1. Introduction
Epoxy resins (EPs) are known for their outstanding properties, including solvent resistance, low shrinkage, excellent electrical insulation, favorable mechanical characteristics, and strong adhesion. These attributes make EPs widely applicable in various fields such as the automotive industry, packaging, textiles, and biomedical equipment [
1,
2,
3,
4]. However, due to the composition of carbon, hydrogen, and oxygen elements in EPs, its limiting oxygen index (LOI) value is relatively low at only 19.8%. Consequently, EPs exhibit highly flammable characteristics. When subjected to heat decomposition, EPs generate flammable vapors that ignite upon contact with a heated surface in the presence of oxygen [
5]. The combustion process of EPs results in the release of an amount of smoke that restricts their application across various fields [
6,
7]. Therefore, enhancing the fire resistance properties of epoxy materials poses a challenging and essential task.
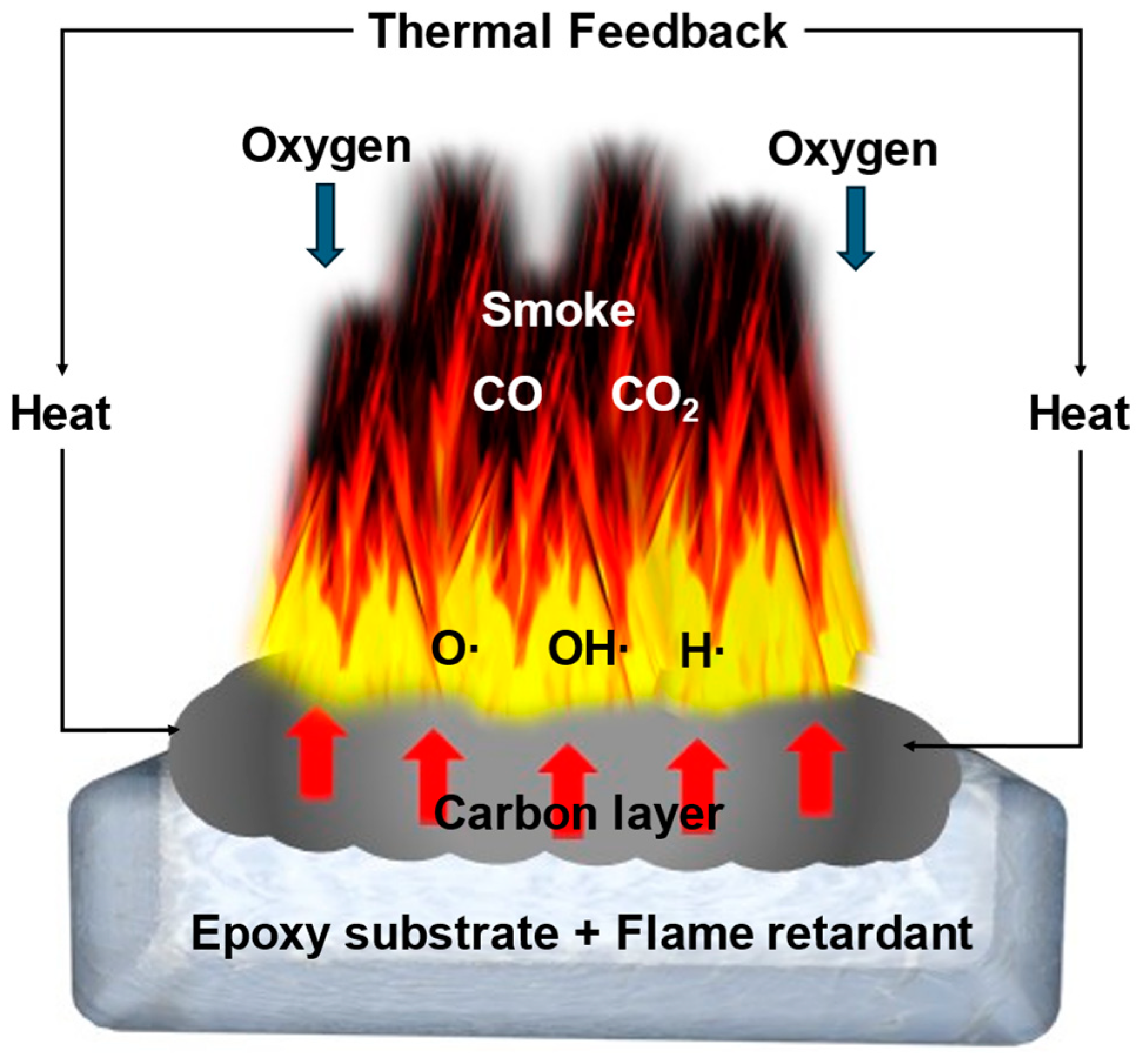
The flame retardancy of EPs can be effectively enhanced by incorporating flame retardants into the material. The fire prevention mechanism primarily involves two approaches (
Figure 1). Firstly, the addition of flame retardants forms a carbon layer, which creates a barrier effect. The released volatiles may play critical roles in trapping free radicals and releasing gas to dilute combustible gases to realize fire retardancy [
8]. Secondly, flame retardants are utilized to absorb or dissipate heat during the condensation stage, while dripping and isolation measures are employed for fire suppression [
9]. These mechanisms have led to the proposal of various methods for enhancing fire prevention in EPs. Traditional flame retardants such as halogen-containing compounds, ammonium polyphosphate (APP), aluminum trihydroxide (ATH), magnesium hydroxide, organic phosphonates, and red phosphorus [
10,
11,
12] have demonstrated favorable outcomes. However, conventional flame retardants often emit toxic and corrosive fumes, leading to environmental concerns [
13]. Consequently, the pursuit of environmentally friendly systems remains a crucial research objective.
Natural flame retardant minerals, composed of metallic elements (such as aluminum, magnesium, calcium, iron, and titanium) and non-metallic atoms (including silicon, phosphorus, oxygen, and carbon), offer a promising alternative. These minerals exhibit exceptional flame-retardant properties due to the synergistic effects of their diverse elements. Specifically, minerals containing silicon and phosphorus demonstrate enhanced flame retardancy through their synergistic interactions [
14]. The incorporation of metal elements into EPs can occur through specific reactions or dispersions, resulting in the formation of covalent bonds with inorganic clusters [
15]. Lewis’s acid metals, in particular, exhibit catalytic activity for oxidative dehydrogenation in the gas phase. Additionally, integrating metals into EPs leads to the development of a more thermally stable phase and facilitates cross-linking, thereby enhancing thermal stability [
16].
Due to the diverse range of raw material sources, environmental friendliness, and excellent compatibility, natural mineral flame retardants present a promising option for epoxy resin applications that necessitate stringent safety measures, have minimal environmental impact, and strive for high-performance standards. This review systematically introduces the methods of combining EPs with mineral flame retardants. Subsequently, detailed explanations are given on the interaction mechanism between EPs and several typical natural mineral flame retardants, including clay materials, silicon materials, and metal hydroxides. Lastly, an analysis is conducted on the current challenges and future development prospects.
2. Methods for Integrating Natural Mineral Flame Retardants with EPs
The flame retardant performance of EP composite is influenced not only by the flame retardant itself but also by how the flame retardants are combined with EPs. Based on current research, there are primarily four types of methods for integrating natural mineral flame retardants with EPs: direct doping, surface modification, composite techniques, and advanced processing methods.
2.1. Physical Method
Physically integrating natural mineral flame retardants into the EP matrix represents an early strategy for enhancing flame resistance. This integration is achieved through the uniform dispersion of mineral particles within the EP matrix using mechanical mixing or high-speed shear blending techniques. This method does not require altering the polymer synthesis process, but the ratios, morphologies, and crystalline structures of the flame retardant have an impact on its effectiveness. Lee et al. [
17] investigated the application of different wolframite dopants in EP coatings. Increasing the content of wolframite resulted in an 11.8% improvement in fire resistance and a 57.2% enhancement in the thermal stability of the coating. Wang et al. [
18] utilized manganese dioxide particles with varying morphologies and crystal structures to mitigate fire risks associated with EPs. The findings indicate that α-nanosheets exhibit superior comprehensive properties compared to β-nanorods and nanospheres, displaying excellent fire resistance characteristics as evidenced by lower peak heat release rate, total heat release rate, tar production rate, and total tar production value. TG-IR results demonstrate that α-nanoparticles exert optimal inhibitory effects on volatile gas permeation. However, physical blending methods may result in uneven distribution within the EPs matrix and weak interface adhesion between the matrix and flame retardant, leading to compromised mechanical properties of composites, particularly at higher fill volumes, which can adversely affect resin digital integrity and fluidity.
2.2. Surface Modification Method
Surface modification of natural mineral flame retardants can substantially enhance their compatibility and integration within epoxy resins, thereby improving the overall performance of EPs. Common surface modification techniques include silane coupling and polymer grafting [
19]. For instance, silane-treated halloysite nanotubes (HNT) exhibited significant improvements in interfacial adhesion with EPs. The silyl group forms a stronger chemical bond with the epoxy resin and enhances surface polarity, resulting in better dispersion of HNTs within the resin and prevention of agglomeration, thus improving the mechanical properties of the EP composite [
20]. Similarly, introducing a phosphorus-containing flame retardant into the structure of silicone EPs can greatly enhance the toughness, water resistance, dielectric properties, and flame retardant properties of the cured resin. The results showed that the EP composite meets the V-0 requirements of the ul-94 test and exhibits a high oxygen limit index of 29.8% due to its phosphorus and silicon content [
21]. However, the surface modification method does have certain limitations. For instance, it has the potential to impact the chemical structure of the EP matrix. Furthermore, under high temperatures or wet conditions, the stability of the composite obtained through surface modification may deteriorate due to the instability of the modifier. Additionally, performing surface modification often requires additional steps, thereby increasing both complexity and cost in the manufacturing process.
2.3. Compounding Methods
2.3.1. Blending Method
Integrating natural mineral flame retardants with various chemical flame retardants, such as phosphates and aluminum hydroxide, elicits a synergistic response that substantially enhances the flame retardant efficacy of EPs. This approach allows for the adaptive modification of the flame retardant composition to meet specific requirements, achieving a multifaceted and comprehensive enhancement of EP properties. Kong et al. [
22] successfully prepared a novel epoxy composite material with exceptional thermal conductivity and robust fire resistance using boron nitride, talc, and ammonium polyphosphate composites. The filler mesh composite exhibited an LOI value of 37.8%, which was 1.9 times higher than that of pure EPs. The BN-based network structure played a crucial role in facilitating heat transfer, while the APP component generated phosphoric acid at elevated temperatures, combining with talc and other residues to form ceramic residues on the surface. This enhanced the isolation effect of the carbon layer and improved the fire resistance properties of the composite materials significantly. Furthermore, phosphate-based fire retardants interacted synergistically with talc to create a robust high-temperature barrier that not only enhanced the fire resistance capabilities but also improved the mechanical properties of the epoxy digital signal processor. However, the precise control of component proportions is challenging during the mixing phase. Moreover, the interaction between different flame retardants can potentially trigger side reactions, both of which may compromise the efficacy of flame retardants.
2.3.2. Layered Composite Method
Natural mineral flame retardants with layered structures are used to implement lamination composite technology with EPs. This layered structure provides excellent flame retardant properties and mechanical strength, optimizing the overall properties of the composite by adjusting the layer thickness and arrangement. Currently, prevalent layered minerals include montmorillonite, mica, and their analogs. The fusion of Al
2O
3 nanolayers with graphene has yielded a novel composite material. Due to its meticulous microstructure design, superior interfacial cohesion, covalently bonded interfaces, and the synergistic reinforcing impact of the graphene framework upon the alumina mineral nanolayers, the covalently bonded interface between the three-dimensional (3D) ordered graphene-alumina (GA) template demonstrates considerable mutual reinforcement, excellent compatibility in deformation, and a linear relationship between density and Young’s modulus. Notably, the dimensions of the mineral nanolayers significantly influence the mechanical properties of such mineral-based metamaterials [
23]. However, the layered composite method may suffer from inadequate interfacial bonding strength, which can result in delamination between layers. Additionally, the manufacturing process is intricate and has the potential to induce brittleness in the material.
2.4. Advanced Processing Methods
2.4.1. Microencapsulation Method
When the concentration of flame retardants within a composite material is elevated, a marked decrease in thermoplasticity is observed [
24]. To mitigate this issue, advanced microencapsulation techniques, such as emulsion polymerization or solvent evaporation methods, are employed to envelop natural mineral flame retardants within microcapsules. This encapsulation strategy enhances their dispersion and stability within the resin matrix, preventing the agglomeration of mineral particles during processing. Recently, the development of a novel layered nanostructured flame retardant, ZIF-8@PA@SiO
2, has been reported. This material not only effectively disperses within an epoxy resin matrix but also forms a robust interfacial bond with the matrix, significantly enhancing the thermal stability and flame retardancy of the composite. The enhancement is primarily attributed to the barrier effect established by the multilayer P/N/Si nanostructures, in conjunction with its superior free radical scavenging and dilution capabilities [
25]. The microencapsulation method is susceptible to rupture under high temperatures or mechanical stress, leading to the premature release of flame retardants. Moreover, the processing involved in this technique is intricate and costly.
2.4.2. Nanocomposite Method
By diminishing the particle size, the material can be imbued with distinctive characteristics. However, a primary constraint of nanoparticles is their propensity to aggregate, which underscores the criticality of improving their dispersion within matrices [
26]. The commonly used dispersion methods are ultrasonic treatment, ball milling, electrostatic spray, and surfactant treatment. Nano-scale natural mineral flame retardants, including nano-clay and nano-wollastonite, can be integrated into EPs via solution blending or in-situ polymerization to fabricate nano-composite materials. Such nanoparticles not only enhance flame retardant efficacy but also exert minimal influence on the mechanical integrity of the composites [
27]. However, the nanocomposite approaches encounter the issue of nanoparticle agglomeration and dispersion difficulty, along with significant challenges pertaining to high costs and potential risks to the environment.
3. Classification and Mechanisms of Natural Minerals in Enhancing Flame Retardancy of EPs Composites
Natural minerals play a crucial role in enhancing the flame retardancy of EP. These minerals are classified based on their chemical composition and structural characteristics into clay minerals, silica-based materials, metal hydroxides, and other types. During combustion, these minerals can form protective layers or barriers that inhibit the transfer of heat and mass. Their application in epoxy resin composites ensures effective dispersion and interaction within the resin matrix, thereby optimizing their flame-retardant properties. A thorough understanding of these mechanisms and methods is essential for fully harnessing the potential of natural minerals in advancing flame retardant technologies for epoxy resin composites.
3.1. Clay Materials
In the field of flame retardant materials research, kaolin, montmorillonite, and bentonite are significant clay minerals known for their unique layered structures and chemical properties, demonstrating considerable flame retardant potential and wide-ranging application prospects. This chapter provides an overview of the structures, flame retardant mechanisms, and applications of these three important clay minerals in EPs.
3.1.1. Kaolinite
Kaolinite, a 1:1 type clay mineral, is composed of two-layer structures: a siloxane layer and a gibbsite layer. The siloxane layer consists of silicon tetrahedra arranged in a hexagonal pattern, while the gibbsite layer is composed of octahedral aluminum connected to four hydroxyl groups and two oxygen atoms. These layers are linked by apex oxygen atoms, forming a stable structure (
Figure 2) [
28,
29,
30].
In terms of flame retardancy, the layered structure of kaolinite creates a barrier during combustion, limiting the transmission of oxygen and heat. At high temperatures, kaolinite promotes the charring of the polymer matrix, forming a dense char layer that isolates the flame from the underlying material. It also has good thermal stability, reducing the rate of thermal decomposition and combustion [
32,
33,
34,
35].
However, the delamination or direct intercalation with inorganic and/or organic molecules is challenging for kaolinite, making surface functionalization difficult and leading to poor dispersion in epoxy resin matrices. Su et al. [
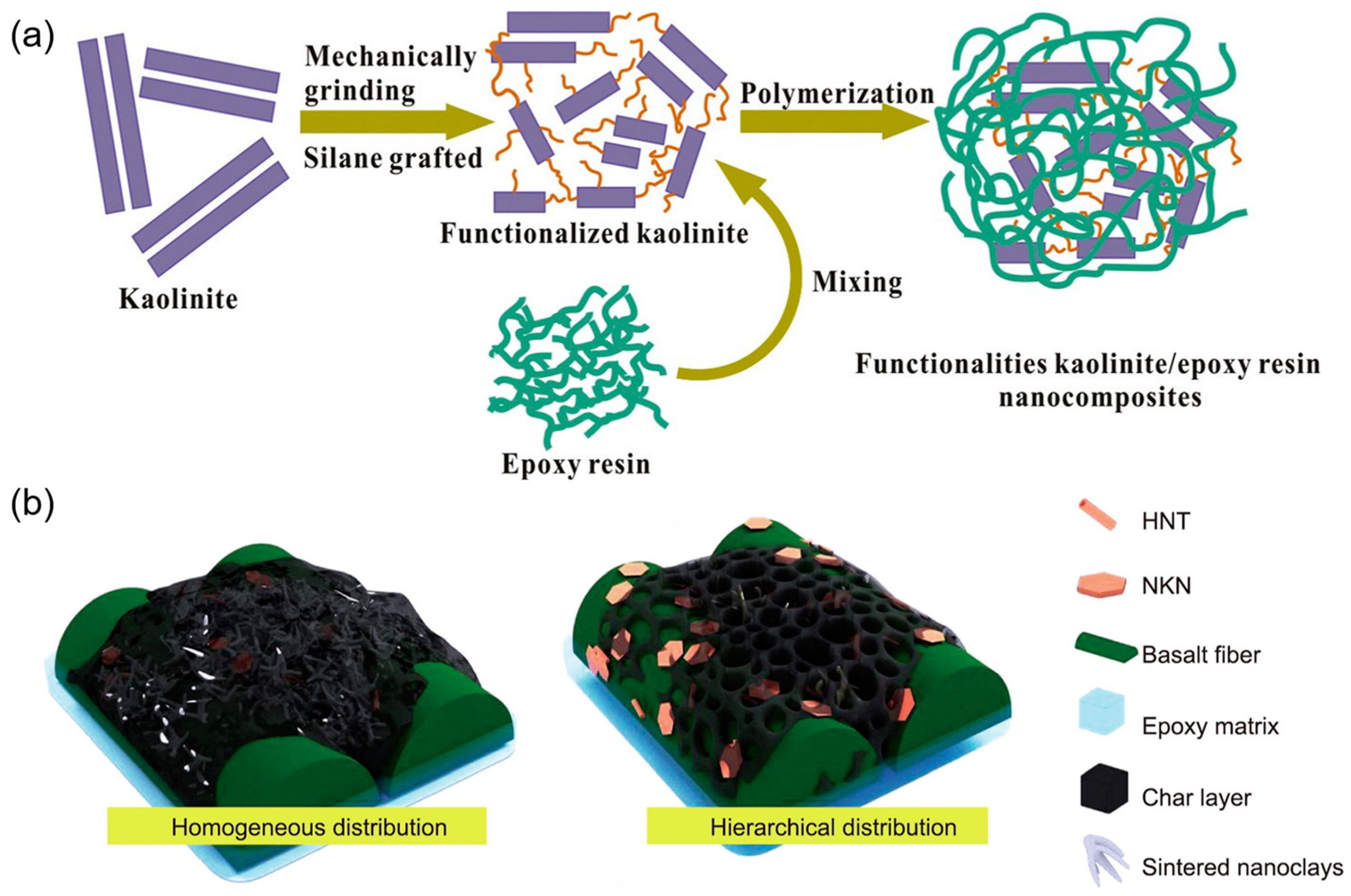
36] used 3-aminopropyltriethoxysilane to modify the surface of kaolinite through mechanical grinding, preparing functionalized kaolinite/epoxy resin(KGS/EP) nanocomposites,
Figure 3a. The results showed that physical grinding disordered the interlayers of kaolinite and exposed surface hydroxyl groups, providing active sites for silane under mild conditions. The thermal stability of KGS/EP was higher than that of kaolinite/EP, indicating improved compatibility between silane-grafted kaolinite and epoxy resin, with the glass transition temperature increasing by 8 °C compared to the pure epoxy resin. This suggests that KGS enhanced the interface with the epoxy resin matrix, restricting chain mobility.
Additionally, researchers have investigated the effect of the hierarchical distribution of kaolinite nanofillers on flame retardancy. The hierarchical distribution of nano additives, referring to the specific dispersion of nano additives in the interfacial region between fibers and the matrix, has been shown to improve the interfacial and interlayer strength of fiber-matrix composites. Chen et al. [
37] designed a 1D/2D hybrid kaolinite nano clay system,
Figure 3b. Emphasizing the synergistic flame retardancy of the 1D/2D hybrid kaolinite nano clay system, they found that 2D nano kaolinite (NKN) impeded heat transfer, while 1D halloysite nanotubes (HNT) reduced van der Waals forces between NKN, thus bridging and enhancing the char layer. The full interaction between the two maximized the synergistic effect, and the addition of nano clay further enhanced flame retardancy and smoke suppression due to the significant synergistic flame retardant performance of 1D/2D kaolinite nano clay.
3.1.2. Montmorillonite
Montmorillonite (MMT) has gained widespread attention in the flame retardant field in recent years due to its affordability, abundance, non-toxicity, and smoke suppression capabilities [
38,
39]. Its unique layered structure and excellent thermal stability provide significant advantages in flame retardancy. MMT mainly consists of layered silicate minerals, presenting a 2:1 layered structure with the chemical formula (Na, Ca)
0.33(Al, Mg)
2(Si
4O
10)(OH)
2·nH
2O. Structurally, it consists of two tetrahedral silicate layers sandwiching an octahedral aluminum or magnesium oxide layer, held together by weak van der Waals forces (
Figure 4). This layered structure endows MMT with a high cation exchange capacity, facilitating the adsorption of alkaline earth metal ions between layers and enabling exchange with ions in the environment, thereby achieving good modification and dispersion effects [
38,
40,
41,
42].
During combustion, MMT releases water and gases, helping to dilute the oxygen concentration and reduce flame spread. Additionally, the SiO
2 layer formed during MMT combustion can insulate and shield the polymer, blocking the transmission of heat, oxygen, and matter, altering the polymer’s degradation pathway, and limiting the mobility of polymer chains. Thus, MMT effectively reduces the heat release, smoke production, and toxic gas emission rates of composite materials during combustion [
39,
44,
45,
46].
Due to its layered structure, montmorillonite tends to exhibit strong interlayer interactions, leading to aggregation within polymer matrices, which is detrimental to enhancing the flame-retardant properties of epoxy resins. To address this issue, Maznah Kabeb et al. [
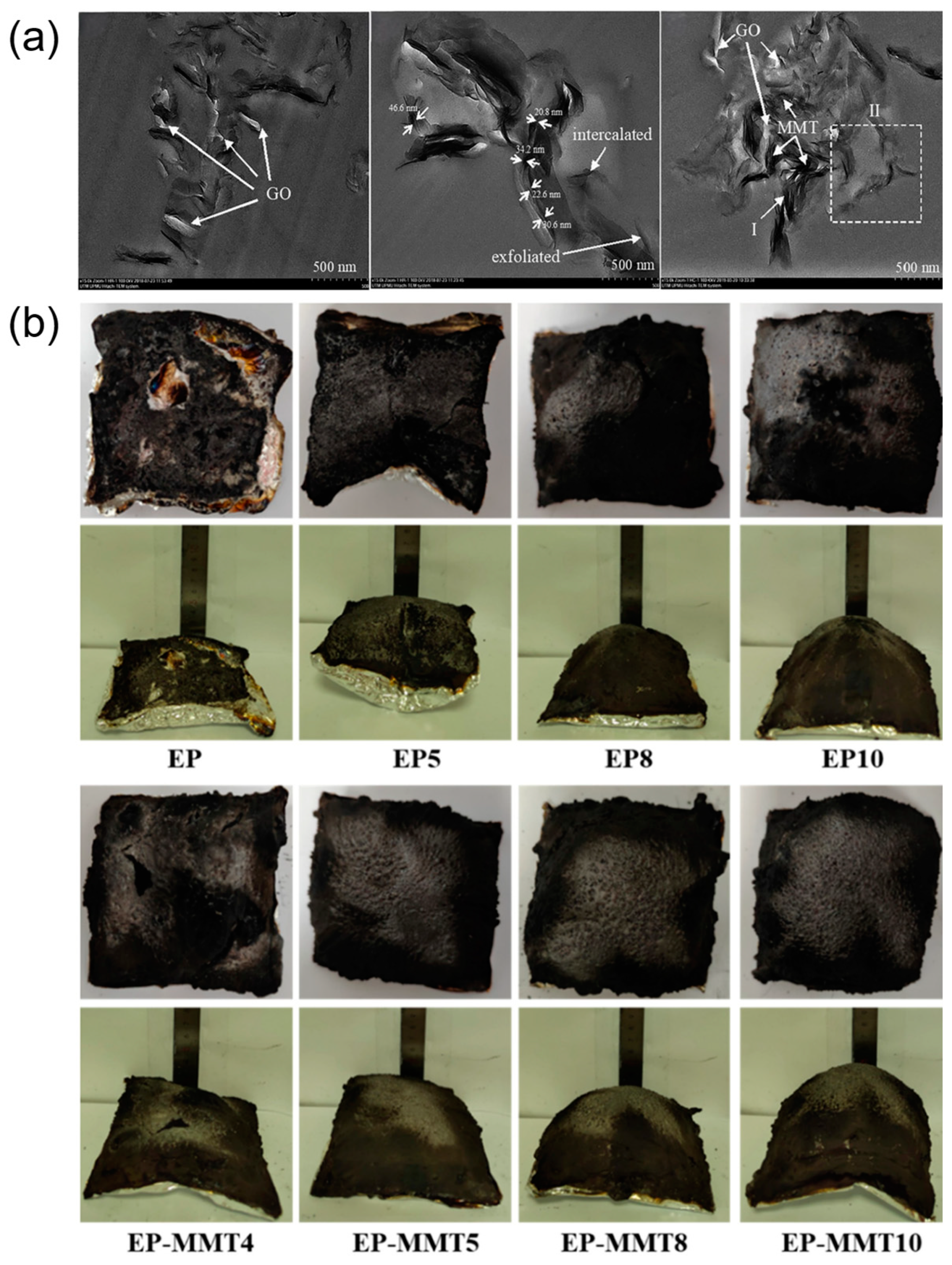
47] proposed the use of graphene oxide (GO) to effectively prevent the aggregation of montmorillonite,
Figure 5a. Compared with the LOI of neat epoxy coating (21.0%) and MMT/EP composites (22.0%), the best LOI value of GO/MMT/EP composites can reach 23.5%. Their research demonstrated that the incorporation of GO not only achieved more uniform dispersion within the polymer matrix but also synergistically enhanced the flame retardancy and thermal stability of the epoxy coatings. To further improve the exfoliation of montmorillonite and form thinner nanosheets, Mi et al. [
48] utilized a phosphorus and nitrogen-containing macromolecule (THPSU) to interact with and fully intercalate into montmorillonite. As a result, this method significantly improved the flame retardancy of the material through the synergistic effect of MMT and THPSU. THPSU-MMT can induce the formation of a continuous and dense char residue in EP composites, making the microscopic surface more complete,
Figure 5b.
3.1.3. Bentonite
Bentonite, mainly composed of montmorillonite, has a similar layered structure,
Figure 6a. Bentonite’s structural characteristics provide the highest surface area for cation exchange and heat resistance [
49,
50,
51]. The flame retardant effect of bentonite is similar to that of montmorillonite. The evaporated water molecules form a steam layer on the material’s surface, diluting the oxygen concentration in the flame zone and reducing flame spread. Simultaneously, at high temperatures, bentonite can expand and form an insulating char layer.
Introducing cationic clays like bentonite into epoxy resin generally shows significant effects. However, using these clays alone is not very effective for enhancing flame retardancy. Typically, ion exchange is performed to combine these clays with nitrogen-containing compounds. This modification can significantly improve flame retardancy even at very low filler densities. Benelli et al. [
52] used 11-amino-N-(pyridine-2-yl)undecanamide (APUA), a nitrogen-rich compound, to promote the exfoliation of montmorillonite; we observed that the bentonite/APUA (Bento-APUA) nanocomposite, with a montmorillonite filler loading of only 3% by weight, significantly reduced the peak heat release rate (pHRR), flame propagating index (FPI), and fire growth rate index (FIGRA) compared to neat resin (NEAT) and Bento-APUA. This demonstrates that the synergy between APUA and bentonite effectively enhances flame retardancy. De et al. [
53] utilized poly(amido-amine) modified bentonite to interact with hyperbranched epoxy resin, achieving uniform dispersion of bentonite within the epoxy resin matrix,
Figure 6b. It demonstrated that the improvement in dispersion effectively enhances interactions, increasing synergistic effects and thereby improving thermal stability.
Figure 6.
(
a) Structural model of the surface of montmorillonite [
54] and (
b) TEM and SEM images of Epoxy resin composites (MNC3) [
53].
Figure 6.
(
a) Structural model of the surface of montmorillonite [
54] and (
b) TEM and SEM images of Epoxy resin composites (MNC3) [
53].
The unique layered structure of kaolinite, bentonite, and montmorillonite clays enhances flame retardancy through heat absorption, insulation, physical shielding, and char promotion. These layered structures act as thermal barriers, reducing heat transfer to the substrate and forming protective char layers. This layered structure not only provides effective insulation but also serves as a structural bridge, further enhancing flame protection. Due to their abundance, low cost, and excellent flame-retardant properties, these clay materials are widely used in various flame-retardant materials and composites.
Table 1 summarizes the typical flame-retardant performance parameters of clay/epoxy resin composites, providing a comprehensive reference for evaluating their flame-retardant properties.
3.2. Silicon Materials
In addition to clay materials, silicon materials play a significant role in enhancing fire resistance, thermal stability, and mechanical properties due to their diverse structures and excellent performance. This section will delve into three main silicon materials, talc, wollastonite, and mica, including their structural characteristics, flame retardant mechanisms, applications in epoxy resins, and their impacts on material performance.
3.2.1. Talc
Talc is a magnesium silicate mineral with the chemical formula Mg
3Si
4O
10(OH)
2. It has a layered structure consisting of two siloxane tetrahedral layers sandwiching a magnesium oxide octahedral layer, connected only by van der Waals forces,
Figure 7a. Talc has a specific surface area of 3–35 m
2/g, a density of approximately 2.8 g/cm
3, and a particle size of 1.4–19 μm [
55,
56,
57,
58].
The large specific surface area and small particle size facilitate the efficiency of the capture of free radicals during combustion, thus promoting the breakdown of organic polymers and improving the fire resistance and thermal stability of polymer composites, with these factors being one of several important contributors. Adding talc powder increases the initial decomposition temperature and char yield of coatings, forming an expanded physical barrier that effectively prevents flame penetration, delays the combustion process, and lowers the burning temperature, exhibiting excellent fire resistance and smoke suppression performance [
59,
60,
61,
62]. This can be attributed to the fact that under high-temperature sintering conditions, talc coatings are more likely to fuse into the flow phase, filling the pores of the substrate material and forming a dense barrier layer by connecting the loose substrate material [
63].
The stability of the barrier formed by talc combustion during heating significantly affects the flame retardancy of the epoxy resin matrix. To enhance this stability, researchers have focused on mineralization strategies. For instance, Liu et al. [
64] used APP to bind talc powder, resulting in a crystallization reaction that forms a mineral-like residue. This not only increases the stability of the residue but also improves the flame retardancy of the epoxy resin. Compared to epoxy resin (with an LOI of 20.0%), the LOI of Ep/Talc/APP composite (ETA5) can reach 29.2%. Epoxy resin composites formed a dense char layer structure (
Figure 7b), showing a significant enhancement in flame retardancy. Similarly, Shahidi et al. [
61] demonstrated that the synergistic effect between multi-layered graphene oxide (MLGO) and talc powder enhances flame retardancy. To prevent oxidation at high temperatures, they used silica fume to improve the adhesion and cohesion of the epoxy resin matrix, resulting in improved thermal stability and flame retardancy at high temperatures. The values of PHRR, HRR, and total heat release (THR) of the composite material (IFR4) were 293.85 kW/m
2, 98.60 kW/m
2, and 58.14 MJ/m
2. Cone calorimeter tests show that MLGO and talc powder make the epoxy resin matrix form a uniform, dense, and crack-free char,
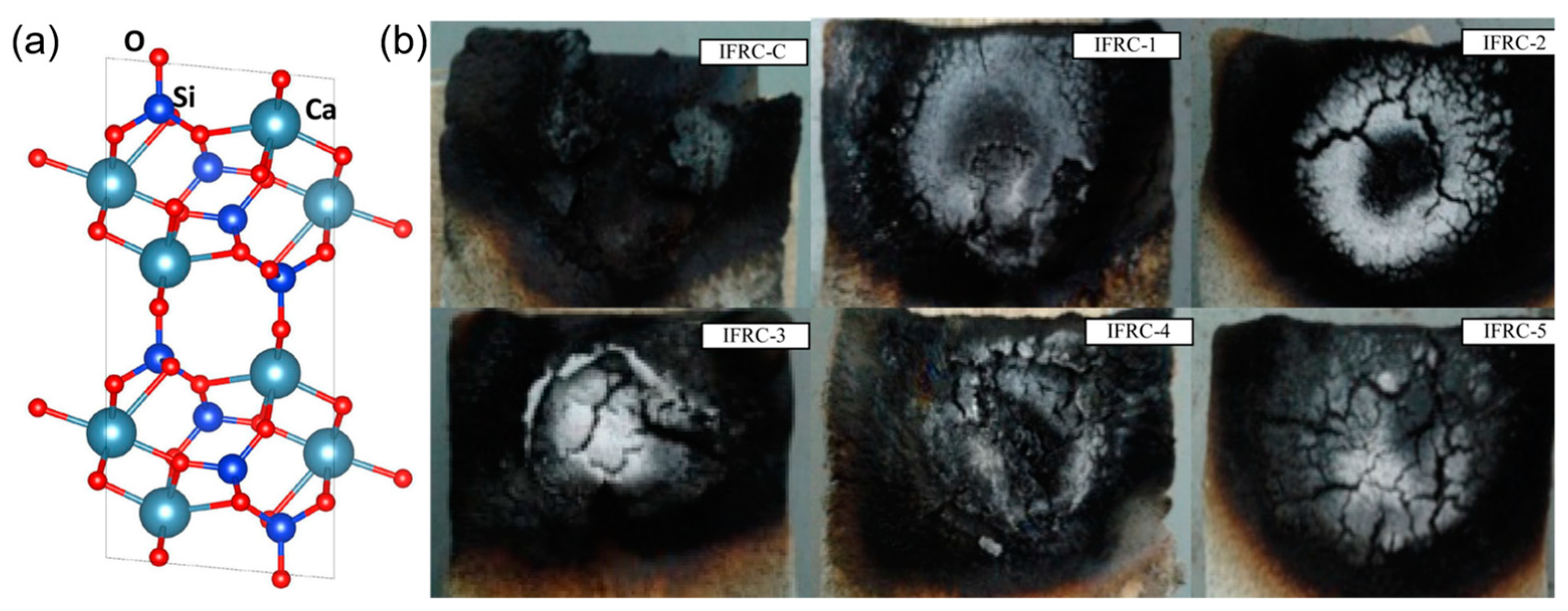
Figure 7c.
Figure 7.
(
a) Structural model of the surface of talc [
65]; The images of cone calorimetry (
b) EP and EP composites (ETA) [
64]; (
c) EP and MLGO/talc powder/EP composites (IFR) [
61].
Figure 7.
(
a) Structural model of the surface of talc [
65]; The images of cone calorimetry (
b) EP and EP composites (ETA) [
64]; (
c) EP and MLGO/talc powder/EP composites (IFR) [
61].
3.2.2. Wollastonite
Wollastonite, also known as calcium metasilicate (CaSiO
3), is a natural calcium silicate with abundant reserves. As an inorganic filler belonging to the silicate family, wollastonite is widely used to enhance the mechanical and thermal properties of polymer matrices due to its unique needle-like crystal structure and good nucleation ability [
66,
67],
Figure 8a. Furthermore, wollastonite has good thermal stability and high silicon content, making it a promising synergist. Industrially, micron-sized wollastonite is obtained from natural ore through beneficiation processes, but it can also be synthesized into nano-sized wollastonite through sol-gel reactions or other chemical methods. Different particle sizes of wollastonite have a significant impact on flame retardancy [
68,
69]. Importantly, wollastonite has been proven not to pose health risks to humans and is an environmentally friendly material without chemical pollution [
70].
Regarding flame retardant properties, wollastonite significantly enhances flame retardancy by promoting char formation and serving as a heat and mass transfer barrier between the flame zone and the underlying sample. The char formation is primarily facilitated by the physical presence of wollastonite, which remains in the condensed phase during combustion. Wollastonite contributes to the stability and density of the char layer by acting as a filler and thermal barrier, which limits polymer chain movement, thereby enhancing the thermal stability and heat resistance of composites [
71,
72].
Karle et al. [
73] conducted a study where they enhanced the flame retardancy of epoxy resin by incorporating wollastonite. The results showed that, compared to the neat epoxy resin matrix (which had a char content of 10%), the addition of wollastonite significantly increased the char content. When the wollastonite content was 5 wt%, the char content of the composite rose to 17%. This indicates that the incorporation of wollastonite particles into epoxy resin can markedly improve its thermal stability. Additionally, the mechanical properties of the composite were also improved with the addition of just 1–2% wollastonite. Similarly, Zia-ul-Mustafa et al. [
71] demonstrated that the synergistic effect of kaolin clay and wollastonite improves thermal stability. When the filler content is 5%, a hole-free char structure forms (
Figure 8b), effectively blocking the transfer of heat and flammable compounds, thereby significantly enhancing the flame retardancy of the composite.
Figure 8.
(
a) Structural model of the surface of wollastonite [
74]; (
b) SEM images of Epoxy resin composites [
71].
Figure 8.
(
a) Structural model of the surface of wollastonite [
74]; (
b) SEM images of Epoxy resin composites [
71].
3.2.3. Mica
Mica is a natural layered material composed of metal oxides such as Al
2O
3 and SiO
2, with the chemical formula Kal
2(Si
3AlO
10(OH)
2). It is low-cost and exhibits good thermal conductivity and chemical stability. Additionally, It is widely available in nature and has a large specific surface area for the physical adsorption of some water [
75,
76,
77,
78],
Figure 9a. Its layered structure interacts with the resin polymer matrix surface, thus enhancing damping effects [
79].
During heating, mica forms a clay-rich barrier that effectively inhibits heat diffusion, slows the release of combustible volatiles, and delays flame spread. It has a good catalytic ability to promote char formation, creating a coherent and dense protective layer that effectively blocks heat transfer [
80,
81,
82].
Jouyandeh et al. [
83] modified mica with N-octadecyl-N′-octadecyl imidazolium iodide (IM) (Mica-IM) to improve thermal stability of EP. The IM facilitated the uniform dispersion of mica, and the interaction between IM and EP formed a dense network (
Figure 9b), restricting polymer chain movement and making decomposition more difficult. Kinetic analysis based on the Friedman, Flynn–Wall–Ozawa (FWO), Kissinger–Akahira–Sunose (KAS), and modified Coats–Redfern (m-CR) conversion methods confirmed that the synergistic effect of Mica-IM enhanced the thermal stability of the epoxy resin matrix, with the best results observed at a 2% filler content. Similarly, He et al. [
82] enhanced the thermal stability of epoxy resin matrices using a synergistic combination of carbon nanotubes and mica. They modified the mica with a silane coupling agent (KH560) and used it as a carrier to uniformly load the carbon nanotubes onto the mica. Thermogravimetric analysis results showed that the thermal degradation rate of the mica-MWCNTs/epoxy composite was the lowest, indicating that the carbon nanotubes and mica improved the high-temperature resistance of the epoxy resin matrix.
Figure 9.
(
a) Structural model of the surface of mica [
84]; (
b) Diagram of the interaction between functionalized Mica (Mica-IM) and EP [
83].
Figure 9.
(
a) Structural model of the surface of mica [
84]; (
b) Diagram of the interaction between functionalized Mica (Mica-IM) and EP [
83].
In conclusion, talc, wollastonite, and mica act as effective flame retardants and reinforcing agents, forming dense physical barriers through different mechanisms that effectively prevent flame penetration. This significantly enhances the fire resistance, thermal stability, and mechanical properties of composite materials.
Table 2 summarizes the typical flame-retardant performance parameters of silicon/epoxy resin composites, providing a comprehensive reference for evaluating their flame-retardant properties.
3.3. Metal Hydroxides
Magnesium hydroxide and aluminum hydroxide, as significant inorganic flame retardants, demonstrate unique advantages and potential in fire-resistant materials. This section will introduce the structures, flame retardant mechanisms, and applications of these two metal oxides in epoxy resins.
Magnesium hydroxide (MH) and aluminum hydroxide (ATH) are widely used as a flame retardant in various polymer materials due to their wide availability, low cost, environmental friendliness, non-toxicity, and good flame retardant and smoke suppression properties [
85,
86,
87,
88,
89].
MH and ATH exhibit similar flame-retardant mechanisms. Both decompose at high temperatures to produce their respective metal oxides (MgO and Al
2O
3) and water vapor. The water vapor released during decomposition enters the flame zone and dilutes the concentration of combustible gases and oxygen. The resulting MgO and Al
2O
3 form a protective layer on the polymer surface, blocking the transfer of gases and heat, thereby reducing the supply of fuel. This process slows down the degradation of the polymer, effectively suppresses the release of pyrolysis products from the epoxy resin, and promotes the formation of a dense char layer. Additionally, the reduction in volatile products during decomposition significantly decreases smoke production. These mechanisms indicate their effectiveness in flame retardancy and fire risk reduction [
90,
91,
92,
93].
However, using single-component magnesium hydroxide (MH) or aluminum hydroxide (ATH) as flame retardants has certain limitations. The MgO barrier layer formed by the thermal decomposition of MH is fragile, and the inner layer of carbon residue has large honeycomb-like pores, which cannot effectively suppress the migration of heat and combustible volatiles [
94]. Another limitation is the high loading required; typically, the content of MH or ATH at least exceeds 50% to achieve desirable flame retardancy, which leads to a decline in mechanical properties and limits the applicability of epoxy resins [
95,
96,
97]. These factors inevitably impair the flame-retardant efficiency of the composite materials.
To address these issues, MH or ATH is often organically functionalized, such as by altering particle size or structure. Zhao et al. [
98] explored the impact of the microscopic morphology of MH on flame retardancy and found that when MH exhibited a sheet-like morphology, agglomeration was significant, resulting in poor dispersion within the polymer matrix. In contrast, organically modified MH with a rod-like morphology showed no significant agglomeration, greatly improved compatibility with the epoxy resin matrix, and increased flexural modulus, flexural strength, and flame retardancy. Könnicke et al. [
99] demonstrated that particle size is related to flame retardancy, with nano-scale ATH exhibiting stronger flame retardant properties than micron-scale ATH.
Alternatively, MH or ATH can be mixed with other types of flame retardants, such as ammonium polyphosphate [
100]. When used in combination with ATH to prepare epoxy resin composites, a good synergistic flame retardant effect is observed. The degradation products of APP and ATH or MH react in the condensed phase to form a three-dimensional network and a more compact carbon layer, which can effectively reduce the filler content of MH while achieving higher flame retardancy efficiency.
In conclusion, through organic modification or combination with other flame retardants, the filling amount of magnesium hydroxide and aluminum hydroxide can be significantly reduced while maintaining excellent flame retardant performance, making them competitive flame retardants.
Table 3 summarizes the typical flame-retardant performance parameters of metal hydroxides/epoxy resin composites, providing a comprehensive reference for evaluating their flame-retardant properties.
3.4. Others
In addition to clay materials, mineral materials, and metal oxides and hydroxides, which are the most common types of natural minerals used as flame retardants for epoxy resins, hydromagnesite and barite can also be used as flame retardants. Since the usage of these minerals is far less than the three types mentioned above, their flame-retardant mechanisms are briefly introduced here.
Hydromagnesite, with the chemical formula 4MgCO
3·Mg(OH)
2·4H
2O or 5MgO·4CO
2·5H
2O, contains the highest magnesium content among magnesium ores. During combustion, it decomposes at higher temperatures (300 °C) to form MgO and H
2O. The released water dilutes the concentration of combustible gases. Compared to aluminum trihydrate and magnesium dihydroxide, hydromagnesite releases water and carbon dioxide over a wider temperature range. MgO then forms an effective protective barrier between the substrate material and the flame [
101,
102].
Barite is a barium-based mineral (BaSO
4) that is non-toxic, unlike BaCrO
4 [
103]. Barite acts as a physical barrier by forming a protective layer on the char surface, which limits the penetration of surrounding heat. This heat-resistant layer provides an interface between the substrate and the flame, improving the material’s flame retardancy.
4. Summary and Outlook
With the implementation of global zero-carbon emission policies, the development and refinement of relevant regulations and standards are crucial for promoting the application of natural flame-retardant minerals in epoxy composites. Therefore, the prospects for using natural minerals flame retardants in epoxy resin composites are becoming increasingly promising. This paper systematically reviews the research progress of natural minerals as flame retardants for epoxy resin composites, introducing their classifications, interaction mechanisms with epoxy resin, and composite methods, and clarifying the underlying flame retardant mechanisms.
Methods for Integrating Natural Mineral Flame Retardants with EPs. In order to cater to the diverse requirements of different applications, various approaches have been developed for integrating natural mineral flame retardants with epoxy resins. These methods range from simple physical and mechanical mixing to intricate surface modification and structural design, encompassing the utilization of single-component mineral flame retardants as well as a variety of flame retardant ratios. Moreover, these techniques span across macroscopic, nanoscopic, and microscopic levels of design. Each method possesses its own advantages and disadvantages within specific aspects and levels. Taking into account objective factors such as synthesis conditions and product objectives, it is possible to effectively enhance the flame retardancy of EPs at minimal cost.
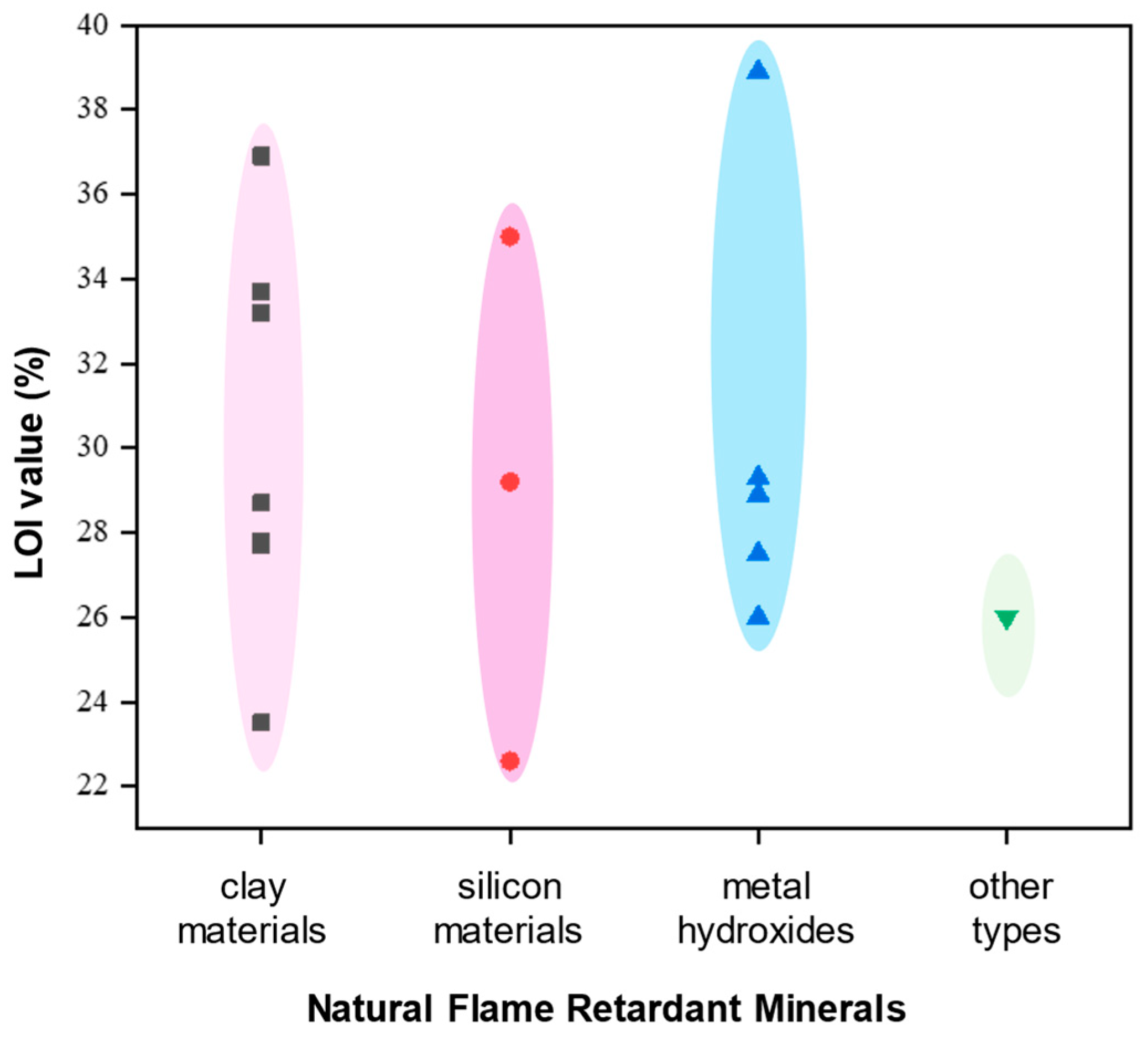
Application of natural minerals in epoxy resin. Natural mineral flame retardants, including clay materials (kaolin, montmorillonite, bentonite, etc.), silicon materials (talc, wollastonite, mica, etc.), metal hydroxides, and other natural mineral materials, play a crucial role in enhancing the flame retardancy of epoxy resins. This chart compares the Limiting Oxygen Index (LOI) values of different types of natural flame-retardant mineral materials,
Figure 10. Among these materials, metal hydroxides, such as aluminum hydroxide and magnesium hydroxide, stand out. They have the highest number of studies and the highest LOI values, indicating excellent flame-retardant properties. These materials are widely used in epoxy resins and other applications, offering reliable flame retardancy across various scenarios. Clay materials have LOI values that are second to metal hydroxides, also showing a degree of flame retardancy. These materials typically achieve flame-retardant properties through nano-composites with polymer matrices, utilizing layered structures. Common types include montmorillonite and kaolin. Although their flame-retardant effectiveness is not as high as that of metal hydroxides, they still have advantages in specific applications. Silicon-based materials have relatively fewer research data, and their LOI values indicate a moderate level of flame retardancy. Despite potentially not being as effective as other inorganic materials, these materials still offer unique advantages in epoxy resin applications, such as high-temperature resistance and flexibility, making them irreplaceable in certain uses. Other materials, such as hydromagnesite and barite, have fewer studies and lower LOI values. The actual effectiveness of these materials requires further verification and research.
Future perspective. Further optimizing the dispersibility, stability, and synergistic flame retardant effects of mineral flame retardants in epoxy resin matrices is an important direction for future research. Surface modification and nanocomposite technology have great potential. Surface modification, by introducing functional groups on the surface of mineral flame retardants, can improve their compatibility with the epoxy resin matrix and promote uniform dispersion. Additionally, nanocomposite technology, by dispersing nanoscale mineral particles into the matrix, can significantly improve the mechanical properties, thermal stability, and flame retardancy of the materials. Moreover, exploiting the synergistic effects of different natural mineral materials to develop new composite materials is another effective way to enhance flame retardancy and mechanical properties. Alongside technological innovation, exploring potential new application areas and market demands remains crucial for future research endeavors. For instance, given the growing demand for environmentally friendly and high-performance materials, natural mineral fire retardants find extensive applications in sustainable building materials, lightweight automotive components, and electronic devices.
In summary, through surface modification, nanocomposite technology, and the synergistic effects of natural mineral materials, significant progress is expected in improving the dispersibility, stability, and synergistic flame retardancy of mineral flame retardants in epoxy resin matrices in the future. Although natural mineral flame retardants have broad application prospects in epoxy resin composites, their large-scale application still faces some challenges. Continuous research on synergistic flame retardant mechanisms and technological innovations is expected to achieve the comprehensive commercialization of these materials shortly, contributing positively to the goal of carbon neutrality.
Author Contributions
Conceptualization, Y.L., X.Z. and W.W.; methodology, Y.L and L.X.; software, J.L.; validation, Y.L., X.Z. and W.W.; formal analysis, H.L.; investigation, Y.L.; resources, X.Z.; data curation, Y.L.; writing—original draft preparation, Y.L. and X.Z.; writing—review and editing, Y.L., X.Z. and W.W.; visualization, L.X.; supervision, L.X. and W.W.; project administration, W.W.; funding acquisition, G.H.Y. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported under the Australian Research Council/Discovery Early Career Researcher Award (DECRA) funding scheme (DE230100180) and ARC Research Hub for Fire Resilience Infrastructure, Assets, and Safety Advancements (FRIASA) in Urban, Resources, Energy, and Renewables Sectors (IH220100002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, S.; Yan, H.; Fang, Z.; Wang, H. Effect of graphene nanosheets on morphology, thermal stability and flame retardancy of epoxy resin. Compos. Sci. Technol. 2014, 90, 40–47. [Google Scholar] [CrossRef]
- Spontón, M.; Ronda, J.C.; Galià, M.; Cádiz, V. Flame retardant epoxy resins based on diglycidyl ether of (2,5-dihydroxyphenyl)diphenyl phosphine oxide. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 2142–2151. [Google Scholar] [CrossRef]
- Wang, C.S.; Lin, C.H. Synthesis and properties of phosphorus-containing epoxy resins by novel method. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3903–3909. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Yang, R. The degradation and charring of flame retarded epoxy resin during the combustion. J. Appl. Polym. Sci. 2013, 130, 4119–4128. [Google Scholar] [CrossRef]
- Wilkie, C.A.; Morgan, A.B. Fire Retardancy of Polymeric Materials; CRC Press: Boca Raton, FL, USA, 2024. [Google Scholar]
- Wang, W.; Kan, Y.; Pan, Y.; Yuan, Y.; Liew, K.M.; Hu, Y. Urchinlike shells of TiO2 hollow spheres for improving the fire safety of epoxy resin. Ind. Eng. Chem. Res. 2017, 56, 1341–1348. [Google Scholar] [CrossRef]
- Sienkiewicz, A.; Czub, P. Flame Retardancy of Biobased Composites-Research Development. Materials 2020, 13, 5253. [Google Scholar] [CrossRef]
- Vahabi, H.; Michely, L.; Moradkhani, G.; Akbari, V.; Cochez, M.; Vagner, C.; Renard, E.; Saeb, M.R.; Langlois, V. Thermal Stability and Flammability Behavior of Poly(3-hydroxybutyrate) (PHB) Based Composites. Materials 2019, 12, 2239. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Luda di Cortemiglia, M.P. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Suihkonen, R.; Nevalainen, K.; Orell, O.; Honkanen, M.; Tang, L.; Zhang, H.; Zhang, Z.; Vuorinen, J. Performance of epoxy filled with nano- and micro-sized Magnesium hydroxide. J. Mater. Sci. 2011, 47, 1480–1488. [Google Scholar] [CrossRef]
- Gérard, C.; Fontaine, G.; Bellayer, S.; Bourbigot, S. Reaction to fire of an intumescent epoxy resin: Protection mechanisms and synergy. Polym. Degrad. Stab. 2012, 97, 1366–1386. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, Y.-K.; Zhang, K.; Shen, M.-M.; Hu, Y. Effect of microencapsulation on thermal properties and flammability performance of epoxy composite. J. Anal. Appl. Pyrolysis 2012, 94, 196–201. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X. Synthesis, characterization, thermal properties and flame retardancy of a novel nonflammable phosphazene-based epoxy resin. Polym. Degrad. Stab. 2009, 94, 617–624. [Google Scholar] [CrossRef]
- Chao, P.; Li, Y.; Gu, X.; Han, D.; Jia, X.; Wang, M.; Zhou, T.; Wang, T. Novel phosphorus–nitrogen–silicon flame retardants and their application in cycloaliphatic epoxy systems. Polym. Chem. 2015, 6, 2977–2985. [Google Scholar] [CrossRef]
- Kickelbick, G. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar] [CrossRef]
- Fina, A.; Abbenhuis, H.C.L.; Tabuani, D.; Frache, A.; Camino, G. Polypropylene metal functionalised POSS nanocomposites: A study by thermogravimetric analysis. Polym. Degrad. Stab. 2006, 91, 1064–1070. [Google Scholar] [CrossRef]
- Lee, Y.X.; Ahmad, F.; Karuppanan, S.; Sumby, C.; Shahed, C.A.; Ramli, S.H. Thermo-mechanical performance of wolframite mineral reinforced siloxane-modified epoxy-based intumescent coating for structural steel. Polym. Adv. Technol. 2024, 35, e6279. [Google Scholar] [CrossRef]
- Wang, W.; Kan, Y.; Yu, B.; Pan, Y.; Liew, K.M.; Song, L.; Hu, Y. Synthesis of MnO2 nanoparticles with different morphologies and application for improving the fire safety of epoxy. Compos. Part A Appl. Sci. Manuf. 2017, 95, 173–182. [Google Scholar] [CrossRef]
- Jiang, J.; Cheng, Y.; Liu, Y.; Wang, Q.; He, Y.; Wang, B. Intergrowth charring for flame-retardant glass fabric-reinforced epoxy resin composites. J. Mater. Chem. A 2015, 3, 4284–4290. [Google Scholar] [CrossRef]
- Rajaei, M.; Kim, N.K.; Bickerton, S.; Bhattacharyya, D. A comparative study on effects of natural and synthesised nano-clays on the fire and mechanical properties of epoxy composites. Compos. Part B Eng. 2019, 165, 65–74. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, T.; Li, J.; Tan, J.; Zhu, X. Enhancing toughness, flame retardant, hydrophobic and dielectric properties of epoxy resin by incorporating multifunctional additive containing phosphorus/silicon. Mater. Des. 2023, 225, 111529. [Google Scholar] [CrossRef]
- Kong, L.; Zhao, D.; Jiang, G.; Jiang, G.; Shen, Y.; Wang, T. Constructing integrated thermal conductive and flame-retardant filler network in ceramifiable epoxy composites. J. Reinf. Plast. Compos. 2023. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, D.; Deng, B.; Xu, X.; Nian, Q.; Jin, S.; Leedy, K.D.; Li, H.; Cheng, G.J. Flyweight, Superelastic, Electrically Conductive, and Flame-Retardant 3D Multi-Nanolayer Graphene/Ceramic Metamaterial. Adv. Mater. 2017, 29, 1605506. [Google Scholar] [CrossRef]
- Yan, W.; Xie, P.; Yang, Z.; Luo, G.; Huang, W.; Tian, Q.; Tu, C.; Zhang, C.; Yang, C.; Wang, K. Flame-retardant behaviors of aluminum phosphates coated sepiolite in epoxy resin. J. Fire Sci. 2020, 39, 3–18. [Google Scholar] [CrossRef]
- Yin, L.; Gong, K.; Pan, H.; Qian, X.; Shi, C.; Qian, L.; Zhou, K. Novel design of MOFs-based hierarchical nanoarchitecture: Towards reducing fire hazards of epoxy resin. Compos. Part A Appl. Sci. Manuf. 2022, 158, 106957. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Manzano, C.V.; Reinosa, J.J.; Lorite, I.; Romero, J.J.; Fernández, J.F.; Martín-González, M.S. Modification of optical properties in ZnO particles by surface deposition and anchoring of NiO nanoparticles. J. Alloys Compd. 2011, 509, 2891–2896. [Google Scholar] [CrossRef]
- Wang, W.; Lin, B.; Yuen, A.C.Y.; Yeoh, G.H. One-dimensional nanomaterials for flame retardant epoxy thermosets and composites. In Non-Halogenated Flame-Retardant Technology for Epoxy Thermosets and Composites; Woodhead Publishing: Cambridge, UK, 2024; pp. 291–322. [Google Scholar]
- Fan, H.; Zhao, J.; Zhang, J.; Li, H.; Zhang, S.; Sun, J.; Xin, F.; Liu, F.; Qin, Z.; Tang, W. TiO2/SiO2/kaolinite hybrid filler to improve the flame retardancy, smoke suppression and anti-aging characteristics of epoxy resin. Mater. Chem. Phys. 2022, 277, 125576. [Google Scholar] [CrossRef]
- Anam, A.; Gamit, N.; Prajapati, V.; Dholakiya, B.Z. An overview of kaolin and its potential application in thermosetting polymers. Mater. Today Commun. 2023, 36, 106827. [Google Scholar] [CrossRef]
- Yuan, B.; Zhao, H.; Wang, S. Surface modification of ammonium polyphosphate by kaolinite and the study on thermal decomposition behavior and flame-retardant performance. J. Therm. Anal. Calorim. 2022, 147, 7311–7321. [Google Scholar] [CrossRef]
- Liu, L.; Kong, C.; Zhao, H.; Lu, F. Elucidating the enhancement of kaolinite flotation by iron content through density functional theory: A study on sodium oleate adsorption efficiency. Int. J. Min. Sci. Technol. 2024; in press. [Google Scholar]
- de Oliveira, C.R.S.; Batistella, M.A.; Guelli Ulson de Souza, S.M.d.A.; Ulson de Souza, A.A. Functionalization of cellulosic fibers with a kaolinite-TiO2 nano-hybrid composite via a solvothermal process for flame retardant applications. Carbohydr. Polym. 2021, 266, 118108. [Google Scholar] [CrossRef] [PubMed]
- Ingtipi, K.; Choudhury, B.J.; Moholkar, V.S. Kaolin-embedded cellulose hydrogel with tunable properties as a green fire retardant. Carbohydr. Polym. 2023, 313, 120871. [Google Scholar] [CrossRef]
- Tang, W.; Liao, X.; Qin, Z.; Zeng, Y.; Chen, C.; Zhu, Q.; Mo, Z.; Jin, X. Improving the flame retardancy of epoxy resin by incorporating a bio-based flame retardant and kaolinite. Polym. Degrad. Stab. 2024, 227, 110895. [Google Scholar] [CrossRef]
- Yan, L.; Guan, J.; Wei, Z.; Tang, X.; Xu, Z. Combination effect of ADP-intercalated kaolinite and ammonium polyphosphate on simultaneously enhancing the flame retardancy and smoke suppression of epoxy resins. J. Vinyl Addit. Technol. 2024, 30, 868–879. [Google Scholar] [CrossRef]
- Su, L.; Zeng, X.; He, H.; Tao, Q.; Komarneni, S. Preparation of functionalized kaolinite/epoxy resin nanocomposites with enhanced thermal properties. Appl. Clay Sci. 2017, 148, 103–108. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, T.; Yang, Z.; Wei, Z.; Li, Y.; Yang, W.; Yu, T. Superior Mechanical Behavior and Flame Retardancy FRP via a Distribution Controllable 1D/2D Hybrid Nanoclay Synergistic Toughening Strategy. Engineering, 2024; in press. [Google Scholar] [CrossRef]
- Xu, B.-T.; Jin, D.-Z.; Yu, Y.; Zhang, Q.; Weng, W.-J.; Ren, K.-X.; Tai, Y.-L. Nanoclay-reinforced alginate aerogels: Preparation and properties. RSC Adv. 2024, 14, 954–962. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Yuan, D.; Wang, Z.; Xie, H. Use of montmorillonite to improve flame retardancy, thermal stability and reducing smoke toxicity of chicken feather protein-based rigid polyurethane foam. J. Vinyl Addit. Technol. 2024, 30, 483–498. [Google Scholar] [CrossRef]
- Uddin, M.N.; Hossain, M.T.; Mahmud, N.; Alam, S.; Jobaer, M.; Mahedi, S.I.; Ali, A. Research and applications of nanoclays: A review. SPE Polym. 2024, 1–29. [Google Scholar] [CrossRef]
- Chee, S.S.; Jawaid, M.; Alothman, O.Y.; Yahaya, R. Thermo-oxidative stability and flammability properties of bamboo/kenaf/nanoclay/epoxy hybrid nanocomposites. RSC Adv. 2020, 10, 21686–21697. [Google Scholar] [CrossRef]
- Yang, R.; Fang, Y.; Zhu, J.; Ren, G.; Lin, B.; Wang, F.; Ou, R.; Wang, Q.; Song, Y. Fabrication ZnO functionalized nano-montmorillonite for enhancing the fire-safety and mechanical properties of wood flour/poly (vinyl chloride) composites. Constr. Build. Mater. 2024, 420, 135522. [Google Scholar] [CrossRef]
- Ge, W.; Mao, H.; Chen, J.; Min, F.; Liu, H.; Song, S. Understanding the thermal activation behavior of montmorillonite and kaolinite at atomic level by ReaxFF molecular dynamics simulations. Appl. Clay Sci. 2024, 251, 107313. [Google Scholar] [CrossRef]
- Kaynak, C.; Nakas, G.I.; Isitman, N.A. Mechanical properties, flammability and char morphology of epoxy resin/montmorillonite nanocomposites. Appl. Clay Sci. 2009, 46, 319–324. [Google Scholar] [CrossRef]
- Tang, S.; Wachtendorf, V.; Klack, P.; Qian, L.; Dong, Y.; Schartel, B. Enhanced flame-retardant effect of a montmorillonite/phosphaphenanthrene compound in an epoxy thermoset. RSC Adv. 2017, 7, 720–728. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, Y.; Qian, L.; Chen, Y.; Xu, B. Strengthen flame retardancy of epoxy thermoset by montmorillonite particles adhering phosphorus-containing fragments. J. Appl. Polym. Sci. 2020, 137, 47500. [Google Scholar] [CrossRef]
- Maznah Kabeb, S.; Hassan, A.; Mohamad, Z.; Sharer, Z.; Mokhtar, M.; Ahmad, F. Sustainable flame retardant coating based graphene oxide and montmorillonite. Mater. Today Proc. 2022, 51, 1327–1331. [Google Scholar] [CrossRef]
- Mi, Z.; Chu, F.; Hu, W.; Hu, Y.; Song, L. Eco-friendly preparation of advanced epoxy composites and their pyrolysis and flame retardant mechanisms. Polym. Degrad. Stab. 2024, 224, 110749. [Google Scholar] [CrossRef]
- Ahmad, F.; Ullah, S.; Merican, N.H.b.H.; Oñate, E.; Al-Sehemi, A.G.; Yeoh, G.H. An investigation on thermal performance of wollastonite and bentonite reinforced intumescent fire-retardant coating for steel structures. Constr. Build. Mater. 2019, 228, 116734. [Google Scholar] [CrossRef]
- Chen, G.; Chen, C.; Pei, Y.; He, S.; Liu, Y.; Jiang, B.; Jiao, M.; Gan, W.; Liu, D.; Yang, B.; et al. A strong, flame-retardant, and thermally insulating wood laminate. Chem. Eng. J. 2020, 383, 123109. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Soltani, F.; Shabanian, M.; Maleki, M.; Khonakdar, H.A.; Kruppke, B. Plasticized polyvinyl chloride/melamine-cyanurate modified Mg(OH)2@bentonite nanocomposites; mechanical, thermal, and flame retardant properties. J. Vinyl Addit. Technol. 2024, 30, 114–129. [Google Scholar] [CrossRef]
- Benelli, T.; Mazzocchetti, L.; D’Angelo, E.; Lanzi, M.; Saraga, F.; Sambri, L.; Franchini, M.C.; Giorgini, L. New nitrogen-rich heterocycles for organo-modified bentonites as flame retardant fillers in epoxy resin nanocomposites. Polym. Eng. Sci. 2017, 57, 621–630. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Tough hyperbranched epoxy/poly(amido-amine) modified bentonite thermosetting nanocomposites. J. Appl. Polym. Sci. 2014, 131, 40327. [Google Scholar] [CrossRef]
- Almajed, A.; Dafalla, M.; Lemboye, K. The plastic behavior and compression of bentonite clay under heating effect. Case Stud. Therm. Eng. 2024, 59, 104543. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, X.; Chen, Z.; Wan, M.; Wu, Q. Thermal Stability and Flame Resistance of the Coextruded Wood-Plastic Composites Containing Talc-Filled Plastic Shells. Int. J. Polym. Sci. 2020, 2020, 1435249. [Google Scholar] [CrossRef]
- Younis, A.A.; Mohamed, S.A.A.; El-Samahy, M.A.; Abdel Kader, A.H. Novel fire-retardant bagasse papers using talc/cyclodiphosphazane and nanocellulose as packaging materials. Egypt. J. Pet. 2021, 30, 25–32. [Google Scholar] [CrossRef]
- Podkościelna, B.; Klepka, T.; Podkościelny, P.; Bocho-Janiszewska, A.; Wasilewski, T.; Klapiszewski, Ł. Structural, Mechanical and Flammability Characterization of Crosslinked Talc Composites with Different Particle Sizes. Materials 2022, 15, 4492. [Google Scholar] [CrossRef]
- Antosik, A.K.; Grajczyk, A.; Półka, M.; Zdanowicz, M.; Halpin, J.; Bartkowiak, M. Influence of Talc on the Properties of Silicone Pressure-Sensitive Adhesives. Materials 2024, 17, 708. [Google Scholar] [CrossRef]
- Yan, L.; Tang, X.; Xu, Z.; Xie, X. Fabrication of talc reinforced transparent fire-retardant coating towards excellent fire protection, antibacterial, mechanical and anti-ageing properties. Polym. Degrad. Stab. 2022, 203, 110074. [Google Scholar] [CrossRef]
- Goller, S.M.; Schartel, B.; Krüger, S. Phosphorus features halogen –calcium hypophosphite replaces antimony trioxide, reduces smoke, and improves flame retardancy. Thermochim. Acta 2024, 737, 179764. [Google Scholar] [CrossRef]
- Shahidi, S.; Mohammadi, S. Synergistic effect of nano hybrid multi-layered graphene oxide/talc and silica fume on the fire and water -resistance of intumescent coatings. Prog. Org. Coat. 2023, 183, 107736. [Google Scholar] [CrossRef]
- Yan, L.; Huang, D.; Xu, Z.; Xie, X. Influence of talc particle size on the optical transparency, flame retardancy, and smoke release suppression of waterborne transparent fireproof coatings applied on wood surface. J. Vinyl Addit. Technol. 2024, 30, 557–569. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Yu, Z.; Zhao, Y.; Zhang, Z.X. Lightweight and flame retardant silicone rubber foam prepared by supercritical nitrogen: The influence of flame retardants combined with ceramicizable fillers. Constr. Build. Mater. 2023, 370, 130735. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, D.; Pan, Z.; Shen, Y.; Wang, T. Enhanced residue stability and strength of epoxy-based coating for fire protection via ceramifiable strategy. Prog. Org. Coat. 2021, 154, 106211. [Google Scholar] [CrossRef]
- Luo, Y.; Ou, L.; Chen, J.; Zhou, H.; Yin, C.; Yang, H. Insights into the adsorption mechanism of water at different coverage rates on talc (Mg3Si4O10(OH)2) (001) basal surface: A first-principles study. J. Mol. Liq. 2022, 363, 119879. [Google Scholar] [CrossRef]
- Zhou, R.; Lai, X.; Li, H.; Tang, S.; Zeng, X. Enhancement of wollastonite on flame retardancy and mechanical properties of PP/IFR composite. Polym. Compos. 2014, 35, 158–166. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Hassan, A.; Othman, N.; Razak, J.A.; Nirmal, U.; Hashim, S.; Ching, Y.C.; Yunos, M.Z.; Yahaya, R.; et al. Synthetic wollastonite nanofiber for polybutylene terephthalate nanocomposite: Mechanical, thermal, tribological and flammability properties. Polymer 2022, 256, 125259. [Google Scholar] [CrossRef]
- Chan, J.X.; Wong, J.F.; Hassan, A.; Mohamad, Z.; Othman, N. Mechanical properties of wollastonite reinforced thermoplastic composites: A review. Polym. Compos. 2020, 41, 395–429. [Google Scholar] [CrossRef]
- Wong, J.F.; Chan, J.X.; Hassan, A.; Mohamad, Z.; Hashim, S.; Abd Razak, J.; Ching, Y.C.; Yunos, Z.; Yahaya, R. Use of synthetic wollastonite nanofibers in enhancing mechanical, thermal, and flammability properties of polyoxymethylene nanocomposites. Polym. Compos. 2022, 43, 7845–7858. [Google Scholar] [CrossRef]
- Taghiyari, H.R.; Militz, H.; Antov, P.; Papadopoulos, A.N. Effects of Wollastonite on Fire Properties of Particleboard Made from Wood and Chicken Feather Fibers. Coatings 2021, 11, 518. [Google Scholar] [CrossRef]
- Zia-ul-Mustafa, M.; Ahmad, F.; Ullah, S.; Amir, N.; Gillani, Q.F. Thermal and pyrolysis analysis of minerals reinforced intumescent fire retardant coating. Prog. Org. Coat. 2017, 102, 201–216. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Wang, Q. The investigation of melamine polyphosphate flame retardant polyamide-6/inorganic siliciferous filler with different geometrical form. J. Appl. Polym. Sci. 2009, 113, 2046–2051. [Google Scholar] [CrossRef]
- Karle, A.H.; Nukulwar, M.R.; Tungikar, V.B. Evaluation of mechanical and thermal properties of epoxy composites reinforced with CaSiO3 particulate fillers. Mater. Today Proc. 2021, 46, 325–330. [Google Scholar] [CrossRef]
- Hoang, N.-H.; Ha, D.T.; Trinh, T.T. A first-principles insight into thermodynamic and mechanical properties of Xonotlite and Wollastonite phases of high temperature geothermal well cement. Results Mater. 2023, 20, 100454. [Google Scholar] [CrossRef]
- Guo, S.; Yu, Z.; Peng, B.; Chen, H.; Guo, Y.; Chen, J. Preparation and corrosion resistance study of two-dimensional mica-based composite coatings modified with UIO-66 and polyethyleneimine. J. Appl. Polym. Sci. 2023, 140, e54497. [Google Scholar] [CrossRef]
- Pan, C.; Wu, F.; Fan, G.; Long, Y.; Yang, H.; Yang, G.; Li, X. A multifunctional flexible composite film with excellent insulation flame retardancy, thermal management and solar-thermal conversion properties based on CNF-modified mica/electrospun fibrous networks structure. Sol. Energy Mater. Sol. Cells 2023, 261, 112530. [Google Scholar] [CrossRef]
- Soykok, I.F.; Taş, H. Effects of natural mica particle fillers on the mechanical properties of glass fiber/epoxy composite plates. J. Braz. Soc. Mech. Sci. Eng. 2023, 46, 18. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, J.; Wang, D.; Yang, J.; Zhang, H.; Wang, B. Reinforcement of mica on phthalonitrile resin and composites: Curing, thermal, mechanical and dielectric properties. Compos. Sci. Technol. 2023, 244, 110289. [Google Scholar] [CrossRef]
- Wu, J.; Bi, J.; Xu, B.; Fu, L.; Hao, W. Enhanced Flame Retardancy of Styrene-Acrylic Emulsion Based Damping Composites Based on an APP/EG Flame-Retardant System. Materials 2023, 16, 3894. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, S.; Zhao, J.; Chen, X. Flame-retarded mechanism of SEBS/PPO composites modified with mica and resorcinol bis(diphenyl phosphate). Polym. Degrad. Stab. 2013, 98, 2765–2773. [Google Scholar] [CrossRef]
- Ryu, H.-J.; Lee, J.-H.; Choi, J.Y.; Choi, G.; Rejinold, N.S.; Choy, J.-H. Composite nanoarchitectonics with ionic clay nanofillers-embedded polypropylene for efficient flame retardance. Appl. Clay Sci. 2023, 246, 107181. [Google Scholar] [CrossRef]
- He, Y.; Fan, Y.; Chen, C.; Zhong, F.; Qing, D. Synthesis of mica-multiwalled carbon nanotube (MWCNT) hybrid material and properties of mica-MWCNT/epoxy composites coating research. High Perform. Polym. 2015, 27, 191–199. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Akbari, V.; Paran, S.M.R.; Livi, S.; Lins, L.; Vahabi, H.; Saeb, M.R. Epoxy/Ionic Liquid-Modified Mica Nanocomposites: Network Formation–Network Degradation Correlation. Nanomaterials 2021, 11, 1990. [Google Scholar] [CrossRef]
- Oliveira, L.H.; França, D.B.; Moraes, A.I.S.; Medina-Carrasco, S.; Fonseca, M.G.; Osajima, J.A.; da Silva-Filho, E.C.; Orta, M.d.M. An overview about synthetic high charge micas and their uses. Appl. Clay Sci. 2024, 251, 107325. [Google Scholar] [CrossRef]
- Li, P.; Li, L.; Ji, L.; Dang, L.; Lan, S.; Zhu, D. Functionalized magnesium hydroxide with zinc borate and 3-aminopropyltriethoxysilane for enhanced flame retardant and smoke suppressant properties of epoxy resins. J. Appl. Polym. Sci. 2023, 140, e53941. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, Y.; Cai, L.; Li, L. Functionalized graphene with Platelet-like magnesium hydroxide for enhancing fire safety, smoke suppression and toxicity reduction of Epoxy resin. Appl. Surf. Sci. 2022, 578, 152052. [Google Scholar] [CrossRef]
- Jiao, L.-L.; Zhao, P.-C.; Liu, Z.-Q.; Wu, Q.-S.; Yan, D.-Q.; Li, Y.-L.; Chen, Y.-N.; Li, J.-S. Preparation of Magnesium Hydroxide Flame Retardant from Hydromagnesite and Enhance the Flame Retardant Performance of EVA. Polymers 2022, 14, 1567. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.K.; Do, T.D.; Nguyen, B.T.; Tran, C.K.; Nguyen, T.A.; Nguyen, D.M.; Pham, L.H.; Nguyen, T.D.; Nguyen, T.-D.; Hoang, D. Effect of metal oxide nanoparticles and aluminum hydroxide on the physicochemical properties and flame-retardant behavior of rigid polyurethane foam. Constr. Build. Mater. 2022, 356, 129268. [Google Scholar] [CrossRef]
- Qin, Z.; Li, D.; Li, Q.; Yang, R. Effect of nano-aluminum hydroxide on mechanical properties, flame retardancy and combustion behavior of intumescent flame retarded polypropylene. Mater. Des. 2016, 89, 988–995. [Google Scholar] [CrossRef]
- Baskaran, M.; Hashim, R.; Leong, J.Y.; Ong, Y.N.; Yhaya, M.F.; Sulaiman, O. Flame retardant properties of oil palm trunk particleboard with addition of epoxy resin as a binder and aluminium hydroxide and magnesium hydroxide as additives. Bull. Mater. Sci. 2019, 42, 138. [Google Scholar] [CrossRef]
- Basnayake, A.P.; Hidalgo, J.P.; Heitzmann, M.T. A flammability study of aluminium hydroxide (ATH) and ammonium polyphosphate (APP) used with hemp/epoxy composites. Constr. Build. Mater. 2021, 304, 124540. [Google Scholar] [CrossRef]
- Staszko, S.; Półka, M.; Kozikowski, P. Analysis of the Influence of Organophosphorus Compounds and of Aluminium and Magnesium Hydroxides on Combustion Properties of Epoxy Materials. Energies 2022, 15, 6696. [Google Scholar] [CrossRef]
- Dun, L.; Ouyang, Z.; Sun, Q.; Yue, X.; Wu, G.; Li, B.; Kang, W.; Wang, Y. A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin. Polymers 2024, 16, 1471. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Yang, X.; Jin, Y.; Lv, Z.; Lan, S.; Zhu, D.; Dang, L. Self-emulsification synthesis of epoxy phosphate ester and its flame-retardant mechanism in flexible poly(vinyl chloride)/magnesium hydroxide composites. J. Appl. Polym. Sci. 2024, 141, e55354. [Google Scholar] [CrossRef]
- Thi, N.H.; Nguyen, T.N.; Oanh, H.T.; Trang, N.T.T.; Tham, D.Q.; Nguyen, H.T.; Van Nguyen, T.; Hoang, M.H. Synergistic effects of aluminum hydroxide, red phosphorus, and expandable graphite on the flame retardancy and thermal stability of polyethylene. J. Appl. Polym. Sci. 2021, 138, 50317. [Google Scholar] [CrossRef]
- Xu, S.; Li, J.; Ye, Q.; Shen, L.; Lin, H. Flame-retardant ethylene vinyl acetate composite materials by combining additions of aluminum hydroxide and melamine cyanurate: Preparation and characteristic evaluations. J. Colloid Interface Sci. 2021, 589, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Yao, D.; Yin, G.-Z.; You, J.; Liu, X.-Q.; Wang, N.; Wang, D.-Y. Surface engineering of magnesium hydroxide via bioinspired iron-loaded polydopamine as green and efficient strategy to epoxy composites with improved flame retardancy and reduced smoke release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Zhao, P.; Zeng, W.; Yang, Z.; Yang, Y.; Li, J.; Shi, J.; Wen, N.; Li, H.; Guan, J.; Lei, Z.; et al. Preparation of a novel functionalized magnesium-based curing agent as an intrinsic flame retardant for epoxy resin. Chemosphere 2021, 273, 129658. [Google Scholar] [CrossRef]
- Könnicke, D.; Kühn, A.; Mahrholz, T.; Sinapius, M. Polymer nanocomposites based on epoxy resin and ATH as a new flame retardant for CFRP: Preparation and thermal characterisation. J. Mater. Sci. 2011, 46, 7046–7055. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, Z.; Zhu, J. Synergistic flame retardant effect of aluminum hydroxide and ammonium polyphosphate on epoxy resin. J. Appl. Polym. Sci. 2022, 139, e53168. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Xu, K.; Yuan, Z.; Zhang, J.; Chen, R.; Xie, H.; Cheng, R. Brucite modified epoxy mortar binders: Flame retardancy, thermal and mechanical characterization. Constr. Build. Mater. 2015, 93, 1089–1096. [Google Scholar] [CrossRef]
- Aziz, H.; Ahmad, F. Effects from nano-titanium oxide on the thermal resistance of an intumescent fire retardant coating for structural applications. Prog. Org. Coat. 2016, 101, 431–439. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).