Research and Application of CO2 Fire Prevention Mechanism and Key Technologies in Mines: A Review
Abstract
1. Introduction
2. History of CO2 Fire Prevention Technology Development
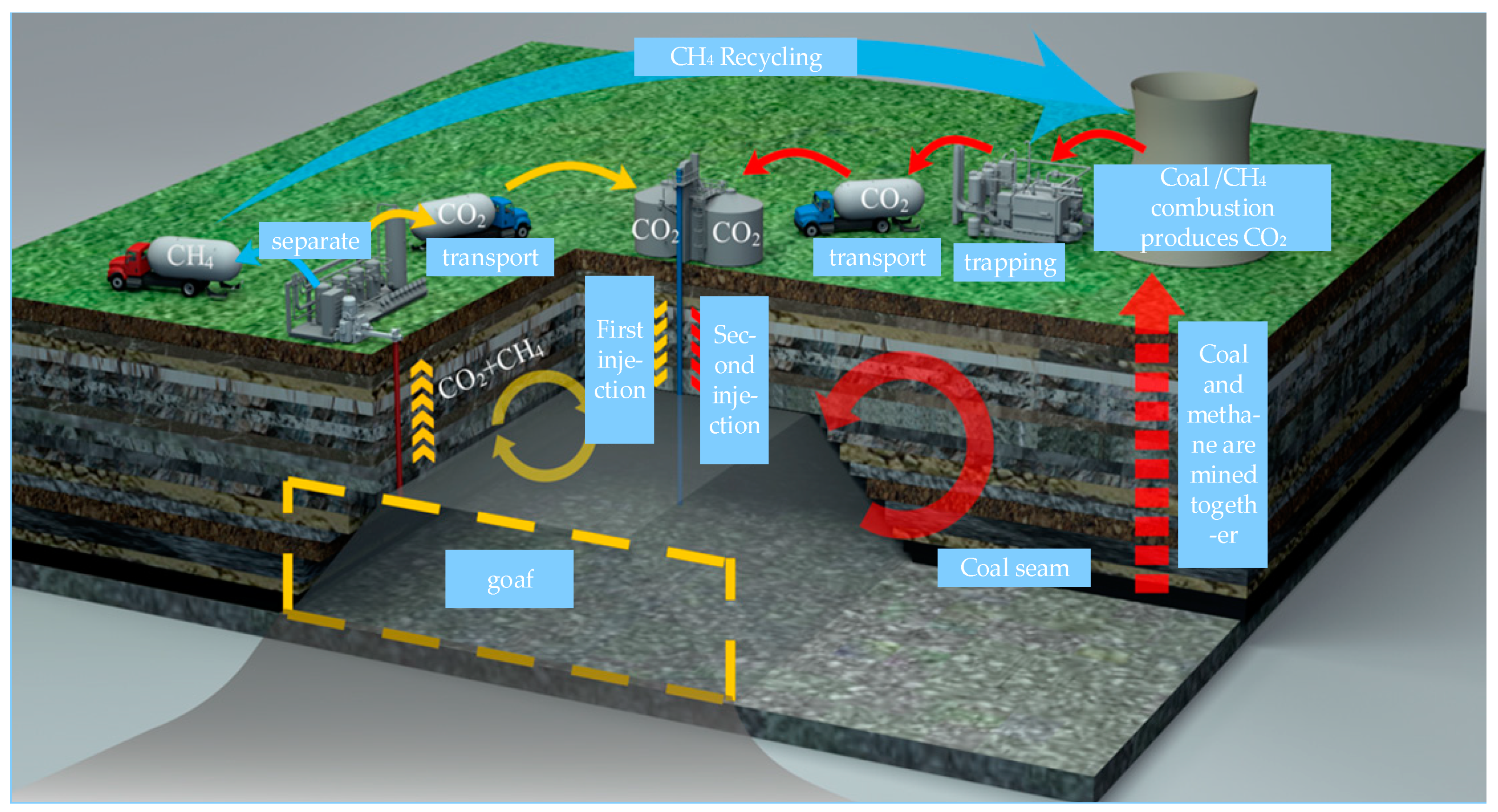
2.1. Sources of CO2
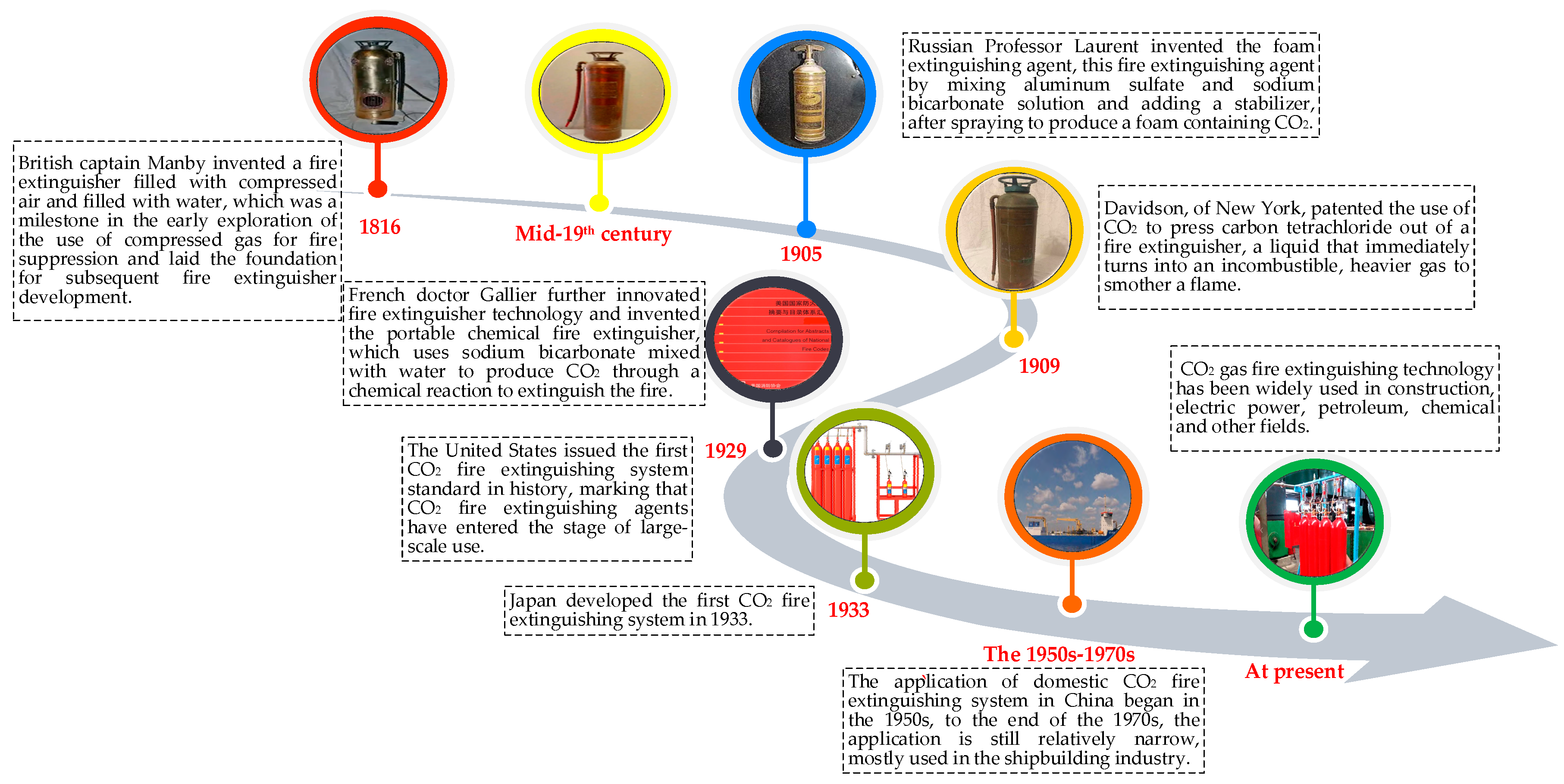
2.2. History of CO2 Fire Extinguishing Technology Development
2.3. Coal Mine CO2 Fire Prevention Technology
3. Physicochemical Properties of CO2 and Fire Prevention Mechanism
3.1. Physicochemical Properties and Phase Characteristics of CO2
3.1.1. Physical and Chemical Properties of CO2
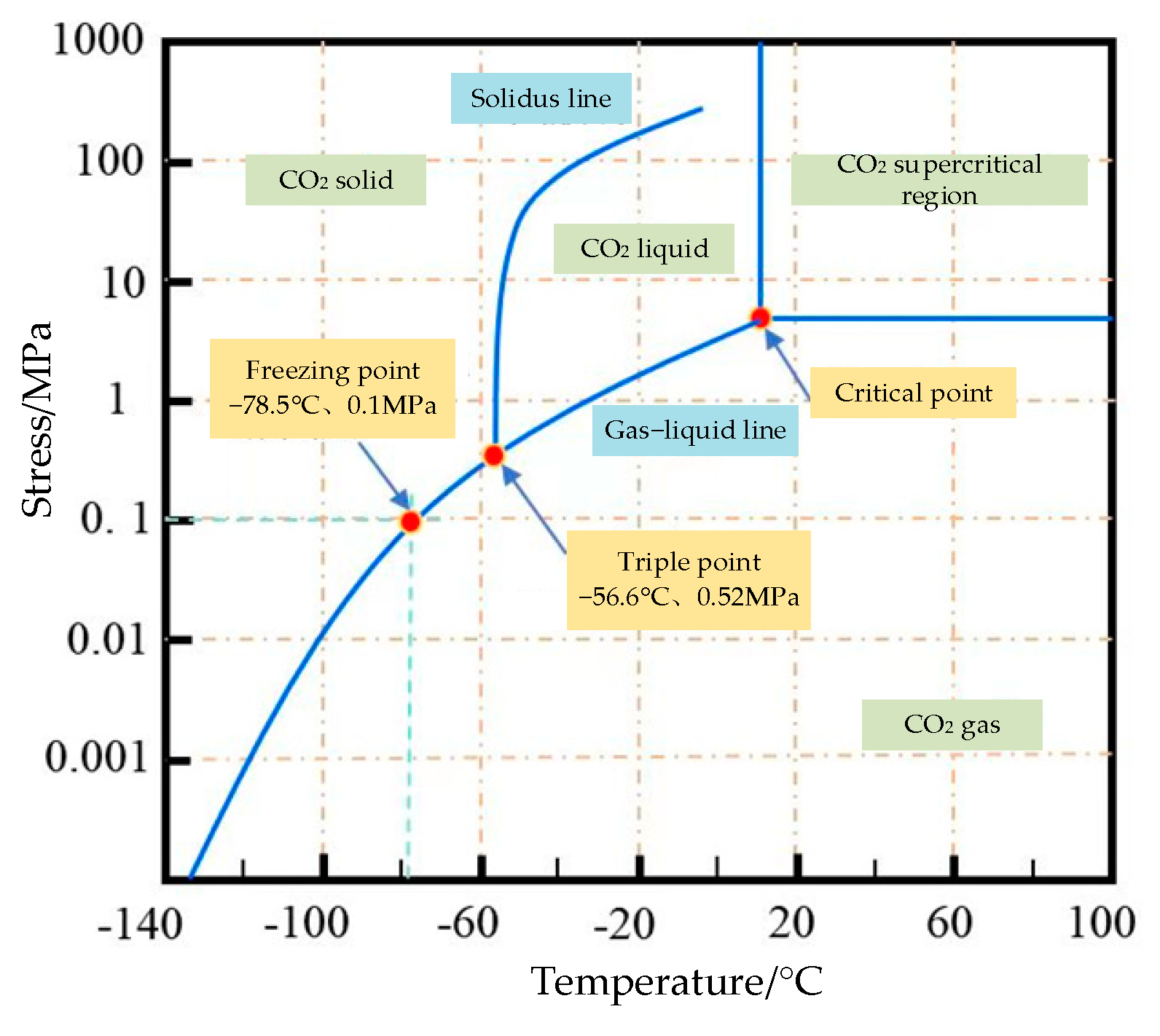
3.1.2. Phase Characteristics of CO2
3.2. Mechanism Underlying CO2 Fire Prevention
- (1)
- Isolation effect of asphyxia
- (2)
- Inerting explosion suppression effect
- (3)
- Cooling effect
3.3. Analysis of CO2 Fire Prevention Performance
- (1)
- Comparison adsorption comparison
- (2)
- Comparison of critical oxygen concentration
- (3)
- Comparison of inert coverage in fire zones
- (4)
- Comparison of separation purity
3.4. Studies on the Influencing Factors of CO2 Fire Prevention Performance
3.4.1. Effect of CO2 Concentration on Coal Oxidation Rate
3.4.2. Effect of CO2 Injection Position on Coal Oxidation Rate
4. CO2 Fire Prevention Technology
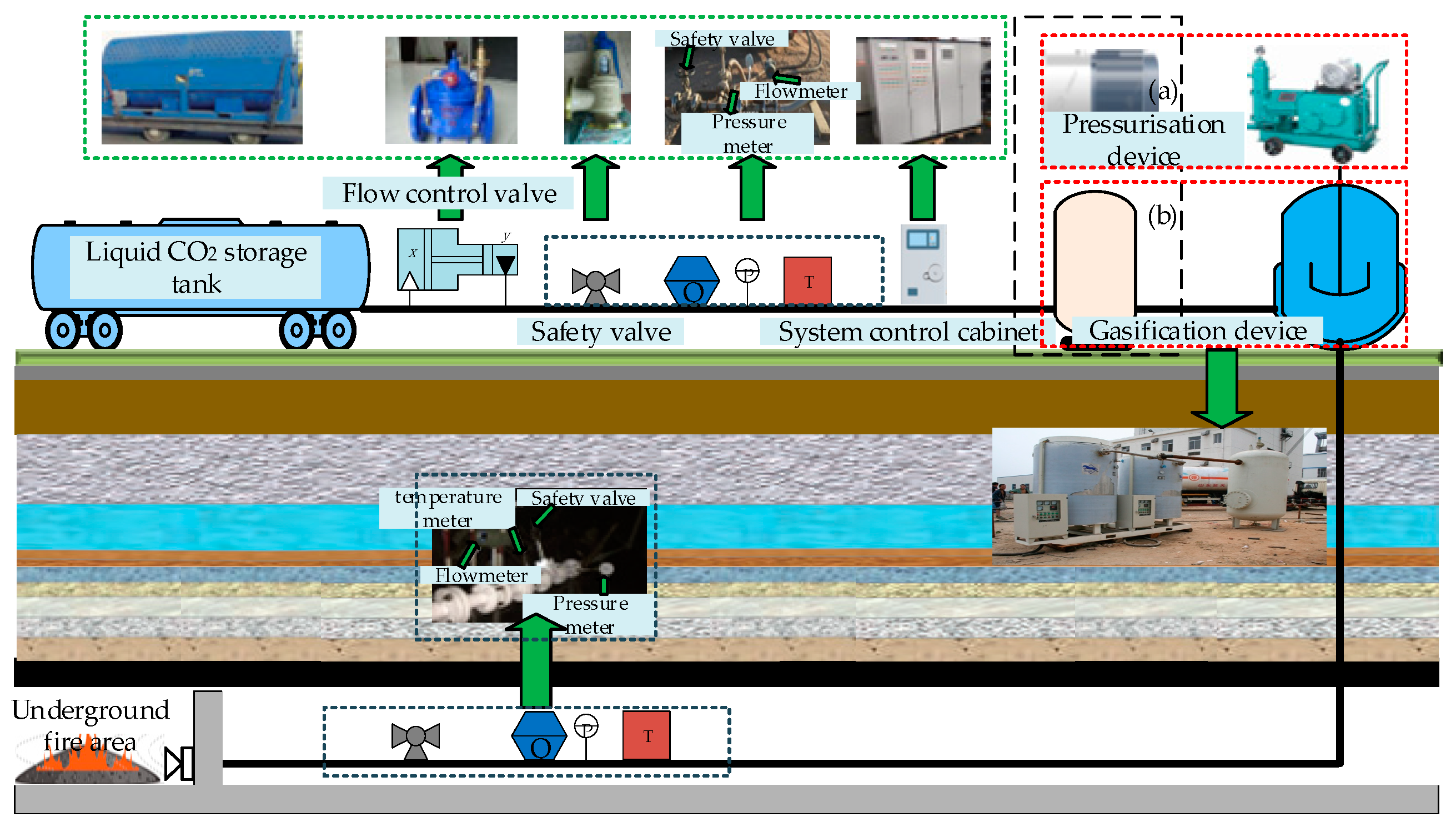
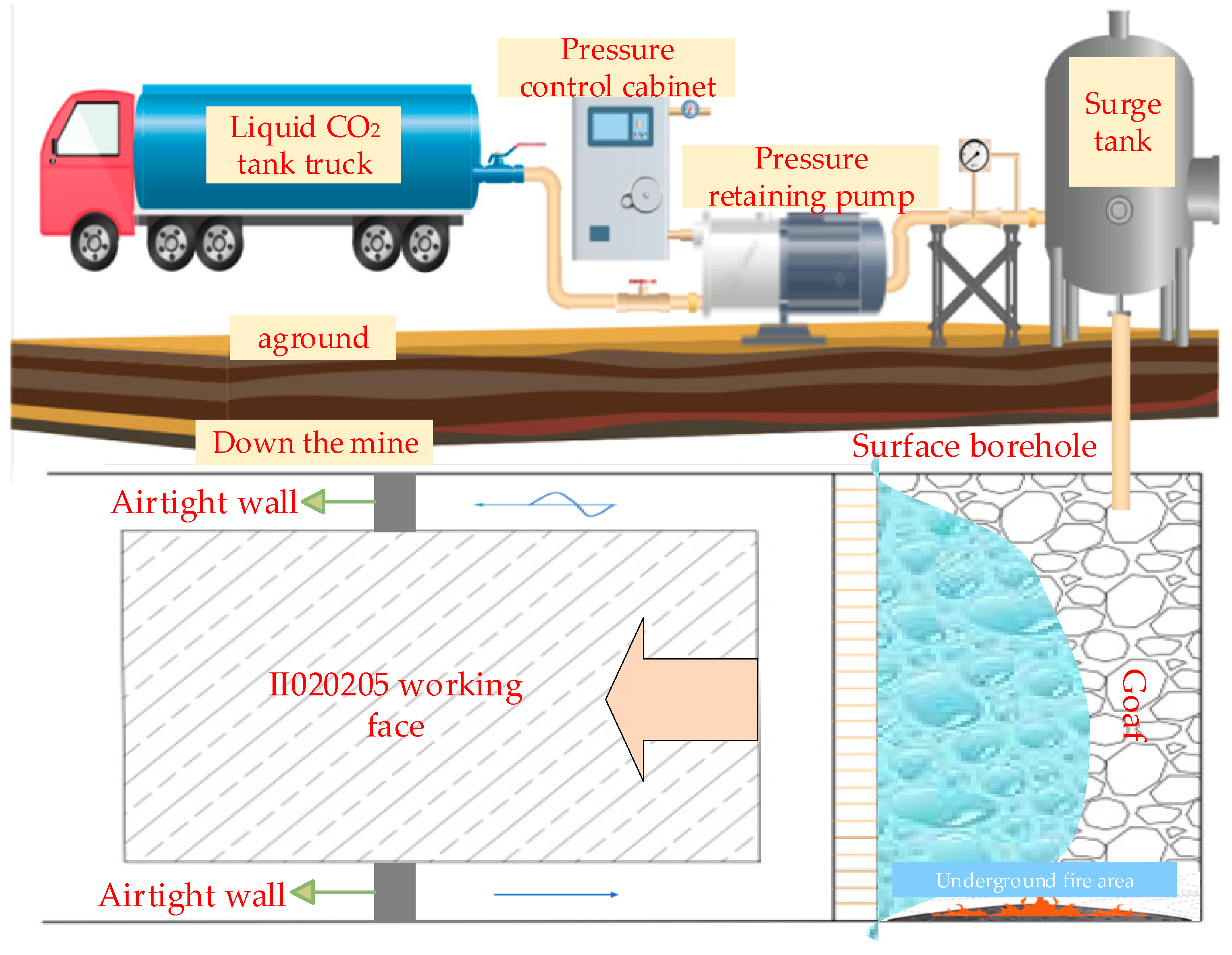
4.1. Surface Injection
4.2. Downhole Injection
4.3. Dry Ice Phase-Change Fire Prevention
5. Field Application and Effect
6. Shortcomings and Prospects of CO2 Fire Prevention Technology
6.1. Shortcomings
6.2. Prospects
- (1)
- Studying material and crack propagation of CO2 transport pipeline
- (2)
- Intelligent monitoring and control system for CO2 transmission pipelines
- (3)
- Multifactor coupling analysis of the inert mechanism
- (4)
- Microscopic mechanism of CO2 inerting
- (5)
- Comprehensive fire prevention technology
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Liu, X.W.; Patouillard, L.; Margni, M.; Bulle, C.; Hua, H.; Yuan, Z.W. Remarkable spatial disparity of life cycle inventory for coal production in China. Environ. Sci. Technol. 2023, 57, 15443–15453. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, C.M.; Wen, H.; Cai, G.B.; Liu, Y. Prediction model of goaf coal temperature based on PSO-GRU deep neural network. Case Stud. Therm. Eng. 2024, 53, 103813. [Google Scholar] [CrossRef]
- Wei, D.Y.; Du, C.F.; Lei, B.; Lin, Y.F. Prediction and prevention of spontaneous combustion of coal from goafs in workface: A case study. Case Stud. Therm. Eng. 2020, 21, 100668. [Google Scholar] [CrossRef]
- Wang, B.; Zuo, S.; Zuo, X.X.; Ma, X.M. Experimental investigation on the influencing factors of preparing three-phase foam. J. Serb. Chem. Soc. 2023, 88, 199–209. [Google Scholar] [CrossRef]
- Biswal, S.S.; Gorai, A.K. Change detection analysis in coverage area of coal fire from 2009 to 2019 in Jharia Coalfield using remote sensing data. Int. J. Remote Sens. 2020, 24, 9545–9564. [Google Scholar] [CrossRef]
- Muduli, L.; Jana, P.K.; Mishra, D.P. Wireless sensor network based fire monitoring in underground coal mines: A fuzzy logic approach. Process Saf. Environ. 2018, 113, 435–447. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Sun, L.L.; Song, X.; Liu, Q.Q.; Xu, H.; Du, W.Z. Study on the Low-Temperature Oxidation Law in the Co-Mining Face of Coal and Oil Shale in a Goaf A Case Study in the Liangjia Coal Mine, China. Energies 2018, 11, 174. [Google Scholar] [CrossRef]
- Qin, B.T.; Li, L.; Ma, D.; Lu, Y.; Zhong, X.X.; Jia, Y.W. Control technology for the avoidance of the simultaneous occurrence of a methane explosion and spontaneous coal combustion in a coal mine: A case study. Process Saf. Environ. 2016, 103, 203–211. [Google Scholar] [CrossRef]
- Ren, W.X.; Guo, Q.; Yang, H.H. Analyses and prevention of coal spontaneous combustion risk in gobs of coal mine during withdrawal period. Geomat. Nat. Hazards Risk 2019, 10, 353–367. [Google Scholar] [CrossRef]
- Yang, Y.L.; Li, Z.H.; Si, L.L.; Hou, S.S.; Zhou, Y.B.; Qi, Q.Q. Consolidation grouting technology for fire prevention in mined-out areas of working face with large inclined angle and its application. Fire Mater. 2017, 41, 700–715. [Google Scholar] [CrossRef]
- Kong, B.; Li, Z.H.; Yang, Y.L.; Liu, Z.; Yan, D.C. A review on the mechanism, risk evaluation, and prevention of coal spontaneous combustion in China. Environ. Sci. Pollut. 2017, 24, 23453–23470. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.B.; Hu, S.H.; Wang, H.E. Using P-Cl inorganic ultrafine aerosol particles to prevent spontaneous combustion of low-rank coal in an underground coal mine. Fire Saf. J. 2020, 115, 103140. [Google Scholar] [CrossRef]
- Huang, Z.A.; Sun, C.W.; Gao, Y.K.; Ji, Y.C.; Wang, H.; Zhang, Y.H.; Yang, R. R&D of colloid components of composite material for fire prevention and extinguishing and an investigation of its performance. Process Saf. Environ. 2018, 113, 357–368. [Google Scholar]
- Gao, F.; Bai, Q.H.; Jia, Z.; Zhang, X.; Li, Y.D. Influence and inerting mechanism of inert gas atmospheres on the characteristics of oxidative spontaneous combustion in coal. Energy 2024, 293, 130470. [Google Scholar] [CrossRef]
- Liu, H.W.; Wang, F. Thermal characteristics and kinetic analysis of coal-oxygen reaction under the condition of inert gas. Int. J. Coal Prep. Util. 2019, 42, 846–862. [Google Scholar] [CrossRef]
- Szurgacz, D.; Tutak, M.; Brodny, J.; Sobik, L.; Zhironkina, O. The method of combating coal spontaneous combustion hazard in goafs—A case study. Energies 2020, 12, 4538. [Google Scholar] [CrossRef]
- Tang, L.; Wang, G.; Wang, E.M.; Li, X.M.; Cheng, Q. The fire inhibition characteristics of composite inert gas and its application potential analysis. Energy Sources Part A 2021, 1940387. [Google Scholar] [CrossRef]
- Hu, X.Q.; Kraaijeveld, A. Experimental and numerical investigation of extinguishing effectiveness of inert-gas agents in a leaky enclosure. Energies 2022, 15, 4323. [Google Scholar] [CrossRef]
- Tang, L.; Qin, Y.D.; Li, X.M.; Wang, J.Z. Coal fire prevention in large areas over long term with a composite inert gas—A case study in Tangkou coal mine, China. Energy Sources Part A 2019, 1684600. [Google Scholar] [CrossRef]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.W.; Bakker, D.C.E.; Hauck, J.; Landschutzer, P. Global carbon budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Biermann, M.; Normann, F.; Johnsson, F.; Skagestad, R. Partial carbon capture by absorption cycle for reduced specific capture cost. Ind. Eng. Chem. Res. 2018, 57, 15411–15422. [Google Scholar] [CrossRef]
- Ding, Y.; Li, S.G.; Zhu, B.; Lin, H.F.; Zhang, J.F.; Tan, J.H.; Chen, W.B. Research on the feasibility of storage and estimation model of storage capacity of CO2 in fissures of coal mine old goaf. Int. J. Min. Sci. Technol. 2023, 33, 675–689. [Google Scholar] [CrossRef]
- Lei, B.W.; He, B.B.; Xiao, B.W.; Du, P.Y.; Wu, B. Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 2020, 265, 116976. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Yang, K.; Li, R.J.; Wang, J.W.; Tang, L. Research progress and development trend of mine fire prevention and extinguishing materials. Saf. Coal Mines 2023, 54, 82–91. [Google Scholar]
- Mohalik, N.K.; Singh, R.V.K.; Pandey, J.; Singh, V.K. Application of nitrogen as preventive and controlling subsurface fire—Indian context. J. Sci. Ind. Res. India 2005, 64, 273–280. [Google Scholar]
- Zhou, F.B.; Shi, B.B.; Cheng, J.W.; Ma, L.J. A new approach to control a serious mine fire with using liquid nitrogen as extinguishing media. Fire Technol. 2015, 51, 325–334. [Google Scholar]
- Jürgen, F.B.; Saqib, A.S. Prevention of gob ignitions and explosions in longwall mining using dynamic seals. Int. J. Min. Sci. Technol. 2017, 27, 999–1003. [Google Scholar]
- Xian, X.F.; Wang, H.T.; Jiang, D.Y.; Liu, B.X. Research summary of fire prevention technology in coal mine in China. Strateg. Study CAE 2001, 3, 28–32. [Google Scholar]
- Gao, G.W. Present situation and future of nitrogen gas fire prevention in coal mines in China. J. China Coal Soc. 1999, 24, 50–53. [Google Scholar]
- Zhang, S.L.; Guo, D.T. Dynamics and development of fire prevention and control in coal mines in China. Saf. Coal Mines 1991, 4, 1–6. [Google Scholar]
- Watkinson, M.; Liddell, K.; Muller, S.; Gido, M.; Nissen, J. A Practical Research Study into the Environmental and Physical Impacts during an Underground Mine Inertisation with the GAG Jet. Available online: https://acarp.com.au/abstracts.aspx?repId=C2300 (accessed on 14 July 2014).
- Bell, S.; Cliff, D.; Harrison, P.; Hester, C. Recent developments in coal mine inertisation in Australia. Proceeding of the 1998 Coal Operators’ Conference, University of Wollongong, Wollongong, NSW, Australia, 18–20 February 2019. [Google Scholar]
- Hansen, R. Retardation of an IGG gas flow along a mine drift due to condensation and temperature decrease. Int. J. Min. Sci. Technol. 2019, 29, 831–839. [Google Scholar] [CrossRef]
- Mucho, T.P.; Houlison, I.R.; Smith, A.C.; Trevits, M.A. Coal mine inertisation by remote application. In Proceedings of the National Coal Show, Pittsburgh, PA, USA, 7–9 June 2005. [Google Scholar]
- Phillips, J.; Hanrahan, C. QMRS Jet operations at Newlands. GAG Inertisation Seminar. Available online: https://sc.panda985.com/scholar?q=+++QMRS+Jet+operations+at+Newlands.+GAG+Inertisation+Seminar. (accessed on 1 December 2006).
- Wu, J.X. Current situation and prospect of inert gas fire extinguishing technology in coal mine in China. Saf. Coal Mines 1998, 10, 20–22. [Google Scholar]
- Xiao, D.C.; Xu, Z.Y.; Wang, F.Q. DQ-500 type inert gas fire extinguishing device. China Saf. Sci. J. 1991, 3, 35–40. [Google Scholar]
- Hatakeyama, T.; Aida, E.; Yokomori, T.; Ohmura, R.; Ueda, T. Fire extinction using carbon dioxide hydrate. Ind. Eng. Chem. Res. 2009, 8, 4083–4087. [Google Scholar] [CrossRef]
- Zhu, J.F.; Geng, Y.; Li, D.M.; Jiao, Y.J.; Liang, Q. Research progress of coal mine fire prevention and control technology by CO2. Saf. Coal Mines 2021, 52, 197–203. [Google Scholar]
- Westfield, J.; Brumbaugh, H.S.; Whittaker, R.W. Extinguishing Fire with Carbon Dioxide in the Valier Mine; USBM Information Cireular; Valier Coal Co.: Valier, IL, USA, 1950; p. 7563. [Google Scholar]
- Moriss, R.E. A review of experiences on the use of inert gases in mine fires. Int. J. Min. Sci. Technol. 1987, 6, 37–69. [Google Scholar] [CrossRef]
- Zeng, X.K. Study on the Characteristics of Long Distance Transportation of Liquid CO2 for Coal Mine Fire Prevention. Master’s Thesis, Liaoning Technical University, Fuxin, China, 2021. [Google Scholar]
- Inagaki, F.; Okada, Y.; Matsumoto, C.; Yamada, M.; Nakazawa, K.; Mukai, C. Energy less CO2 Absorption, Generation, and Fixation Using Atmospheric CO2. Chem. Pharm. Bull. 2016, 64, 8–13. [Google Scholar] [CrossRef]
- Kim, J.D.; Sunku, H.; Ho, R.K. Comparison of the physical properties for alternative fire extinguishing of pure and mixture component of inert gases. Fire Sci. Eng. 2004, 18, 12–19. [Google Scholar]
- Chen, H.; Cao, Y.F.; Xing, X.S.; Zou, M.H.; Yu, J.F.; Du, X.Y.; Wang, Y.; Peng, J.L. Experimental study on the variation of physical property parameters of multi-component gases with high CO2 content. In Proceedings of the 2023 International Conference on Oil and Gas Exploration and Development, Wuhan, China, 20 September 2023. [Google Scholar]
- Yang, H.; Dejam, M.; Tan, S.P.; Adidharma, H. Experimental study on phase transitions of carbon dioxide confined in nanopores: Evaporation, melting, sublimation, and triple point. Langmuir 2023, 39, 16060–16068. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.L.; Ma, Y.T.; Zeng, X.Y.; Liu, S.C. Study on the properties of CO2 fluid at supercritical pressure. Fluid Mach. 2008, 1, 53–57. [Google Scholar]
- Pan, J.N.; He, H.X.; Li, G.F.; Wang, X.L.; Hou, Q.L.; Liu, L.L.; Cheng, N.N. Anisotropic strain of anthracite induced by different phase CO2 injection and its effect on permeability. Energy 2023, 284, 128619. [Google Scholar] [CrossRef]
- Li, Y.F.; Dong, X.Y.; Wang, W.S.; Liu, L.; Zhao, Y.F. Numerical study on heat transfer of dry ice sublimation spray cooling on the surface of micro-ribbed plate. CIESC J. 2024, 75, 1830–1842. [Google Scholar]
- Fang, X.Y.; Wang, H.Y.; Tan, B.; Wang, F.R.; Shao, Z.Z.; Cheng, G.; Yao, H.F. Experimental comparison study of CO2 and N2 inerted loose coal based on atmospheric pressure gas replacement. Fuel 2022, 328, 125347. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z.X.; Wang, J.R.; Lu, B.; Gao, D.M. Effects of inert gas CO2/N2 injection on coal low- temperature oxidation characteristic: Experiments and simulations. Arab. J. Chem. 2022, 16, 104510. [Google Scholar] [CrossRef]
- Jin, Z.X.; Wu, S.Y.; Deng, C.B.; Dai, F.W. Competitive adsorption behavior and mechanism of different flue gas proportions in coal. J. China Coal Soc. 2017, 42, 1201–1206. [Google Scholar]
- Zheng, Y.N.; Li, Q.Z.; Zhang, G.Y.; Zhao, Y.; Zhu, P.F.; Ma, X.; Liu, X.X. Effect of multi-component gases competitive adsorption on coal spontaneous combustion characteristics under goaf conditions. Fuel Process Technol. 2020, 208, 106510. [Google Scholar] [CrossRef]
- Abunowara, M.; Bustam, M.A.; Sufian, S.; Babar, M.; Eldemerdash, U.; Mukhtar, A.; Ullah, S. High pressure CO2 adsorption onto Malaysian mukah-balingian coals: Adsorption isotherms, thermodynamic and kinetic investigations. Environ. Res. 2022, 218, 114905. [Google Scholar] [CrossRef]
- Day, S.; Duffy, G.; Sakurovs, R.; Weir, S. Effect of coal properties on CO2 sorption capacity under supercritical conditions. Int. J. Greenh. Gas Control 2008, 2, 342–352. [Google Scholar] [CrossRef]
- Zhai, X.W.; Wang, T.Y. Experimental study on cooling laws of liquid CO2 on high temperature coal. Saf. Coal Mines 2018, 49, 30–33. [Google Scholar]
- Ma, L.; Deng, J.; Wang, W.F.; Wang, Z.P. Experimental study of effect of CO2 on low temperature oxidation reaction process for coal. J. Xi’an Uni. Sci. Technol. 2014, 34, 379–383. [Google Scholar]
- Ma, L.; Yu, W.C.; Ren, L.F.; Qin, X.Y.; Wang, Q.H. Micro-characteristics of low-temperature coal oxidation in CO2/O2 and N2/O2 atmospheres. Fuel 2019, 246, 259–267. [Google Scholar] [CrossRef]
- Miao, J.R.; Yang, S.Q.; Jiang, X.Y.; Hou, Z.S.; Shao, H.D. Effect of the sudden change of ambient atmosphere on free radicals in coal body by CO2 fire prevention gas injection. Energy Sources Part A 2023, 45, 829–840. [Google Scholar] [CrossRef]
- Zhang, C.S.; Zhang, X.H. Fire Prevention and Extinguishing Technology with Canned Liquid Carbon Dioxide. Saf. Coal Mines 2016, 47, 82–84. [Google Scholar]
- Cao, N.F.; Liang, Y.T. Mechanism of fire prevention with liquid carbon dioxide and application of long-distance pressure-holding transportation technology based on shallow buried and near-horizontal goaf geological conditions. J. Chem. 2021, 2021, 5572963. [Google Scholar] [CrossRef]
- Ou, S.N.; Liu, J.G.; Jin, L.Z.; Sun, Z.C. Inhibition effect of CO2 and N2 on the explosion and spontaneous combustion performance of silo mixed coal. J. China Univ. Min. Technol. 2020, 2, 387–394. [Google Scholar]
- Zhang, F.J.; Liu, W.; Qin, Y.P.; Han, D.Y.; Guo, M.Y.; Chu, X.Y. Numerical investigation of gas emission and extraction under the action of coal-oxygen recombination in longwall gobs. J. Nat. Gas Sci. Eng. 2024, 124, 205258. [Google Scholar] [CrossRef]
- Qi, X.Y.; Li, Q.Z.; Zhang, H.J.; Xin, H.H. Thermodynamic characteristics of coal reaction under low oxygen concentration conditions. J. Energy Inst. 2017, 90, 544–555. [Google Scholar] [CrossRef]
- Lin, L.; Li, Z.X.; Zhang, M.Q.; Miao, C.T.; Jia, N.; Liu, Y. Study on Spontaneous Combustion Law and Prevention Technology of Abandoned Coal in Goaf of Coal Mine. Geofluids 2023, 2023, 5535774. [Google Scholar] [CrossRef]
- Cheng, G.; Wang, H.Y.; Tan, B.; Fu, S.H. Carbon dioxide prevents oxygen adsorption at low-temperature oxidation stage of low-rank coal: Laboratory study and molecular simulation. Processes 2023, 11, 2504. [Google Scholar] [CrossRef]
- Ge, L.; Shao, Y.M.; Wang, Y.J.; Zhang, G.X.; Zhang, Z.J.; Liu, L. Experimental research on inerting characteristics of carbon dioxide used for fire extinguishment in a large sealed space. Process Saf. Environ. 2020, 142, 174–190. [Google Scholar] [CrossRef]
- Luo, Z.M.; Su, B.; Cheng, F.M.; Wang, T.; Shu, C.M.; Li, Y.Q. Influences of ethane on the flammable limits and explosive oxygen concentration of methane with nitrogen dilution. J. Loss Prevent. Proc. 2018, 56, 478–485. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, X.S.; Deng, J.; Wen, H.; Xiao, Y.; Jia, H. Research on coal spontaneous combustion period based on pure oxygen adiabatic oxidation experiment. Fuel 2021, 288, 119651. [Google Scholar] [CrossRef]
- Zhai, X.W.; Ge, H.; Obracaj, D. The cooling range of liquid CO2 on loose coal through experimental investigation. Int. J. Oil Gas Coal Technol. 2021, 27, 54–77. [Google Scholar] [CrossRef]
- Zhou, B.Z.; Yang, S.Q.; Yang, W.M.; Jiang, X.Y.; Song, W.X.; Cai, J.W.; Xu, Q.; Tang, Z.Q. Variation characteristics of active groups and macroscopic gas products during low-temperature oxidation of coal under the action of inert gases N2 and CO2. Fuel 2021, 307, 121893. [Google Scholar] [CrossRef]
- Wang, F.; Liu, H.W. Comparative experiment study on fire prevention and extinguishing in goaf by N2-water mist and CO2-water mist. Arab. J. Geosci. 2020, 13, 856. [Google Scholar] [CrossRef]
- Baran, P.; Jodlowski, G.S.; Zarebska, K. Sorption of CO2 in lignites from Polish coal mines: Measurements and thermodynamic analysis. Adsorption 2016, 22, 839–846. [Google Scholar] [CrossRef]
- Zhu, H.Q.; Wang, W.; Huo, Y.J.; He, X.; Zhao, H.R.; Wang, H.R. Molecular Simulation Study on Adsorption and Diffusion Behaviors of CO2/N2 in Lignite. ACS Omega 2020, 5, 29416–29426. [Google Scholar] [CrossRef]
- Fang, X.Y.; Tan, B.; Wang, H.Y.; Wang, F.R.; Li, T.Z.; Wan, B.; Xu, C.F.; Qi, Q.J. Experimental study on the displacement effect and inerting differences of inert gas in loose broken coal. Energy 2024, 289, 130102. [Google Scholar] [CrossRef]
- Wu, W.Y.; Huang, W.X.; Wei, A.Z.; Schmidt, M.; Krause, U.; Wu, D.J. Inhibition effect of N2/CO2 blends on the minimum explosion concentration of agriculture and coal dusts. Powder Technol. 2022, 399, 117195. [Google Scholar] [CrossRef]
- Yu, Z.J.; Gu, Y.; Yang, S.; Deng, J. Temperature characteristic of crushed coal under liquid coolant injection: A comparative investigation between CO2 and N2. J. Therm. Anal. Calorim. 2020, 144, 363–372. [Google Scholar] [CrossRef]
- Li, D.D.; Zhou, Y.; Shen, Y.H.; Sun, W.N.; Fu, Q.; Yan, H.Y.; Zhang, D.H. Experiment and simulation for separating CO2/N2 by dual-reflux pressure swing adsorption process. Chem. Eng. J. 2016, 297, 315–324. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Shimizu, G.K.H.; Rajendran, A. CO2/N2 separation by vacuum swing adsorption using a metal-organic framework, CALF-20: Multi-objective optimization and experimental validation. Chem. Eng. J. 2023, 452, 139550. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, S.Q.; Xu, Y.; Yao, Q. Experimental and theoretical analyses on ignition and surface temperature of dispersed coal particles in O2/N2 and O2/CO2 ambients. Fuel 2016, 201, 93–98. [Google Scholar] [CrossRef]
- Zhu, H.Q.; He, X.; Xie, Y.Y.; Guo, S.; Huo, Y.J.; Wang, W. A Study on the Effect of Coal Metamorphism on the Adsorption Characteristics of a Binary Component System: CO2 and N2. ACS Omega 2021, 6, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Cao, Y.J.Z.; Tien, C.J. Method for prevention and control of spontaneous combustion of coal seam and its application in mining field. Int. J. Min. Sci. Technol. 2017, 5, 839–846. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, G.; Liao, S.Y.; Cheng, Q.; Xiang, C.X.; Yuan, C. Comparative study on the effects of nitrogen and carbon dioxide on methane/air flames. Energy 2016, 106, 431–442. [Google Scholar] [CrossRef]
- Cui, Y.J.; Zhang, Q.; Zhang, H.; Zhang, Q.L. Adsorption of CH4, N2 and CO2 by different coal grades. Nat. Gas Ind. 2005, 25, 61–65. [Google Scholar]
- Si, J.H.; Li, L.; Cheng, G.Y.; Shao, H.; Wang, Y.Q.; Li, Z.Q. Characteristics and Safety of CO2 for the fire prevention technology with gob-side entry retaining in goaf. ACS Omega 2021, 6, 18518–18526. [Google Scholar] [CrossRef]
- Pang, L.; Zhao, Y.; Yang, K.; Zhai, H.; Lv, P.F.; Sun, S.H. Law of variation for low density polyethylene dust explosion with different inert gases. J. Loss Prevent. Proc. 2019, 58, 42–50. [Google Scholar] [CrossRef]
- Wang, Z.P.; Ma, L.; Wen, H.; Wang, X.Y. A Experiment contrast study of CO2 and N2 inhibited oxidation of spontaneous combustion of coal. Saf. Coal Mines 2010, 41, 14–17. [Google Scholar]
- Ma, L.; Gao, Y.; Yang, H.S. Application of inert gas technology in prevention and control of local fire in tail lane. Saf. Coal Mines. 2020, 51, 145–149. [Google Scholar]
- Liu, W.; Chu, X.Y.; Xu, H.; Chen, W.; Ma, L.W.; Qin, Y.P.; Wei, J. Oxidation reaction constants for coal spontaneous combustion under inert gas environments: An experimental investigation. Energy 2022, 247, 123457. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Z.; Zhai, X.W.; Wen, H. Thermodynamic behaviors of coal spontaneous combustion under different CO2 concentration. Int. J. Coal Prep. Util. 2022, 43, 1583–1596. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Wang, D.M. Experimental study on the inhibition effects of nitrogen and carbon dioxide on coal spontaneous combustion. Energies 2022, 12, 5256. [Google Scholar] [CrossRef]
- Li, S.Y. Experimental Study on Performance That Carbon Dioxide Inhibits Coal Oxidation and Spontaneous Combustion. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2008. [Google Scholar]
- Liu, S.M. Experimental study on CO2 concentration affected to low temperature coal oxidation. Coal Sci. Technol. 2014, 42, 149–151. [Google Scholar]
- Wang, K.; Du, Y.T.; Zhai, X.W.; Deng, J.; Gao, P. Migration and cooling characteristics of low-temperature gaseous CO2 in high-temperature loose coal. Energy 2024, 301, 131741. [Google Scholar] [CrossRef]
- Hao, C.Y.; Wang, J.R.; Huang, G.; He, F. Study on inject position of low temperature CO2 in gob based on coupling effect of inerting cooling. J. Saf. Sci. Technol. 2015, 11, 17–23. [Google Scholar]
- Wang, J.R.; Zhang, Y.; Hao, C.Y. Numerical simulation of fire prevention in gob area with half O-ring risked falling by CO2 injection. China Saf. Sci. J. 2015, 25, 48–54. [Google Scholar]
- Li, Z.X.; Liu, Y.; Wang, Z.; Lin, L. Study on numerical simulation of CO2 injection fire prevention and extinguishing technique in goaf of Jiudaoling Mine. Coal Sci. Technol. 2018, 46, 153–157. [Google Scholar]
- Bai, G.; Ling, H.J.S.; Li, Y.Q.; Zhou, X.H.; Tang, X.Y. Numerical simulation of CO2 fire preventing and extinguishing based on Box-Behnken design. Saf. Coal Mines 2023, 54, 117–122. [Google Scholar]
- Song, X.L.; Wu, C.L.; Liu, C.; Li, Y.L.; Wang, Z.Y.; Shi, B.B. Study on the inerting effect and migration law of nitrogen and carbon dioxide in large inclined goaf by physical simulation model. Geofluids 2022, 2022, 9295411. [Google Scholar] [CrossRef]
- Yao, H.F.; Hu, J.; Zhang, L.; Hu, S.P.; Wang, Y.Q.; Mao, Y.Q.; Mao, X.O.W.; Liu, D.Y.; Cao, K.B.; Zhao, Y.X. Study on inhibition of spontaneous combustion of coal by liquid CO2. Solid Fuel Chem. 2023, 57, 513–518. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, Z.J.; Wang, Y.J.; Zhang, S.T.; Chen, Y.J. Investigation on a mobile fire extinguishing approach using liquid carbon dioxide as inert medium for underground mine. PLoS ONE 2024, 19, e0299940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, X.X.; Wen, H.; Zhang, S.S.; Wang, H.Q.; Sun, Y.; Feng, H. Effect of nucleating agents on fire prevention of dry ice from compound inert gas. Energy 2024, 286, 129635. [Google Scholar]
- Yan, X.Q.; Guo, X.L.; Liu, Z.G.; Yu, J.L. Release and dispersion behaviour of carbon dioxide released from a small-scale underground pipeline. J. Loss. Prevent. Proc. 2016, 43, 165–173. [Google Scholar] [CrossRef]
- Lv, Y.H. Application of Liquid Carbon Dioxide Injection in Ground Drilling for Fire Preventing and Extinguishing in Goaf. Saf. Coal Mines 2020, 51, 179–183. [Google Scholar]
- Qin, B.T.; Zhong, X.X.; Wang, D.M.; Xin, H.H.; Shi, Q.L. Research progress of coal spontaneous combustion process characteristics and prevention technology. Coal Sci. Technol. 2021, 49, 66–99. [Google Scholar]
- Wang, G. Application and analysis of liquid CO2 infusion technology in mine fire prevention and control. Coal Sci. Technol. 2017, 45, 89–93. [Google Scholar] [CrossRef]
- Dieken, D. Fighting Fire with CO2. Power Eng. 2008, 112, 80–82. [Google Scholar]
- Liu, C.S. Fire Fighting of Wind Extinguisher with CO2 Gas Assisted. Appl. Mech. Mater. 2011, 130–134, 1054–1057. [Google Scholar] [CrossRef]
- Zhao, M.C. Application of large flow gasous CO2 fire control technology in control of spontaneous combustion fire in goaf. Saf. Coal Mines 2021, 52, 122–126. [Google Scholar]
- Wang, S.M.; Shen, Y.J.; Sun, Q.; Liu, L.; Shi, Q.M.; Zhu, M.B.; Zhang, B.; Cui, S.D. Underground CO2 storage and technical problems in coal mining area under the “dual carbon” target. J. China Coal Soc. 2022, 47, 45–60. [Google Scholar]
- Ge, L. Underground and mobile liquid carbon dioxide fire extinguishing device. Saf. Coal Mines 2016, 47, 98–101. [Google Scholar]
- Zagorscak, R.; Thomas, H.R. Dynamic transport and reaction behaviour of high-pressure gases in high-rank coal. J. Nat. Gas Sci. Eng. 2019, 71, 102978. [Google Scholar] [CrossRef]
- Hirsch, D. Carbon dioxide fire suppressant concentration needs for international space station environments. J. Fire Sci. 2002, 20, 391–399. [Google Scholar] [CrossRef]
- Deng, J.; Xi, H.J.; Zhai, X.W.; Zhang, Y.N. Key parameters of liquid CO2 perfusion for fire control in coal mine goaf. J. Xi’an Univ. Sci. Technol. 2017, 37, 605–609. [Google Scholar]
- Fan, S.X.; Wen, H.; Chen, X.J.; Zhang, C.R.; Wei, G.M. Research and application of a complete set equipment of permeability enhancements induced by high-pressure L—CO2 fracturing. J. China Coal Soc. 2020, 45, 801–812. [Google Scholar]
- Fan, S.X.; Wen, H.; Jin, Y.F.; Chen, J.; Ton, X.Z. Initiation pressure model for liquid CO2 fracturing through upward penetrating boreholes and its engineering verification. Chin. J. Rock Mech. Eng. 2021, 40, 703–712. [Google Scholar]
- Liu, W.; Qin, Y.P.; Yang, X.B.; Wang, W.Q.; Chen, Y.Q. Early extinguishment of spontaneous combustion of coal underground by using dry-ice’s rapid sublimation: A case study of application. Fuel 2018, 217, 544–552. [Google Scholar] [CrossRef]
- Qin, Y.P.; Guo, W.J.; Xu, H.; Song, Y.P.; Chen, Y.Q.; Ma, L.W. A comprehensive method to prevent top-coal spontaneous combustion utilizing dry ice as a fire extinguishing medium: Test apparatus development and field application. Environ. Sci. Pollut. Res. 2021, 29, 19741–19751. [Google Scholar] [CrossRef]
- Zeng, C.L.; Shan, M.T. Application of dry ice fire-extinguishing technology in erjing coal mine. Saf. Coal Mines. 2018, 49, 125–127. [Google Scholar]
- Dong, W. Application of liquid CO2 high efficiency fire prevention technology in Yangchangwan Coal Mine. Coal Safty/Green Mining Disaster Prevention and Control technology. In Proceedings of the 2016 National Coal Mine Safety Academic Conference, Ji’an, China, 3 November 2016. [Google Scholar]
- National Mine Safety Supervision Bureau. Coal Mine Fire Prevention and Extinguishing Rules, 2021st ed; Emergency Management Publishing House: Beijing, China, 2021. [Google Scholar]
- State Administration of Work Safety; State Coal Mine Safety Supervision Bureau. Coal Mine Safety Regulations, 2022nd ed.; Coal Industry Publishing House: Beijing, China, 2022. [Google Scholar]
- Zhou, G.H. Study on Mechanism of Preventing Coal Spontaneous Combustion with Liquid Carbon Dioxide and the Effective Fire-Fighting Technology in Goaf. Master’s Thesis, Xi’an University of Science and Technology, Xi’an, China, 2019. [Google Scholar]
- Jing, J.D. Research on Fire Prevention and Extinguishing Technology of Liquid Carbon Dioxide in Yangchangwan Coal Mine. Master’s Thesis, China University of Mining and Technology, Beijing, China, 2019. [Google Scholar]

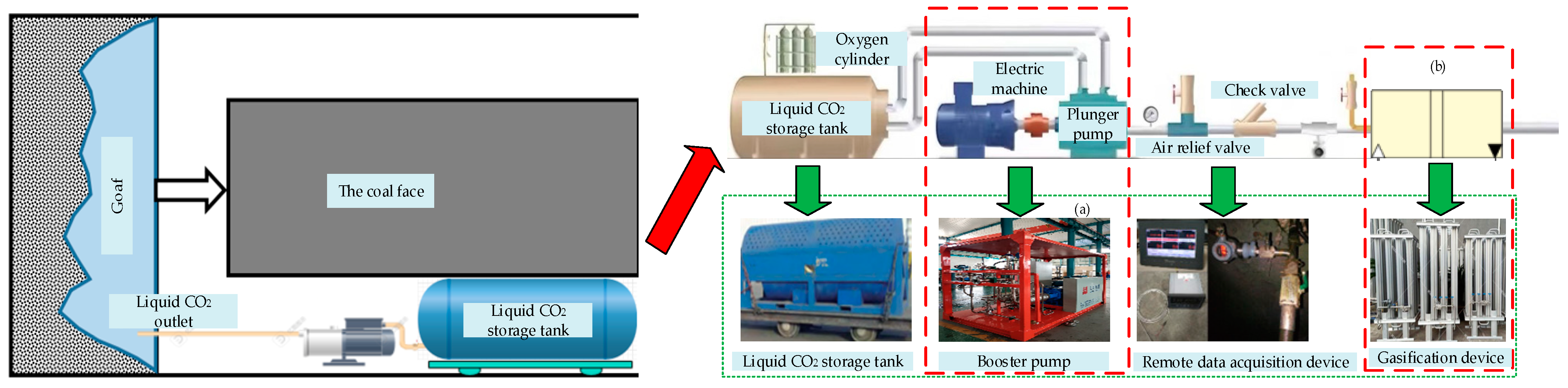
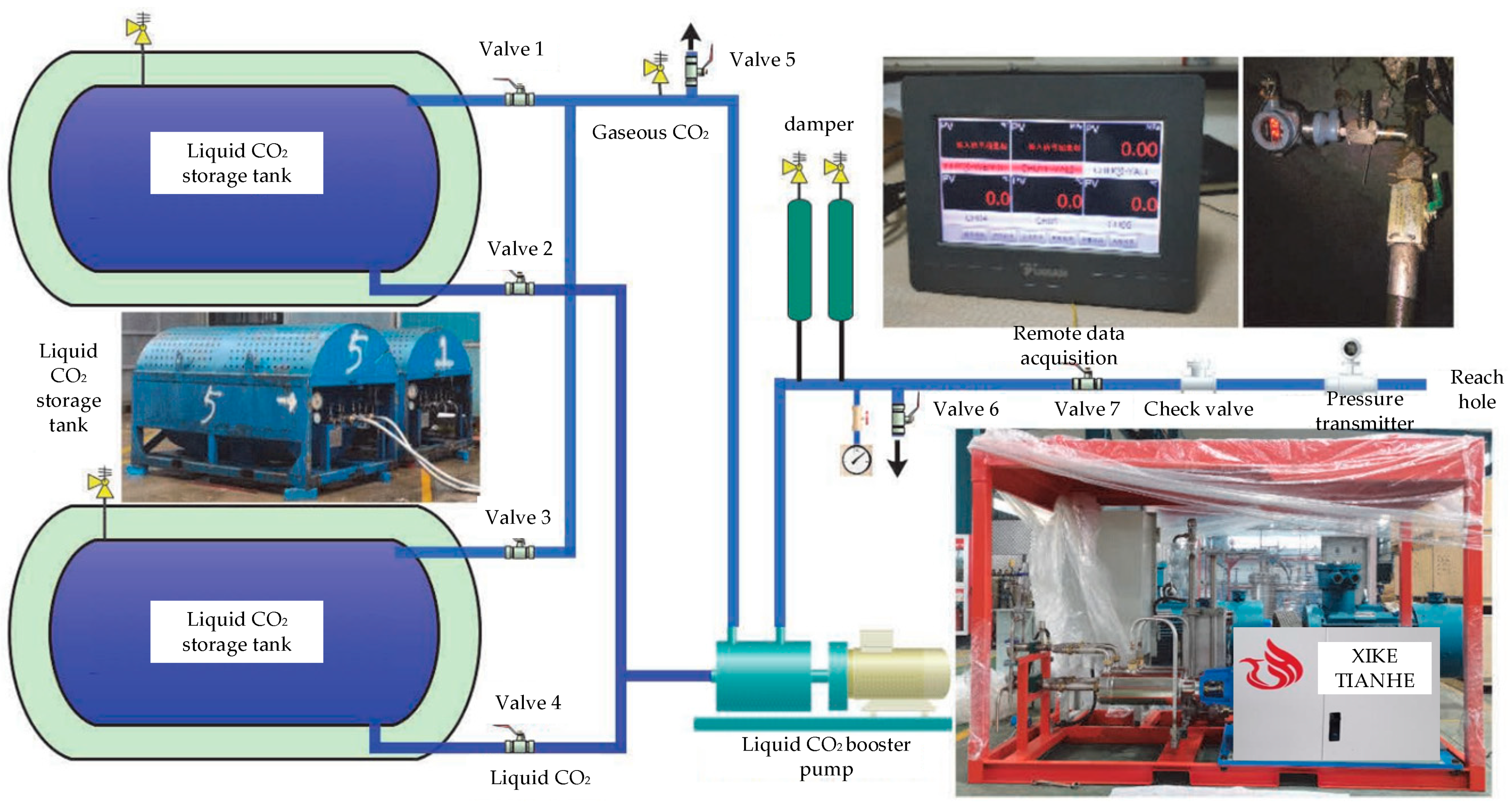
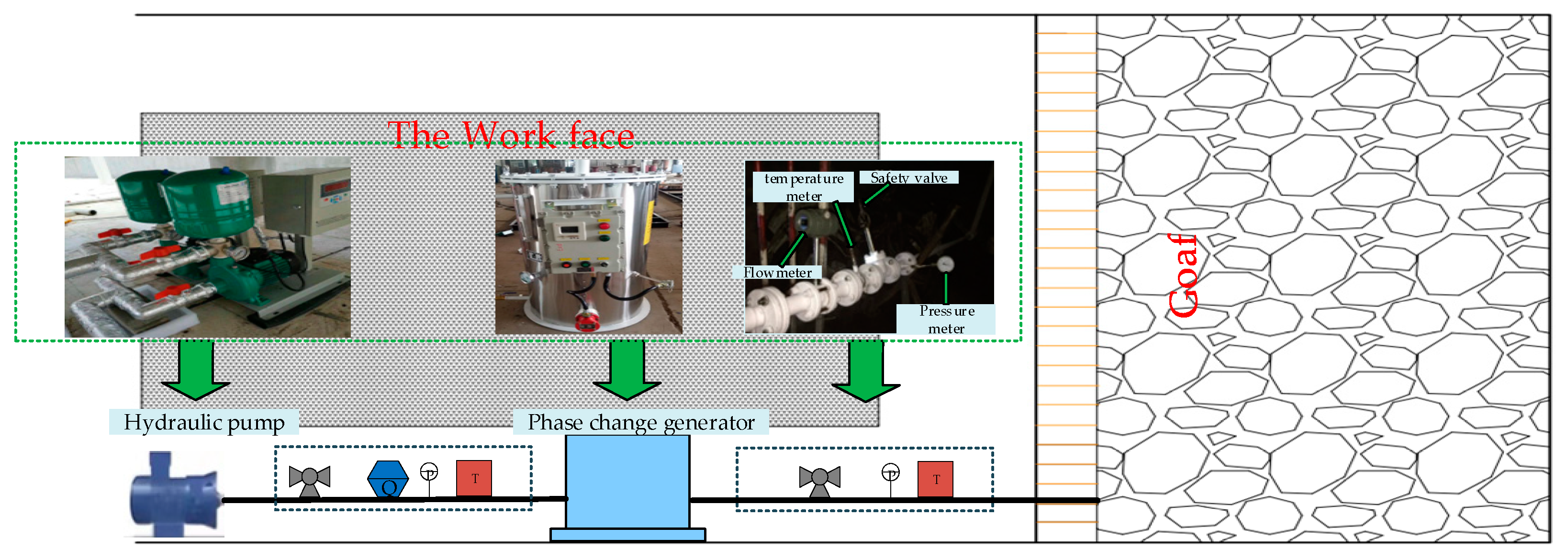
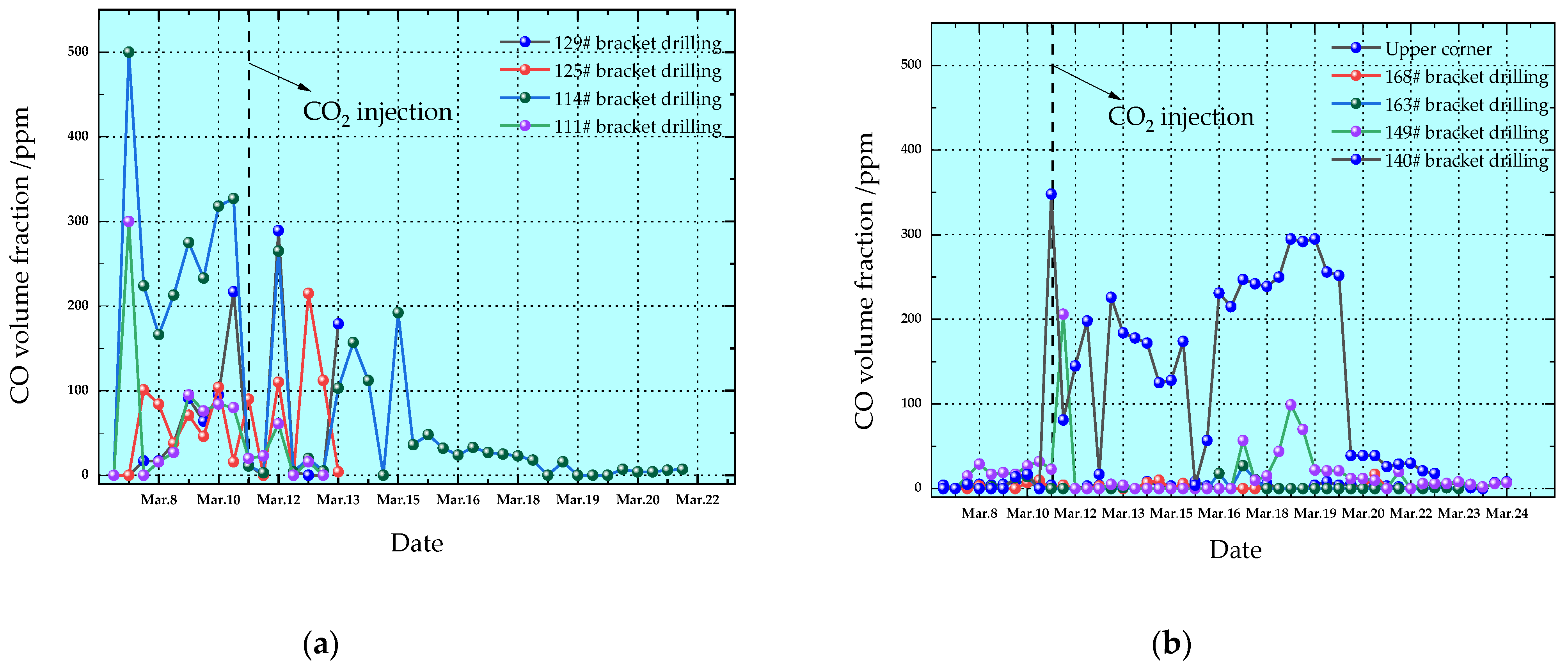

| Parameter Name | Parameter Value |
|---|---|
| Relative molecular mass | 44.01 |
| Density | 1.97 kg/m3 |
| Relative density | 1.53 |
| Melting point (0.52 MPa) | −56.6 °C |
| Boiling point (0.1 MPa) | −78.5 °C |
| Heat of gasification | 573.6 kJ/kg |
| Heat of melting | 198.9 kJ/kg |
| Heat of Sublimation | 151.6 kJ/kg |
| Specific volume (21.1 °C, l atm) | 0.5457 m3/kg |
| Vapor pressure (20 °C) | 7.17 MPa |
| Contrast Parameters | CO2 | N2 | Differences between CO2 and N2 |
|---|---|---|---|
| Liquid purity (%) | Close to 100 | Close to 97 | All conform to the coal mine safety regulations |
| Relative density | 1.53 | 0.967 | The submergence effect of CO2 is good. |
| Adsorption capacity of coal (L/kg) | 48 | 8 | The adsorption capacity of CO2 is 6 times that of N2. |
| Maximum oxygen concentration in inert gas (%) | 0 | 2~3 | Separation of liquid CO2 with high purity |
| Critical oxygen concentration for explosion prevention (%) | 14.6 | 11.5 | The critical oxygen concentration of CO2 is high |
| Critical oxygen concentration for extinguishing an open flame (%) | 12 | 9.5 | The critical oxygen concentration of the extinguished flame of CO2 is high. |
| Gas volume expansion | 600 | 700 | The volume expansion of CO2 gas is slightly small |
| Application scenario | Pole tilt face fire | Fire on the upper stratified face | The application scenarios are different and the fire prevention and extinguishing gas is selected according to the conditions of the field working face. |
| Fire of Extinguishing and Preventing System | Ground Liquid CO2 Pressure Maintaining Direct Injection | Ground Liquid CO2 Gasification Pressure Injection | Downhole Mobile Liquid CO2 Pressure Injection | Downhole Mobile Liquid CO2 Gasification Pressure Injection | Dry Ice Phase-Change Fire Prevention |
|---|---|---|---|---|---|
| Device | Liquid CO2 storage tank car, gasification device, pressurization device, flow control valve, pressure meter (P), temperature meter (T), flow meter (Q), etc. | Gasification device, gas storage tank, gas pressure control unit, safety valve, pressure meter (P), temperature meter (T), flow meter (Q), control valve, etc. | Liquid storage tank, booster pump, safety valve, pressure meter (P), temperature meter (T), flow meter (Q), control valve, etc. | Liquid storage tank, gasification device, safety valve, pressure meter (P), temperature meter (T), control valve, etc | Dry ice phase-change generator, injection pipe safety valve, pressure meter (P), temperature meter (T), flow meter (Q), control valve, etc. |
| Pipeline | High-pressure stainless-steel pipe, sealing, pressure control and other conditions are required | High-pressure hose or steel pipe | High-pressure hose or steel pipe | High-pressure hose or steel pipe | High-pressure hose or steel pipe |
| Ease of operation | Very demanding | High demand | High demand | High demand | High demand |
| Pressure injection capacity | Continuous injection, up to 60 t/h | It can reach 5–10 t/h | It can reach 5–10 t/h | It can reach 5–10 t/h | It can reach 5–10 t/h |
| Cooling effect | The outlet temperature is −25 to 10 °C, and the dry ice formed can reach −60 °C | Outlet temperature −2 to 5 °C | Outlet temperature −1 to 0 °C | Outlet temperature −5 to 5 °C | Outlet temperature −5 to 0 °C |
| Line clogging | The possibility of a pressure-holding section is low | The ground may partially appear | The ground may partially appear | It may appear at the end of the pipe | It may appear at the end of the pipe |
| Scope of application | Prevent coal spontaneous combustion in key underground areas; Control and direct extinguishing of large fire areas | Large area underground fire prevention | Small fire area control and emergency response | Small fire area control and emergency response | Large area underground fire prevention |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Gao, B.; Liu, Y.; Chen, C.; Cai, G.; Wang, L. Research and Application of CO2 Fire Prevention Mechanism and Key Technologies in Mines: A Review. Fire 2024, 7, 353. https://doi.org/10.3390/fire7100353
Guo J, Gao B, Liu Y, Chen C, Cai G, Wang L. Research and Application of CO2 Fire Prevention Mechanism and Key Technologies in Mines: A Review. Fire. 2024; 7(10):353. https://doi.org/10.3390/fire7100353
Chicago/Turabian StyleGuo, Jun, Bo Gao, Yin Liu, Changming Chen, Guobin Cai, and Lei Wang. 2024. "Research and Application of CO2 Fire Prevention Mechanism and Key Technologies in Mines: A Review" Fire 7, no. 10: 353. https://doi.org/10.3390/fire7100353
APA StyleGuo, J., Gao, B., Liu, Y., Chen, C., Cai, G., & Wang, L. (2024). Research and Application of CO2 Fire Prevention Mechanism and Key Technologies in Mines: A Review. Fire, 7(10), 353. https://doi.org/10.3390/fire7100353






