Self-Heating Risk of Coals and Metal Powders: A Comparison
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
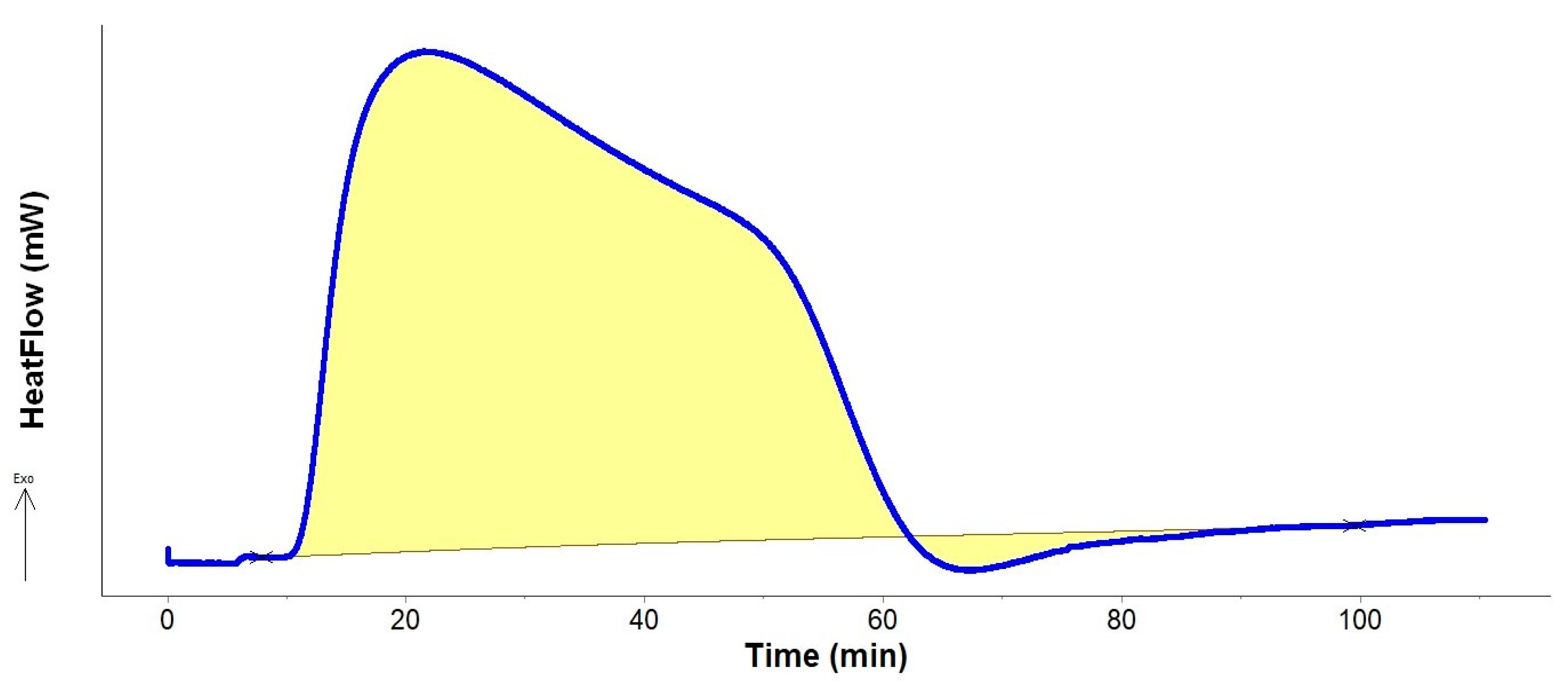
2.2. Oxidation Heat Measurements
2.3. Pre-Treatment of the Samples
2.4. Chemical Characterisation
2.5. Grain Size Measurements
3. Results and Discussion
3.1. Texture of the Samples
3.2. Oxidation Heat
3.3. Consideration of Comprehensive Self-Heating Risk Indicator
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Wen, H.; Chen, C.; Guo, J.; Jin, Y.; Zheng, X.; Cheng, X.; Li, D. Research Status and Development Trend of Coal Spontaneous Combustion Fire and Prevention Technology in China: A Review. ACS Omega 2024, 9, 21727–21750. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Kuenzer, C. Coal Fires in China over the Last Decade: A Comprehensive Review. Int. J. Coal Geol. 2014, 133, 72–99. [Google Scholar] [CrossRef]
- Ivanov, V.G.; Gavrilyuk, O.V. Specific Features of the Oxidation and Self-Ignition of Electroexplosive Ultradisperse Metal Powders in Air. Combust. Explos. Shock Waves 1999, 35, 648–655. [Google Scholar] [CrossRef]
- Dufaud, O.; Bideau, D.; Le Guyadec, F.; Corriou, J.-P.; Perrin, L.; Caleyron, A. Self Ignition of Layers of Metal Powder Mixtures. Powder Technol. 2014, 254, 160–169. [Google Scholar] [CrossRef]
- Kawatra, S.K.; Hess, M.J. Environmental Beneficiation of Machining Wastes—Part II: Measurement of the Effects of Moisture on the Spontaneous Heating of Machining Swarf. J. Air Waste Manag. Assoc. 1999, 49, 477–481. [Google Scholar] [CrossRef]
- Le Guyadec, F.; Génin, X.; Bayle, J.P.; Dugne, O.; Duhart-Barone, A.; Ablitzer, C. Pyrophoric Behaviour of Uranium Hydride and Uranium Powders. J. Nucl. Mater. 2010, 396, 294–302. [Google Scholar] [CrossRef]
- Azhagurajan, A.; Prakash, L.; Jeyasubramanian, K. Prevention of Explosion Accidents by Employing Boron Instead of Aluminium in Flash Powder. Process Saf. Environ. Prot. 2019, 131, 160–168. [Google Scholar] [CrossRef]
- Taraba, B.; Podstawka, T.; Maršálek, R. Case Study of Spontaneous Heating of Metal Powder—Calorimetric Solution. Powder Technol. 2021, 393, 74–76. [Google Scholar] [CrossRef]
- Joseph, G. Combustible Dusts: A Serious Industrial Hazard. J. Hazard. Mater. 2007, 142, 589–591. [Google Scholar] [CrossRef]
- Pei, Q.; Jia, Z.; Liu, J.; Wang, Y.; Wang, J.; Zhang, Y. Prediction of Coal Spontaneous Combustion Hazard Grades Based on Fuzzy Clustered Case-Based Reasoning. Fire-Switz. 2024, 7, 107. [Google Scholar] [CrossRef]
- Sen, R.; Srivastava, S.; Singh, M. Aerial Oxidation of Coal-Analytical Methods, Instrumental Techniques and Test Methods: A Survey. Indian J. Chem. Technol. 2009, 16, 103. [Google Scholar]
- Beamish, B.B.; Hamilton, G.R. Effect of Moisture Content on the R70 Self-Heating Rate of Callide Coal. Int. J. Coal Geol. 2005, 64, 133–138. [Google Scholar] [CrossRef]
- Leslie, G.; Pollard, A.; Matovic, D. Calorimetric and Heat Transfer Studies of the Spontaneous Combustion of Two Low Carbon Fuels. J. Loss Prev. Process Ind. 2014, 32, 44–51. [Google Scholar] [CrossRef]
- Zarrouk, S.J.; O’Sullivan, M.J.; St George, J.D. Modelling the Spontaneous Combustion of Coal: The Adiabatic Testing Procedure. Combust. Theory Model. 2006, 10, 907–926. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Shen, D.; Liu, H.; Song, D.; Li, Y. Reactions and Morphologies of Mg and Mg/Teflon/Viton Particles during Oxidation. Metals 2023, 13, 417. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Lin, Z.-S. Thermal Incompatibility Analysis of Cu-Etching Solution in a Metal-Etching System Using Adiabatic Calorimetry. J. Therm. Anal. Calorim. 2023, 148, 4681–4688. [Google Scholar] [CrossRef]
- Nie, H.; Schoenitz, M.; Dreizin, E.L. Calorimetric Investigation of the Aluminum–Water Reaction. Int. J. Hydrogen Energy 2012, 37, 11035–11045. [Google Scholar] [CrossRef]
- Taraba, B.; Maršálek, R.; Podstawka, T. Two Aspects of Water in Self-Heating Risk of Aluminium Powders: Calorimetric Study. J. Therm. Anal. Calorim. 2022, 147, 11671–11677. [Google Scholar] [CrossRef]
- Taraba, B. Reversible and Irreversible Interaction of Oxygen with Coal Using Pulse Flow Calorimetry. Fuel 1990, 69, 1191–1199. [Google Scholar] [CrossRef]
- Karsner, G.; Perlmutter, D. Reaction Regimes in Coal Oxidation. AIChE J. 1981, 27, 920–927. [Google Scholar] [CrossRef]
- Taraba, B.; Pavelek, Z. Investigation of the Spontaneous Combustion Susceptibility of Coal Using the Pulse Flow Calorimetric Method: 25 Years of Experience. Fuel 2014, 125, 101–105. [Google Scholar] [CrossRef]
- Chen, X.; Stott, J. The Effect of Moisture-Content on the Oxidation Rate of Coal During Near-Equilibrium Drying and Wetting at 50-Degrees-C. Fuel 1993, 72, 787–792. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Wang, J.; Zhang, X.; Wang, J.; Zhou, C. Study on the Effect of Extraneous Moisture on the Spontaneous Combustion of Coal and Its Mechanism of Action. Energies 2020, 13, 1969. [Google Scholar] [CrossRef]
- Liotta, R.; Brons, G.; Isaacs, J. Oxidative Weathering of Illinois No-6 Coal. Fuel 1983, 62, 781–791. [Google Scholar] [CrossRef]
- Wang, H.H.; Dlugogorski, B.Z.; Kennedy, E.M. Coal Oxidation at Low Temperatures: Oxygen Consumption, Oxidation Products, Reaction Mechanism and Kinetic Modelling. Prog. Energy Combust. Sci. 2003, 29, 487–513. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Qi, X.; Dou, G.; Qi, G.; Ma, L. Reaction Pathway of Coal Oxidation at Low Temperatures: A Model of Cyclic Chain Reactions and Kinetic Characteristics. Combust. Flame 2016, 163, 447–460. [Google Scholar] [CrossRef]
- Gai, W.-Z.; Deng, Z.-Y. Effect of Initial Gas Pressure on the Reaction of Al with Water. Int. J. Hydrog. Energy 2014, 39, 13491–13497. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Gai, W.-Z.; Zhang, X.-Y.; Pan, H.-Y.; Cheng, Z.; Xu, P.; Deng, Z.-Y. Effect of Storage Environment on Hydrogen Generation by the Reaction of Al with Water. RSC Adv. 2017, 7, 2103–2109. [Google Scholar] [CrossRef]



| Sample | Description (Technology Type) | Grain Size/ d50 (μm) | Content of Main Element (%) | Original State | Dried State | Wetted State | |||
|---|---|---|---|---|---|---|---|---|---|
| W (%) | q30 (W kg−1) | W (%) | q30 (W kg−1) | W (%) | q30 (W kg−1) | ||||
| Coals | |||||||||
| B1 | Bituminous | 60–150 | Cdaf = 87.6 | 1.5 | 0.52 ** | <0.1 | 0.25 | 3.6 | 0.30 |
| B2 | Bituminous | 60–150 | Cdaf = 92.5 | 1.1 | 0.36 ** | <0.1 | 0.18 | 2.5 | 0.25 |
| SB1 | Subbituminous | 60–150 | Cdaf = 72.6 | 25.4 | 4.1 ** | <0.2 | 1.5 | 14 | 3.0 |
| SB2 | Subbituminous | 60–150 | Cdaf = 71.3 | 28.4 | 3.0 ** | <0.5 | 2.3 | 17 | 3.9 |
| Metals | |||||||||
| Al_1 | Aluminium (grinding) | 190 | Al = 85 | 0.4 | 0.12 * | ~0 | 0.011 | 2.8 | 11.7 |
| Al_2 | Aluminium (welding) | 16 | Al = 53 | 2.3 | 0.083 * | ~0 | 0.040 | 13 | 2.8 |
| Mg | Magnesium (cutting) | - | Mg = 95 | 0.6 | 0.045 * | ~0 | ~ 0.01 | 2.0 | 0.32 |
| Sn/Zn | Sn-Zn (metal coating) | 11 | Sn = 50; Zn = 44 | 0.8 | 0.083 * | ~0 | 0.043 | 7 | 1.3 |
| Fe | Iron (grinding-lab) | 170 | Fe = 93 | 0.8 | 0.47 ** | ~0 | 0.15 | 19 | 31.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taraba, B.; Maršálek, R. Self-Heating Risk of Coals and Metal Powders: A Comparison. Fire 2024, 7, 378. https://doi.org/10.3390/fire7110378
Taraba B, Maršálek R. Self-Heating Risk of Coals and Metal Powders: A Comparison. Fire. 2024; 7(11):378. https://doi.org/10.3390/fire7110378
Chicago/Turabian StyleTaraba, Boleslav, and Roman Maršálek. 2024. "Self-Heating Risk of Coals and Metal Powders: A Comparison" Fire 7, no. 11: 378. https://doi.org/10.3390/fire7110378
APA StyleTaraba, B., & Maršálek, R. (2024). Self-Heating Risk of Coals and Metal Powders: A Comparison. Fire, 7(11), 378. https://doi.org/10.3390/fire7110378







