Impacts of Fire Frequency on Net CO2 Emissions in the Cerrado Savanna Vegetation
Abstract
1. Introduction
2. Materials and Methods
2.1. Immediate Emissions
2.2. AGB Recovery from Field Data
2.3. Fire Return Interval (FRI) Index
2.4. Biomass Change Map
2.5. Net CO2 Emissions
3. Results
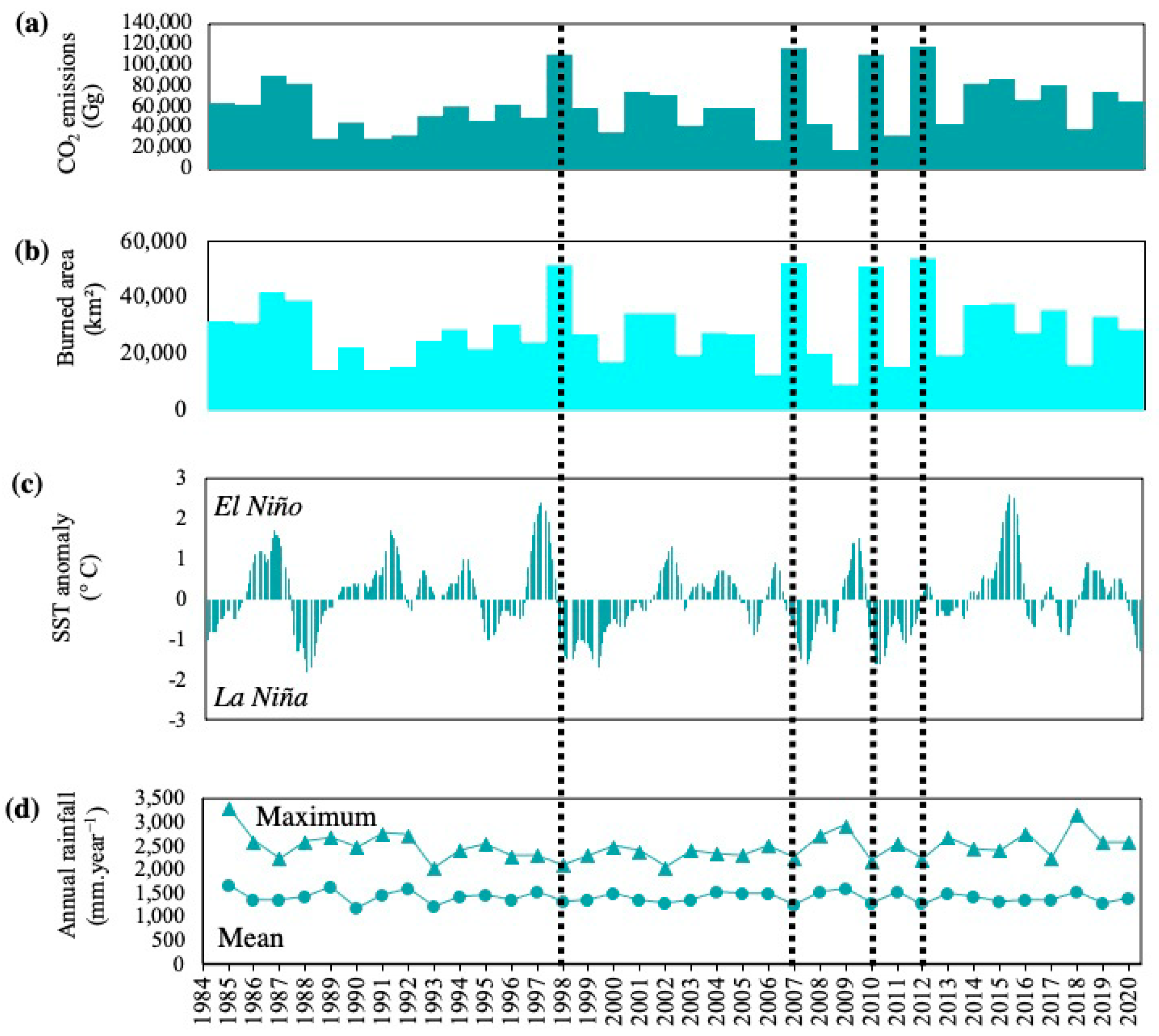
3.1. Immediate Emissions
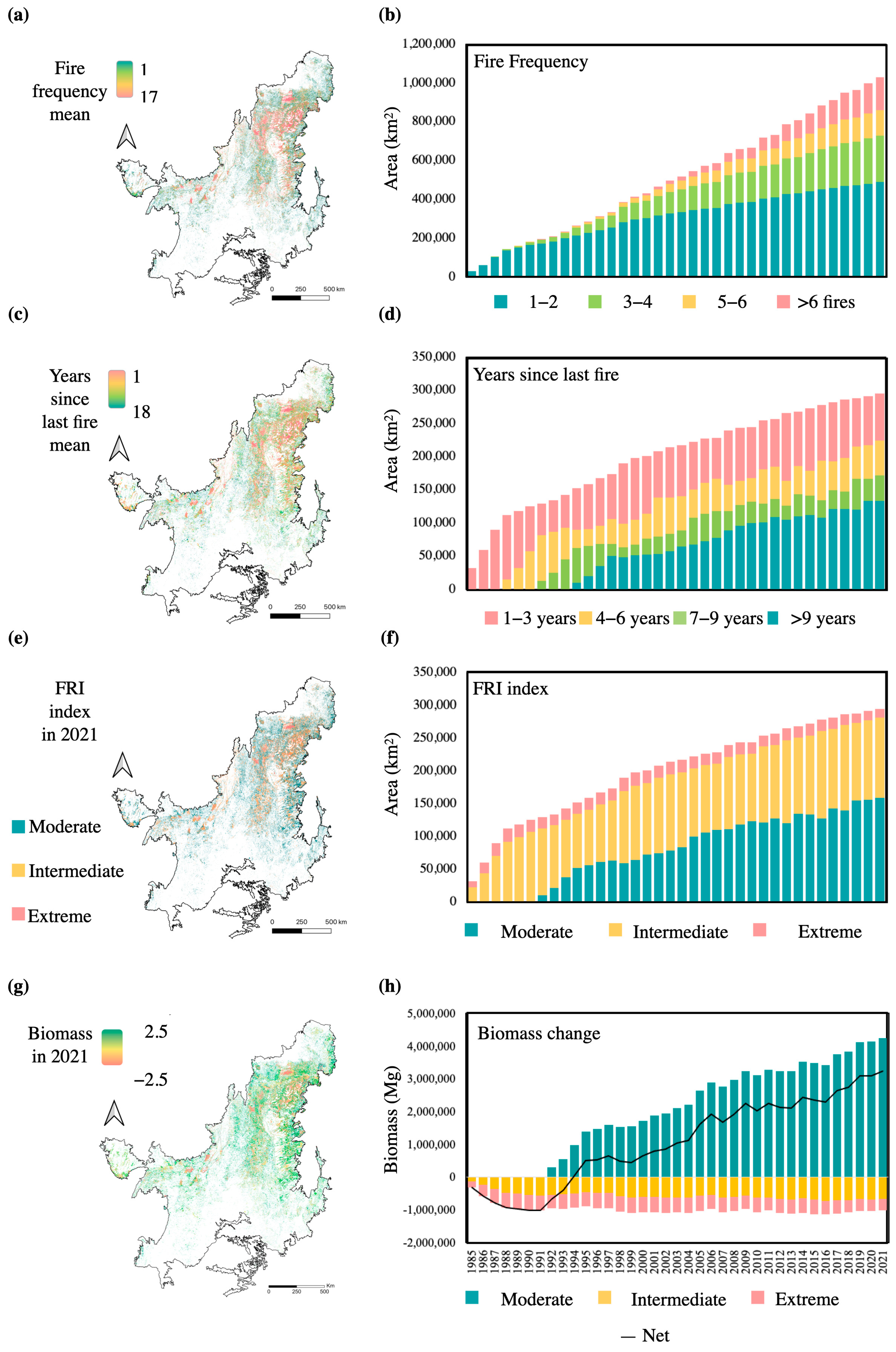
3.2. Fire Frequency Patterns and Fire Return Interval Index
3.3. Biomass Change Model
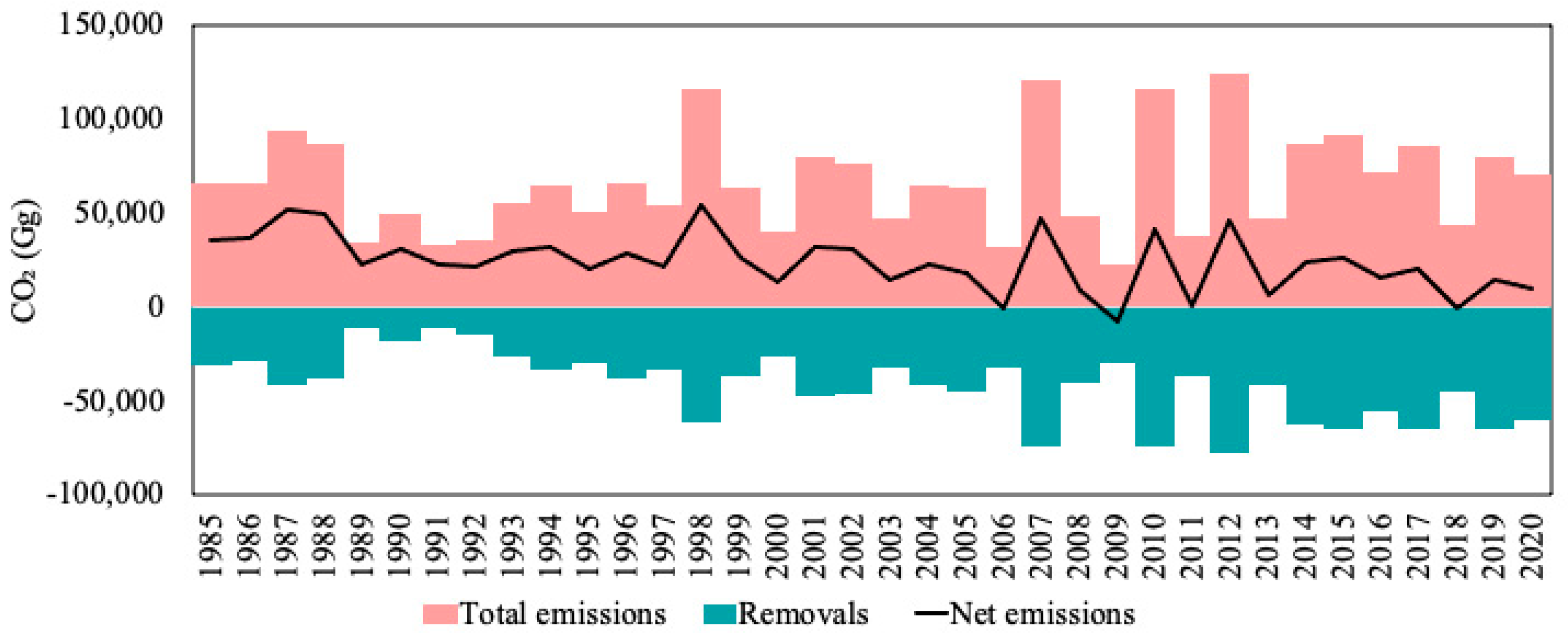
3.4. Net CO2 Emissions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poth, M.; Anderson, I.C.; Miranda, H.S.; Miranda, A.C.; Riggan, P.J. The magnitude and persistence of soil NO, N2O, CH4, and CO2 fluxes from burned tropical savanna in Brazil. Glob. Biogeochem. Cycles 1995, 9, 503–513. [Google Scholar] [CrossRef]
- Van Der Werf, G.R.; Randerson, J.T.; Giglio, L.; Collatz, G.J.; Mu, M.; Kasibhatla, P.S.; Morton, D.C.; DeFries, R.S.; Jin, Y.; van Leeuwen, T.T. Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos. Chem. Phys. 2010, 10, 11707–11735. [Google Scholar] [CrossRef]
- Freitas, S.R.; Longo, K.M.; Dias, M.A.F.S.; Dias, P.L.S. Emissões de queimadas em ecossistemas da América do Sul. Estud. Avançados 2005, 19, 167–185. [Google Scholar] [CrossRef]
- van der Werf, G.R.; Randerson, J.T.; Giglio, L.; van Leeuwen, T.T.; Chen, Y.; Rogers, B.M.; Mu, M.; van Marle, M.J.E.; Morton, D.C.; Collatz, G.J.; et al. Global fire emissions estimates during 1997–2016. Earth Syst. Sci. Data 2017, 9, 697–720. [Google Scholar] [CrossRef]
- Le Quéré, C.; Raupach, M.R.; Canadell, J.G.; Marland, G.; Bopp, L.; Ciais, P.; Conway, T.J.; Doney, S.C.; Feely, R.A.; Foster, P.; et al. Trends in the sources and sinks of carbon dioxide. Nat. Geosci. 2009, 2, 831–836. [Google Scholar] [CrossRef]
- Reichstein, M.; Bahn, M.; Ciais, P.; Frank, D.; Mahecha, M.D.; Seneviratne, S.I.; Zscheischler, J.; Beer, C.; Buchmann, N.; Frank, D.C.; et al. Climate extremes and the carbon cycle. Nature 2013, 500, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Malhi, Y. The carbon balance of tropical forest regions, 1990–2005. Curr. Opin. Environ. Sustain. 2010, 2, 237–244. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andres, R.J.; Boden, T.; Conway, T.; Houghton, R.A.; House, J.I.; Marland, G.; Peters, G.P.; van der Werf, G.R.; Ahlström, A.; et al. The global carbon budget 1959–2011. Earth Syst. Sci. Data 2013, 5, 165–185. [Google Scholar] [CrossRef]
- van der Werf, G.R.; Randerson, J.T.; Collatz, G.J.; Giglio, L.; Kasibhatla, P.S.; Arellano, A.F.; Olsen, S.C.; Kasischke, E.S. Continental-Scale Partitioning of Fire Emissions During the 1997 to 2001 El Niño/La Niña Period. Science 2004, 303, 73–76. [Google Scholar] [CrossRef]
- Ma, X.; Huete, A.; Cleverly, J.; Eamus, D.; Chevallier, F.; Joiner, J.; Poulter, B.; Zhang, Y.; Guanter, L.; Meyer, W.; et al. Drought rapidly diminishes the large net CO2 uptake in 2011 over semi-arid Australia. Sci. Rep. 2016, 6, 37747. [Google Scholar] [CrossRef]
- Villalobos, Y.; Rayner, P.J.; Silver, J.D.; Thomas, S.; Haverd, V.; Knauer, J.; Loh, Z.M.; Deutscher, N.M.; Griffith, D.W.T.; Pollard, D.F. Was Australia a sink or source of CO2 in 2015? Data assimilation using OCO-2 satellite measurements. Atmos. Chem. Phys. 2021, 21, 17453–17494. [Google Scholar] [CrossRef]
- SEEG. Análise das Emissões Brasileiras de Gases de Efeito Estufa e Suas Implicações Para as Metas Climáticas do Brasil 1970–2020. In Obs. do Clima; SEEG: Brasília, Brazil, 2021; Available online: https://seeg-br.s3.amazonaws.com/Documentos Analiticos/SEEG_9/OC_03_relatorio_2021_FINAL.pdf (accessed on 20 June 2024).
- Bustamante, M.M.C.; Silva, J.S.O.; Cantinho, R.Z.; Shimbo, J.Z.; Oliveira, P.V.C.; Santos, M.M.O.; Ometto, J.P.H.B.; Cruz, M.R.; Mello, T.R.B.; Godiva, D.; et al. Engagement of scientific community and transparency in C accounting: The Brazilian case for anthropogenic greenhouse gas emissions from land use, land-use change and forestry. Environ. Res. Lett. 2018, 13, 055005. [Google Scholar] [CrossRef]
- Grace, J.; José, J.S.; Meir, P.; Miranda, H.S.; Montes, R.A. Productivity and carbon fluxes of tropical savannas. J. Biogeogr. 2006, 33, 387–400. [Google Scholar] [CrossRef]
- Zhou, Y.; Singh, J.; Butnor, J.R.; Coetsee, C.; Boucher, P.B.; Case, M.F.; Hockridge, E.G.; Davies, A.B.; Staver, A.C. Limited increases in savanna carbon stocks over decades of fire suppression. Nature 2022, 603, 445–449. [Google Scholar] [CrossRef]
- Gomes, L.; Maracahipes, L.; Reis, S.M.; Marimon, B.S.; Marimon-Junior, B.H.; Lenza, E. Dynamics of the woody vegetation of two areas of Cerrado sensu stricto located on different substrates. Rodriguésia 2016, 67, 859–870. [Google Scholar] [CrossRef]
- Gomes, L.; Maracahipes, L.; Marimon, B.S.; Reis, S.M.; Elias, F.; Maracahipes-Santos, L.; Marimon-Junior, B.H.; Lenza, E. Post-fire recovery of savanna vegetation from rocky outcrops. Flora—Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 201–208. [Google Scholar] [CrossRef]
- Machida, W.S.; Gomes, L.; Moser, P.; Castro, I.B.; Miranda, S.C.; da Silva-Júnior, M.C.; Bustamante, M.M. Long term post-fire recovery of woody plants in savannas of central Brazil. For. Ecol. Manag. 2021, 493, 119255. [Google Scholar] [CrossRef]
- Almeida, R.F.; Fagg, C.W.; De Oliveira, M.C.; Munhoz, C.B.R.; de Lima, A.S.; de Oliveira, L.S.B. Mudanças florísticas e estruturais no cerrado sensu stricto ao longo de 27 anos (1985–2012) na Fazenda Água Limpa, Brasília, DF. Rodriguésia 2014, 65, 1–19. [Google Scholar] [CrossRef]
- Rios, M.N.S. Dinâmica de Comunidades Vegetais em Cerrado Típico com Histórico de fogo no Distrito Federal. Ph.D. Thesis, Universidade de Brasilia, Brasília, Brazil, 2016. [Google Scholar]
- Gomes, L.; Miranda, H.S.; Soares-Filho, B.; Rodrigues, L.; Oliveira, U.; Bustamante, M.M.C. Responses of Plant Biomass in the Brazilian Savanna to Frequent Fires. Front. For. Glob. Chang. 2020, 3, 507710. [Google Scholar] [CrossRef]
- Gomes, L.; Miranda, H.S.; Silvério, D.V.; Bustamante, M.M. Effects and behaviour of experimental fires in grasslands, savannas, and forests of the Brazilian Cerrado. For. Ecol. Manag. 2020, 458, 117804. [Google Scholar] [CrossRef]
- Gomes, L.; Miranda, H.S.; Bustamante, M.M.d.C. How can we advance the knowledge on the behavior and effects of fire in the Cerrado biome? For. Ecol. Manag. 2018, 417, 281–290. [Google Scholar] [CrossRef]
- Gomes, L.; Lenza, E.; Souchie, F.F.; Pinto, J.R.R.; Maracahipes-Santos, L.; Furtado, M.T.; Maracahipes, L.; Silvério, D. Long-term post-fire resprouting dynamics and reproduction of woody species in a Brazilian savanna. Basic Appl. Ecol. 2021, 56, 58–71. [Google Scholar] [CrossRef]
- MapBiomas. Dados anuais de cobertura e uso da terra para o período de 1985 a 2021 (Coleção 6). 2023. Available online: https://brasil.mapbiomas.org/ (accessed on 4 May 2023).
- MapBiomas Fogo. Mapeamento das cicatrizes de fogo do Brasil de 1985 a 2020 (Coleção 1). 2023. Available online: https://brasil.mapbiomas.org/metodo-mapbiomas-fogo/ (accessed on 5 May 2021).
- Alencar, A.A.C.; Arruda, V.L.S.; da Silva, W.V.; Conciani, D.E.; Costa, D.P.; Crusco, N.; Duverger, S.G.; Ferreira, N.C.; Franca-Rocha, W.; Hasenack, H.; et al. Long-Term Landsat-Based Monthly Burned Area Dataset for the Brazilian Biomes Using Deep Learning. Remote Sens. 2022, 14, 2510. [Google Scholar] [CrossRef]
- Lipsett-Moore, G.J.; Wolff, N.H.; Game, E.T. Emissions mitigation opportunities for savanna countries from early dry season fire management. Nat. Commun. 2018, 9, 2247. [Google Scholar] [CrossRef] [PubMed]
- Keeley, J.; Pausas, J. Evolutionary Ecology of Fire. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 203–225. [Google Scholar] [CrossRef]
- Bond, W.J. What Limits Trees in C4 Grasslands and Savannas? Annu. Rev. Ecol. Evol. Syst. 2008, 39, 641–659. [Google Scholar] [CrossRef]
- Pausas, J.G.; Lamont, B.B.; Paula, S.; Appezzato-Da-Glória, B.; Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. New Phytol. 2018, 217, 1435–1448. [Google Scholar] [CrossRef]
- Simon, M.F.; Pennington, T. Evidence for Adaptation to Fire Regimes in the Tropical Savannas of the Brazilian Cerrado. Int. J. Plant Sci. 2012, 173, 711–723. [Google Scholar] [CrossRef]
- Moreira, A.G. Effects of fire protection on savanna structure in Central Brazil. J. Biogeogr. 2000, 27, 1021–1029. [Google Scholar] [CrossRef]
- Pinto, A.S.; Bustamante, M.M.C. Emissões de gases-traço (CO2, CO, NO e N2O) do solo para a atmosfera em fitofisionomias de Cerrado sob diferentes regimes de queima. In Efeitos do Regime do Fogo Sobre a Estrutura de Comunidades de Cerrado: Resultados do Projeto Fogo; IBAMA: Brasília, Brazil, 2010; pp. 50–57. [Google Scholar]
- Miranda, J.d.R.; da Silva, R.G.; Juvanhol, R.S. Forest fire action on vegetation from the perspective of trend analysis in future climate change scenarios for a Brazilian savanna region. Ecol. Eng. 2022, 175, 106488. [Google Scholar] [CrossRef]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.J.S.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Aquino, A.R.; Paletta, F.C.; Silva, A.C.M.; Galvão, A.S.; Bordon, I.C.A.; Ribeiro, L.S.S.; Stecher, L.; Oliveira, M.J.A.; Silva, M.V.; Santos, R.M.; et al. Sustentabilidade Ambiental, 1st ed.; Rede Sirius: Rio de Janeiro, Brazil, 2015. [Google Scholar]
- Andrade, S.M.A. Dinâmica do Combustível Fino e Produção Primária do Estrato Rasteiro de áreas de campo sujo de Cerrado Submetidas a Diferentes Regimes de Queimas. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 1998. [Google Scholar]
- Montenegro, S.R. Efeitos do Manejo do fogo na Estrutura de Comunidades Lenhosas em Formações Savânicas do Cerrado. Master’s Thesis, Universidade de Brasília, Brasília, Brazil, 2019. [Google Scholar]
- Rodrigues, C.A.; Zirondi, H.L.; Fidelis, A. Fire frequency affects fire behavior in open savannas of the Cerrado. For. Ecol. Manag. 2021, 482, 118850. [Google Scholar] [CrossRef]
- Sano, E.E.; Rosa, R.; Brito, J.L.S.; Ferreira, L.G. Land cover mapping of the tropical savanna region in Brazil. Environ. Monit. Assess. 2010, 166, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.M.; Ribeiro, J.F. Diversidade fitofisionômica e o papel do fogo no bioma Cerrado. In Efeitos do Regime do Fogo Sobre a Estrutura de Comunidades de Cerrado: Resultados do Projeto Fogo; Miranda, H.S., Ed.; IBAMA: Brasília, Brazil, 2010; pp. 59–76. [Google Scholar]
- Brazil. Fourth National Communication of Brazil to, IV; UNFCCC: Brasilia, Brazil, 2022. [Google Scholar]
- Ottmar, R.D.; Vihnanek, R.E.; Miranda, H.S. Séries de Estéreo-Fotografias Para Quantificar a Biomassa da Vegetação do Cerrado do Brasil Central; Floresta: Curitiba, Brazil, 2001. [Google Scholar]
- Ribeiro, S.C.; Fehrmann, L.; Soares, C.P.B.; Jacovine, L.A.G.; Kleinn, C.; Gaspar, R.d.O. Above- and belowground biomass in a Brazilian Cerrado. For. Ecol. Manag. 2011, 262, 491–499. [Google Scholar] [CrossRef]
- SEEG. Nota Metodológica: SEEG 9 Setor Mudança de Uso da Terra e Florestas. 2021. Available online: https://seeg-br.s3.amazonaws.com/2019-v7.0/notas-metodologicas/SEEG8_NOTA_METODOLOGICA_MUT_2020.11.03.pdf (accessed on 20 June 2024).
- Kauffman, J.B.; Cummings, D.L.; Ward, D.E. Relationships of fire, biomass and nutrient dynamics along a vegetation gradient in the Brazilian Cerrado. J. Ecol. 1994, 82, 519–531. [Google Scholar] [CrossRef]
- Guild, L.S.; Kauffman, J.B.; Ellingson, L.J.; Cummings, D.L.; Castro, E.A.; Babbitt, R.E.; Ward, D.E. Dynamics associated with total aboveground biomass, C, nutrient pools, and biomass burning of primary forest and pasture in Rondônia, Brazil during SCAR-B. J. Geophys. Res. Atmos. 1998, 103, 32091–32100. [Google Scholar] [CrossRef]
- Ward, D.E.; Susott, R.; Kauffman, J.B.; Babbitt, R.E.; Cummings, D.L.; Dias, B.; Holben, B.N.; Kaufman, Y.J.; Rasmussen, R.A.; Setzer, A.W. Smoke and Fire Characteristics for Cerrado and Deforestation Burns in Brazil: BASE-B Experiment. J. Geophys. Res. 1992, 97, 14601–14619. [Google Scholar] [CrossRef]
- Schmidt, I.B.; Fonseca, C.B.; Ferreira, M.C.; Sato, M.N. Implementação do programa piloto de Manejo Integrado do Fogo em três Unidades de Conservação do Cerrado. Biodiversidade Bras. 2016, 6, 55–70. [Google Scholar]
- dos Santos, A.C.; Montenegro, S.d.R.; Ferreira, M.C.; Barradas, A.C.S.; Schmidt, I.B. Managing fires in a changing world: Fuel and weather determine fire behavior and safety in the neotropical savannas. J. Environ. Manag. 2021, 289, 112508. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.N. Efeito a Longo Prazo de Queimadas Prescritas na Estrutura de Comunidade de Lenhosas da Vegetação do Cerrado Sensu Stricto. Ph.D. Thesis, Universidade de Brasília, Brasilia, Brazil, 2003. [Google Scholar]
- MCTI (Ministério da Ciência e Tecnologia e Inovação). Relatório de Referência: Setor Uso da Terra, Mudança do Uso da Terra e Florestas. Terceiro Inventário Brasileiro de Emissões e Remoções Antrópicas de Gases de Efeito Estufa; Relatório Técnico; Ministério da C: Brasília, Brasil, 2015. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). IPCC Guidelines for National Greenhouse Gas Inventories; Prepared by the National Greenhouse Gas Inventories Programme; Institute for Global Environmental Strategies: Genebra, Switzerland, 2006. [Google Scholar]
- MCTI. Quarta Comunicação Nacional do Brasil à Convenção-Quadro das Nações Unidas sobre Mudança do Clima. 2020. Available online: https://repositorio.mctic.gov.br/handle/mctic/4782 (accessed on 12 December 2023).
- IPCC. Generic Methodologies Applicable to Multiple Land-Use Categories (Chapter 2). In Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2006; pp. 1–59. [Google Scholar]
- NOAA. National Oceanic and Atmospheric Administration. 2023. Available online: https://www.noaa.gov/ (accessed on 20 June 2024).
- Filho, W.L.F.C.; de Oliveira-Júnior, J.F.; Junior, C.A.d.S.; Santiago, D.d.B. Influence of the El Niño–Southern Oscillation and the sypnotic systems on the rainfall variability over the Brazilian Cerrado via Climate Hazard Group InfraRed Precipitation with Station data. Int. J. Clim. 2022, 42, 3308–3322. [Google Scholar] [CrossRef]
- Gomes, L.; Lenza, E.; Maracahipes, L.; Marimon, B.S.; de Oliveira, E.A. Comparações florísticas e estruturais entre duas comunidades lenhosas de cerrado típico e cerrado rupestre, Mato Grosso, Brasil. Acta Bot. Bras. 2011, 25, 865–875. [Google Scholar] [CrossRef]
- Maracahipes, L.; Lenza, E.; Marimon, B.S.; de Oliveira, E.A.; Pinto, J.R.R.; Junior, B.H.M. Estrutura e composição florística da vegetação lenhosa em cerrado rupestre na transição Cerrado-Floresta Amazônica, Mato Grosso, Brasil. Biota Neotrop. 2011, 11, 133–141. [Google Scholar] [CrossRef][Green Version]
- de Miranda, S.D.C.; Júnior, M.C.d.S.; de Carvalho, P.S. O efeito da proteção do fogo na estrutura da vegetação lenhosa de uma área de cerrado sentido restrito no Brasil Central. Heringeriana 2014, 7, 61–72. [Google Scholar] [CrossRef]
- de Miranda, S.D.C.; Bustamante, M.; Palace, M.; Hagen, S.; Keller, M.; Ferreira, L.G. Regional variations in biomass distribution in Brazilian Savanna Woodland. Biotropica 2014, 46, 125–138. [Google Scholar] [CrossRef]
- Lenza, E.; Abadia, A.C.; Menegat, H.; Lúcio, N.W.; Maracahipes-Santos, L.; Mews, H.A.; dos Santos, J.O.; Martins, J. Does fire determine distinct floristic composition of two Cerrado savanna communities on different substrates? Acta Bot. Bras. 2017, 31, 250–259. [Google Scholar] [CrossRef]
- Passos, F.B.; Marimon, B.S.; Phillips, O.L.; Morandi, P.S.; das Neves, E.C.; Elias, F.; Reis, S.M.; de Oliveira, B.; Feldpausch, T.R.; Júnior, B.H.M. Savanna turning into forest: Concerted vegetation change at the ecotone between the Amazon and “Cerrado” biomes. Braz. J. Bot. 2018, 41, 611–619. [Google Scholar] [CrossRef]
- Miranda, S.C. Variação Espacial e Temporal da Biomassa Vegetal em áreas de Cerrado. Ph.D. Thesis, Universidade de Brasilia, Brasilia, Brazil, 2012. [Google Scholar]
- Almeida, R.F. O Cerrado Sensu Stricto da Fazenda Água Limpa, Brasília, DF: Mudanças florísticas em 27 anos de Monitoramento. Master’s Thesis, Universidade de Brasilia, Brasilia, Brazil, 2013. [Google Scholar]
- Rios, M.N.d.S.; Sousa-Silva, J.C. Grupos funcionais em áreas com histórico de queimadas em Cerrado sentido restrito no Distrito Federal. Pesqui. Florest. Bras. 2017, 37, 285. [Google Scholar] [CrossRef]
- Rios, M.; Sousa-Silva, J.; Malaquias, J. Mudanças pós-fogo na florística e estrutura da vegetação arbórea-arbustiva de um cerrado sentido restrito em Planaltina-DF. Ciência Florest. 2018, 28, 469–482. [Google Scholar] [CrossRef]
- Roitman, I.; Bustamante, M.M.C.; Haidar, R.F.; Shimbo, J.Z.; Abdala, G.C.; Eiten, G.; Fagg, C.W.; Felfili, M.C.; Felfili, J.M.; Jacobson, T.K.B.; et al. Optimizing biomass estimates of savanna woodland at different spatial scales in the Brazilian Cerrado: Re-evaluating allometric equations and environmental influences. PLoS ONE 2018, 13, e0196742. [Google Scholar] [CrossRef]
- Phillips, O.L.; Baker, T.R.; Arroyo, L.; Higuchi, N.; Killeen, T.J.; Laurance, W.F.; Lewis, S.L.; Lloyd, J.; Malhi, Y.; Monteagudo, A.; et al. Pattern and process in Amazon tree turnover, 1976–2001. Philos. Trans. R. Soc. B Biol. Sci. 2004, 359, 381–407. [Google Scholar] [CrossRef] [PubMed]
- Govender, N.; Trollope, W.S.W.; VAN Wilgen, B.W. The effect of fire season, fire frequency, rainfall and management on fire intensity in savanna vegetation in South Africa. J. Appl. Ecol. 2006, 43, 748–758. [Google Scholar] [CrossRef]
- Hoffmann, W.A.; Solbrig, O.T. The role of topkill in the differential response of savanna woody species to fire. For. Ecol. Manag. 2003, 180, 273–286. [Google Scholar] [CrossRef]
- Sato, M.N.; Miranda, H.S.; Maia, J.M.F. O fogo e o estrato arbóreo do Cerrado: Efeitos imediatos e a longo prazo. In Efeitos do Regime do Fogo Sobre a Estrutura de Comunidades de Cerrado: Resultados do Projeto Fogo; Miranda, H.S., Ed.; Ibama: Brasília, Brazil, 2010; pp. 77–91. [Google Scholar]
- Pellegrini, A.F.A.; Hoffmann, W.A.; Franco, A.C. Carbon accumulation and nitrogen pool recovery during transitions from savanna to forest in central Brazil. Ecology 2014, 95, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Laris, P. On the problems and promises of savanna fire regime change. Nat. Commun. 2021, 12, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Fearnside, P.M. Fogo e emissão de gases de efeito estufa dos ecossistemas florestais da Amazônia brasileira. Estud. Avançados 2002, 16, 99–123. [Google Scholar] [CrossRef]
- SEEG. Análise das Emissões Brasileiras de Gases de Efeito Estufa e Suas Implicações Para as Metas de Clima do Brasil 1970–2019. In Obs. do Clima; SEEG: Brasilia, Brazil, 2020; Available online: http://www.atmosphere.mpg.de/enid/2__Estufa__luz_e_biosfera/-_Gases_estufa_2mb.html (accessed on 20 June 2024).
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Rocha, G.F.; Ferreira, L.G.; Ferreira, N.C.; Ferreira, M.E. Detecção de desmatamento no bioma Cerrado entre 2002 e 2009: Padrões, Tendência e Impactos. Rev. Bras. Cartogr. 2011, 63, 341–349. [Google Scholar] [CrossRef]
- de Araújo, F.M.; Ferreira, L.G.; Arantes, A.E. Distribution patterns of burned areas in the Brazilian Biomes: An analysis based on satellite data for the 2002–2010 period. Remote Sens. 2012, 4, 1929–1946. [Google Scholar] [CrossRef]
- Oliveira, U.; Soares-Filho, B.; Costa, W.L.d.S.; Gomes, L.; Bustamante, M.; Miranda, H. Modeling fuel loads dynamics and fire spread probability in the Brazilian Cerrado. For. Ecol. Manag. 2021, 482, 118889. [Google Scholar] [CrossRef]
- Alencar, A.; Shimbo, J.Z.; Lenti, F.; Marques, C.B.; Zimbres, B.; Rosa, M.; Arruda, V.; Castro, I.; Ribeiro, J.P.F.M.; Varela, V.; et al. Mapping Three Decades of Changes in the Brazilian Savanna Native Vegetation Using Landsat Data Processed in the Google Earth Engine Platform. Remote Sens. 2020, 12, 924. [Google Scholar] [CrossRef]
- Marengo, J.A.; de Oliveira, G.S. Impactos do fenômeno La niña no tempo e clima do Brasil: Desenvolvimento e intensificação do La niña 1998/99. In Proceedings of the 10th Brazilian Congress Meteorol, Belém, Brasil, June 1998; Volume 43, pp. 1–9. [Google Scholar]
- Marengo, J.A.; Alves, L.M.; Soares, W.R.; Rodriguez, D.A.; Camargo, H.; Riveros, M.P.; Pabló, A.D. Two contrasting severe seasonal extremes in tropical South America in 2012: Flood in Amazonia and drought in Northeast Brazil. J. Clim. 2013, 26, 9137–9154. [Google Scholar] [CrossRef]
- Filho, W.L.F.C.; De Oliveira-Júnior, J.F.; Santiago, D.D.B.; Terassi, P.M.D.B.; Teodoro, P.E.; De Gois, G.; Blanco, C.J.C.; Souza, P.H.D.A.; Costa, M.d.S.; Gomes, H.B.; et al. Rainfall variability in the Brazilian northeast biomes and their interactions with meteorological systems and ENSO via CHELSA product. Big Earth Data 2019, 3, 315–337. [Google Scholar] [CrossRef]
- Borges, P.d.A.; Bernhofer, C.; Rodrigues, R. Extreme rainfall indices in Distrito Federal, Brazil: Trends and links with El Nino southern oscillation and Madden-Julian oscillation. Int. J. Clim. 2018, 38, 4550–4567. [Google Scholar] [CrossRef]
- Heinemann, A.B.; Ramirez-Villegas, J.; Stone, L.F.; Silva, A.P.G.A.; da Matta, D.H.; Diaz, M.E.P. The impact of El Niño Southern Oscillation on cropping season rainfall variability across Central Brazil. Int. J. Clim. 2020, 41, E283–E304. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.; Schüler, J.; Silva, C.; Alencar, A.; Zimbres, B.; Arruda, V.; Silva, W.V.d.; Souza, E.; Shimbo, J.; Marimon, B.S.; et al. Impacts of Fire Frequency on Net CO2 Emissions in the Cerrado Savanna Vegetation. Fire 2024, 7, 280. https://doi.org/10.3390/fire7080280
Gomes L, Schüler J, Silva C, Alencar A, Zimbres B, Arruda V, Silva WVd, Souza E, Shimbo J, Marimon BS, et al. Impacts of Fire Frequency on Net CO2 Emissions in the Cerrado Savanna Vegetation. Fire. 2024; 7(8):280. https://doi.org/10.3390/fire7080280
Chicago/Turabian StyleGomes, Letícia, Jéssica Schüler, Camila Silva, Ane Alencar, Bárbara Zimbres, Vera Arruda, Wallace Vieira da Silva, Edriano Souza, Julia Shimbo, Beatriz Schwantes Marimon, and et al. 2024. "Impacts of Fire Frequency on Net CO2 Emissions in the Cerrado Savanna Vegetation" Fire 7, no. 8: 280. https://doi.org/10.3390/fire7080280
APA StyleGomes, L., Schüler, J., Silva, C., Alencar, A., Zimbres, B., Arruda, V., Silva, W. V. d., Souza, E., Shimbo, J., Marimon, B. S., Lenza, E., Fagg, C. W., Miranda, S., Morandi, P. S., Marimon-Junior, B. H., & Bustamante, M. (2024). Impacts of Fire Frequency on Net CO2 Emissions in the Cerrado Savanna Vegetation. Fire, 7(8), 280. https://doi.org/10.3390/fire7080280






