Study on Liquid Hydrogen Leakage and Diffusion Behavior in a Hydrogen Production Station
Abstract
1. Introduction
2. Modeling and Validation
2.1. Governing Equations
2.1.1. Fluid Flow Model
2.1.2. Phase Transition Model
2.1.3. Turbulence Model
2.1.4. Component Transport Model
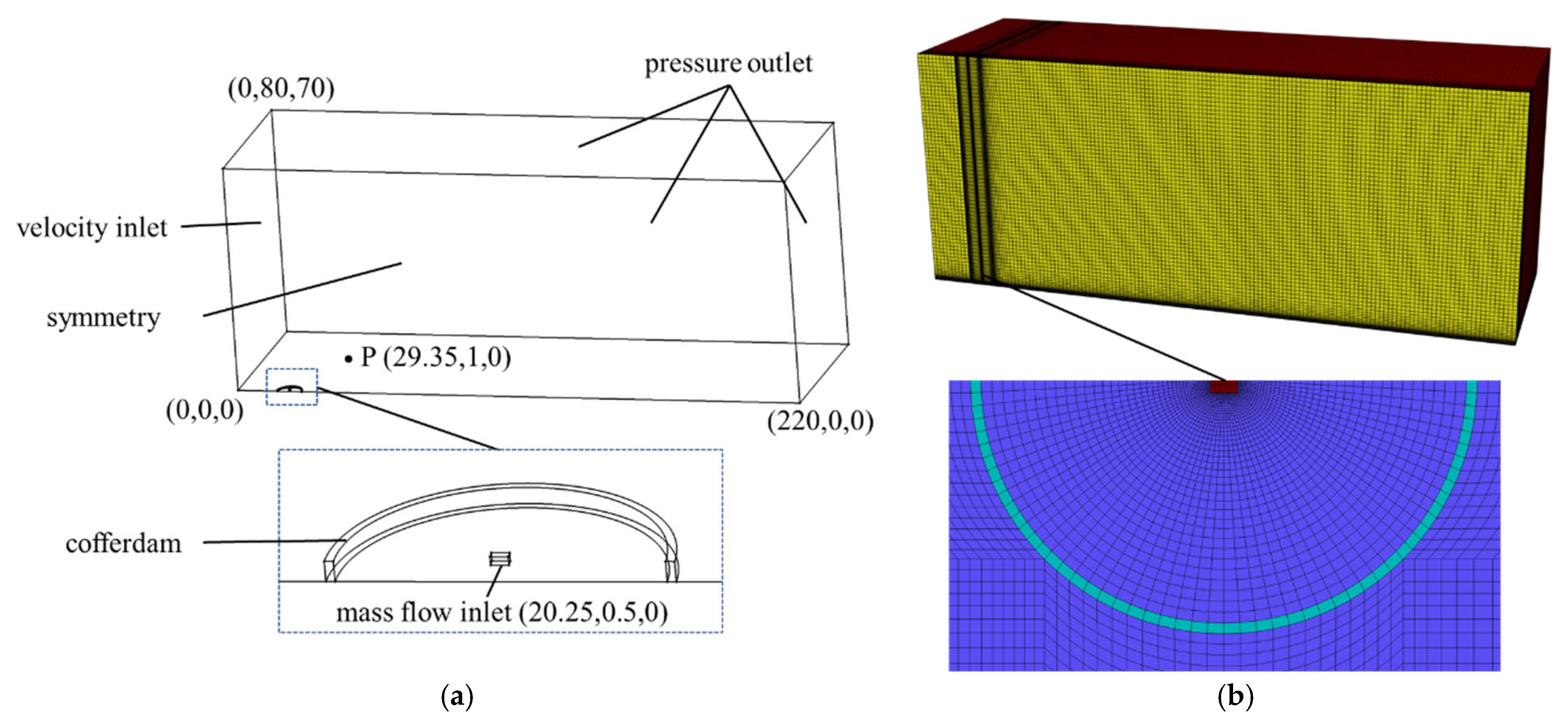
2.2. Model Validation
3. Results and Analysis
3.1. Grid-Independent Verification
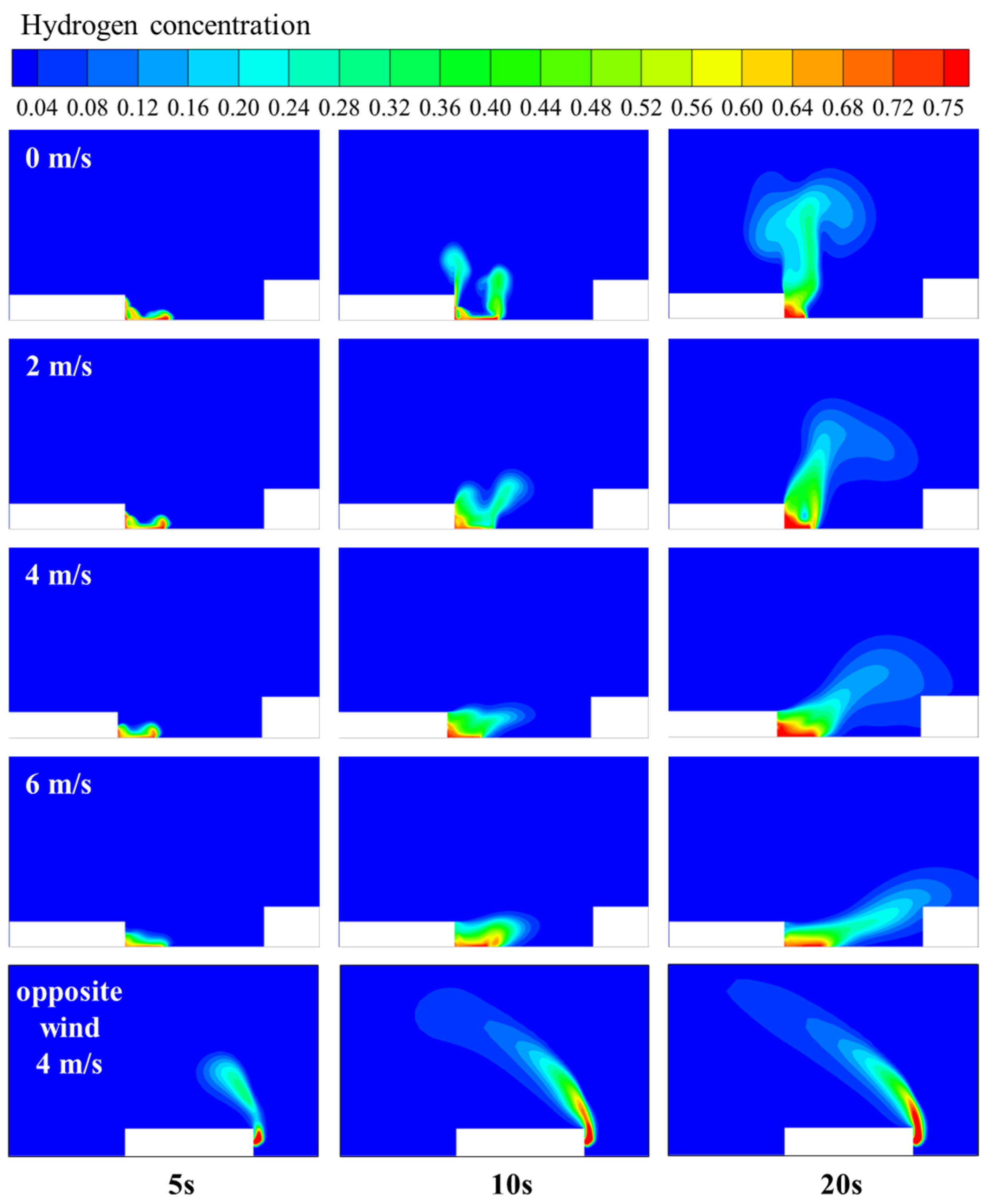
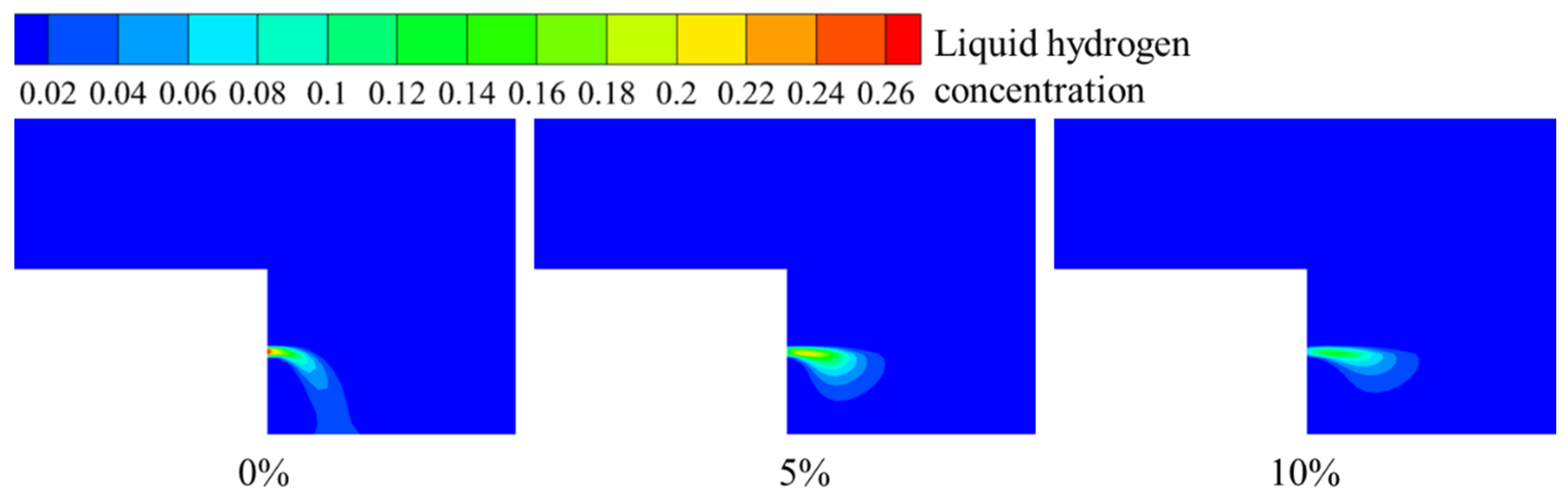
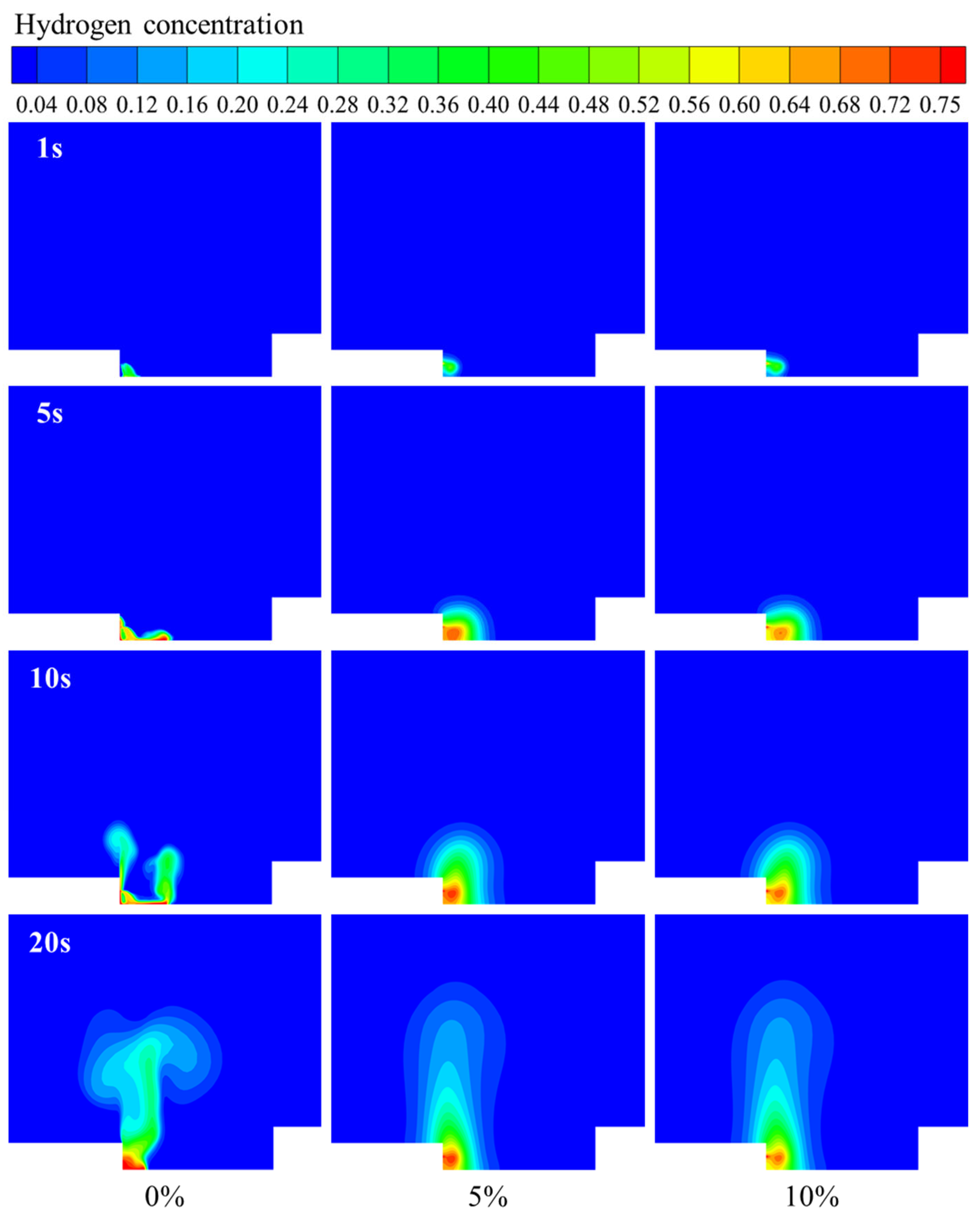
3.2. Hydrogen Leakage and Diffusion Behavior in Windless Environments
3.3. Influence of Wind Direction and Speed on Hydrogen Leakage and Diffusion Behavior
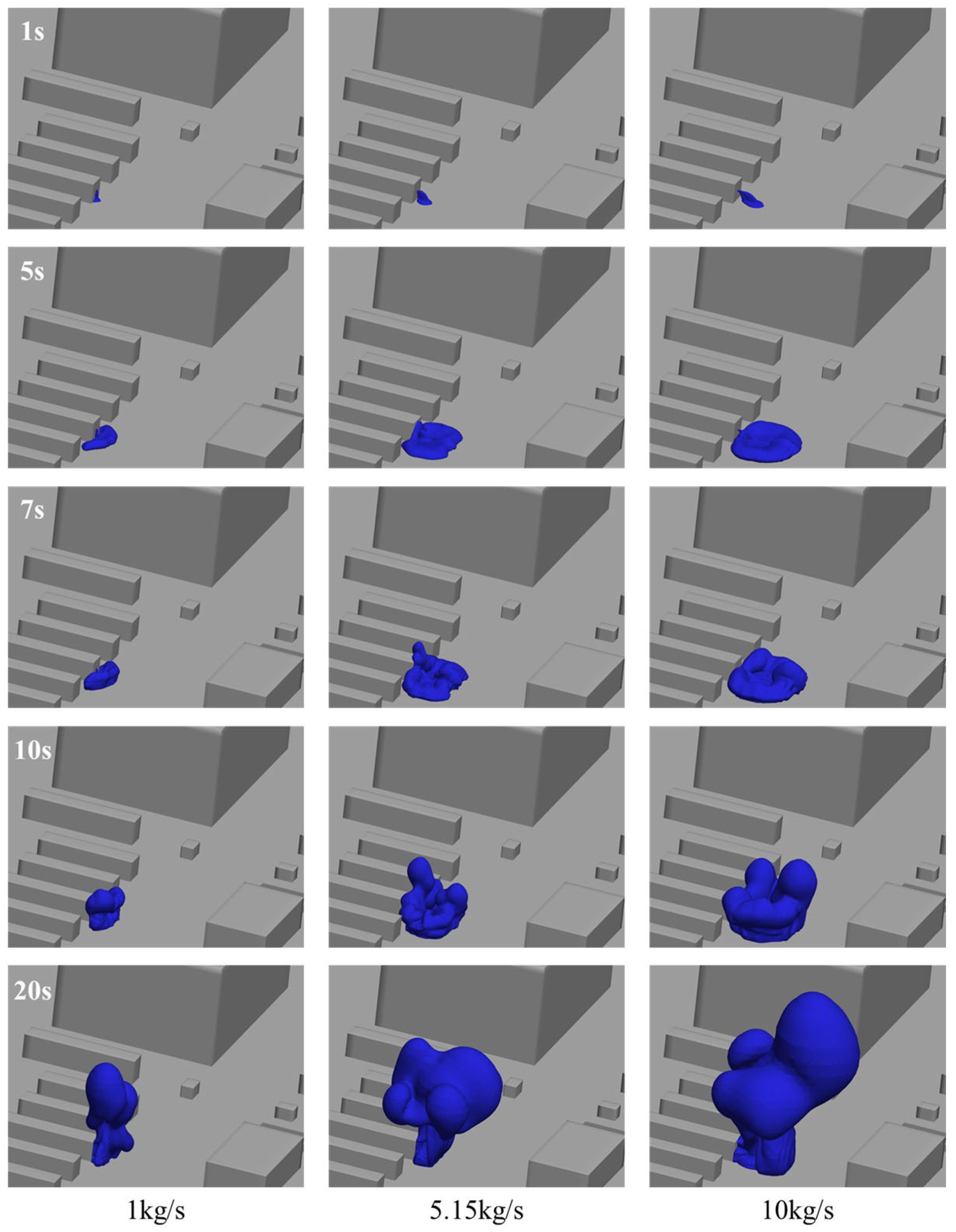
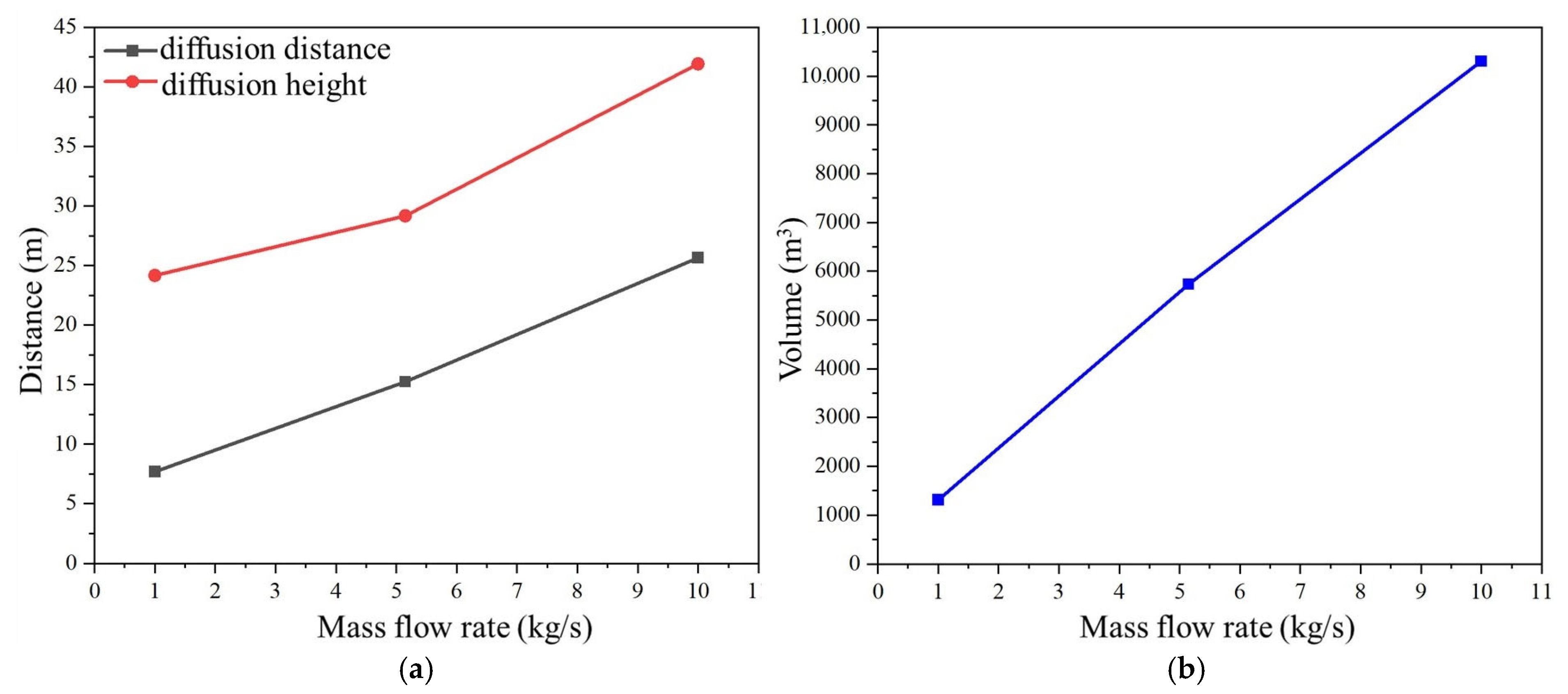
3.4. Influence of Leakage Mass Flow Rate on Hydrogen Leakage and Diffusion Behavior
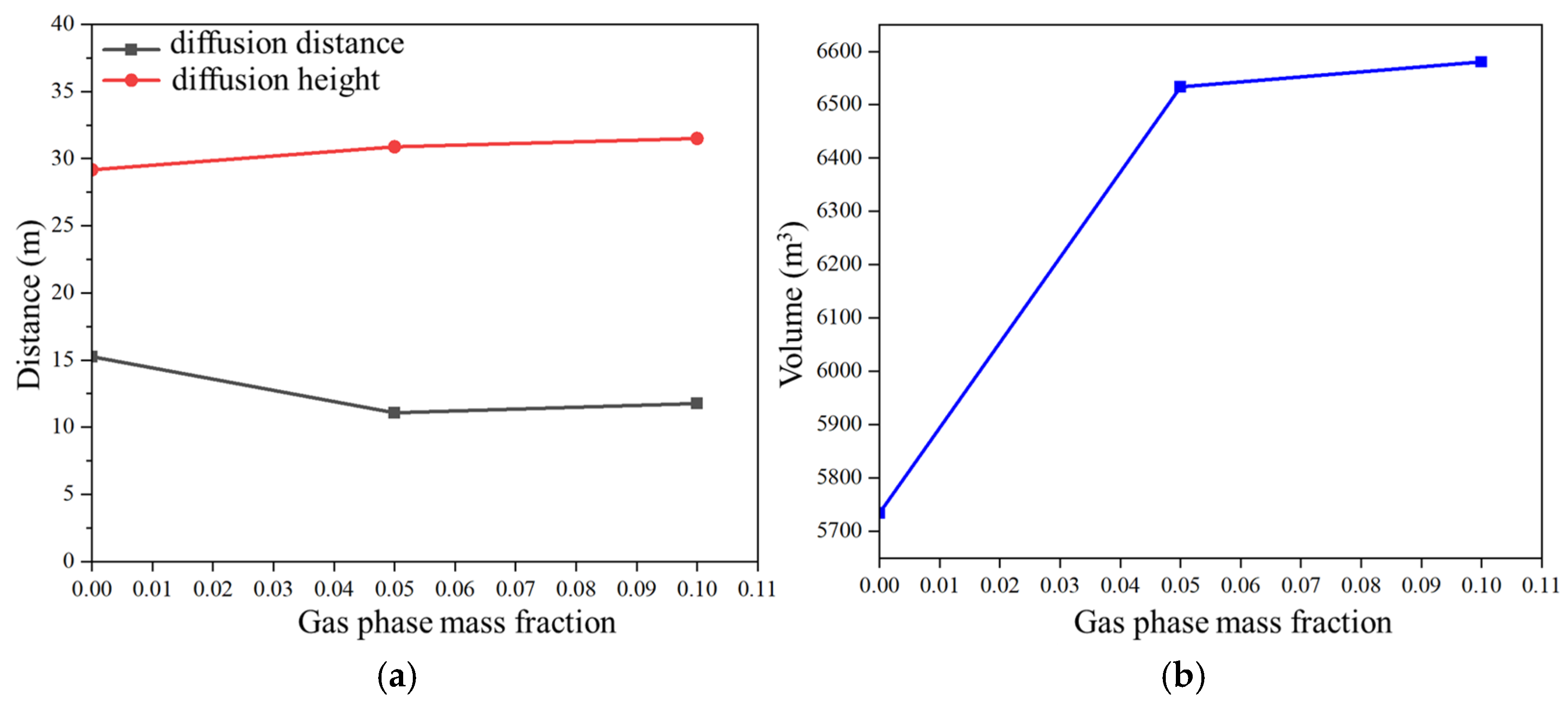
3.5. Influence of Leakage Gas Phase Mass Fraction on Hydrogen Leakage and Diffusion Behavior
3.6. Influence of Barrier Walls on Hydrogen Leakage and Diffusion Behavior
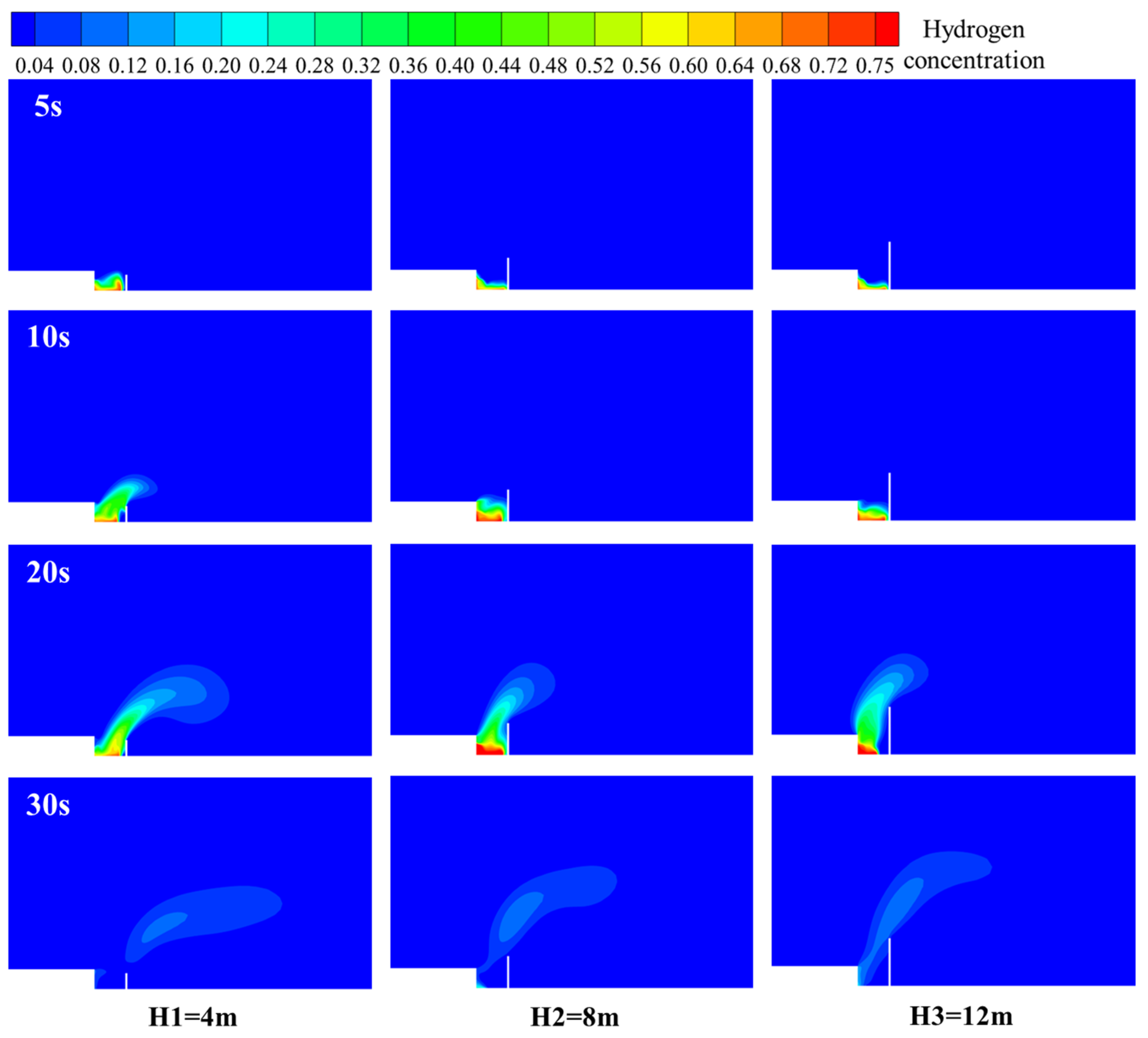
3.6.1. Barrier Wall Distance
3.6.2. Barrier Wall Height
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Density, kg/m3 | |
| Velocity, m/s | |
| Kinetic viscosity, N·s/m3 | |
| Temperature, K | |
| Pressure, Pa | |
| Drift velocity, m/s | |
| Effective thermal conductivity, W/(m·K) | |
| Enthalpy of phase k, J | |
| Volume fraction | |
| Number of substance species in the g phase | |
| Mass transfer from substance i in the gas phase to the liquid phase, kg/s | |
| Mass transfer from the liquid phase to substance i in the gas phase, kg/s | |
| Relaxation time, s | |
| Acceleration vector, m/s2 | |
| Trailing force | |
| Saturation temperature of liquid hydrogen, K | |
| Turbulent kinetic energy, m2/s2 | |
| Turbulent viscosity coefficient | |
| Prandtl number of the k equation | |
| Prandtl number of the equation | |
| Turbulence kinetic energy affected by the laminar velocity gradient | |
| Turbulence kinetic energy affected by buoyancy force kinetic energy | |
| Net production rate of the ith component | |
| Mass fraction of the ith component | |
| Production rate provided by the source phase | |
| Diffusion velocity vector | |
| Diffusive flux of the ith component | |
| Turbulent Schmidt number | |
| Diffusion coefficient of the ith component in the mixture |
References
- Zhao, J.; Dong, K.; Dong, X.; Shahbaz, M. How renewable energy alleviate energy poverty? A global analysis. Renew. Energy 2022, 186, 299–311. [Google Scholar] [CrossRef]
- Witcofski, R.D.; Chirivella, J.E. Experimental and analytical analyses of the mechanisms governing the dispersion of flammable clouds formed by liquid hydrogen spills. Int. J. Hydrogen Energy 1984, 9, 425–435. [Google Scholar] [CrossRef]
- Hooker, P.; Willoughby, D.B.; Royle, M. Experimental releases of liquid hydrogen. In Proceedings of the International Conference on Hydrogen Safety, Southport, UK, 12–15 November 2012; Institution of Chemical Engineers Symposium Series: Warwickshire, UK, 2012; pp. 496–504. [Google Scholar]
- Anaeby, J.; Gjesdal, T.; Voie, Ø. Large scale leakage of liquid hydrogen (LH2)-Tests related to bunkering and maritime use of liquid hydrogen. Nor. Def. Res. Establ. Rep. 2021, 20/03101. Available online: https://www.ffi.no/en/publications-archive/large-scale-leakage-of-liquid-hydrogen-lh2-tests-related-to-bunkering-and-maritime-use-of-liquid-hydrogen (accessed on 1 June 2024).
- Van Wingerden, K.; Kluge, M.; Habib, A.K.; Skarsvåg, H.L.; Ustolin, F.; Paltrinieri, N.; Odsæter, L.H. Experimental Investigation into the Consequences of Release of Liquified Hydrogen onto and under Water. Chem. Eng. Trans. 2022, 90, 541–546. [Google Scholar]
- Sun, R.; Pu, L.; Yu, H.; Dai, M.; Li, Y. Modeling the diffusion of flammable hydrogen cloud under different liquid hydrogen leakage conditions in a hydrogen refueling station. Int. J. Hydrogen Energy 2022, 47, 25849–25863. [Google Scholar] [CrossRef]
- Holborn, P.G.; Benson, C.M.; Ingram, J.M. Modelling hazardous distances for large-scale liquid hydrogen pool releases. Int. J. Hydrogen Energy 2020, 45, 23851–23871. [Google Scholar] [CrossRef]
- Jin, T.; Wu, M.; Liu, Y.; Lei, G.; Chen, H.; Lan, Y. CFD modeling and analysis of the influence factors of liquid hydrogen spills in open environment. Int. J. Hydrogen Energy 2017, 42, 732–739. [Google Scholar] [CrossRef]
- Wu, N.-N.; Duan, C.-D.; Jiang, Y.-C.; Lian, J. Simulation and safety analysis of the leakage of liquefied hydrogen from high-pressure container. Therm. Sci. 2019, 23 (Suppl. S3), 829–836. [Google Scholar] [CrossRef]
- Pu, L.; Shao, X.; Zhang, S.; Lei, G.; Li, Y. Plume dispersion behaviour and hazard identification for large quantities of liquid hydrogen leakage. Asia-Pac. J. Chem. Eng. 2019, 14, e2299. [Google Scholar] [CrossRef]
- Yuan, W.; Li, J.; Zhang, R.; Li, X.; Xie, J.; Chen, J. Numerical investigation of the leakage and explosion scenarios in China’s first liquid hydrogen refueling station. Int. J. Hydrogen Energy 2022, 47, 18786–18798. [Google Scholar] [CrossRef]
- Statharas, J.; Venetsanos, A.; Bartzis, J.; Würtz, J.; Schmidtchen, U. Analysis of data from spilling experiments performed with liquid hydrogen. J. Hazard. Mater. 2000, 77, 57–75. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, J.; Lei, G.; Lan, Y.; Chen, H.; Gao, X.; Wang, T.; Jin, T. Numerical investigation on the effects of dike around liquid hydrogen source on vapor cloud dispersion. Int. J. Hydrogen Energy 2019, 44, 5063–5071. [Google Scholar] [CrossRef]
- Tang, X.; Pu, L.; Shao, X.; Lei, G.; Li, Y.; Wang, X. Dispersion behavior and safety study of liquid hydrogen leakage under different application situations. Int. J. Hydrogen Energy 2020, 45, 31278–31288. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhao, H.; Xiong, Y. Study on the influence of barriers in the diffusion process of liquid hydrogen leakage. IOP Conf. Ser. Earth Environ. Sci. 2020, 546, 042048. [Google Scholar] [CrossRef]
- Busini, V.; Lino, M.; Rota, R. Influence of Large Obstacles and Mitigation Barriers on Heavy Gas Cloud Dispersion: A Liquefied Natural Gas Case-Study. Ind. Eng. Chem. Res. 2012, 51, 7643–7650. [Google Scholar] [CrossRef]
- Busini, V.; Rota, R. Influence of the shape of mitigation barriers on heavy gas dispersion. J. Loss Prev. Process Ind. 2014, 29, 13–21. [Google Scholar] [CrossRef]
- Rong, Y.; Peng, J.; Gao, J.; Zhang, X.; Li, X.; Pan, X.; Chen, J.; Chen, S. Numerical Investigation on the Liquid Hydrogen Leakage and Protection Strategy. Processes 2023, 11, 1173. [Google Scholar] [CrossRef]
- Xiao, J.; He, P.; Li, X.; Bénard, P.; Yang, T.; Chahine, R. Computational fluid dynamics model based artificial neural network prediction of flammable vapor clouds formed by liquid hydrogen releases. Int. J. Energy Res. 2022, 46, 11011–11026. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, H.; Hwang, Y.; Radermacher, R.; Jeong, H.M. Optimization of two-phase R600a ejector geometries using a non-equilibrium CFD model. Appl. Therm. Eng. 2016, 109, 272–282. [Google Scholar] [CrossRef]
- Manninen, M.; Taivassalo, V.; Kallio, S. On the Mixture Model for Multiphase Flow. VTT Publ. 1996, 288. Available online: http://www.vtt.fi/inf/pdf/publications/1996/P288.pdf (accessed on 1 June 2024).

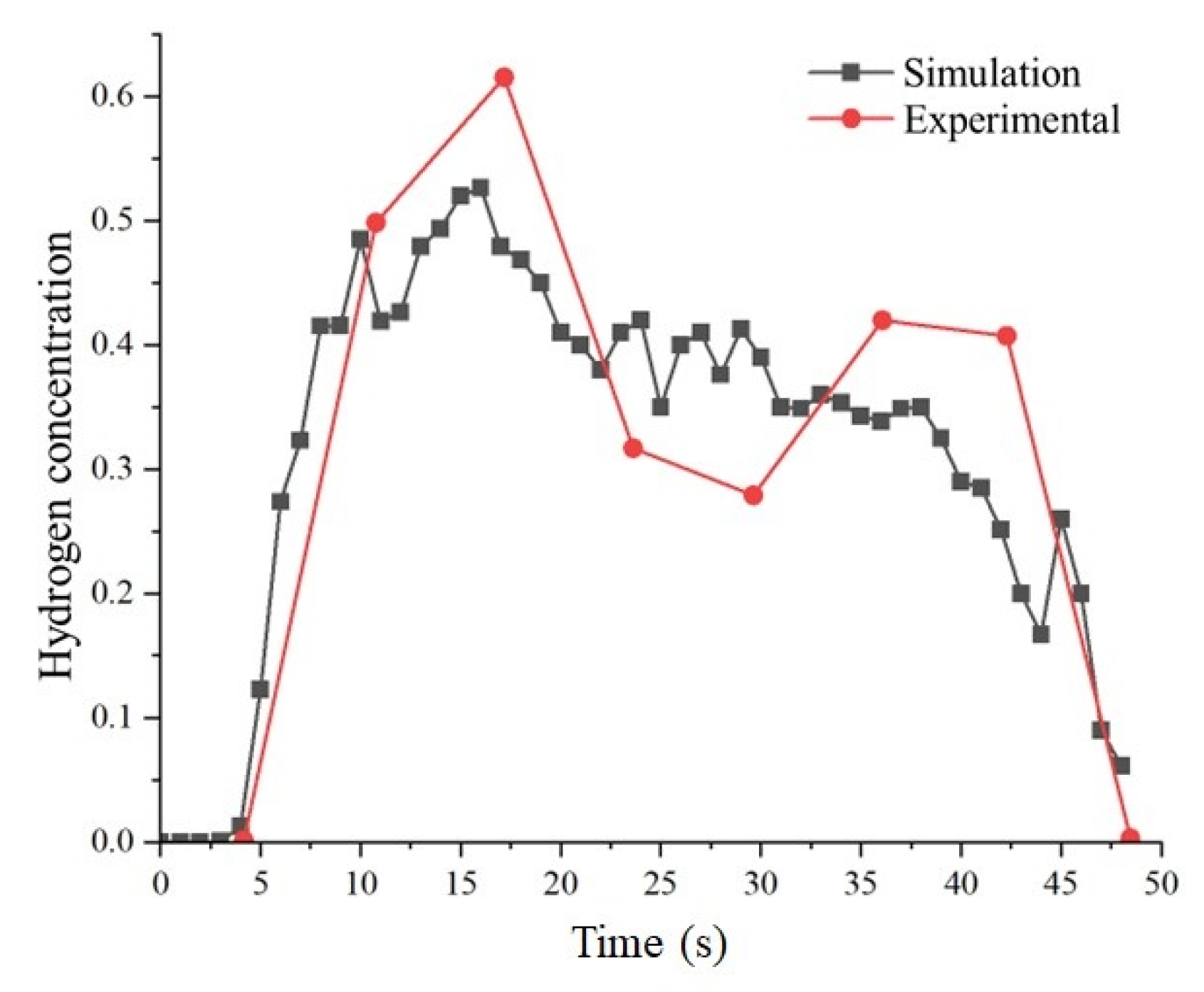












| Parameters | Simulation Values | Experimental Values [2] | Relative Error |
|---|---|---|---|
| Diffusion height | 18.9 m | 20 m | −5.5% |
| Horizontal diffusion distance | 39.1 m | 37 m | +5.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, X.; Li, G.; Chen, S.; Song, C.; Xiao, Z.; Luo, H.; Wan, J.; Yang, T.; Xu, N.; Xiao, J. Study on Liquid Hydrogen Leakage and Diffusion Behavior in a Hydrogen Production Station. Fire 2024, 7, 217. https://doi.org/10.3390/fire7070217
Fu X, Li G, Chen S, Song C, Xiao Z, Luo H, Wan J, Yang T, Xu N, Xiao J. Study on Liquid Hydrogen Leakage and Diffusion Behavior in a Hydrogen Production Station. Fire. 2024; 7(7):217. https://doi.org/10.3390/fire7070217
Chicago/Turabian StyleFu, Xiang, Guodong Li, Shiyu Chen, Chunyan Song, Zhili Xiao, Hao Luo, Jiaqi Wan, Tianqi Yang, Nianfeng Xu, and Jinsheng Xiao. 2024. "Study on Liquid Hydrogen Leakage and Diffusion Behavior in a Hydrogen Production Station" Fire 7, no. 7: 217. https://doi.org/10.3390/fire7070217
APA StyleFu, X., Li, G., Chen, S., Song, C., Xiao, Z., Luo, H., Wan, J., Yang, T., Xu, N., & Xiao, J. (2024). Study on Liquid Hydrogen Leakage and Diffusion Behavior in a Hydrogen Production Station. Fire, 7(7), 217. https://doi.org/10.3390/fire7070217






