 17 | 10 | 90 | 60 | 1 | Not detected |
| 18 | 21 | 30 | 60 | 5 | Not detected |
| 19 | 17 | 90 | 3 | 5 | 80.0 |
| 20 | 13 | 30 | 3 | 5 | 83.0 |
| 21 | 22 | 90 | 60 | 5 | Not detected |
| 22 | 12 | 90 | 60 | 1 | Not detected |
| 23 | 23 | 90 | 60 | 5 | Not detected |
| 24 | 1 | 30 | 3 | 1 | 69.9 |
| Hydro Magnesite/Brucite Yield (Interaction Factors A, B, C) | Run 1 | Run 2 | Run 3 | Sum |
|---|---|---|---|---|
| 1 (L, L, L) | 70.3 | 70.1 | 69.9 | 210.0 |
| 2 (H, L, L) | 14.0 | 14.9 | 14.9 | 45.0 |
| 3 (L, H, L) | 2.0 | 2 | 1 | 5.0 |
| 4 (H, H, L) | 0 | 0 | 0 | 0 |
| 5 (L, L, H) | 83.0 | 85.0 | 83.0 | 251.0 |
| 6 (H, L, H) | 13.6 | 80.0 | 15.0 | 109.0 |
| 7 (L, H, H) | 0 | 0 | 0 | 0 |
| 8 (H, H, H) | 0 | 0 | 0 | 0 |
| Name of Solid Precipitate Samples | Phase | Na | Mg | Ca |
|---|---|---|---|---|
| ppm | ppm | ppm | ||
| Calcium Oxide CaO (s) | Solid | 55 | 4700 | 11,880 |
| Magnesium compounds at 90 °C_#1 | Solid | 2440 | 4899 | 3620 |
| Magnesium compounds at 90 °C_#2 | Solid | 587 | 4513 | 3934 |
| Magnesium compounds at 90 °C_#3 | Solid | 4040 | 10,815 | 3707 |
| Magnesium compounds at 90 °C_#4 | Solid | 718 | 7279 | 3820 |
| Magnesium compounds at 90 °C_#5 | solid | 283 | 11,792 | 1889 |
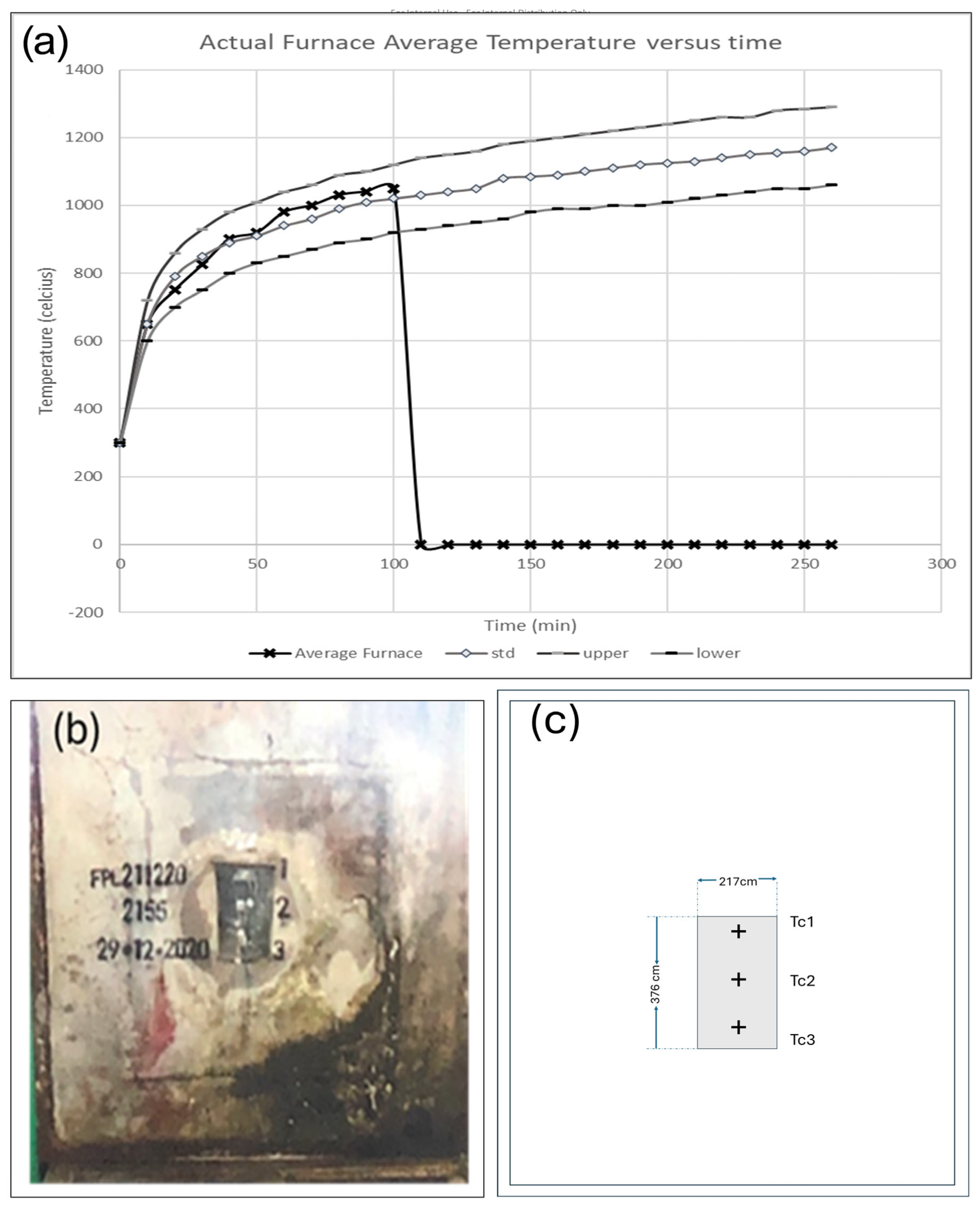
| Time (min) | Thermocouples | Mean Temperature | Temperature Rise Above Mean | Observation | |||
|---|---|---|---|---|---|---|---|
| TC1 | TC2 | TC3 | (°C) | Mean Temp | Max Temp | ||
| 0 | 27 | 28 | 28 | 27 | 0 | 1 | Test commences |
| 1 | 27 | 28 | 28 | 27 | 0 | 1 | |
| 2 | 27 | 28 | 28 | 28 | 1 | 1 | |
| 3 | 27 | 28 | 28 | 28 | 1 | 1 | |
| 4 | 27 | 28 | 28 | 29 | 2 | 5 | |
| 5 | 28 | 41 | 29 | 33 | 6 | 14 | |
| 6 | 39 | 51 | 32 | 41 | 14 | 24 | |
| 7 | 59 | 60 | 43 | 54 | 27 | 33 | Observed water moisture appeared at the top surface of the sample at 7 min test |
| 8 | 73 | 67 | 60 | 67 | 40 | 46 | |
| 9 | 81 | 72 | 67 | 73 | 46 | 54 | |
| 10 | 85 | 76 | 74 | 78 | 51 | 58 | |
| 12 | 89 | 81 | 79 | 83 | 56 | 62 | |
| 14 | 91 | 85 | 82 | 86 | 59 | 64 | |
| 16 | 92 | 86 | 83 | 87 | 60 | 65 | |
| 18 | 93 | 88 | 85 | 89 | 62 | 66 | |
| 20 | 93 | 88 | 86 | 89 | 62 | 66 | |
| 22 | 94 | 89 | 87 | 90 | 63 | 67 | |
| 24 | 94 | 89 | 88 | 90 | 63 | 67 | |
| 26 | 95 | 90 | 90 | 91 | 64 | 68 | |
| 28 | 94 | 89 | 88 | 91 | 64 | 67 | |
| 30 | 95 | 89 | 90 | 91 | 64 | 68 | |
| 40 | 96 | 90 | 90 | 92 | 65 | 69 | All temperature readings still below 100 °C |
| 50 | 98 | 93 | 93 | 94 | 67 | 71 | |
| 60 | 99 | 95 | 95 | 96 | 69 | 72 | |
| 70 | 99 | 96 | 96 | 97 | 70 | 72 | TC1 reached 101 °C at 75 min |
| 80 | 100 | 97 | 97 | 98 | 71 | 73 | |
| 90 | 129 | 101 | 97 | 109 | 82 | 102 | |
| 96 | 211 | 148 | 97 | 152 | 125 | 184 | Small hole appeared on top of the sample at 100 min |
| 100 | 248 | 184 | 97 | 177 | 150 | 221 | |
| 104 | 272 | 214 | 97 | 194 | 167 | 245 | Test discontinued |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, M.G.M.; Yuhana, N.Y.; Jumali, M.H.b.H. Sustainable Synthesis of Hydro Magnesite Fire Retardants Using Seawater: Characterization, Yield Modeling and Process Optimization. Fire 2025, 8, 409. https://doi.org/10.3390/fire8100409
Noh MGM, Yuhana NY, Jumali MHbH. Sustainable Synthesis of Hydro Magnesite Fire Retardants Using Seawater: Characterization, Yield Modeling and Process Optimization. Fire. 2025; 8(10):409. https://doi.org/10.3390/fire8100409
Chicago/Turabian StyleNoh, Mohammad Ghaddaffi Mohd, Nor Yuliana Yuhana, and Mohammad Hafizuddin bin Hj Jumali. 2025. "Sustainable Synthesis of Hydro Magnesite Fire Retardants Using Seawater: Characterization, Yield Modeling and Process Optimization" Fire 8, no. 10: 409. https://doi.org/10.3390/fire8100409
APA StyleNoh, M. G. M., Yuhana, N. Y., & Jumali, M. H. b. H. (2025). Sustainable Synthesis of Hydro Magnesite Fire Retardants Using Seawater: Characterization, Yield Modeling and Process Optimization. Fire, 8(10), 409. https://doi.org/10.3390/fire8100409







