1. Introduction
Mixed conifer forests are a diverse, prominent habitat type covering approximately 20% of forested lands in the Southwestern United States, at elevations 2270–3030 m [
1,
2]. The disturbance regimes, habitat characteristics, and species present in mixed conifer forests represent a transition zone between lower elevation ponderosa pine forests and higher elevation spruce–fir forests [
1]. Mixed conifer forests may be categorized as warm–dry to cool–moist [
1]. Warm–dry mixed conifer forests occur predominantly on south-facing aspects at lower elevations and, in the Southwest, are composed primarily of ponderosa pine (
Pinus ponderosa), Douglas fir (
Pseudotsuga menziesii), white fir (
Abies concolor), aspen (
Populus tremuloides), and Gambel oak (
Quercus gambelii) [
1]. These forests are valuable ecological systems that are home to dozens of resident and migratory birds, including species of management concern, such as the Mexican Spotted Owl, Northern Goshawk, Williamson’s Sapsucker, Dusky Flycatcher, and the Olive-Sided Flycatcher [
3]. Avian species contribute to the trophic interactions within a forest ecosystem and provide a suite of ecosystem services such as nutrient transport, seed dispersal, habitat creation, and insect regulation [
4,
5]. These birds form communities that vary in response to the structure and composition of the forest, which may be drastically altered by fire, the predominant disturbance in the Southwest [
6,
7,
8,
9]. Recognizing the variation in avian community assemblages within a post-fire environment may aid in understanding the resilience of mixed conifer ecosystems following fire, as avian diversity is often correlated with the diversity of other taxa [
10,
11].
Fires in warm–dry mixed conifer forests were historically of mixed severity: low- to moderate-intensity surface fires burned at multi-decadal frequencies, with occasional high-severity patches of crown fires [
1,
12]. Low- to moderate-severity burn areas generally are characterized by removal and then regrowth of resprouting surface fuels (grass, forbs and shrubs), with losses of the tree canopy in areas with moderate burn severity and long burning residence times. High-severity fires cause tree mortality and create open areas with snags and regrowth of herbaceous plants and shrubs. Mixed-severity fires generate a heterogeneous mosaic of stands that vary in their vertical and horizontal structure and composition [
13,
14]. Following European settlement in the Southwest, fire suppression altered the recurrent mixed-severity fire regime [
15]. The absence of fire promoted the growth of shade-tolerant, mesic species such as
Pseudotsuga menziesii and
Abies concolor in warm–dry mixed conifer forests, altering stand characteristics such as density and canopy cover, serving to homogenize the horizontal and vertical structure of mixed conifer forests on a landscape scale [
16,
17]. The homogenization of forests and increased drought conditions in the Southwest have increased fire severity, frequency, and area burned, influencing avian species assemblages post-fire [
15,
18,
19,
20,
21,
22,
23].
Post-fire conditions vary based on the severity of the fire and provide alternate resources used by different bird species. A greater complexity of post-fire habitat in a region should correlate to a greater diversity of birds, assuming the habitat is suitable for local species [
11,
14,
24,
25]. In regions with prominent deciduous forests, diversity is often positively correlated with the presence of broadleaf trees, due to their structural complexity and foraging opportunities [
11]. In western conifer ecosystems, this same principle may be tested in post-fire successional stands that gain complexity from shrub cover, snags, and the resprouting of aspen. Quantifying post-fire diversity is increasingly relevant due to warming climate trends and increased fire frequency in the Southwest, necessitating active forest management strategies [
22,
23]. Diversity is an important measure of ecosystem and community health; however, it is important to consider other measures when evaluating communities, as an area with high diversity may not have high ecological value [
26]. Likewise, areas with low diversity may have distinct features that are utilized by unique specialist species. Specialists often have narrow habitat requirements and may be significantly impacted by successional changes in post-fire vegetation and forest management activities [
9]. These specialist species may be considered positive indicator species, species that have special habitat needs and are representative of the habitat in which they are found [
26,
27]. For wildfire, a species may be considered a positive indicator of a post-burn habitat if they are strongly associated with one burn severity. In addition to diversity indices and indicator species, the composition of avian communities is of interest, to illustrate how interrelated taxa form assemblages in a habitat. Variations in environmental conditions in different burn areas may drive divergent community assemblages with unique compositions [
9].
To provide a generalized approach in understanding avian communities, species may be grouped into functional guilds based on a variety of life history habits such as feeding substrate, feeding technique, nesting sites, and migratory patterns [
6]. These are useful ways to measure how changes in habitat influence not just species, but community dynamics [
6]. The variety of habitats in post-burn forests provides diverse opportunities for functional guilds. High-burn severity areas typically have a high snag density, which favors cavity-nesting species such as woodpeckers and insectivorous species that eat insects associated with recently deceased trees [
28]. Aerial insectivores, such as flycatchers, are known to respond favorably to moderately open canopies that favor their hunting strategies [
29]. The successional growth of shrubs and aspen following low- and moderate-severity fire favors shrub-nesting species [
29]. In low- and moderate-severity fires, large trees may produce more cones when released from pressure from surrounding small trees. This increase in cone production may favor granivorous species such as Clark’s Nutcracker and Red Crossbill [
29]. The evaluation of functional guilds can illuminate the type and quantity of resources available in different post-fire environments, and how different resources reflect avian community structure.
Avian communities in post-burn mixed conifer forests have been studied extensively in some regions of the country, but there is little recent information about these systems in Southwest Colorado [
3,
9,
13,
28,
30]. When researchers first began studying the effects of fire on birds in USA, studies often focused on the difference between burned and unburned forest [
30,
31]. It quickly became clear that some birds respond favorably to fire and others unfavorably. Indeed, Bock and Lynch reported more species unique to burned areas than unburnt in 1970 [
30]. However, the lack of distinction between burn severities caused many birds to be listed as mixed responders [
28,
31]. Around 2004, researchers began to include burn severity into studies, making the response of avian species more predictable and informative [
28,
32]. This study addresses the variation in burn severities by stratifying burn areas by change in percent canopy cover, according to RAVG data (Rapid Assessment of Vegetation Condition after Wildfire). The time since fire has also been revealed to be a crucial component to post-burn avian communities, as many groups follow successional trajectories initiated by fire and continue to be influenced by fire up to a decade post-burn [
24,
29,
33]. Many studies in mixed conifer areas post-fire have been conducted three years or less following a burn, with studies five years post-fire lacking in the Southwest [
9,
25]. Five years post-fire is sufficient time for shrub regeneration and the secondary colonization of snags, following the dispersal of wood-boring insects [
13,
31].
Previous studies in mixed conifer forests have demonstrated changes in the abundance and/or density of avian species in response to fire [
25,
28]. This study aims to build on previous work by evaluating avian community differentiation and changes in functional guild abundance across burn severities, as well as identifying indicator species. This can inform management decisions, such as forest thinning, prescribed fire treatments, and species monitoring, to help forest managers promote biodiversity when considering the effects of fire in the Southwest. We used the 416 Fire in Southwest Colorado as a model to quantify the differences in avian community composition following mixed severity fire in warm–dry mixed conifer forests. The objectives were as follows: (1) to determine how avian richness, abundance, and diversity vary among burn severities (unburned, low, moderate, and high); and (2) to quantify variations in avian community assemblages and functional guild associations and to identify indicator species (e.g., species that are uniquely associated with burn severity) for different burn severities in warm–dry mixed conifer forests in Southwest Colorado. We hypothesized that, firstly, high burn severities would significantly differ in their community and functional guild composition from other burn areas, due to the greater density of snags, lower density of live trees, and increased presence of herbaceous plants; and, secondly, that indicator species would be found in unburned and high-severity areas, as some birds rely on snags and others on undisturbed old growth.
2. Materials and Methods
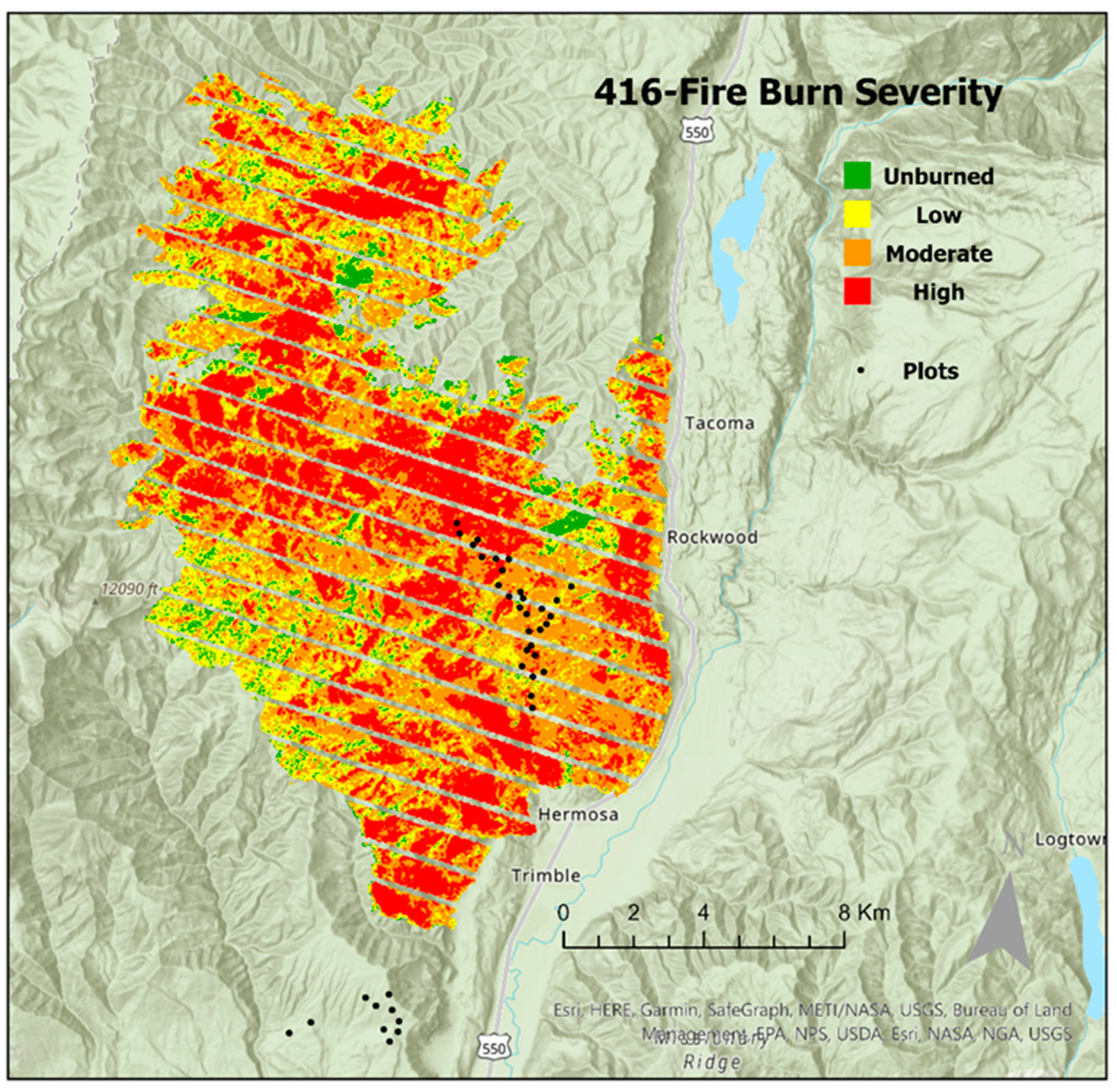
The 416 Fire was ignited on 1 June 2018, and burned 223
in the Southern San Juan National Forest in the Hermosa Special Management Area and Hermosa Wilderness [
33]. The area burned was primarily mixed conifer [
33]. The approximate distribution of burn severities was 44% low, 20% moderate, 19% high, and 17% unburned [
34]. The study site is located approximately 21 km north of Durango, Colorado, in the southern portion of the San Juan National Forest adjacent to Hermosa Creek within the Hermosa Special Management Area and Hermosa Wilderness [
33]. The study area ranges in elevation from 2277 m to 2470 m on slopes that range from 30 to 45 degrees with diverse aspects. The average daily temperatures range from a maximum of 30 °C in July to a minimum of −9.7 °C in January [
35]. The average annual precipitation is 53.2 cm, with the greatest amounts occurring in July and August due to summer thunderstorm activity [
35]. Precipitation from November to March is dominated by snowfall. Forest types in the study area vary from pine oak forest and warm–dry mixed conifer in the southern section of the burn to cool–moist mixed conifer and subalpine in the northern section of the burn area [
34]. Aspen is present in the study area and continuous stands of aspen exist adjacent to plots; however, aspen is only a minor component of the overstory trees present in plots. The study area has never been logged and has a high proportion of large diameter trees for all species present, with many stands having old growth characteristics [
34].
Pinus ponderosa,
Pseudotsuga menziesii,
Abies concolor,
Abies lasiocarpa (subalpine fir),
Pinus flexilis (limber pine),
Picea pungens (blue spruce), and
Populus tremuloides are the common tree species. Common sprouting shrubs include
Quercus gambelii,
Symphoricarpos oreophilus (snowberry),
Prunus virginiana (chokecherry), and
Amelanchier alnifolia (Utah serviceberry). In 2008, portions of the study area were burned in a broadcast prescribed fire using aerial ignitions [
34]. Ten years later in 2018, the study area was burned by an unplanned, artificial ignition that burned a total of 223
(the 416 Fire). The 416 Fire burned during an extreme drought year, resulting in mixed burn severities from overall moderate fire behavior, driven primarily by available fuels and topography. Suppression efforts focused on the wildland urban interface and no slurry drops or direct attack measures were taken in the study area (from communication with the Incident Section Chief).
Forty random points were stratified across burn severities in the southern area of the burn using RAVG data (low, moderate, and high). Ten plots in each burn severity were established in burned patches no less than 180 m from burn severity boundaries, as well as ten unburned control plots adjacent to the 416 Fire burn perimeter in the Junction Creek drainage area (
Figure 1). Unburned plots were established outside of the burn perimeter due to a lack of suitable, accessible unburned warm–dry mixed conifer sites within the 416 Fire perimeter. While stringent experimental design is ideal, it is not always possible in ecological studies of isolated large-scale disturbance, such as the 416 Fire [
36]. The absence of pre-fire data in the area eliminates our ability to draw inference on the effect of divergent environmental variables observed between burned and unburned plots. To compare burned areas with unburned areas, we established unburned plots as spatially segregated pseudoreplicates and reported environmental variables that were significantly different. Plots were spaced, on average, 409 m apart, with a standard error of 19.5 m [
37]. Points were selected within a 100 m buffer of existing trails to ensure accessibility to sites, given the steep slopes of the drainage. Plots were aggregated near accessible trailheads and roads due to the steep, inaccessible terrain within the burn perimeter and the temporal limitations of the study.
Starting in May 2023, we established 40 plots, and collected vegetation data. We counted live trees and snags (>2.64 m height) within a 22.6 m diameter or 400
circle plot [
34]. Gambel oak with a diameter at breast height (dbh) of >3 cm and a height of >1.5 m were considered trees [
38,
39]. Aspen with a dbh of >10 cm were considered trees. We estimated basal area using 15 and 20 Basal Area Factor (BAF) wedge prisms and averaged the prism scores. We measured the understory cover and aspen regeneration on a 30 m belt transect along the elevational gradient, 15 m above and below the center point of the plot. We divided the understory cover into two classes: greater than and less than 1.4 m. We counted conifer saplings (<2.64 m height) and seedlings along the 30 m transect, extending 5 m to both sides of the belt (300
) [
33]. We established four
subplots evenly along the 30 m transect to quantify herbaceous cover and divided the cover into grass and forbs [
34]. We recorded slope, aspect, and elevation for each plot.
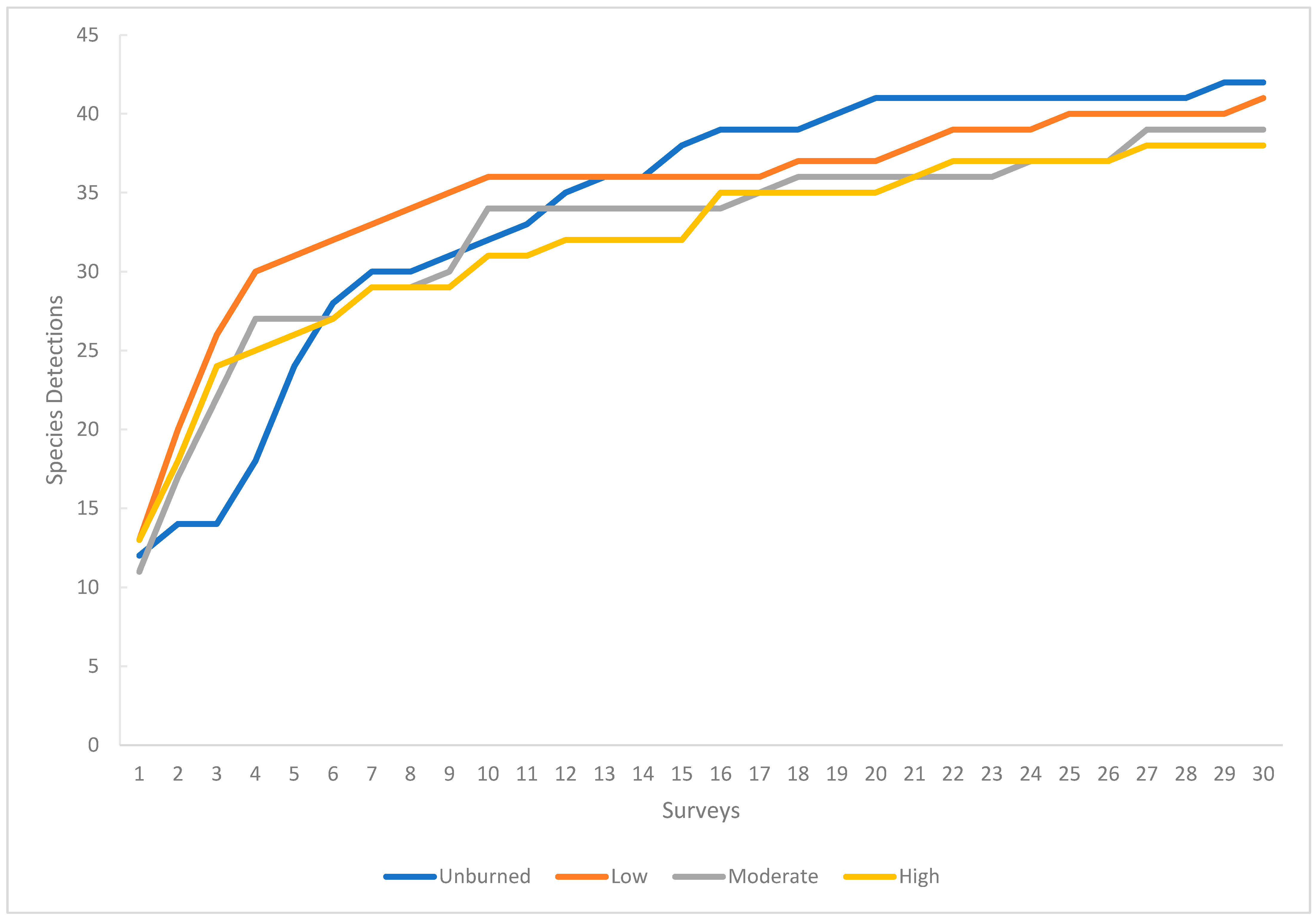
From May 25 to July 15, we conducted standard point count surveys at the center of each plot for 10 min [
40,
41]. We identified species and individuals by visual and vocal detection. We began our surveys 30 min before sunrise and ended five hours later [
40]. We visited each of the 40 plots three times to ensure suitable sample size (
Appendix A,
Figure A1) [
42].
We converted shrubs, aspen regeneration, forbs, and grasses to percentage surface cover. We summed point count data for each plot and grouped species into functional guilds based on foraging technique, foraging substrate, nest placement, and migratory pattern [
6,
11,
14,
43,
44] (see
Appendix A,
Table A2). Species that fell into more than two guilds were classified as generalists; species that fell into two guilds were classified on a per-species basis, based on the best available information. We compared environmental, vegetation structure, avian functional guilds, abundance, richness, and diversity data among the four study sites with a Kruskal–Wallis test (
p < 0.05), followed by a post hoc Bonferroni pairwise test [
45]. We used non-metric multi-dimensional scaling (NMDS) to examine avian community assemblages among all four study sites in R versions 4.2.1 using the metaMDS function in the package vegan [
46,
47,
48]. We ran the NMDS ordination using a Bray–Curtis distance measure, random starting configurations, and a minimum of 50 runs. Differences in avian assemblages among the four burn severities were determined using a permutational multivariate analysis of variance (Permanova), using adonis in the R package vegan [
47,
49,
50]. Permanova uses common ecological distance measures (Bray–Curtis for this study) to examine multivariate data sets and calculate
p-values using permutations, rather than tabled
p-values that assume normality. We used a one fixed factor design with burn severity as our main effect [
51]. We performed Pearson and Kendall correlation tests between avian and environmental/vegetation data using combined Permanova in PC-ORD software version 5.10 [
52]. We performed an indicator species analysis, which uses richness and associated abundance values of species, to identify species that were particularly faithful indicators for a particular burn severity [
52]. A comparison between the maximum indicator value (0–100) and random trials for occurrence of a given species (1000 Monte Carlo randomizations) provided an approximate
p-value [
51]. Species with
p < 0.05 and indicator values (INDVAL) > 25 (INDVAL = relative abundance × relative frequency; INDVAL ranges from 0 to 100) were accepted as indicator species for a particular burn severity [
26].
4. Discussion
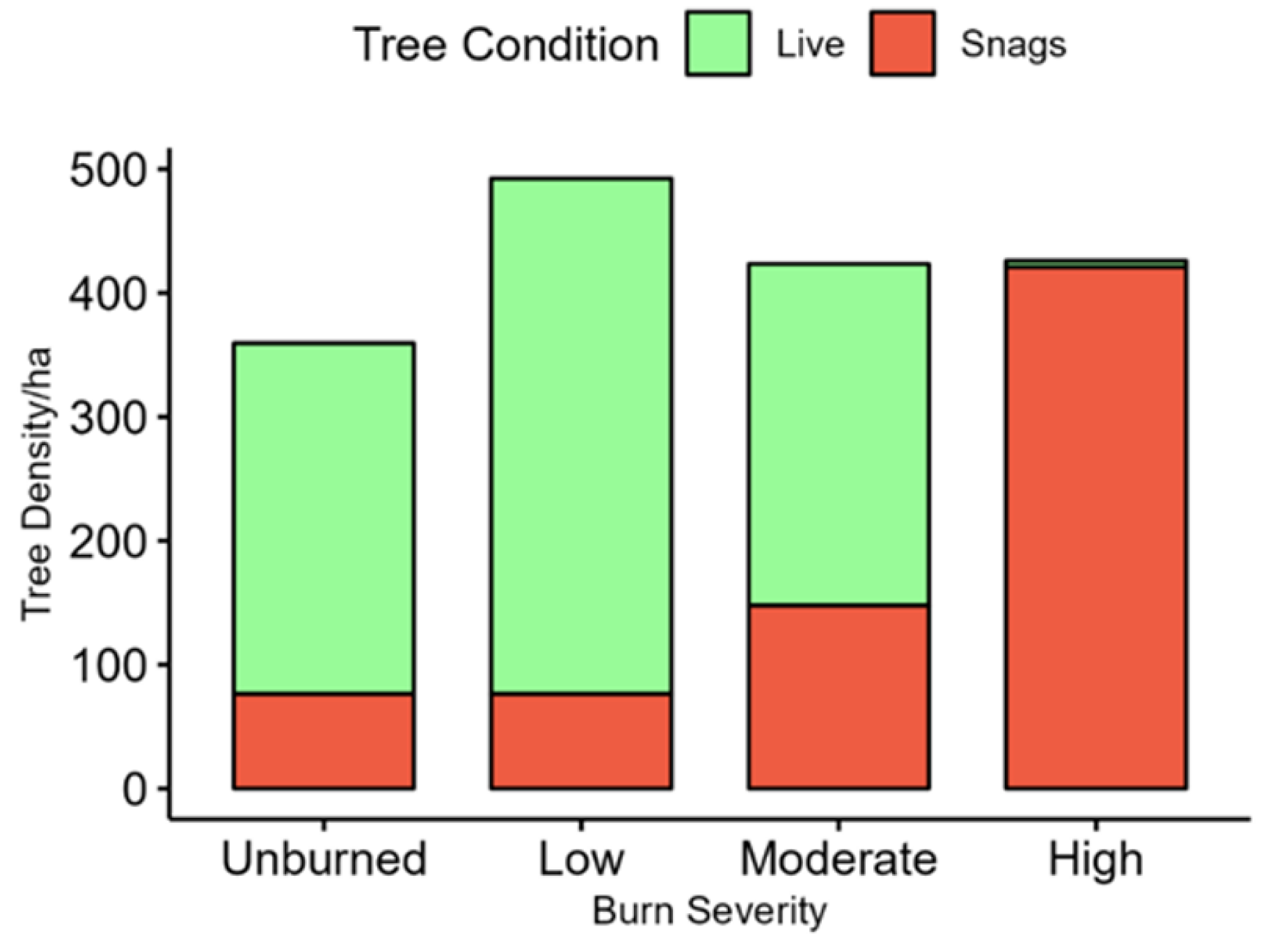
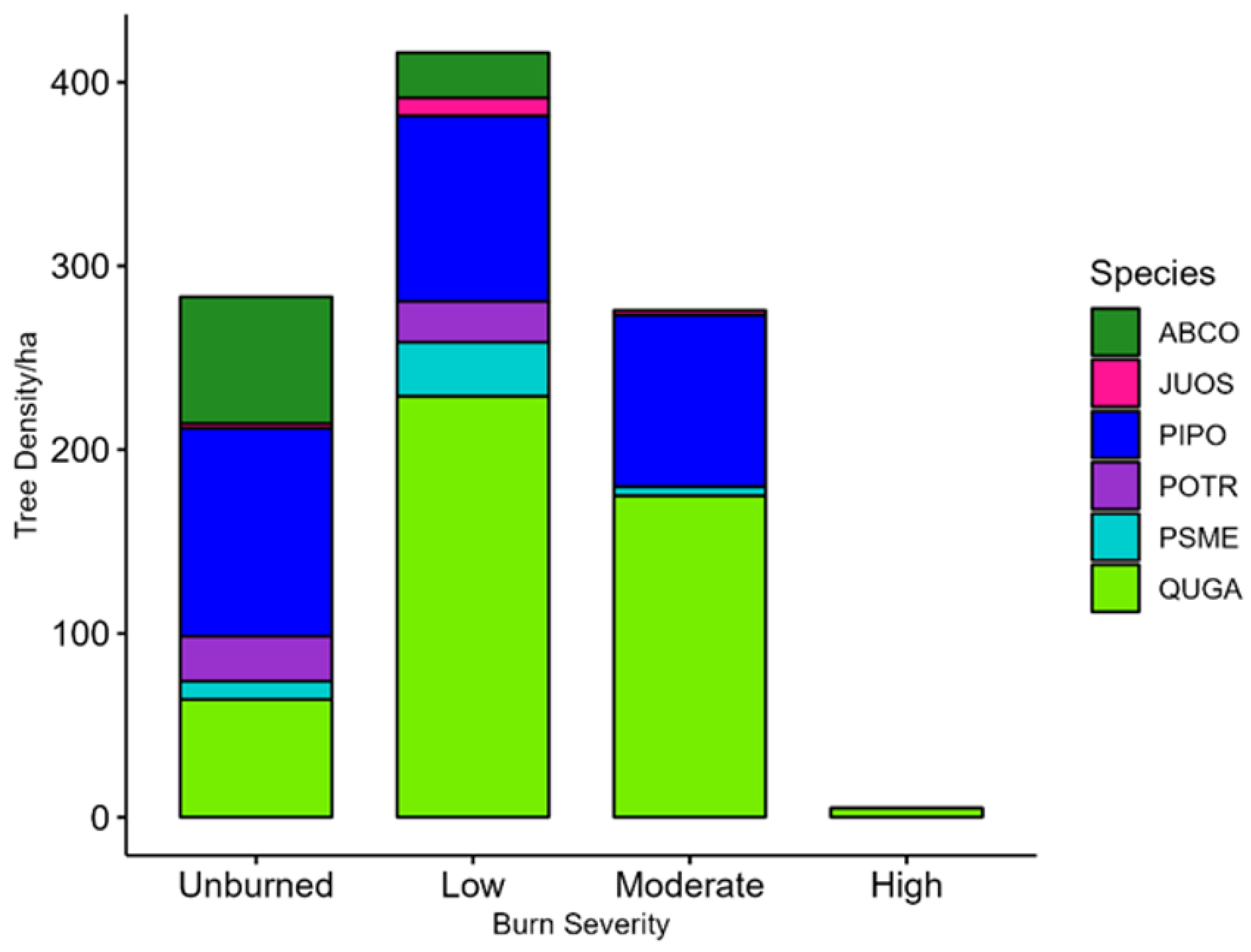
The varying vegetation characteristics of the different burn severity areas indicate ecological succession is occurring five years post-fire; the five years were ample time for the regeneration of
Q. gambelii throughout the study area, irrespective of burn severity, as well as other low-growing shrub species. Low shrub cover (<1.4 m) averaged 30–50% in all burn areas, benefiting the diversity of shrub-using birds in all burn severities. Aspen regeneration was more prevalent in high-burn areas, while mesic tree species were absent from these areas, illustrating that differential succession patterns are creating spatially heterogenous habitats. Live tree species were predominantly xeric-adapted species, including
P. ponderosa and
Q. gambelii. Conifer regeneration was not significantly different among burn severities but was more prominent in unburned and low-burn areas, which is likely associated with the predominance of
P. ponderosa in the overstory for these two burn severities [
53].
Univariate measures of richness, diversity, and abundance were not significantly different across burn severities. In this study, abundance was not considered per-species, but as a measure of total birds observed; this study focused on community composition, as opposed to other studies that have shown fine-scale changes in relative abundance, occupancy, or density pre- and post-fire [
8,
25,
32]. One study that compared average species abundance identified four response patterns that correlated to burn severity, reinforcing that individual species’ response to burn severity, as well as community structure, may change, while overall abundance may not, as demonstrated in our study [
8]. Other recent studies on avian species’ richness responses have reported similar results within burn severities [
25,
54]. A 1970 study found that species richness was greater in burned forest than unburned, without considering burn severity; this is unsurprising, given the complexity of post-fire habitat in mixed conifer forests [
30]. A study in montane forests in California found that, at a landscape scale, a greater diversity of fire behavior (pyrodiversity) promoted avian diversity, while, within a single fire, diversity tended to decrease with increasing fire severity [
24]. The lack of significant difference in avian species diversity across burn severities in our study reinforces the importance of uniquely burned habitats and suggests that assessing diversity over larger landscape scales across different wildfires, rather just within one fire, may more accurately reflect the importance of mixed-severity fire in promoting biodiversity.
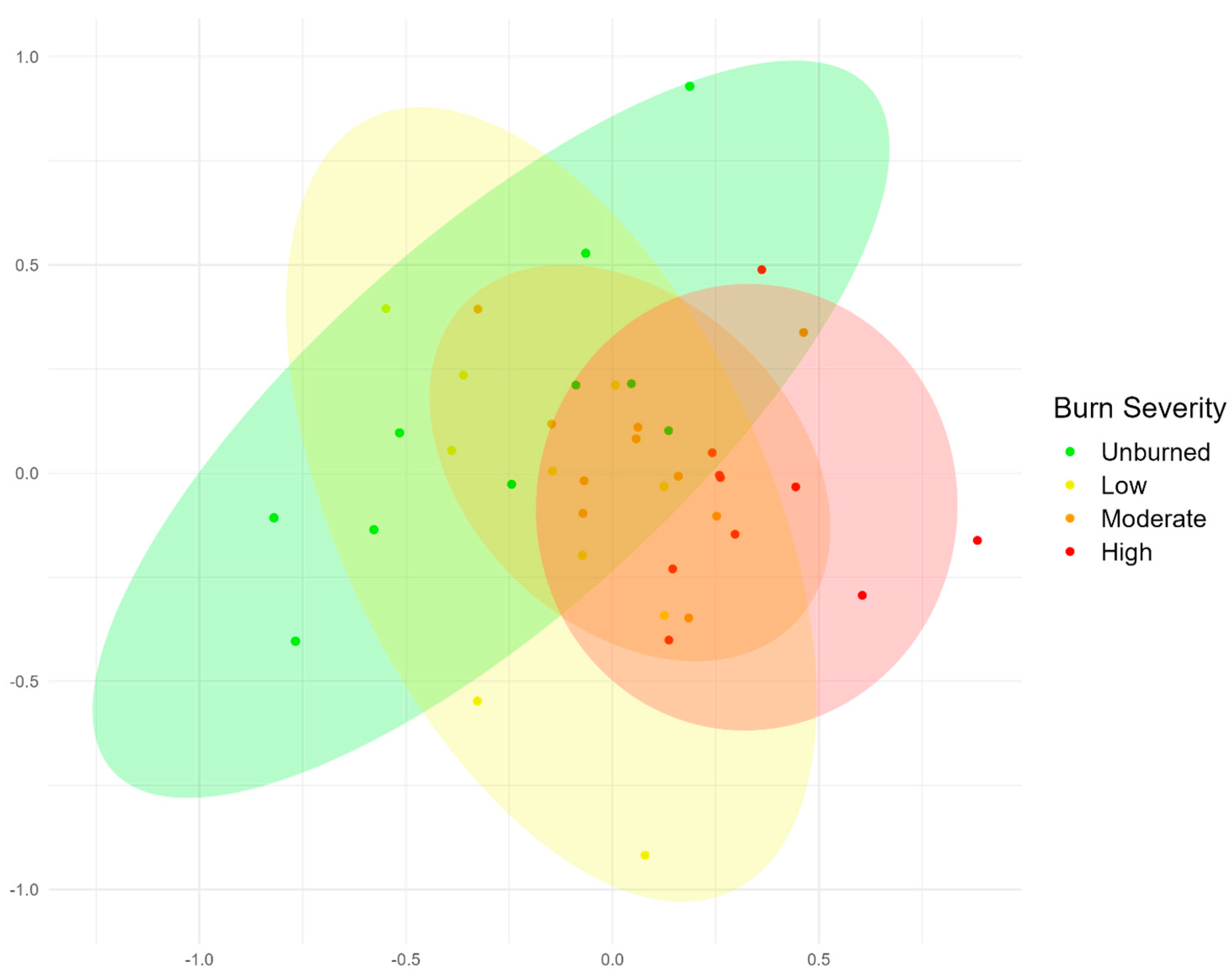
Distinct avian community differentiation between unburned and burned forest was observed in this study, as well as finer scale differences among burn severities established by mixed-severity fire. The significant divergence of species assemblages between burn severities and lack of variation in univariate richness and diversity exemplify the ecological benefits of mixed-severity fire in promoting biodiversity at a landscape scale. Indeed, this study highlights the importance of community analysis at multiple levels; the results of univariate analyses of abundance, richness, and diversity were not different across burn severities, but multivariate community analysis identified significantly divergent species assemblages across all burn severities, except between low- and moderate-burn areas. This is consistent with other studies that found fine-scale patterns of avian response to wildfire when evaluated by burn severity [
8,
24]. While significant divergence was identified, there was some overlap in assemblages that represent a gradient of species present from unburned to high-burn areas. This gradient is most convergent at the low- to moderate-burn severities and most divergent between unburned and high-burn areas. A study of Mediterranean pine forests also observed significantly divergent avian communities between recently burned and unburned areas for >40 years [
55]. The variation in species assemblages was best correlated with the density of live trees and snags, indicating the importance of these variables for promoting avian biodiversity and predicting species’ response to wildfire [
8,
9]. This builds on previous work that demonstrated the importance of snag and live tree density for avian communities three years post-burn and reinforces that these factors are still relevant five years post-burn [
9].
The strong correlation of avian assemblages with live and dead tree density in this study is well supported [
9]. The presence of more indicator species in unburned and high-burn areas than in low- and moderate-burn areas reflects the importance of managing for mixed-severity fire that allows for patchy high-severity burns. Broad-tailed Hummingbird and House Wren were unsurprising indicators of high-burn severities; the two species are known to flourish in areas that experience high tree mortality [
9,
25,
54]. Green-tailed Towhee was another indicator species of high-burn severity areas, whose association with live shrubs has been documented and shown to provide nest sites and foraging opportunities [
56]. Contrary to our findings, one study demonstrated that Green-tailed Towhees were associated with unburned areas following prescribed fire, three to five years post-fire, in montane shrublands [
56]. Differential shrub regeneration between mixed conifer and montane shrubland likely account for the difference in Green-tailed Towhee fire response, as five years post-fire was adequate time for shrub cover to regenerate in high-severity burn areas in our study, such that Green-tailed Towhees were exceedingly associated with high-severity fire. The Dusky Flycatcher’s high-burn severity indicator status is of interest because although they have been found to respond positively to generalized mixed-severity fire, some studies have reported a negative association with fire [
8,
25]. Dusky Flycatchers typically nest in shrubs and given the ubiquitous shrub cover in the study area, other factors such as predation and open canopy space may be influencing their association with the high-severity portions of the 416 Fire area [
57].
The Mountain Chickadee was an expected indicator species for unburned areas as they are associated with live tree density and absence of fire [
31,
54]. Virginia’s Warbler are sometimes associated with low- or moderate-burn severities, but are also a shrub-associated species, and in this study the percentage of shrub cover was consistent across all burn severities, which may have influenced it being an indicator species for unburned areas [
54]. Ruby-crowned Kinglet was another expected indicator of unburned areas, whose reliance on unburned forest has been documented [
25,
31]. Additionally, Evening Grosbeak’s indicator status in unburned areas is consistent with a study that found that this species was more likely to occur in areas with high densities of live trees [
9]. Indicator species of low-severity burn areas are interesting because they accentuate the fine scale differences between unburned and low-burn areas, despite the similar forest structure 5 years post-fire. This is demonstrated by Hammond’s Flycatcher, which was an indicator species for low-burn areas in our study area five years post-fire but is known to have mixed post-fire responses [
28,
31]. The Yellow-rumped Warbler is an expected indicator, as they utilize small forest openings but typically avoid areas with high tree mortality [
8,
54]. The presence of indicator species in low-burn areas emphasizes the importance of analyzing avian communities using multiple methods, especially given the convergent species assemblages in low- and moderate-burn severities. Moderate-burn areas are a unique habitat within mixed conifer systems, in that they have a substantial density of snags and live trees. This combination, however, did not correlate with many indicator species, with the American Robin being the only indicator species. This species is frequently considered a generalist in habitat preference, but has been shown to respond positively to fire, and more specifically, moderate-severity fire [
8,
25,
54]. Indeed, although American Robins were found to be an indicator of moderate-burn areas, they were relatively abundant in all burn severities.
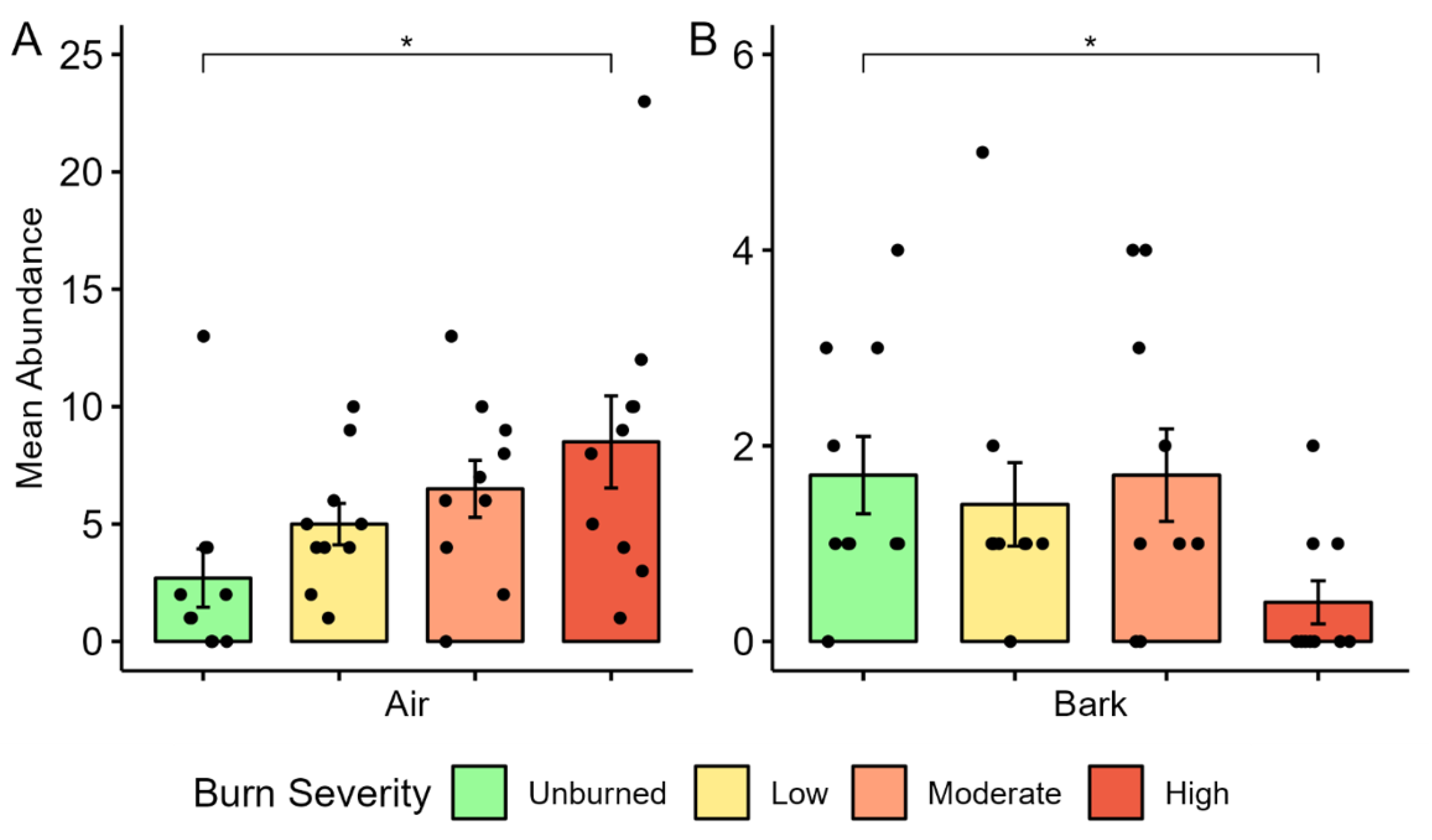
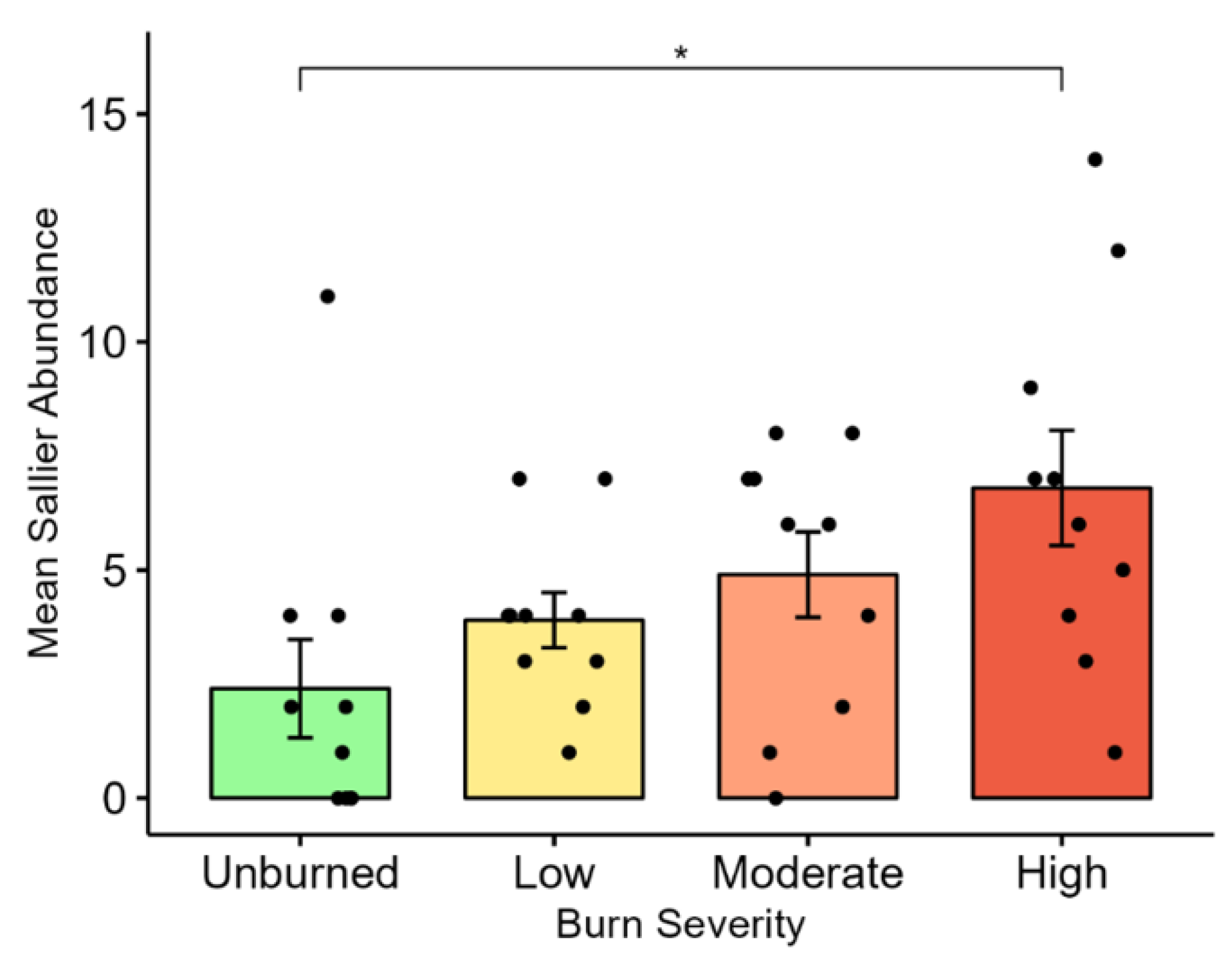
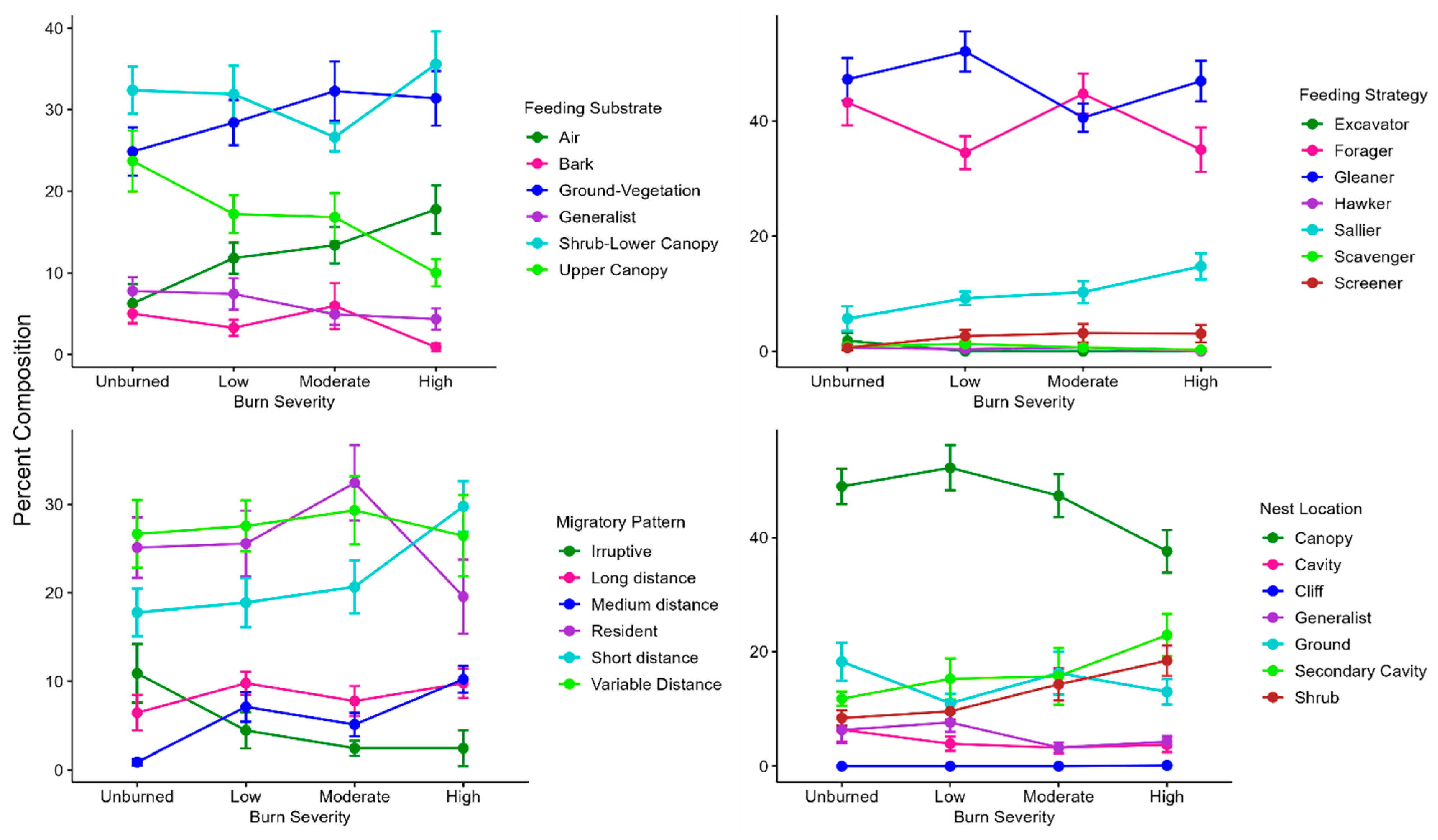
We observed evident trends in avian functional guilds between unburned and high-severity burns. The percent composition of functional guilds within burn severities was significantly different for all burn severities and guild categories, however, some of the differences may be attributed to low abundances of some specialist guild species (cliff nesters, excavators, scavengers, hawkers, and screeners). The prominence of sallier species (flycatchers), such as the Western Wood Pewee, Dusky Flycatcher, and Olive-sided Flycatcher in high-burn areas is consistent with other studies [
8,
25,
54]. These aerial insectivores are known to utilize high-burn areas with ample inter-canopy space for flycatching. An unexpected finding was the association of bark foraging species with unburned habitats. Some bark feeding species, such as woodpeckers, are cavity nesters, which are associated with high-burn areas that provide suitable nest sites and an abundance of wood-boring insects [
9,
31]. High-burn areas are frequently associated with an increase in insects that colonize dead and dying trees immediately following fire, providing food for the bark feeding species [
31,
58]. In our study, five years post-fire was sufficient time for the pulse of insects to subside, followed by the subsequent dispersal of bark feeding species and the colonization of cavities by secondary cavity nesters [
58]. Williamson’s Sapsucker detections account for the association of bark foragers with unburned areas, as Sapsuckers feed primarily on tree sap and are therefore associated with living trees; Williamson’s Sapsucker was also an unburned indicator species in our study [
59]. Other woodpecker species were either generalists (Hairy and Downy) or ground–vegetation foragers (Northern Flicker) and did not contribute to the bark foraging guild. Similarly, bark foraging species have been observed to be more abundant in long-unburned areas of dry Australian woodlands [
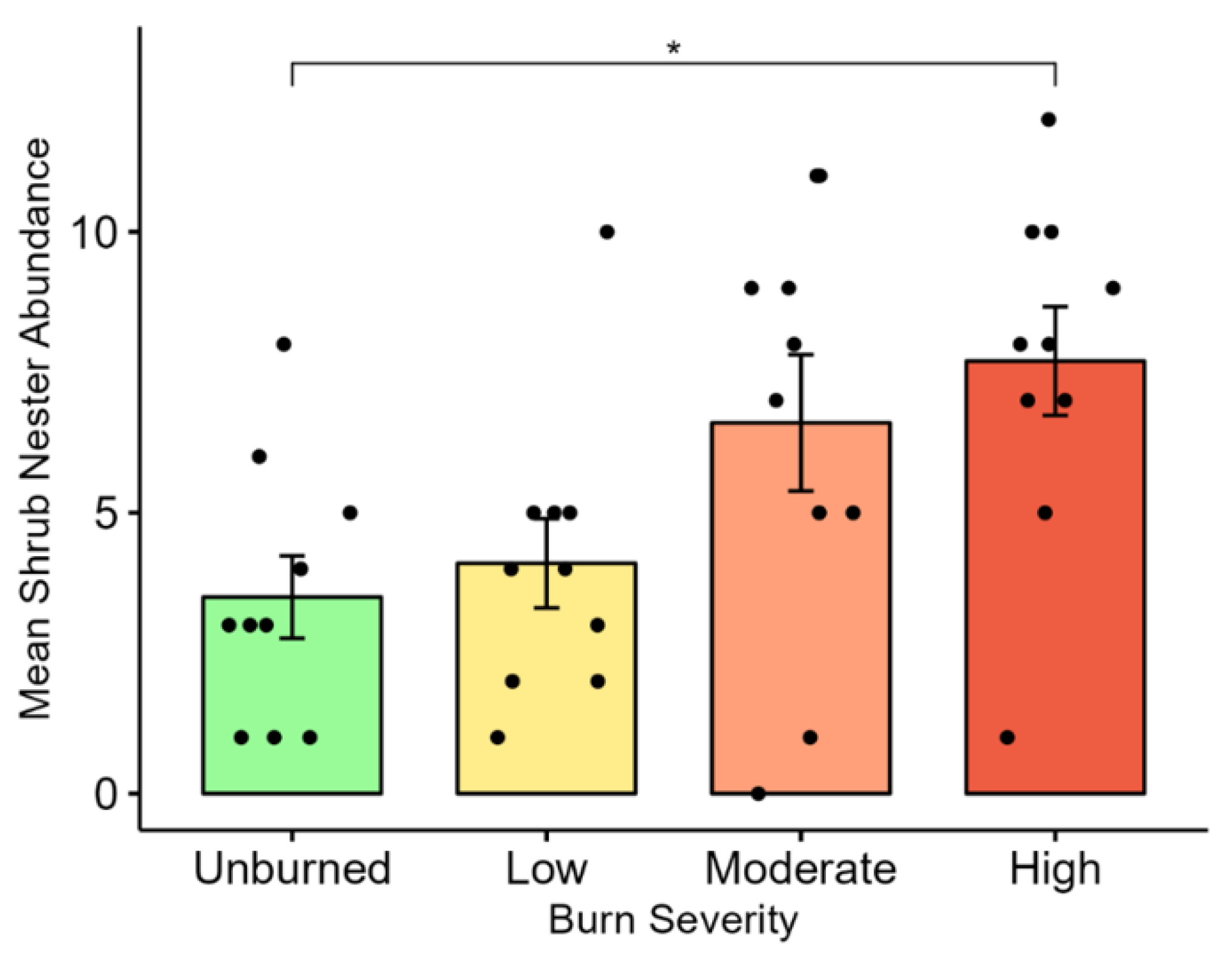
60]. Significantly more shrub nesting species were detected in high-severity burns than unburned areas, largely due to the abundance of Green-tailed Towhees and Dusky Flycatchers in high-burn areas. This is interesting, due to the widespread shrub cover in all burned areas and considering the indicator status of Virginia’s Warbler in unburned areas. As previously expressed, other environmental variables are likely contributing to the association of these species with specific burn severities.
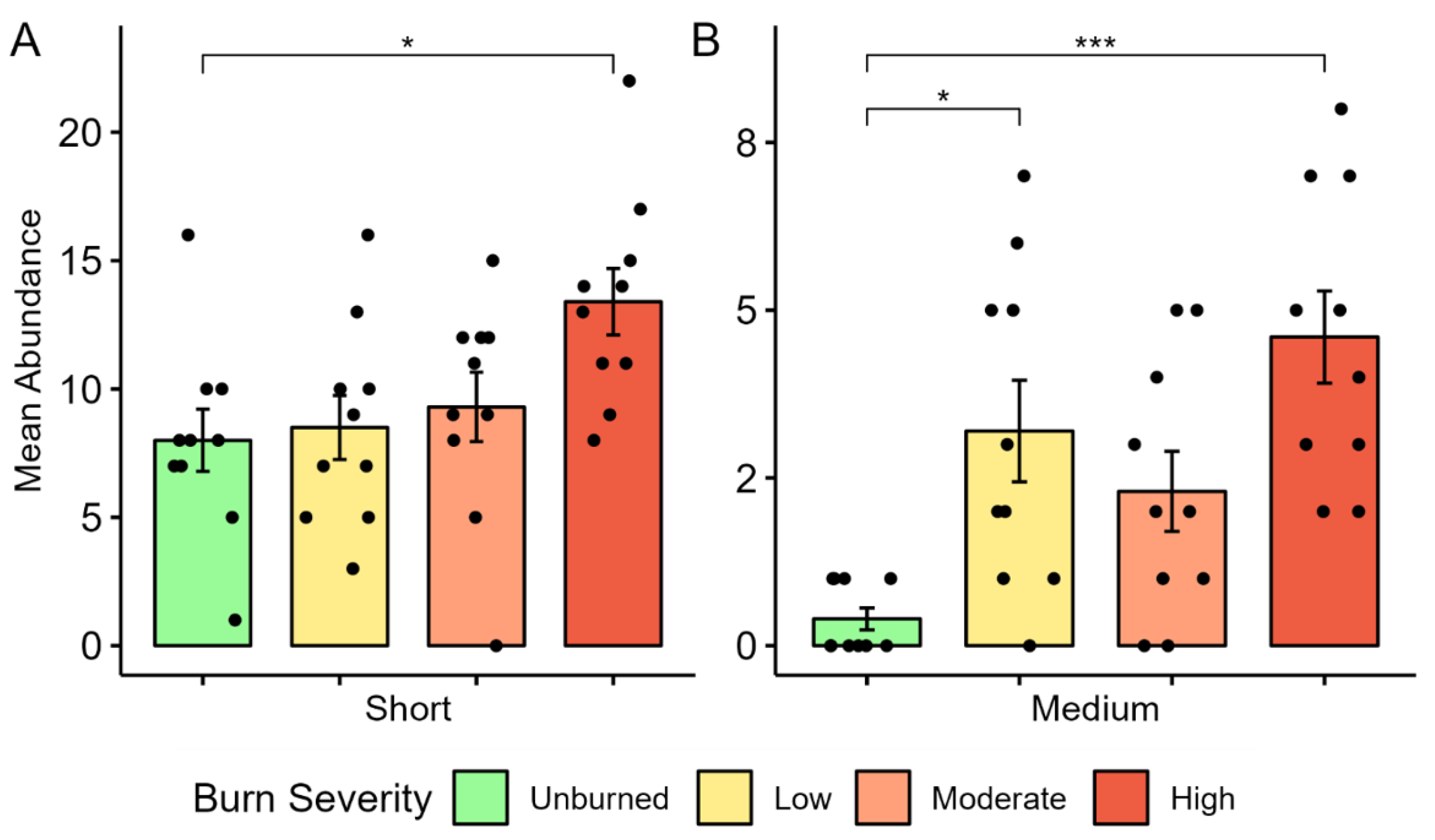
The influence of wildfire on migratory guilds in mixed conifer forests is not well studied, so this study aimed to evaluate migration distance in addition to simply residency status. Short- and medium-distance migrants were both significantly more abundant in high-burn than unburned areas. This is corroborated by a study of Chilean temperate forests that found migrants and partial migrants to be more associated with burned forest than unburned, however, partial migrants required forest that had several years to regenerate post-fire [
14]. Partial migrants may be related to the short- and medium-distance guilds in this study, groups that travel altitudinally or up to several hundred miles, respectively. Interestingly, in this study, medium-distance migrants were significantly more abundant in low-burn than unburned areas and residents were more associated with unburned forest than burned forest [
14]. These findings indicate that migratory birds may be more resilient to disturbance than resident species. However, another study in dry Australian forests found that migrant species were associated with unburned forest [
61]. In our study, the distinction between unburned and burned forest is not sufficient to describe resident species as residents were most abundant in moderate burn severities, least abundant in high burn severities, and equally abundant in unburned and low burn severities. These findings illustrate that distinctions among migratory guilds involving their resiliency and response to fire necessitate further study. We also found irruptive migrants most associated with unburned areas, which may be attributed to the predominance of Red Crossbill and Evening Grosbeak, birds that follow fluctuating tree food-crops and lack site fidelity [
62]. This trend suggests further post-fire avian research at a species-specific scale, including the study of food-crop response to fire severity. The response of migratory guilds to fire is of particular interest, due to its management relevance and fine-scale changes among species. Focusing on this may assist forest managers in predicting migratory stopover and regional movements of species of interest.