Fire-Induced Changes in Geochemical Elements of Forest Floor in Southern Siberia
Abstract
1. Introduction
2. Materials and Methods
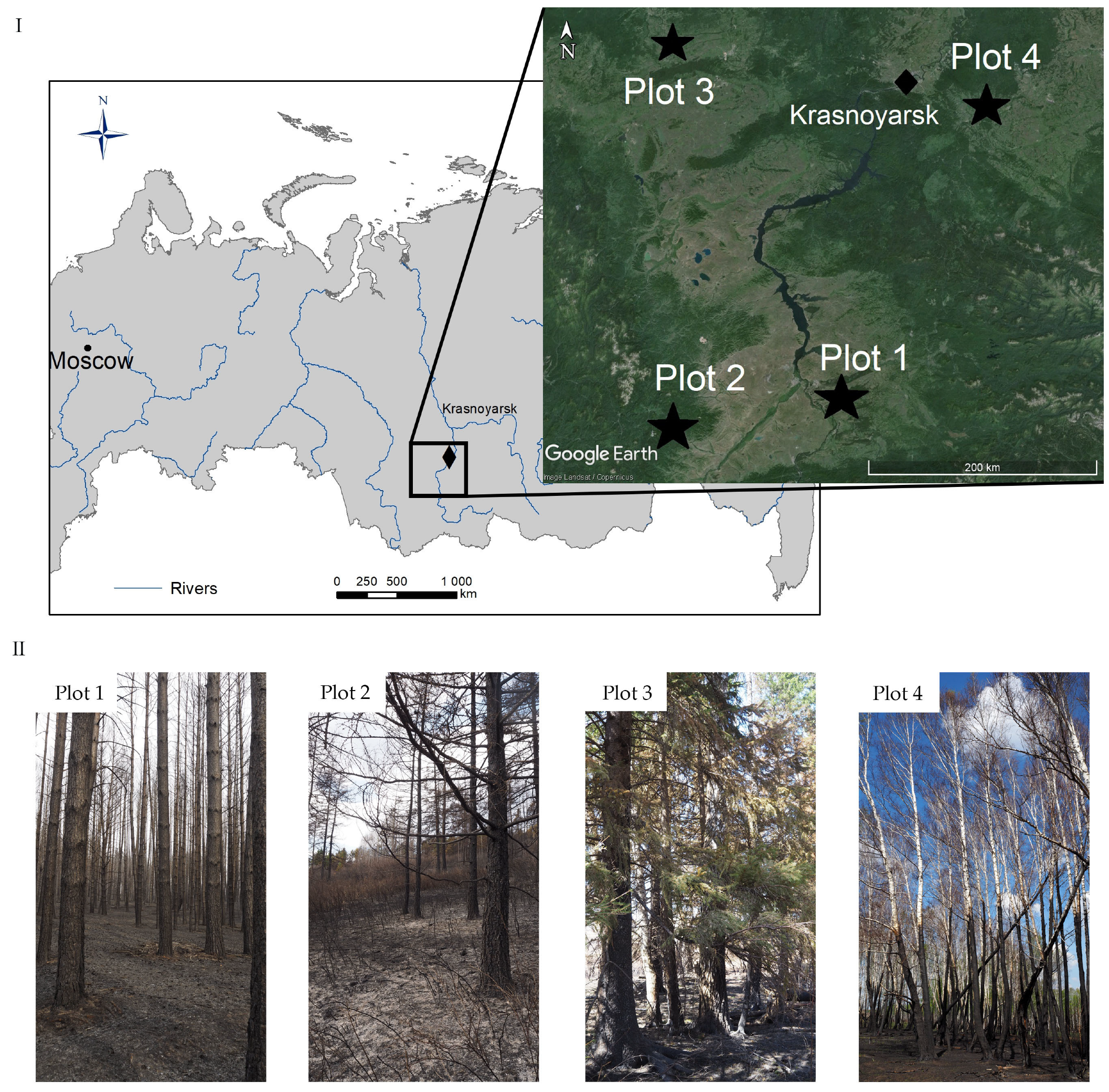
2.1. Study Area and Sampling Procedure
2.2. Analytical Procedures
2.3. Data Analysis
3. Results
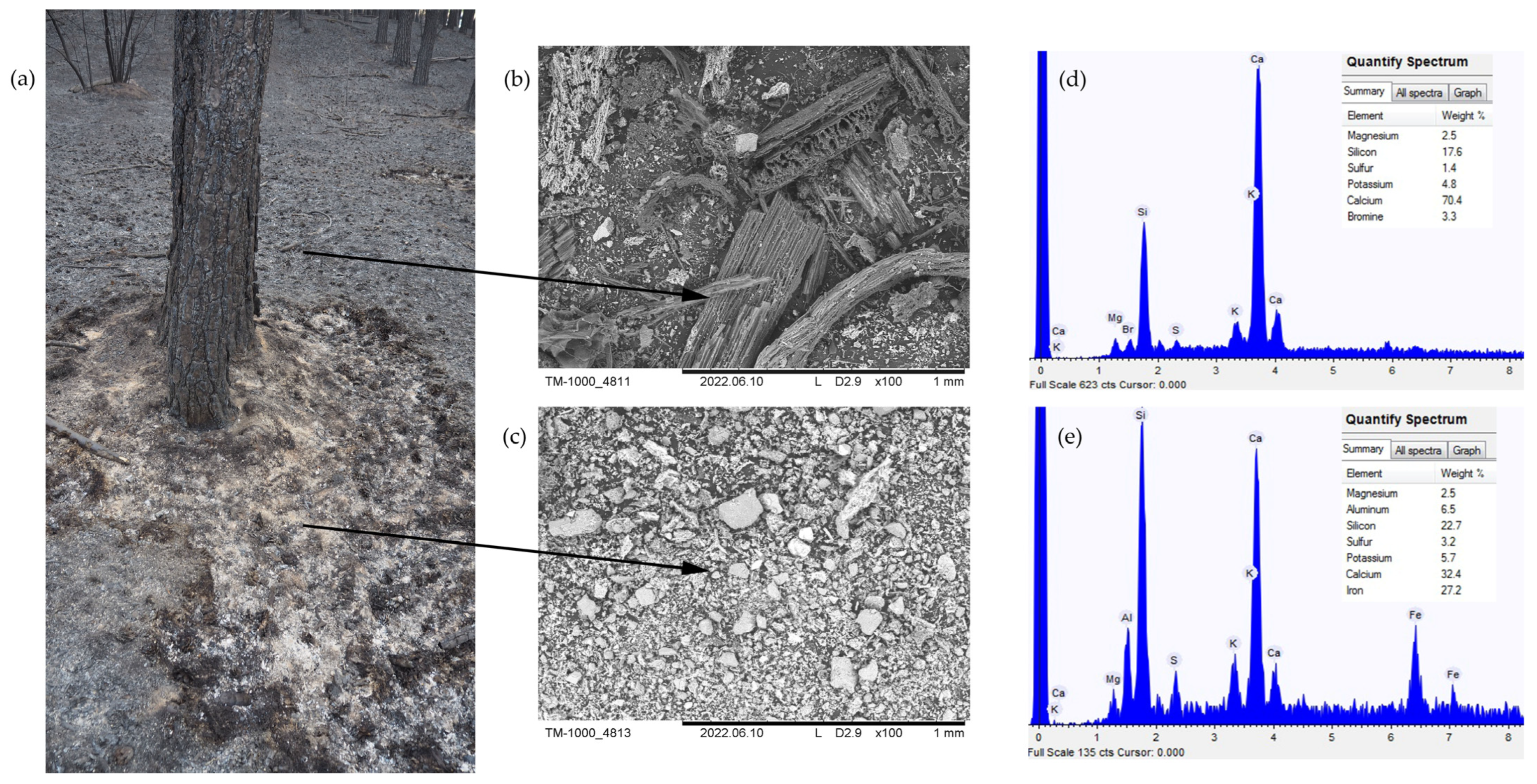
3.1. Physicochemical Characteristics of Forest Floor
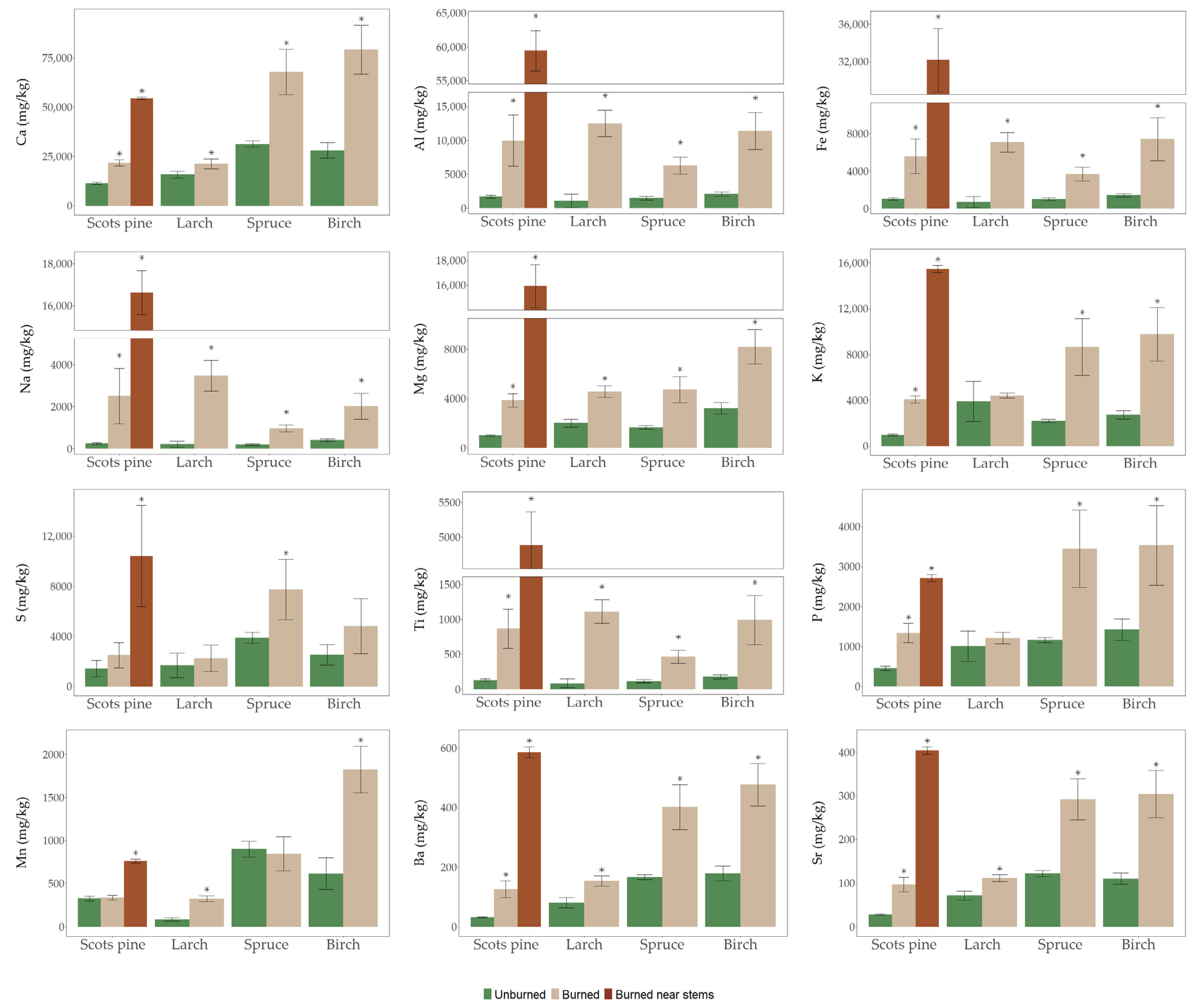
3.2. Major and Minor Elements in Burned and Unburned Forest Floor
3.3. Trace and Ultra-Trace Elements in Burned and Unburned Forest Floors
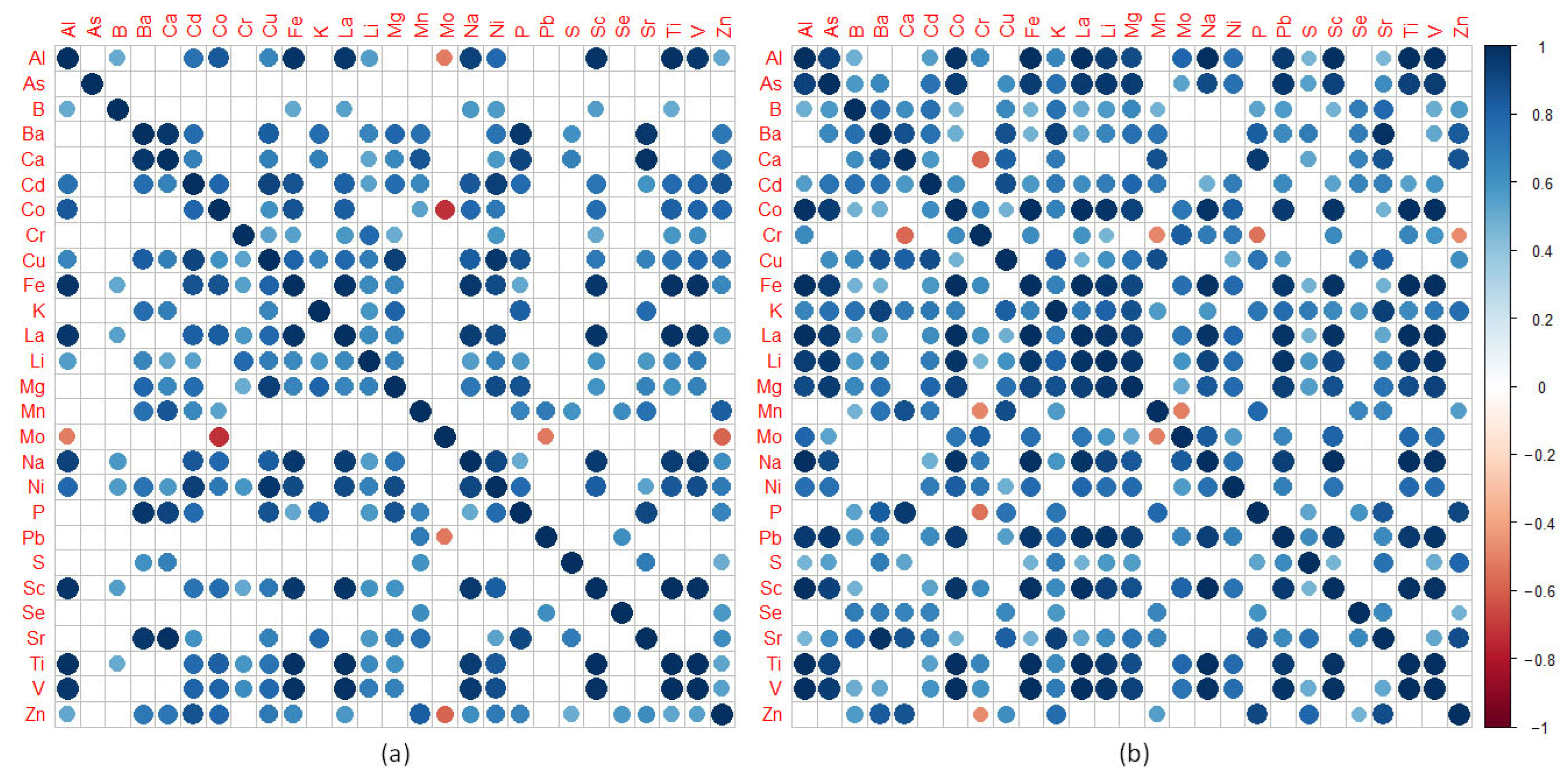
3.4. Correlations among Element Contents and Relationships of Elements with Physiochemical Characteristics of Forest Floor
4. Discussion
4.1. Wildfires Effects on Physicochemical Properties of Forest Floor
4.2. Wildfire Effects on the Chemical Elements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doerr, S.H.; Santín, C. Global trends in wildfire and its impacts: Perceptions versus realities in a changing world. Phil. Trans. R. Soc. 2016, 371, B37120150345. [Google Scholar] [CrossRef]
- McWethy, D.B.; Schoennagel, T.; Higuera, P.E.; Krawchuk, M.; Harvey, B.J.; Metcalf, E.C.; Schultz, C.; Miller, C.; Metcalf, A.L.; Buma, B.; et al. Rethinking resilience to wildfire. Nat. Sustain. 2019, 2, 797–804. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Ponomarev, E.I.; Ivanova, G.A.; Dvinskaya, M.L.; Coogan, S.C.P.; Flannigan, M.D. Wildfires in the Siberian taiga. Ambio 2021, 50, 1953–1974. [Google Scholar] [CrossRef]
- Jones, M.W.; Abatzoglou, J.T.; Veraverbeke, S.; Andela, N.; Lasslop, G.; Forkel, M.; Smith, A.J.P.; Burton, C.; Betts, R.A.; van der Werf, G.R.; et al. Global and regional trends and drivers of fire under climate change. Rev. Geophys. 2022, 60, e2020RG000726. [Google Scholar] [CrossRef]
- Kukavskaya, E.A.; Buryak, L.V.; Shvetsov, E.G.; Conard, S.G.; Kalenskaya, O.P. The impact of increasing fire frequency on forest transformations in southern Siberia. For. Ecol. Manag. 2016, 382, 225–235. [Google Scholar] [CrossRef]
- Tyukavina, A.; Potapov, P.; Hansen, M.C.; Pickens, A.H.; Stehman, S.V.; Turubanova, S.; Parker, D.; Zalles, V.; Lima, A.; Kommareddy, I.; et al. Global trends of forest loss due to fire from 2001 to 2019. Front. Remote Sens. 2022, 3, 825190. [Google Scholar] [CrossRef]
- Burrell, A.L.; Sun, Q.; Baxter, R.; Kukavskaya, E.A.; Zhila, S.; Shestakova, T.; Rogers, B.M.; Kaduk, J.; Barrett, K. Climate change, fire return intervals and the growing risk of permanent forest loss in boreal Eurasia. Sci. Total Environ. 2022, 831, 154885. [Google Scholar] [CrossRef] [PubMed]
- Lapenis, A.G.; Yurganov, L.N. Increase in Arctic Oscillations explains most interannual variability in Russia’s wildfires. Front. For. Glob. Chang. 2023, 6, 1188057. [Google Scholar] [CrossRef]
- Rogers, B.M.; Soja, A.J.; Goulden, M.L.; Randerson, J.T. Influence of tree species on continental differences in boreal fires and climate feedbacks. Nat. Geosci. 2015, 8, 228–234. [Google Scholar] [CrossRef]
- Li, Y.; Janssen, T.A.J.; Chen, R.; He, B.; Veraverbeke, S. Trends and drivers of Arctic-boreal fire intensity between 2003 and 2022. Sci. Total Environ. 2024, 926, 172020. [Google Scholar] [CrossRef]
- Kukavskaya, E.A.; Soja, A.J.; Petkov, A.P.; Ponomarev, E.I.; Ivanova, G.A.; Conard, S.G. Fire emissions estimates in Siberia: Evaluation of uncertainties in area burned, land cover, and fuel consumption. Can. J. For. Res. 2013, 43, 493–506. [Google Scholar] [CrossRef]
- González-Pérez, J.A.; González-Vila, F.J.; Almendros, G.; Knicker, H. The effect of fire on soil organic matter—A review. Environ. Int. 2004, 30, 855–870. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Knicker, H. How does fire affect the nature and stability of soil organic nitrogen and carbon? A review. Biogeochemistry 2007, 85, 91–118. [Google Scholar] [CrossRef]
- Fernandez-Marcos, M.L. Potentially toxic substances and associated risks in soils affected by wildfires: A review. Toxics 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Girona-García, A.; Vieira, D.C.S.; Silva, J.; Fernández, C.; Robichaud, P.R.; Keizer, J.J. Effectiveness of post-fire soil erosion mitigation treatments: A systematic review and meta-analysis. Earth-Sci. Rev. 2021, 217, 103611. [Google Scholar] [CrossRef]
- Abraham, J.; Dowling, K.; Florentine, S. The unquantified risk of post-fire metal concentration in soil: A review. Water Air Soil Pollut. 2017, 228, 175. [Google Scholar] [CrossRef]
- Terzano, R.; Rascio, I.; Allegretta, I.; Porfido, C.; Spagnuolo, M.; Khanghahi, M.Y.; Crecchio, C.; Sakellariadou, F.; Gattullo, C.E. Fire effects on the distribution and bioavailability of potentially toxic elements (PTEs) in agricultural soils. Chemosphere 2021, 281, 130752. [Google Scholar] [CrossRef]
- Roshan, A.; Biswas, A. Fire-induced geochemical changes in soil: Implication for the element cycling. Sci. Total Environ. 2023, 868, 161714. [Google Scholar] [CrossRef]
- Scott, A.C. Charcoal recognition, taphonomy and uses in palaeoenvironmental analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 11–39. [Google Scholar] [CrossRef]
- Dlapa, P.; Bodí, M.B.; Mataix-Solera, J.; Cerdà, A.; Doerr, S.H. FT-IR spectroscopy reveals that ash water repellency is highly dependent on ash chemical composition. Catena 2013, 108, 35–43. [Google Scholar] [CrossRef]
- Bodí, M.B.; Martin, D.A.; Balfour, V.N.; Santín, C.; Doerr, S.H.; Pereira, P.; Cerdà, A.; Mataix-Solera, J. Wildland fire ash: Production, composition and eco-hydro-geomorphic effects. Earth-Sci. Rev. 2014, 130, 103–127. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Otero, X.L.; Chafer, C.J. Quantity, composition and water contamination potential of ash produced under different wildfire severities. Environ. Res. 2015, 142, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Neary, D.G.; Klopatek, C.C.; DeBano, L.F.; Ffolliott, P.F. Fire effects on belowground sustainability: A review and synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Melendez-Perez, J.J.; Fostier, A.H.; Carvalho, J.A.; Windmöller, C.C.; Santos, J.C.; Carpi, A. Soil and biomass mercury emissions during a prescribed fire in the Amazonian rain forest. Atmos. Environ. 2014, 96, 415–422. [Google Scholar] [CrossRef]
- Campos, I.; Vale, C.; Abrantes, N.; Keizer, J.J.; Pereira, P. Effects of wildfire on mercury mobilisation in eucalypt and pine forests. Catena 2015, 131, 149–159. [Google Scholar] [CrossRef]
- Cuypers, F.; De Dobbelaere, C.; Hardy, A.; Van Bael, M.K.; Helsen, L. Thermal behaviour of arsenic trioxide adsorbed on activated carbon. J. Hazard. Mater. 2009, 166, 1238–1243. [Google Scholar] [CrossRef][Green Version]
- Úbeda, X.; Pereira, P.; Outeiro, L.; Martin, D. Effects of fire temperatures on the physical and chemical characteristics of the ash from two plots of cork aok (Quercus suber). Land Degrad. Dev. 2009, 20, 589–608. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Martin, D.; Mataix-Solera, J.; Guerrero, C. Effects of a low severity prescribed fire on water-soluble elements in ash from a cork oak (Quercus suber) forest located in the northeast of the Iberian Peninsula. Environ. Res. 2011, 111, 237–247. [Google Scholar] [CrossRef]
- Tuhý, M.; Ettler, V.; Rohovec, J.; Matoušková, Š.; Mihaljevič, M.; Kříbek, B.; Mapani, B. Metal(loid)s remobilization and mineralogical transformations in smelter-polluted savanna soils under simulated wildfire conditions. J. Environ. Manag. 2021, 293, 112899. [Google Scholar] [CrossRef]
- Harden, J.W.; Neff, J.C.; Sandberg, D.V.; Turetsky, M.R.; Ottmar, R.; Gleixner, G.; Fries, T.L.; Manies, K.L. Chemistry of burning the forest floor during the FROSTFIRE experimental burn, interior Alaska, 1999. Glob. Biogeochem. Cycles 2004, 18, GB3014. [Google Scholar] [CrossRef]
- Hogue, B.A.; Inglett, P.W. Nutrient release from combustion residues of two contrasting herbaceous vegetation types. Sci. Total Environ. 2012, 431, 9–19. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X. Spatial distribution of heavy metals released from ashes after a wildfire. J. Environ. Eng. Landsc. Manag. 2010, 18, 13–22. [Google Scholar] [CrossRef]
- Pacifico, L.R.; Pizzolante, A.; Guarino, A.; Iannone, A.; Esposito, M.; Albanese, S. Wildfires as a source of potentially toxic elements (PTEs) in soil: A case study from Campania Region (Italy). Int. J. Environ. Res. Public Health 2023, 20, 4513. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, C.; Santín, C.; Neris, J.; Sigmund, G.; Otero, X.L.; Manley, J.; González-Rodríguez, G.; Belcher, C.M.; Cerdà, A.; Marcotte, A.L.; et al. Chemical characteristics of wildfire ash across the globe and their environmental and socio-economic implications. Environ. Int. 2023, 178, 108065. [Google Scholar] [CrossRef]
- Campo, J.; Andreu, V.; Gimeno-García, E.; González, O.; Rubio, J.L. Occurrence of soil erosion after repeated experimental fires in a Mediterranean environment. Geomorphology 2006, 82, 376–387. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef]
- De Marco, A.; Gentile, A.E.; Arena, C.; De Santo, A.V. Organic matter, nutrient content and biological activity in burned and unburned soils of a Mediterranean maquis area of southern Italy. Int. J. Wildland Fire 2005, 14, 365–377. [Google Scholar] [CrossRef]
- Sosorova, S.B.; Merkusheva, M.G.; Ubugunov, L.L. Pyrogenic changes in microelement content in soils and plants from the pine forests of western Transbaikal. Contemp. Probl. Ecol. 2013, 6, 499–508. [Google Scholar] [CrossRef]
- Campos, I.; Abrantes, N.; Keizer, J.J.; Vale, C.; Pereira, P. Major and trace elements in soils and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Sci. Total Environ. 2016, 572, 1363–1376. [Google Scholar] [CrossRef]
- Hrelja, I.; Šestak, I.; Delač, D.; Pereira, P.; Bogunović, I. Soil chemical properties and trace elements after wildfire in Mediterranean Croatia: Effect of severity, vegetation type and time-since-fire. Agronomy 2022, 12, 1515. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Martin, D.A. Fire severity effects on ash chemical composition and water-extractable elements. Geoderma 2012, 191, 105–114. [Google Scholar] [CrossRef]
- Harper, A.R.; Santín, C.; Doerr, S.H.; Froyd, C.A.; Albini, D.; Otero, X.L.; Viñas, L.; Pérez-Fernández, B. Chemical composition of wildfire ash produced in contrasting ecosystems and its toxicity to Daphnia magna. J. Int. Assoc. Wildland Fire 2019, 28, 726–737. [Google Scholar] [CrossRef]
- Czimczik, C.I.; Preston, C.M.; Schmidt, M.W.I.; Schulze, E.-D. How surface fire in Siberian Scots pine forests affects soil organic carbon in the forest floor: Stocks, molecular structure, and conversion to black carbon (charcoal). Glob. Biogeochem. Cycles 2003, 17, 1020. [Google Scholar] [CrossRef]
- Krasnoshchekov, Y.N.; Cherednikova, Y.S. Postpyrogenic transformation of soils under Pinus sibirica forests in the southern Lake Baikal basin. Eurasian Soil Sci. 2012, 45, 929–938. [Google Scholar] [CrossRef]
- Krasnoshchekov, Y.N. Postpyrogenic variability of litter in mountain forests of Baikal region. Eurasian Soil Sci. 2019, 52, 258–270. [Google Scholar] [CrossRef]
- Bilichenko, I.N.; Sedykh, S.A.; Kichigina, N.N.; Li, Z. Impact of forest wildfires on components of mountain landscapes of the Baikal region. J. Wildl. Biodivers. 2021, 6, 100–114. [Google Scholar] [CrossRef]
- Chebykina, E.; Polyakov, V.; Abakumov, E.; Petrov, A. Wildfire Effects on Cryosols in Central Yakutia Region, Russia. Atmosphere 2022, 13, 1889. [Google Scholar] [CrossRef]
- Furyaev, V.V. Pyrological regimes and dynamics of the southern taiga forests in Siberia. In Fire in Ecosystems of Boreal Eurasia; Goldammer, J.G., Furyaev, V.V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996; pp. 168–185. Available online: https://link.springer.com/book/10.1007/978-94-015-8737-2 (accessed on 1 March 2024).
- Kurbatsky, N.P. Forest Fire Suppression Procedures and Tactics; Goslesbumizdat: Moscow, Russia, 1962; p. 154. (In Russian) [Google Scholar]
- Rosleskhoz Directive. About Approval of Instructions on Estimating Damage Caused by Forest Fires; No. 53 from 03.04.1998. Available online: https://docs.cntd.ru/document/901863083 (accessed on 1 March 2024).
- Shapchenkova, O.A.; Loskutov, S.R.; Kukavskaya, E.A. Alteration of organic matter during wildfires in the forests of Southern Siberia. Fire 2023, 6, 304. [Google Scholar] [CrossRef]
- Quantitative Chemical Analysis of Soils. Methodology for Measuring the Content of Metals in Solid Objects by Inductively Coupled Plasma Spectrometry Method. State Committee on Environmental Protection: Moscow, Russia, 1998; 28p.
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 March 2024).
- Wickham, H. ggplot2. Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016; 260p. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use ggbreak to effectively utilize plotting space to deal with large datasets and outliers. Front. Genet. 2021, 12, 774846. [Google Scholar] [CrossRef]
- Santín, C.; Doerr, S.H.; Merino, A.; Bryant, R.; Loader, N.J. Forest floor chemical transformations in a boreal forest fire and their correlations with temperature and heating duration. Geoderma 2016, 264 Pt A, 71–80. [Google Scholar] [CrossRef]
- Ivanova, G.A.; Kukavskaya, E.A.; Ivanov, V.A.; Conard, S.G.; McRae, D.J. Fuel characteristics, loads and consumption in Scots pine forests of central Siberia. J. For. Res. 2020, 31, 2507–2524. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quatern. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- Badía, D.; Martí, C.; Aguirre, A.J.; Aznar, J.M.; González-Pérez, J.A.; De la Rosa, J.M.; León, J.; Ibarra, P.; Echeverría, T. Wildfire effects on nutrients and organic carbon of a Rendzic Phaeozem in NE Spain: Changes at cm-scale topsoil. Catena 2014, 113, 267–275. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood-ash composition and soil pH following intense burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Marañón, T.; Navarro-Fernández, C.M.; Domínguez, M.T.; Madejón, P.; Murillo, J.M. How the soil chemical composition is affected by seven tree species planted at a contaminated and remediated site. Web Ecol. 2015, 15, 45–48. [Google Scholar] [CrossRef]
- Erickson, H.E.; Helmer, E.H.; Brandeis, T.J.; Lugo, A.E. Controls on fallen leaf chemistry and forest floor element masses in native and novel forests across a tropical island. Ecosphere 2014, 5, 48. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Hocaoglu-Ozyigit, A.; Genc, B.N. Cadmium in plants, humans and the environment. Front. Life Sci. Relat. Technol. 2020, 1, 12–21. [Google Scholar]
- García-Sánchez, A.; Murciego, A.; Álvarez-Ayuso, E.; Regina, I.S.; Rodríguez-González, M.A. Mercury in soils and plants in an abandoned cinnabar mining area (SW Spain). J. Hazard. Mater. 2009, 168, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Molchanov, V. Biogeochemical accumulation of trace elements in soils and plants of the Russian Far East. BIO Web Conf. 2024, 8, 01002. [Google Scholar] [CrossRef]
- Blackwell, B.D.; Driscoll, C.T. Using foliar and forest floor mercury concentrations to assess spatial patterns of mercury deposition. Environ. Pollut. 2015, 202, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Armiento, G.; Bellatreccia, F.; Cremisini, C.; Ventura, G.D.; Nardi, E.; Pacifico, R. Beryllium natural background concentration and mobility: A reappraisal examining the case of high Be-bearing pyroclastic rocks. Environ. Monit. Assess 2013, 185, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, D.; Goldberg, T.; Frick, D.A.; von Blanckenburg, F. Quantifying beryllium concentrations in plant shoots from forest ecosystems using cation-exchange chromatography and quadrupole ICP-MS. Anal. Sci. Adv. 2020, 1, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Modrzewska, B.; Kosiorek, M.; Wyszkowski, M. Content of some nutrients in Scots pine, silver birch and Norway maple in an urbanized environment. J. Elem. 2016, 21, 149–157. [Google Scholar] [CrossRef]
- Kosiorek, M.; Modrzewska, B.; Wyszkowski, M. Levels of selected trace elements in Scots pine (Pinus sylvestris L.), silver birch (Betula pendula L.), and Norway maple (Acer platanoides L.) in an urbanized environment. Environ. Monit. Assess 2016, 188, 598. [Google Scholar] [CrossRef][Green Version]
- Vesterdal, L.; Raulund-Rasmussen, K. Forest floor chemistry under seven tree species along a soil fertility gradient. Can. J. For. Res. 1998, 28, 1636–1647. [Google Scholar] [CrossRef]
- Bladon, K.D.; Emelko, M.B.; Silins, U.; Stone, M. Wildfire and the future of water Supply. Environ. Sci. Technol. 2014, 48, 8936–8943. [Google Scholar] [CrossRef]
- Doufexi, M.; Gamvroula, D.E.; Alexakis, D.E. Elements’ content in stream sediment and wildfire ash of suburban areas in West Attica (Greece). Water 2022, 14, 310. [Google Scholar] [CrossRef]
- Engle, M.A.; Gustin, M.S.; Johnson, D.W.; Murphy, J.F.; Miller, W.W.; Walker, R.F.; Wright, J.; Markee, M. Mercury distribution in two Sierran forest and one desert sagebrush steppe ecosystems and the effects of fire. Sci. Total Environ. 2006, 367, 222–233. [Google Scholar] [CrossRef]
- Boguta, P.; Skic, K.; Baran, A.; Szara-Bąk, M. The influence of the physicochemical properties of sediment on the content and ecotoxicity of trace elements in bottom sediments. Chemosphere 2022, 287 Pt 4, 132366. [Google Scholar] [CrossRef] [PubMed]
- Kennard, D.K.; Gholz, H.L. Effects of high- and low-intensity fires on soil properties and plant growth in a Bolivian dry forest. Plant Soil 2001, 234, 119–129. [Google Scholar] [CrossRef]
- Pereira, P.; Úbeda, X.; Martin, D.; Mataix-Solera, J.; Cerdà, A.; Burguet, M. Wildfire effects on extractable elements in ash from a Pinus pinaster forest in Portugal. Hydrol. Process. 2014, 28, 3681–3690. [Google Scholar] [CrossRef]
- Kukavskaya, E.A.; Ivanova, G.A.; Conard, S.G.; McRae, D.J.; Ivanov, V.A. Biomass dynamics of central Siberian Scots pine forests following surface fires of varying severity. Int. J. Wildland Fire 2014, 23, 872–886. [Google Scholar] [CrossRef]
- Pinno, B.D.; Landhäusser, S.M.; Chow, P.S.; Quideau, S.A.; MacKenzie, M.D. Nutrient uptake and growth of fireweed (Chamerion angustifolium) on reclamation soils. Can. J. For. Res. 2014, 44, 1–7. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapchenkova, O.A.; Kukavskaya, E.A.; Groisman, P.Y. Fire-Induced Changes in Geochemical Elements of Forest Floor in Southern Siberia. Fire 2024, 7, 243. https://doi.org/10.3390/fire7070243
Shapchenkova OA, Kukavskaya EA, Groisman PY. Fire-Induced Changes in Geochemical Elements of Forest Floor in Southern Siberia. Fire. 2024; 7(7):243. https://doi.org/10.3390/fire7070243
Chicago/Turabian StyleShapchenkova, Olga A., Elena A. Kukavskaya, and Pavel Y. Groisman. 2024. "Fire-Induced Changes in Geochemical Elements of Forest Floor in Southern Siberia" Fire 7, no. 7: 243. https://doi.org/10.3390/fire7070243
APA StyleShapchenkova, O. A., Kukavskaya, E. A., & Groisman, P. Y. (2024). Fire-Induced Changes in Geochemical Elements of Forest Floor in Southern Siberia. Fire, 7(7), 243. https://doi.org/10.3390/fire7070243







