1. Introduction
Wildland fires continue to significantly impact humans and structures in many parts of the world. Understanding the mechanisms and reactions occurring during wildland fires is a pivotal step for predicting fire spread. The complex chemical mechanisms occurring during wildland fires can be categorized into the following: (1) water evaporation; (2) pyrolysis of solid fuel releasing volatiles; (3) combustion of the released volatile compounds; and (4) smoldering and glowing combustion [
1,
2]. Pyrolysis is defined as the thermal degradation of biomass due to exposure to high temperatures that does not require oxygen [
3]. In a propagating wildland fire, due to elevated temperatures, the vegetation undergoes thermal degradation (i.e., pyrolysis) before combustion. Pyrolysis results in three major classes of products: tar, permanent gases and char [
1]. Tar is the mixture of compounds condensable near room temperature. Permanent gases refer to pyrolysis products that do not condense at room temperatures and mainly consist of CO, CO
2, CH
4 and H
2, along with small amounts of other hydrocarbons. Char refers to the carbon-rich solid residue remaining after pyrolysis. The majority of volatile compounds released during pyrolysis later combust in the presence of oxygen and spread the fire [
4,
5]. To have a better understanding of combustion processes and reactions, it is necessary to know the chemical species involved in these reactions and their concentrations.
Since the chemical reactants of combustion processes are generated during pyrolysis of the foliage, detailed analysis of tar and light gases and estimating their energy content are necessary. However, very few studies of the pyrolysis of shrub-like fuels have been performed. Most previous studies of biomass pyrolysis have focused on crushed samples, with the eventual goal of using the pyrolysis products as a fuel. The effects of temperature and heating rate, as the two main operating factors, on pyrolysis products have been explored, as reviewed by Di Blasi [
6]. However, the pyrolysis of vegetation in wildland fires differs from these previous studies in a few ways. First, many biomass fuels consist mainly of cellulose, hemicellulose and lignin, accounting for at least 85% of the fuel. However, shrub foliar fuels have been shown to contain up to 58% extractives such as waxes, lipids, resins, tannins, starches, etc. [
7], which will affect the pyrolysis products. Second, pyrolysis during wildland fires is from intact leaves and small branches rather than crushed or ground samples, meaning that the samples involved in wildland fires are not thermally thin and hence may be impacted by sample thickness. Fragrant smells are evident when some leaf samples are crushed or shredded, indicating that volatiles are released during the crushing. Third, the temperature and heating rates in previous studies have been of the order of 0.5 °C/s [
8,
9,
10,
11,
12,
13]. However, the heating rate during wildland fires ranges between 75 and 5000 °C/s with the highest heating rates observed in tree crown fires [
14,
15,
16,
17,
18,
19]. Temperatures of 477 to 1027 °C were measured in shrubland fires in Portugal [
20]; this range of temperatures is also higher than what is typically used in fundamental pyrolysis experiments.
There have been numerous studies investigating possible correlations between the biomass characteristics and pyrolysis products [
21]. There is some evidence that the ratio of major biopolymers in biomass (i.e., cellulose, hemicellulose and lignin) plays an important role in the pyrolysis behavior of species such as pyrolysis product yields and the concentration of various compounds in tar and permanent gases. The relative proportions of cellulose, hemicellulose and lignin vary in different types of biomass. Pyrolysis of each of the major components of biomass (cellulose, hemicellulose and lignin) occurs through different reaction mechanisms generating different compounds. For example, the pyrolysis of biomass with a higher lignin content may result in the generation of higher molecular weight tar [
21].
High heating values (HHVs) for volatiles released during the pyrolysis of these plants are estimated based on the chemical species present and their relative concentration. The HHVs of volatiles provide valuable information about the potential energy that may be released if these compounds further react with oxygen and combust. The HHVs of pyrolysis volatiles have been studied previously [
10,
22]. However, since the goal of these studies was to investigate the potential use of pyrolysis products as a fuel, the heating rate and temperature applied during the previous studies were much smaller than those observed in wildland fires.
Kelsey [
23] studied the heat content of foliage, bark and branches for nine various species and concluded that in most of the species, foliage and branches generated higher heat content than the corresponding wood. Therefore, as part of the current study, a limited number of experiments were performed to study foliage vs. branches.
Previous studies of the combustion of individual leaves from California chaparral and Utah plant species were conducted to determine time to ignition, flame height, flame duration and leaf temperature in a similar flat flame burner system [
16,
24,
25,
26,
27,
28,
29,
30]. Pyrolysis and the subsequent analysis of pyrolysis products of foliage from shrubs in the southern U.S. were performed at temperatures and heating rates relevant to wildland fires by Safdari et al. [
31,
32].
This study presents the results of pyrolysis experiments conducted on four major plant species from the California chaparral, including yields and speciation of tar and permanent gases. Heating rates were approximately 180 °C/s and the temperature was 725 °C to simulate conditions in a propagating wildland fire in shrubs [
14,
15,
16,
17,
18,
20].
2. Materials and Methods
The equipment and procedures used here were very similar to those described by Safdari et al. [
32] and hence are only described briefly. For this set of tests, live branches with attached foliage were collected in a chaparral stand located on the North Mountain Experimental Area (approximate location 33.855 N, −116.871 W at an elevation of 1170 m), which is located 50 km east of Riverside, California. The chaparral plant species used were scrub oak (
Quercus berberidifolia Liebm.), Eastwood’s manzanita (
Arctostaphylos glandulosa Eastw.), chamise (
Adenostoma fasciculatum Hook. & Am.) and hoaryleaf ceanothus (
Ceanothus crassifolius Torr.). Samples of sparkleberry (
Vaccinium arboreum) native to the southern U.S. were also examined in this study. The freshly cut foliage was shipped to Brigham Young University (in Provo, UT, USA) and stored in closed bags to reduce moisture loss.
The main equipment used during the experiments and analyses were a flat flame burner, a moisture content measurement system, a gas chromatography mass spectroscopy (GC/MS) system and a gas chromatography thermal conductivity detector (GC-TCD) system which are described below.
2.1. Description of the Flat Flame Burner
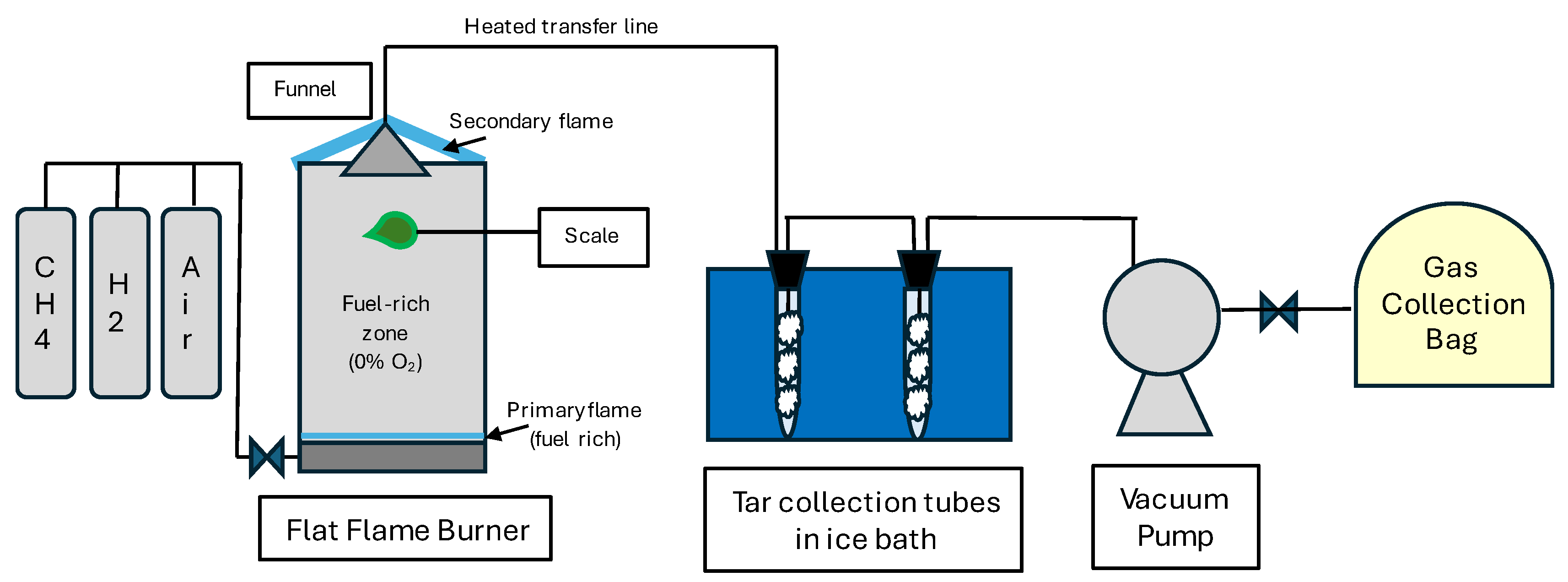
A flat flame burner (FFB) apparatus consisting of a 20 cm × 27 cm perforated metal base enclosed by ceramic glass windows was utilized to maintain an oxygen-free, high-temperature environment essential for the pyrolysis of samples. The ceramic (Neoceram) windows enclosing the base were 30.5 cm high. Samples were placed in an alligator clip connected to a metal rod sitting on a scale (Mettler Toledo XS204, Columbus, OH, USA) [
33]. Complete leaf samples were used to avoid the loss of volatile components. The scale recorded real-time changes in the mass of the sample during pyrolysis.
Fuel supporting the pre-mixed flame consisted of H2 and CH4 with a pre-defined ratio of fuel mixture to air ensuring a fuel-rich primary flame (σ = 1.13). The area between the primary and secondary flames inside the FFB was the oxygen-free and high-temperature zone. The samples were introduced to the high-temperature zone through a 5 cm diameter hole in one of the glass windows. The gas temperature at the location of the sample was 725 °C.
Figure 1 shows a schematic of the pyrolysis products’ collection system. A funnel connected to a vacuum pump was placed over the chamber above the sample. The distance between the foliage sample and the funnel was 15 cm corresponding to a gas residence time of 0.75 s. The pyrolysis products were collected for 15 s, which allowed the majority of the volatiles to be collected. The transfer line was heated to 300 °C to prevent tar condensation. The tar trap consisted of two test tubes filled with glass wool, which collected moisture and tar. The vacuum pump exhaust was collected in 1 L Tedlar
® bags to analyze light gases.
2.2. Moisture Content Measurement System
For each plant species studied, the moisture content of the leaves was measured by a Computrac MAX 1000 moisture analyzer twice per day, at the beginning of the experiments (around 8 a.m.) and the end of the experiments (approximately 5 p.m.). Each sample was approximately 5 g wet weight. The average of these two measurements was reported as the moisture content for the specific samples used that day. The ranges of moisture contents for the plant samples are shown in
Table 1.
2.3. Description of GC-MS
To analyze tar, 1.0 μL of the CH2Cl2–tar mixture was injected into a 1310 Thermo Fisher (Waltham, MA, USA) gas chromatography mass spectroscopy (GC/MS) system equipped with a Restek (Centre County, PA, USA) Rxi-1ms capillary column (60 m × 0.25 mm × 1 μm). The temperature of the injected sample was held at 50 °C for 5 min, then the temperature was increased to 250 °C at 10 °C/min, and finally held at 250 °C for 5 min. Ultra-high purity (UHP) helium was the carrier gas used in the GC/MS system. Each test was conducted three times to ensure that the results were reproducible, and the reported numbers were the averages along with the 95% confidence interval. Tar compounds were identified by comparing their mass spectra with the database provided by NIST. A complete quantitative analysis of tar samples was impossible since many tar compounds had low concentrations resulting in a low signal to noise ratio and hence were below the detection limit. The concentration of identified compounds was reported in the form of the mole fraction, which was calculated as the ratio of the area under the associated peak to the sum of areas under all detected and identified peaks.
2.4. Description of GC-TCD
Post-flame gases were collected in 1 L Tedlar® gas bags and were analyzed immediately by a gas chromatograph (GC, Agilent Technologies 7890A with a Supelco Carboxen 1004 packed column, 2 m × 1.5 mm, Santa Clara, CA, USA) equipped with a thermal conductivity detector. Ultra-high purity (UHP) helium was the carrier gas used in this system. CO, CO2, CH4 and H2 were the major four gases observed, although occasional peaks below the statistical detection limit (~1 mole% of pyrolyzed light gas) were observed for other species such as C2H4 and C2H6. An amount of 1 mL of the desired gas sample was injected manually into the GC. The oven temperature was set to start at 50 °C and was maintained at this value for 5 min, then increased to 225 °C at 20 °C/min. The temperature was then held at 225 °C for 6.5 min. The total run time was 21 min.
2.5. Ash Content and Ultimate Analysis for Species Studied
The chemical composition of wildland fuels mainly consists of carbon, oxygen and hydrogen with nitrogen, sulfur, other micro and macro-nutrients; heavy and alkali metals typically comprise a small proportion of the total mass [
34,
35]. These metals contribute to the ash content of biomass. The ultimate analyses for the plant species studied here (given in
Table 2) were reported by Fletcher et al. [
29] and Matt et al. [
7,
36]. To determine ash content, the sample was placed in an oven that ramped up from 105 to 500 °C in one hour; then its temperature increased to 750 °C over the next hour. The samples remained in the furnace for almost 24 h. The remaining mass in the crucible was reported as the ash content of the original sample. The ash content reported here includes silica, which differs from the silica free ash approach used in fuel descriptions for the Rothermel fire spread model [
37,
38]. The ash contents for the plant species used in these experiments varied between 2.5 and 5% (see
Table 1).
2.6. Tar Extraction
Tar collection was achieved by condensation of pyrolysis products as shown in
Figure 1. The tar trap consisted of two test tubes containing glass wool, which were placed in an ice bath. After the pyrolysis experiment, the test tubes were removed from the ice bath and CH
2Cl
2 was added as a solvent to the test tubes to dissolve the tar. Then, the two pieces of glass wool were removed from the test tubes, and the contents of one tube were combined with the other tube before adding approximately 2 g of anhydrous CaSO
4 to the dissolved tar solution. After a few minutes, two separate phases formed inside the test tube, with tar remaining in the liquid phase and moisture being adsorbed by the CaSO
4.
2.7. Tar Yield
Tar yield was defined as the weight percentage of condensable pyrolysis products on a dry, ash-free basis. After each experiment, tar and moisture were both collected in the test tube that were sitting in the ice bath. Moisture was generated from two sources in this experiment: (a) the moisture present in each sample and (b) the moisture resulting from the flame. The moisture content of the unheated parent biomass was measured by a moisture content analyzer prior to and after the experiments and the average of the two measurements was reported as the moisture content of the sample each day. Moisture generated by the flame was calculated through control experiments; an experiment was conducted without any biomass sample being introduced to the high-temperature zone and the mass of water collected in the test tubes was reported as moisture generated by the flame. The difference between the mass of the test tubes before and after the control experiments was used to determine the yield of moisture and tar collected during experiments. The flame-generated moisture was then subtracted to obtain the tar yield. The tar yield reported here is based on the cumulative amount of tar collected from 10 to 30 separate runs with the same plant species since the amount of tar from individual leaf experiments was low.
2.8. Char Yield
Char yield was defined as the weight percentage of solid pyrolysis products per mass of the initial sample, on a dry, ash-free basis. After each experiment, the mass of the remaining sample on the alligator clip was considered as char and ash for that experiment. Experiments where some of the fragile charred leaf dropped from the alligator clip were omitted from the char yield calculation.
2.9. Gas Yield
Gas yield was defined as the weight percentage of non-condensable light gas products per mass of the initial sample, on a dry, ash-free basis. The gas yield was calculated by difference using the tar and char yields, according to:
2.10. High Heating Value Estimation
The high heating values (HHV) for volatiles released during the pyrolysis of these plants were estimated based on the chemical species present in each of the pyrolysis products (tar and light gases) and their relative concentration. Since yields of both gas and tar species were measured quantitatively in this research, HHVs of both light gas and tar were computed for each plant species, and these values were blended into a composite HHV for volatiles for each plant species.
The HHV of tar was estimated by multiplying the high heating values associated with each individual tar compound by their respective mole percentages. Note that heating value is defined as the negative of the heat of combustion. Two methods were used to estimate the HHV of each individual tar compound. For compounds with known heats of formation (
), the HHV was calculated as the difference between the heats of formation of reactants and that of products for the combustion reaction for that specific compound. For compounds whose heat of formation was not available from the NIST database [
39], the Dulong–Berthelot correlation was used to estimate the HHV [
40]. The Dulong–Berthelot correlation is an adjusted form of the Dulong correlation used to predict the HHV of fuel based on its chemical composition. In this correlation,
,
,
,
and
represent the wt% dry ash-free (daf) of the respective element:
To estimate the HHV of a tar compound using the Dulong–Berthelot correlation, the mass percent of each element, C, H, N, O and S, is determined using molecular weight of that specific tar compound and that of C, H, N, O and S [
41].
The HHV of tar is an estimation because there are many more species in the tar that were below the detection limit of the GC/MS instrument. The HHVs for permanent gases released during pyrolysis were calculated in a similar manner. Values of HHV for each tar species are provided in
Table S1 in the Supplementary Material.
2.11. Statistical Analyses
It has recently been shown that data describing the composition of mixtures of gases from smoke and pyrolysis are multivariate, non-negative and relative in nature, making them compositional data [
42,
43,
44]. Compositional data analysis is a well-developed field of statistics. The common approach to analysis of these data involves transforming the data into log-ratios and then applying familiar statistical tools [
45,
46]. Given that this approach to data analysis of gas and fuel composition is relatively new to the field, the results presented here are based on the more familiar approach. Readers interested in this newer approach that recognizes the unique characteristics of these data are referred to the references above. All concentrations reported in this paper are averages of at least three experiments. The error bars for the reported values are 95% confidence intervals for at least three experiments.
Analysis of variance (ANOVA) was used to test whether the means of independent groups are significantly different from one another. The null hypothesis is that the means of the studied groups are in the same range and are not statistically different from each other. If the calculated p-value is less than 0.05, then the null hypothesis is rejected and there is a significant difference between the means of the investigated groups. The ANOVA test was performed using Microsoft Excel 2017 to calculate p-values.
3. Results and Discussion
In this section, first, the yields of pyrolysis products, tar, light gases and char from various plant species are discussed. Possible similarities between the products’ yields from California species and southern U.S. species are mentioned. Then, compounds identified in tar are thoroughly studied and are classified into aromatic, cyclic or aliphatic groups. Possible explanations for variations in the concentration of these tar species are provided. Based on the concentration of tar species and their relative molecular weight, the average molecular weight for tar from each of the plant species is calculated. Light gases, the major species present in them and variations in the concentration of each of these compounds are discussed. Finally, the high heating value for volatiles released during pyrolysis of each of the plants is estimated and discussed.
3.1. Tar, Gas and Char Yields
Table 3 shows the yields of pyrolysis products for the various plant species studied here. The total yield of volatiles was similar for all samples, ranging from 75 wt% daf (dry, ash-free) for the manzanita branches to 82 wt% daf for the ceanothus and chamise branches with foliage. The amount of collected tar varied between 48 wt% daf (manzanita branches) and 59 wt% daf (chamise branches with leaves), with a corresponding change in the light gas yield. The total yield of volatiles generated from the foliage was compared to the yield from the branches to investigate any possible variations in the pyrolysis behavior of two different parts of one plant species. Results show that the pyrolysis of foliage and branches generated similar amounts of volatiles.
The tar, light gas and total yield of volatiles shown in
Table 3 for the southern California species were in the same range as reported by Safdari et al. [
32] for samples from the southern United States. The average tar collected from the pyrolysis of southern samples ranged between 53 and 62 wt% daf, with light gas yields between 18 and 25 wt% daf. Sparkleberry was retested in this study to compare with data obtained by Safdari. The total yield of volatiles generated by sparkleberry in the current experiment was 75 wt%, while the yield of collected volatiles by Safdari was 78 ± 4.46 wt% daf indicating that the two experiments were in good agreement. The slight differences in the yields of volatiles between the two experiments can be explained by the slightly higher pyrolysis temperatures during the experiments conducted by Safdari.
The tar yields here are fairly high and comparable with the tar yields typically reported for biomass pyrolysis, such as the maximum tar (i.e., oil) yields reported by di Blasi for wood ranging from 45 to 56 wt% [
47]. The fact that the tar yields have remained comparable to experiments at lower temperatures seems to indicate that secondary reactions have not played an important role in these experiments.
3.2. Compounds Present in Tar
Having a knowledge of the compounds present in tar and light gases is crucial for better understanding combustion since most of these volatiles later react with oxygen promoting wildland fires. Tar analysis was conducted to investigate the differences in the species and concentration of condensable compounds released during the pyrolysis of various plant species. A complete list of compounds identified for each plant species is given in
Table S1 in the Supplementary Material. For discussion purposes, tar compounds were classified as aromatic, cyclic or aliphatic (see
Table S2).
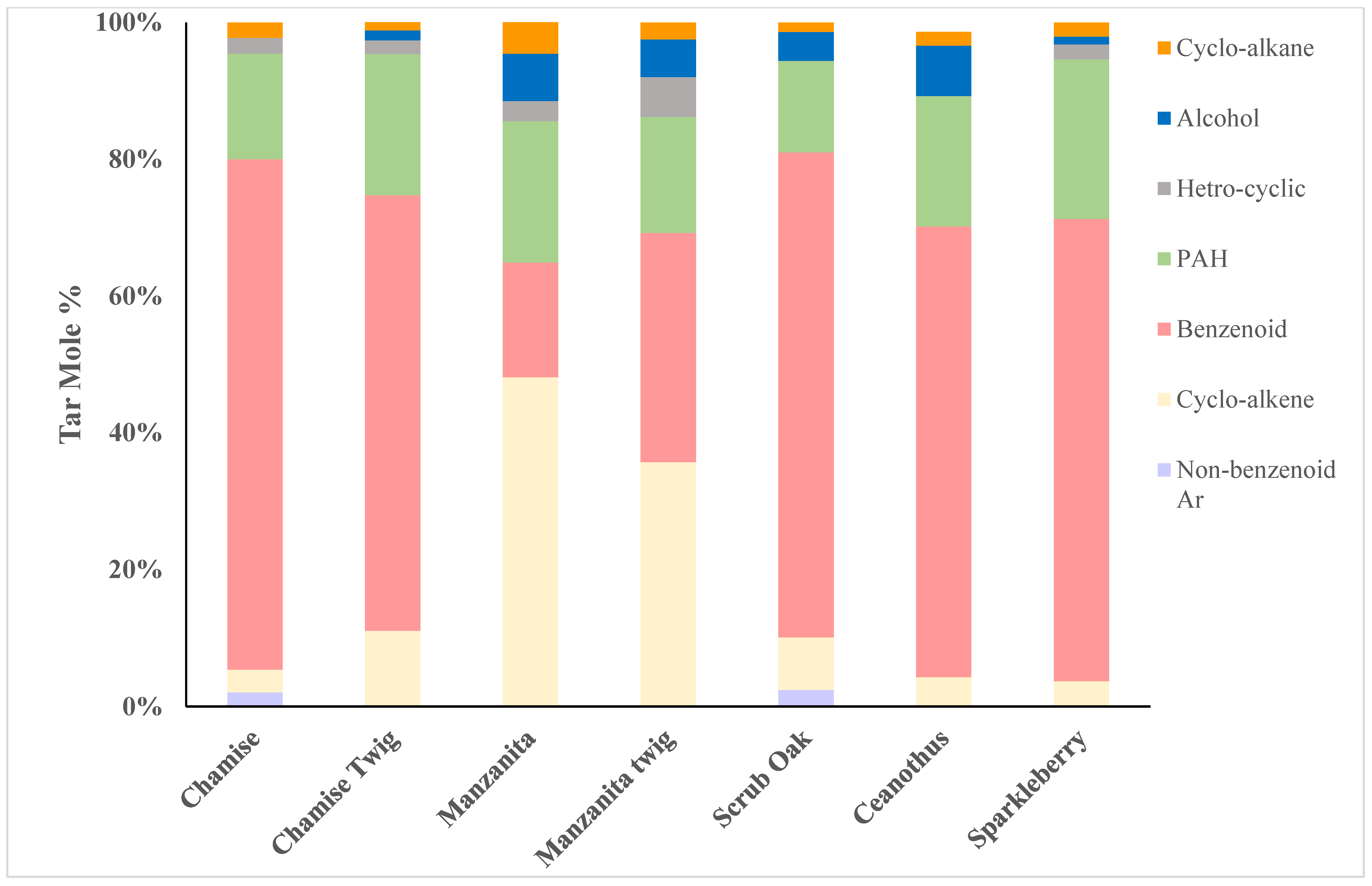
Table 4 shows the classification of species present in tar from the pyrolyzed plant samples. For convenience, these data are illustrated in
Figure 2 as well. Apart from manzanita, benzenoid aromatic compounds (aromatic compounds containing one benzene ring) are the major constituents of tar. The overall concentration of benzenoid aromatics in tar varied from 74.6 mole% for chamise branches with foliage to 16.8 mole% in manzanita. Tar from manzanita and manzanita branches had a higher mole percentage of cycloalkenes.
The only aliphatic compound detected was classified as an alcohol. The total concentrations of benzenoids in tar from scrub oak, ceanothus, sparkleberry and chamise were all much higher than manzanita.
Phenol was the most abundant compound in the benzenoid aromatic group. The other primary tar compounds were 2-methyl phenol, 3-methyl phenol, 4-ethyl-2-methoxy phenol (4-ethyl guaiacol) and hydroquinone (1,4-Benzenediol). Variations in the concentrations of phenolic compounds in tar may be due to several factors. Phenolic compounds are mainly reported to be formed as a result of lignin degradation. Since different plant species have different ratios of lignin and extractables, it is likely that the mole percent of phenolic compounds in tar from pyrolysis of various plants will be different. Moreover, physical differences between various fuels can affect the temperature profile inside the samples. These samples are not thermally thin [
16,
48], resulting in a temperature profile inside the samples. The relatively small diameter of the chamise needles compared to the thickness of other species of biomass affects the heating rate and hence may have affected some of the species detected.
When lignin is exposed to high temperatures, the bond between its monomer units starts to break, releasing compounds with structures close to its monomer constituents. 2-methoxy phenol (also known as guaiacol) is mainly thought to be a pyrolysis product of lignin which acts as an intermediate forming other compounds. Methylated phenols can form quinone methide as an intermediate in the series of reactions leading to coke formation and gas release as shown in
Figure 3 [
49]. The abundance of single-ring aromatic compounds seems to indicate the dominance of primary tar reactions. If the pyrolysis temperature is high enough for further polymerization reactions to occur, a portion of the single-ring aromatics polymerizes and forms polycyclic aromatic hydrocarbons (PAHs) which are important environmental pollutants and are hazardous to human health.
The pathways and mechanisms responsible for the formation of PAHs depend on the chemical structure of the intermediates. Catechols/pyrogallols preferentially generate biphenyl, naphthalenes and phenanthrene, while cresols/xylenols are more likely to transform into xanthene and anthracene. A comparison between tar collected from southern California and the southern U.S. shows similar trends in the major tar compounds. Compounds such as phenol, 4-methyl phenol, 4-ethyl phenol, 2-methoxy phenol (guaiacol) and 3,4-dimethyl phenol were classified as “monocyclic aromatics” [
32]. In tar samples from both southern California and the southern U.S., with the exception of manzanita, these single-ring aromatics, along with multi-ring aromatics, were the major tar compounds.
The presence of furans (and their derivatives such as 2,3-dihydro-Benzofuran) in tar is mainly believed to be from hemicellulose, and the evolution of carbonyl and carboxylic groups are from the pyrolysis of cellulose [
50,
51]. These mechanisms may explain the presence of the tar species with oxygen in the ring or C=O groups.
Hydroquinone, also known as benzene-1,4-diol, is a phenolic compound among pyrolysis products detected in tar from southern California plants. It has two hydroxyl groups attached to a benzene ring in the para position. P-benzoquinone is the oxidized derivative of hydroquinone seen in tar from southern U.S. species. Based on their similar chemical structure, it is highly possible that these two compounds originate from the same intermediate. Some of the species detected in tar from the southern U.S. and southern California have the same backbone structure but different substituents. This may suggest that some of the differences observed in the tar compounds of southern California and the southern U.S. may be due to the secondary reactions involved.
Wildland fuels contain nitrogen as a normal component due to plants’ physiological processes. A portion of these compounds participate in dehydrogenation, dehydration, deamination and condensation reactions generating small amounts of aromatics with nitrogen heteroatoms such as pyridine, indole and quinolone. Samples from southern California and the southern U.S. generated small concentrations of nitrogenated species.
3.3. Average Molecular Weight for Tar
The average molecular weight (MW) of tar is estimated as a weighted average of the mole percentage of each detected tar compound and their corresponding molecular weight. This value can provide some information about the distribution of heavy molecular weight compounds released into tar. The presence of heavy molecular weight compounds in tar, which would increase the average molecular weight, may be due to the prevalence of secondary pyrolysis reactions. All species in these experiments were exposed to similar conditions (temperature, pressure, residence time), meaning the only two factors that affected the molecular weight distribution were heating rate and inherent differences in the composition of each species. Variations in the physical characteristics of each plant, such as the thickness of the leaves, affect the heating rate and temperature profile inside the leaves as they are exposed to elevated temperatures. This may lead to the release of volatiles with different molecular weights. As seen in
Table 5, the molecular weight of the tar from manzanita was much higher than those observed for the other plant species considered here.
3.4. Light Gases
Light gases were analyzed offline using a GC-TCD system. Post-flame gases from FFB were collected and analyzed as background. The exact setup was used for the pyrolysis of biomass and the post-pyrolysis gases were collected. After each pyrolysis experiment, the background spectrum resulting from the flame was subtracted from the spectrum pertaining to the pyrolysis gases to estimate the concentration of gas components resulting from the pyrolysis of samples.
The four major components of pyrolysis gases were CO, CO
2, CH
4 and H
2. Other unspecified peaks were occasionally detected. However, since their concentration was below the detection limit in this experiment, they were not identified. To obtain the mole percentage of each gas component, the area under the peak pertaining to that specific component was divided by the sum of the total identified peak areas. Carbon monoxide was the main component in the light gases on a dry wt% of light gas basis, followed by CO
2, CH
4 and H
2 for all studied plant species. The detailed composition of the gas phase is presented in
Table 6 along with the 95% confidence intervals. More than half of the light gas was CO (on a dry wt% basis) for all plant species, followed by CO
2 (28 to 34%), CH
4 (5 to 9%) and then H
2 (approximately 1.5%).
Statistical analysis shows that there is a significant difference in the concentration of CO released into the gas phase among various pyrolyzed plant species (p = 2.43 × ). A deeper look into the data shows that the concentration of CO in the gas products of sparkleberry is much different to those of all other plant species native to southern California. ANOVA analysis on the concentration of CO in the pyrolysis gases of southern California species produces a higher p-value (p = 2.38 × ). Among all studied species, chamise branches with foliage had the lowest yield of CO (55.0 wt%) and sparkleberry released the highest amount of CO (64.6 wt%).
There is a statistically significant difference in the concentration of CO2 between species native to southern California (p = 0.00008). When sparkleberry is taken into account for an analysis of variations in the concentration of CO2 alongside southern California species, a greater statistical difference between sparkleberry and southern California species is observed (p = 5.99685 .
The statistical evaluation of variations in the wt% of CH4 in the pyrolysis gases of southern California species shows that there is a slight variation in the amount of generated CH4 due to fuel type (p = 0.08). If sparkleberry is taken into account, variations in the concentration of generated CH4 will be statistically significant (p = 0.000784).
3.5. High Heating Value of Tar and Light Gases
High heating value (HHV), sometimes referred to as high calorific value, is the energy released during complete combustion of a unit quantity of fuel (initially at 25 °C) in O2 after the temperature of the products returns to 25 °C. HHV for a specific fuel (such as manzanita) can be measured by a bomb calorimeter in an experimental setup. Estimating the high heating value of a fuel provides researchers with valuable information about the energy content of a specific fuel, enabling researchers to classify and compare various fuel types. Higher values of HHV mean that the energy released during complete combustion of the fuel will be higher.
Table 7 contains the high heating values for volatiles collected from the pyrolysis of various species. Estimated values for the HHVs of volatiles varied between approximately 19 and 23 MJ/kg of daf fuel. These HHV values are close to the heating value of methanol (22.7 MJ/kg). Volatiles from ceanothus had the highest HHV, while those of manzanita branches had the lowest. The tar contained almost three times the energy content of the light gas for each plant sample, which is one of the reasons it is important to study the tar species and include the HHV of tar while estimating the HHVs of volatiles released during the pyrolysis of biomass.
The focus of this paper is to characterize the composition of the volatiles released during the pyrolysis of plants since these compounds may combust and promote fire propagation. Solid residues remaining after pyrolysis do not contribute to fire propagation; as a result, char was not examined after each experiment and only the HHVs of volatiles generated during the pyrolysis of plants are reported here. To estimate the HHV of the parent biomass with the method suggested here, an analysis of the char for each plant species would have been necessary. The reported experimental values for the HHV of biochar from other sources of biomass in the existing literature are in the range of 17 to 35 MJ/kg of biochar [
40,
52].
The values of tar HHV are consistent with the existing literature. There is an extensive amount of research published on the HHV of bio-oil since it has the potential to be used as fuel. The HHV of a bio-oil mainly depends on its composition. Low values of HHV for tar may be due to the relatively high concentrations of oxygenated compounds present in tar [
53]. While operating conditions such as the type of the reactor, temperature and heating rate affect the composition of tar, and as a result its HHV, the reported values for the HHV of tar are in the range of 20 to 35 MJ/kg of tar [
33,
54,
55,
56,
57].
Due to high levels of CO
2, permanent gases generated during the pyrolysis have lower values of HHV. Most of the research on biogas production focuses on the separation of methane from other gases generated during pyrolysis with the aim of improving its HHV. The HHV of gases released from pyrolysis depends on the relative concentration of its combustible components compared to non-combustible ones [
57].
Matt et al. [
7] reported the high heating values for complete combustion of the various plant species (see
Table 8). While the investigated plants in the two studies are different, and the current studies only estimated heating values from the volatiles, Matt’s reported values of HHV for live plants are in the same range as the estimated values for volatiles released during the pyrolysis of southern California species (
Table 7).
The last column of
Table 7 shows the contribution of tar to the total volatiles’ heating value. Tar contributes 82 to 87% of the heating value of the volatiles in these samples, which emphasizes the need to understand tar formation and not restrict modeling emphasis solely on light gas formation.
4. Conclusions
In this study, the characteristics of pyrolysis products (tar and light gas) from selected southern California plant species were investigated at heating conditions representative of a wildland fire [
14,
15,
16,
17,
18]. The main findings are:
Tar yields from the four southern California plant species studied ranged from 48 to 59 wt% of the dry ash-free biomass, which is slightly lower than the 53–62% range reported for southern U.S plant species. Analysis of tar collected from manzanita samples showed that cycloalkenes were the major constituents of tar. However, in all other species from southern California, aromatics are the major constituents of tar. Analysis of aromatics in tar from chamise, scrub oak, ceanothus and sparkleberry showed that benzenoids, especially phenol, were the most abundant tar compound. The other key tar compounds were 2-methyl phenol, 3-methyl phenol, 4-ethyl-2-methoxy phenol (4-ethyl guaiacol) and hydroquinone (1,4-Benzenediol). A comparison between tar collected from southern California and the southern U.S. revealed that similar monocyclic aromatics were the most abundant tar compounds [
32]. Tar from both southern California and southern U.S. samples was rich in single-ring aromatics, along with multi-ring aromatics (PAHs). Higher pyrolysis temperatures likely promoted some secondary pyrolysis reactions generating heavier PAHs.
Light gas yields ranged from 22 to 31 wt% (daf) for the samples studied. The main constituent of the pyrolysis gases was CO followed by CO2, CH4 and H2 on a wt% dry, ash-free basis. The concentration of various gas components varied depending on the species type. Sparkleberry, a plant native to southern U.S., was pyrolyzed to compare its behavior to that of species native to southern California. It was found that the composition of pyrolysis gases from sparkleberry was statistically different from that of the species native to southern California.
High heating values (HHVs) for tar, light gases and total volatiles released during pyrolysis of southern California samples were calculated for each plant species. The estimated values for HHV varied between approximately 19 and 23 kJ/kg of dry volatiles, which is close to the heating value of methanol (22.7 MJ/kg). This range of HHVs for volatiles is consistent with the HHV data from other biomass species, including the HHVs from live plants reported by Matt et al. [
7]. The heating value of the tar was almost three times the heating value of the light gases, and tar contributed to over 80% of the HHVs of the volatiles. Therefore, detailed fire studies should consider the contribution of tar and not solely the light pyrolysis gases.
These data on the yields, composition and high heating values of the light gases and tars from wildland plant species will improve the understanding of fires and provide helpful data for physics-based fire simulation models, especially those models trying to predict emissions.