Fire-Induced Vegetation Dynamics: An In-Depth Discourse on Revealing Ecological Transformations of the Mahaban and Surrounding Forests
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Vegetation Sampling
2.4. Environmental Gradients
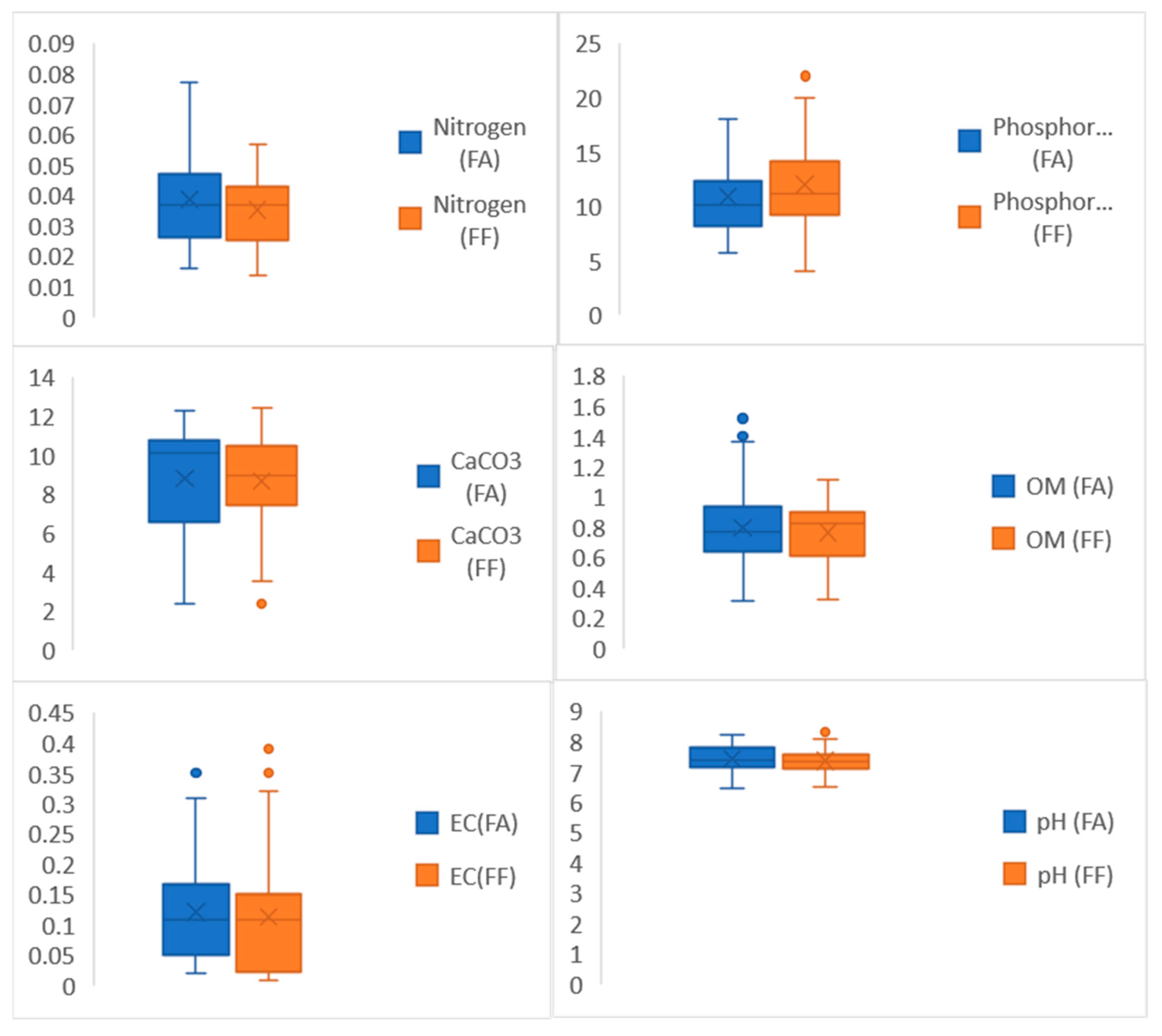
2.5. Soil Analysis
2.6. Data Analysis
3. Results
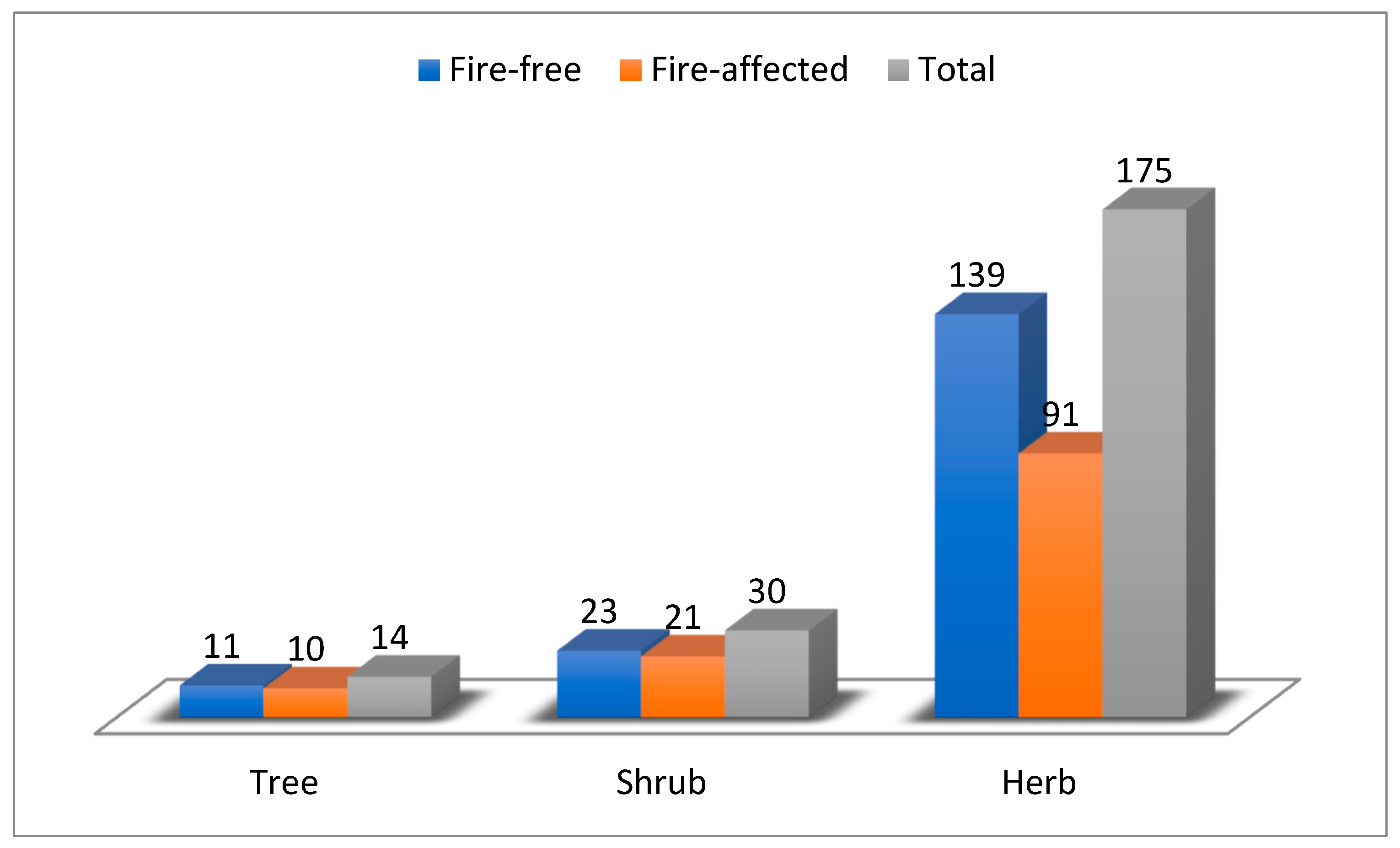
3.1. Floristic Composition
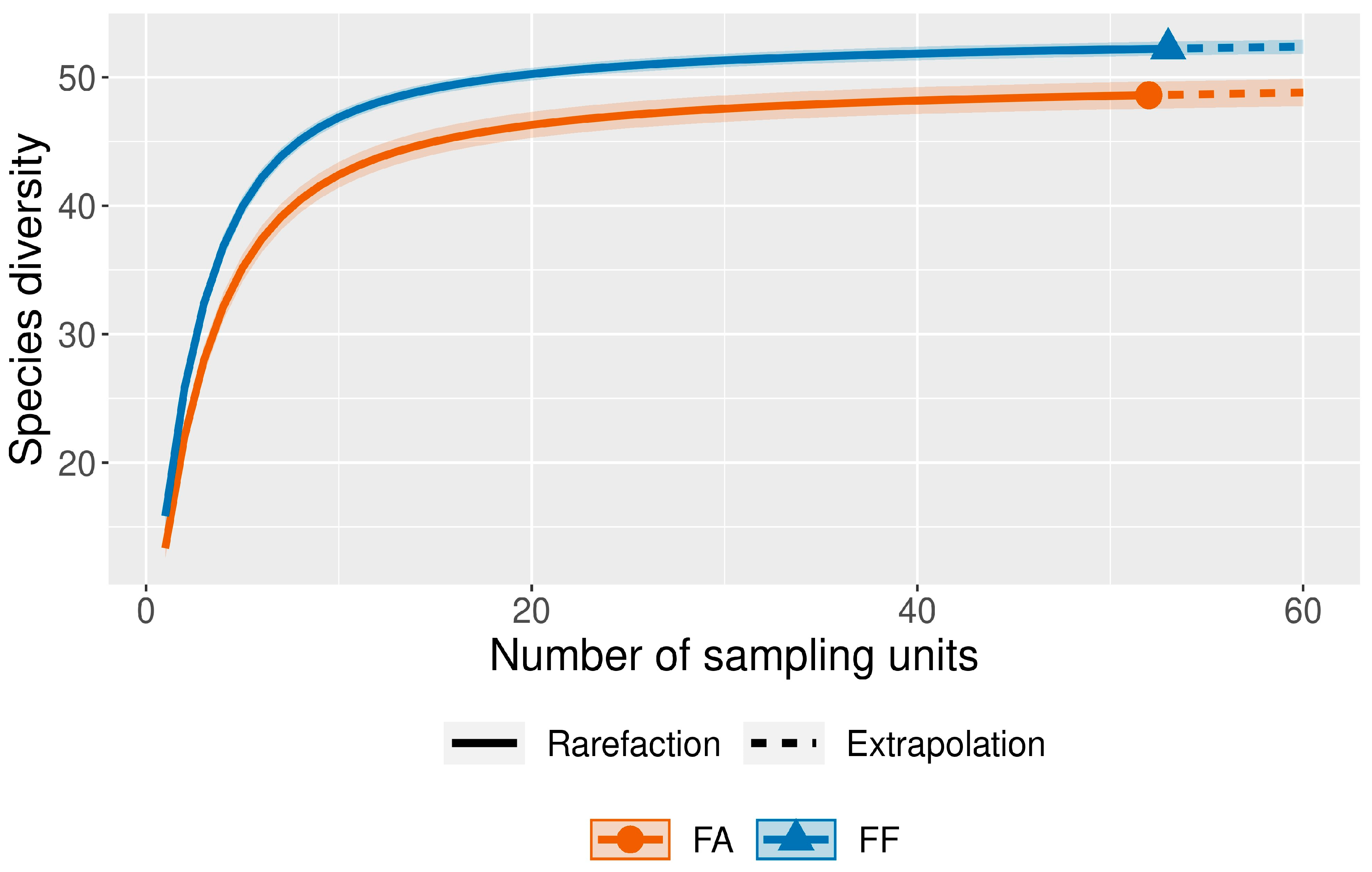
3.2. Species Diversity Indices
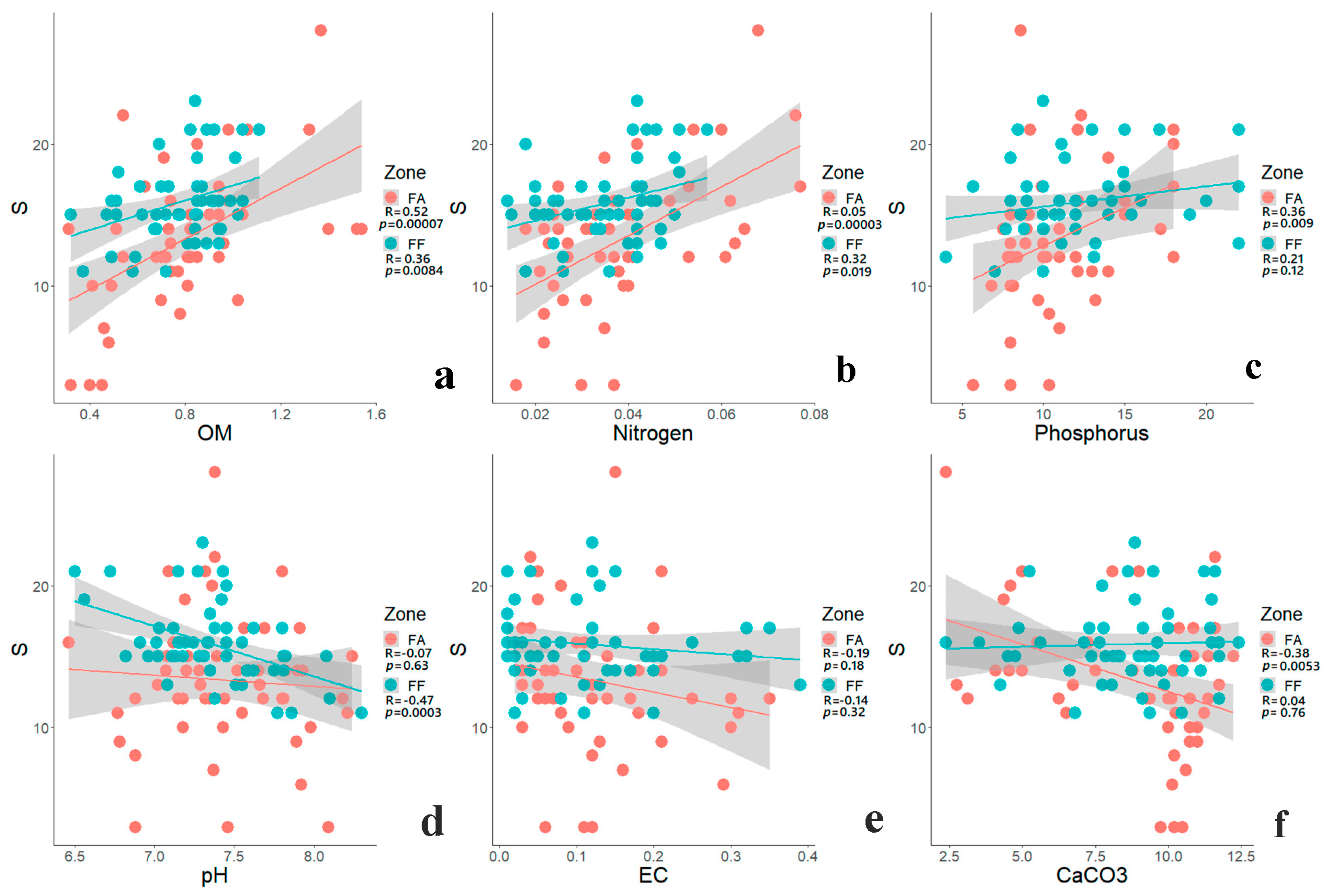
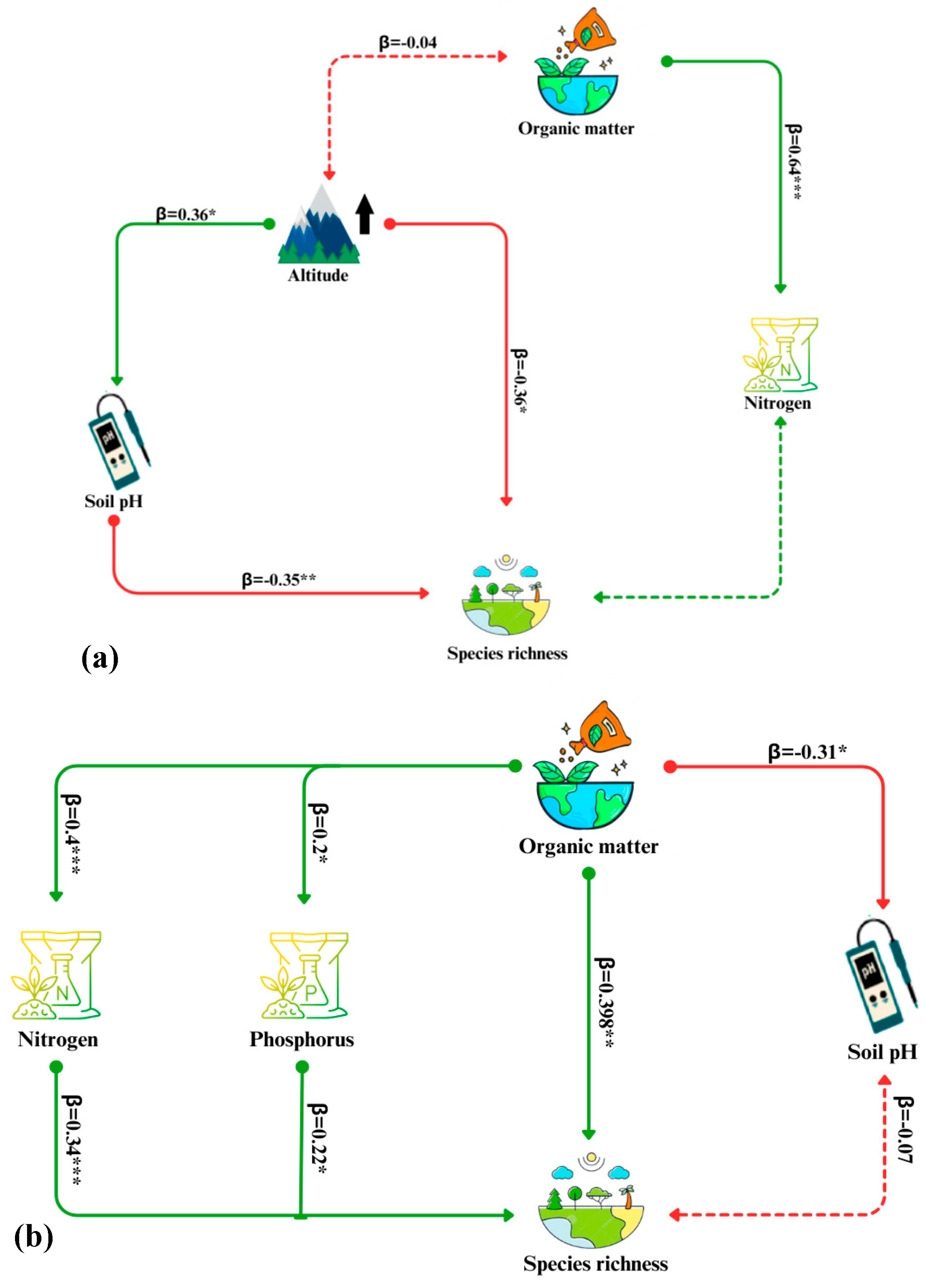
3.3. Impact of Edaphic Factors on Species Richness
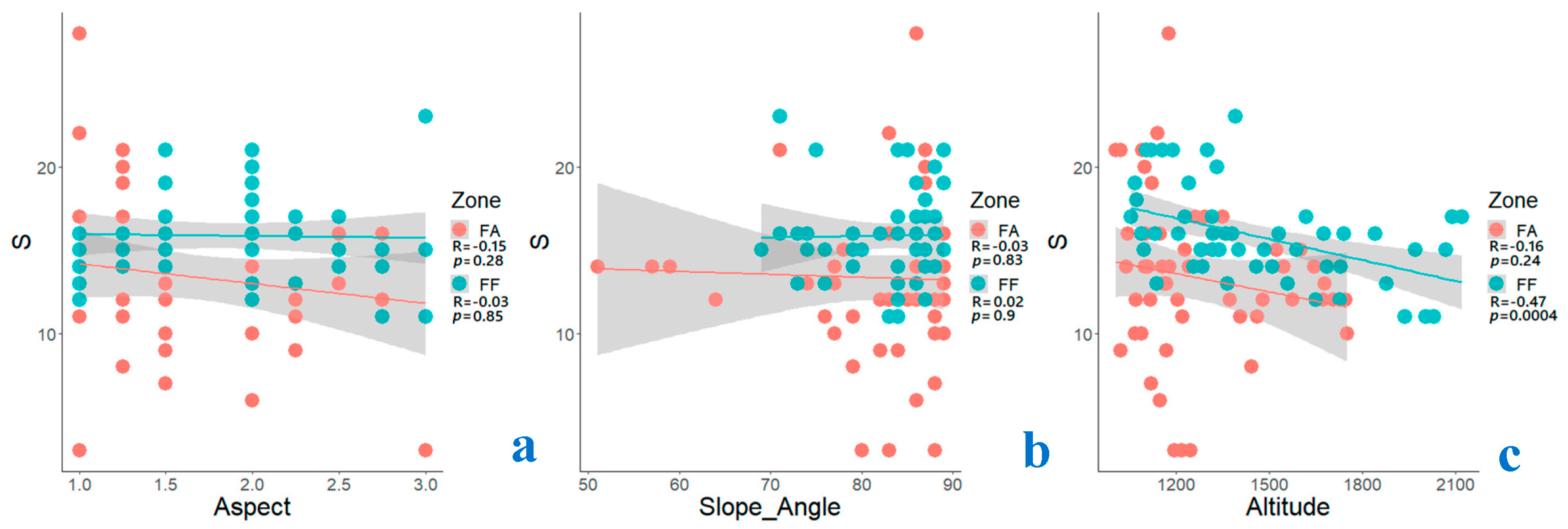
3.4. Impact of Environmental Gradients on Species Richness
3.5. Structure Equation Modeling
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Fire-Free Areas | Fire-Affected Areas | |||||
|---|---|---|---|---|---|---|
| Sr.No. | Plant Name | Habit | D | Plant Name | Habit | D |
| 1 | Pinus roxburghii Sarg. | Tree | 0.23777 | Pinus roxburghii Sarg. | Tree | 0.308946 |
| 2 | Berberis lycium Royle | Shrub | 0.074977 | Cynodon dactylon (L.) Pers. | Herb | 0.073945 |
| 3 | Cynodon dactylon (L.) Pers. | Herb | 0.070309 | Zanthoxylum armatum DC. | Shrub | 0.072871 |
| 4 | Zanthoxylum armatum DC. | Shrub | 0.048806 | Carissa spinarum L. | Shrub | 0.048141 |
| 5 | Quercus leucotrichophora A.Camus | Tree | 0.037879 | Rubus ellipticus Sm. | Shrub | 0.042647 |
| 6 | Dodonaea viscosa (L.) Jacq. | Shrub | 0.034698 | Berberis lycium Royle | Shrub | 0.04145 |
| 7 | Mallotus philippensis (Lam.) Müll.Arg. | Shrub | 0.029749 | Agrostis stolonifera L. | Herb | 0.024808 |
| 8 | Debregeasia saeneb (Forssk.) Hepper and J.R.I.Wood | Shrub | 0.027356 | Dodonaea viscosa (L.) Jacq. | Shrub | 0.022899 |
| 9 | Digitaria sanguinalis (L.) Scop. | Herb | 0.022099 | Digitaria sanguinalis (L.) Scop. | Herb | 0.020579 |
| 10 | Clinopodium serpyllifolium subsp. fruticosum (L.) Bräuchler | Shrub | 0.016923 | Desmostachya bipinnata (L.) Stapf | Herb | 0.015824 |
| 11 | Plantago lanceolata L. | Herb | 0.015587 | Salvia moorcroftiana Wall. ex Benth. | Herb | 0.013698 |
| 12 | Oplismenus compositus P.Beauv. | Herb | 0.015148 | Oxalis corniculata L. | Herb | 0.013586 |
| 13 | Desmostachya bipinnata (L.) Stapf | Herb | 0.01429 | Calendula arvensis M.Bieb. | Herb | 0.013026 |
| 14 | Rubus ellipticus Sm. | Shrub | 0.014026 | Geranium mascatense Boiss. | Herb | 0.012859 |
| 15 | Mimosa rubicaulis subsp. himalayana (Gamble) H.Ohashi | Shrub | 0.01381 | Mimosa rubicaulis subsp. himalayana (Gamble) H.Ohashi | Shrub | 0.011801 |
| 16 | Juncus inflexus L. | Herb | 0.012916 | Gymnosporia royleana M.A.Lawson | Shrub | 0.010727 |
| 17 | Adiantum capillus-veneris L. | Herb | 0.012766 | Rumex hastatus D. Don | Shrub | 0.010598 |
| 18 | Rumex hastatus D. Don | Shrub | 0.012337 | Colebrookea oppositifolia Sm. | Shrub | 0.01038 |
| 19 | Launaea secunda Hook.f. | Herb | 0.010385 | Adiantum pedatum L. | Herb | 0.010187 |
| 20 | Carissa spinarum L. | Shrub | 0.009804 | Achyranthes aspera L. | Herb | 0.010099 |
| 21 | Oxalis corniculata L. | Herb | 0.009693 | Grewia optiva J.R.Drumm. ex Burret | Tree | 0.009993 |
| 22 | Gymnosporia royleana M.A.Lawson | Shrub | 0.009395 | Erigeron floribundus (Kunth) Sch.Bip | Herb | 0.00843 |
| 23 | Ficus carica L. | Tree | 0.008816 | Cyperus alopecuroides Rottb. | Herb | 0.008414 |
| 24 | Melia azedarach L. | Tree | 0.007613 | Avena barbata Pott ex Link | Herb | 0.008146 |
| 25 | Olea ferruginea Wall. ex Aitch. | Tree | 0.007564 | Brachiaria ramosa Stapf | Herb | 0.00736 |
| 26 | Mentha longifolia (L.) L. | Herb | 0.00739 | Eragrostis minor Host | Herb | 0.007223 |
| 27 | Agrostis stolonifera L. | Herb | 0.007087 | Bombax ceiba L. | Tree | 0.005659 |
| 28 | Rhododendron arboreum Sm. | Tree | 0.006693 | Heteropogon contortus Beauv. ex Roem. and Schult. | Herb | 0.004973 |
| 29 | Grewia optiva J.R.Drumm. ex Burret | Tree | 0.006517 | Solanum nigrum L. | Herb | 0.004874 |
| 30 | Rumex dentatus L. | Herb | 0.006235 | Melia azedarach L. | Tree | 0.004398 |
Appendix B
| Fire-Free Areas | Fire Affected Areas | ||||
|---|---|---|---|---|---|
| Quadrats | Species Richness | Shannon Diversity | Quadrats | Species Richness | Shannon Diversity |
| S1Q1 | 18 | 2.487659 | FS1Q1 | 22 | 2.814788 |
| S1Q2 | 15 | 2.396967 | FS1Q2 | 21 | 2.655287 |
| S1Q3 | 16 | 2.324348 | FS1Q3 | 14 | 2.316964 |
| S2Q1 | 16 | 2.381747 | FS1Q4 | 13 | 2.252507 |
| S2Q2 | 17 | 2.346416 | FS1Q5 | 14 | 2.245333 |
| S2Q3 | 16 | 2.347237 | FS1Q6 | 14 | 2.255042 |
| S2Q4 | 14 | 2.260971 | FS2Q1 | 17 | 2.580328 |
| S2Q5 | 24 | 2.741961 | FS2Q2 | 14 | 2.268368 |
| S2Q6 | 16 | 2.420426 | FS2Q3 | 15 | 2.369721 |
| S2Q7 | 17 | 2.260509 | FS2Q4 | 12 | 2.258033 |
| S2Q8 | 17 | 2.431093 | FS2Q5 | 14 | 2.018089 |
| S2Q9 | 16 | 2.44746 | FS2Q6 | 12 | 2.062945 |
| S2Q10 | 16 | 2.513053 | FS2Q7 | 13 | 2.198842 |
| S3Q1 | 20 | 2.620321 | FS2Q8 | 14 | 2.334764 |
| S3Q2 | 19 | 2.597952 | FS2Q9 | 17 | 2.434357 |
| S3Q3 | 16 | 2.548764 | FS2Q10 | 12 | 2.185835 |
| S3Q4 | 22 | 2.794055 | FS2Q11 | 17 | 2.618541 |
| S3Q5 | 14 | 2.335251 | FS3Q1 | 12 | 2.079266 |
| S4Q1 | 18 | 2.668152 | FS3Q2 | 11 | 2.135406 |
| S4Q2 | 17 | 2.488219 | FS3Q3 | 11 | 1.98121 |
| S4Q3 | 22 | 2.837677 | FS3Q4 | 10 | 2.058286 |
| S4Q4 | 17 | 2.520994 | FS3Q5 | 8 | 1.77076 |
| S4Q5 | 22 | 2.720286 | FS3Q6 | 7 | 1.595613 |
| S4Q6 | 22 | 2.783348 | FS3Q7 | 7 | 1.62007 |
| S4Q7 | 17 | 2.54875 | FS3Q8 | 4 | 0.97633 |
| S4Q8 | 20 | 2.709978 | FS3Q9 | 4 | 0.941318 |
| S4Q9 | 15 | 2.383271 | FS3Q10 | 4 | 0.998696 |
| S4Q10 | 16 | 2.442801 | FS4Q1 | 16 | 2.435287 |
| S4Q11 | 22 | 2.864515 | FS4Q2 | 18 | 2.422332 |
| S4Q12 | 18 | 2.565379 | FS4Q3 | 18 | 2.639734 |
| S4Q13 | 21 | 2.665254 | FS4Q4 | 16 | 2.459811 |
| S5Q1 | 15 | 2.417465 | FS4Q5 | 18 | 2.451564 |
| S5Q2 | 16 | 2.466072 | FS4Q6 | 13 | 2.186223 |
| S5Q3 | 15 | 2.294344 | FS4Q7 | 12 | 2.115488 |
| S5Q4 | 17 | 2.48977 | FS4Q8 | 12 | 2.080375 |
| S5Q5 | 14 | 2.272177 | FS4Q9 | 13 | 2.297515 |
| S5Q6 | 16 | 2.504384 | FS4Q10 | 12 | 2.198479 |
| S5Q7 | 18 | 2.619202 | FS5Q1 | 16 | 2.378599 |
| S5Q8 | 13 | 2.154757 | FS5Q2 | 15 | 2.36489 |
| S5Q9 | 17 | 2.589061 | FS5Q3 | 13 | 2.091665 |
| S5Q10 | 15 | 2.538078 | FS5Q4 | 16 | 2.251122 |
| S5Q11 | 15 | 2.272501 | FS5Q5 | 15 | 2.395034 |
| S5Q12 | 13 | 2.053219 | FS5Q6 | 13 | 2.048712 |
| S5Q13 | 17 | 2.332756 | FS5Q7 | 14 | 2.32654 |
| S6Q1 | 17 | 2.328227 | FS5Q8 | 13 | 2.271589 |
| S6Q2 | 14 | 2.239102 | FS5Q9 | 13 | 2.21683 |
| S6Q3 | 12 | 1.89413 | FS5Q10 | 11 | 1.979097 |
| S6Q4 | 16 | 2.517507 | FS6Q1 | 22 | 2.936839 |
| S6Q5 | 12 | 2.202981 | FS6Q2 | 21 | 2.685807 |
| S6Q6 | 12 | 2.031984 | FS6Q3 | 19 | 2.57531 |
| S6Q7 | 16 | 2.507694 | FS6Q4 | 22 | 2.950197 |
| S6Q8 | 18 | 2.576293 | FS6Q5 | 28 | 2.39228 |
| S6Q9 | 18 | 2.338526 | |||
| Sr.No | Stations | Species Richness | CaCO3 | OM | Nitrogen | Phosphorus | pH | EC | Altitude | Slope Angle | Aspect | Region |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S1Q1 | 17 | 7.3 | 0.87 | 0.043 | 5.7 | 7.45 | 0.12 | 1227.134 | 88 | 2.5 | FF |
| 2 | S1Q2 | 14 | 11.12 | 0.94 | 0.042 | 7.7 | 7.59 | 0.2 | 1285.061 | 79 | 2.5 | FF |
| 3 | S1Q3 | 15 | 8.34 | 0.85 | 0.042 | 19 | 7.02 | 0.01 | 1317.988 | 87 | 2.5 | FF |
| 4 | S2Q1 | 15 | 10.62 | 0.62 | 0.027 | 10 | 7.28 | 0.05 | 1282.622 | 86 | 2 | FF |
| 5 | S2Q2 | 16 | 7.66 | 0.49 | 0.024 | 7.99 | 6.91 | 0.15 | 1318.902 | 73 | 2.25 | FF |
| 6 | S2Q3 | 15 | 4.75 | 0.84 | 0.022 | 10 | 6.96 | 0.07 | 1340.244 | 76 | 3 | FF |
| 7 | S2Q4 | 13 | 9.87 | 0.81 | 0.04 | 13.2 | 7.08 | 0.13 | 1364.634 | 73 | 2.25 | FF |
| 8 | S2Q5 | 23 | 8.87 | 0.84 | 0.042 | 10 | 7.3 | 0.12 | 1390.549 | 71 | 3 | FF |
| 9 | S2Q6 | 15 | 8.12 | 0.78 | 0.031 | 15.4 | 8.08 | 0.19 | 1401.524 | 80 | 1.25 | FF |
| 10 | S2Q7 | 16 | 5.62 | 0.87 | 0.043 | 16 | 7.34 | 0.08 | 1380.793 | 71 | 1.25 | FF |
| 11 | S2Q8 | 16 | 11.5 | 0.94 | 0.038 | 9 | 7.16 | 0.01 | 1359.756 | 74 | 1.25 | FF |
| 12 | S2Q9 | 15 | 7.37 | 0.32 | 0.015 | 12 | 7.81 | 0.32 | 1337.5 | 87 | 1.25 | FF |
| 13 | S2Q10 | 15 | 7.87 | 0.51 | 0.02 | 10.71 | 7.11 | 0.31 | 1319.512 | 74 | 2.75 | FF |
| 14 | S3Q1 | 19 | 11.5 | 1.01 | 0.05 | 8 | 6.56 | 0.1 | 1066.463 | 89 | 2 | FF |
| 15 | S3Q2 | 18 | 10 | 0.52 | 0.051 | 14.9 | 7.35 | 0.01 | 1072.866 | 87 | 2 | FF |
| 16 | S3Q3 | 15 | 9.21 | 0.68 | 0.034 | 10 | 7.31 | 0.11 | 1095.427 | 69 | 1.5 | FF |
| 17 | S3Q4 | 21 | 11.62 | 1.04 | 0.057 | 13 | 7.27 | 0.12 | 1117.988 | 75 | 2 | FF |
| 18 | S3Q5 | 13 | 8.06 | 0.89 | 0.024 | 22 | 7.08 | 0.11 | 1138.415 | 76 | 2 | FF |
| 19 | S4Q1 | 17 | 11.45 | 0.73 | 0.02 | 22 | 7.38 | 0.32 | 1053.659 | 88 | 1.5 | FF |
| 20 | S4Q2 | 16 | 3.53 | 0.99 | 0.014 | 8 | 7.33 | 0.02 | 1087.5 | 84 | 2.25 | FF |
| 21 | S4Q3 | 21 | 8.62 | 1.11 | 0.051 | 8.44 | 7.15 | 0.04 | 1104.878 | 89 | 2 | FF |
| 22 | S4Q4 | 16 | 11.75 | 0.85 | 0.024 | 20.01 | 7.15 | 0.25 | 1130.793 | 82 | 1 | FF |
| 23 | S4Q5 | 21 | 9.5 | 0.92 | 0.046 | 22 | 6.72 | 0.15 | 1155.183 | 85 | 1.5 | FF |
| 24 | S4Q6 | 21 | 5.25 | 0.82 | 0.041 | 15 | 6.5 | 0.01 | 1187.805 | 84 | 1.5 | FF |
| 25 | S4Q7 | 16 | 12.43 | 0.51 | 0.02 | 12 | 7.45 | 0.01 | 1206.402 | 84 | 1.5 | FF |
| 26 | S4Q8 | 19 | 8.87 | 0.85 | 0.042 | 11.32 | 7.42 | 0.02 | 1239.939 | 86 | 1.5 | FF |
| 27 | S4Q9 | 14 | 6.62 | 0.84 | 0.042 | 10 | 7.75 | 0.02 | 1255.793 | 84 | 1.5 | FF |
| 28 | S4Q10 | 15 | 11.75 | 0.47 | 0.023 | 11 | 7.45 | 0.02 | 1279.573 | 76 | 1.5 | FF |
| 29 | S4Q11 | 21 | 11.25 | 0.89 | 0.044 | 17.1 | 7.43 | 0.01 | 1300 | 84 | 1.5 | FF |
| 30 | S4Q12 | 17 | 7.8 | 0.85 | 0.042 | 14 | 7.8 | 0.01 | 1315.549 | 88 | 2 | FF |
| 31 | S4Q13 | 20 | 7.75 | 0.69 | 0.018 | 11.1 | 7.45 | 0.13 | 1331.402 | 88 | 2 | FF |
| 32 | S5Q1 | 14 | 10.5 | 0.84 | 0.042 | 12 | 7.62 | 0.17 | 1457.012 | 88 | 1.25 | FF |
| 33 | S5Q2 | 15 | 4.5 | 1.02 | 0.05 | 8.6 | 6.82 | 0.2 | 1484.451 | 79 | 1.25 | FF |
| 34 | S5Q3 | 14 | 8.75 | 0.67 | 0.033 | 10.01 | 7.81 | 0.14 | 1510.366 | 84 | 1 | FF |
| 35 | S5Q4 | 16 | 9.62 | 0.91 | 0.025 | 11 | 7.24 | 0.12 | 1530.488 | 86 | 1 | FF |
| 36 | S5Q5 | 13 | 4.25 | 0.94 | 0.047 | 11.1 | 7.55 | 0.39 | 1558.841 | 84 | 1 | FF |
| 37 | S5Q6 | 15 | 8.25 | 0.51 | 0.03 | 10 | 7.13 | 0.04 | 1586.28 | 89 | 1 | FF |
| 38 | S5Q7 | 17 | 10 | 0.7 | 0.035 | 9 | 7.12 | 0.12 | 1617.988 | 87 | 2 | FF |
| 39 | S5Q8 | 12 | 11.75 | 0.49 | 0.042 | 4 | 7.38 | 0.08 | 1649.085 | 84 | 2 | FF |
| 40 | S5Q9 | 16 | 12.43 | 0.49 | 0.045 | 14.21 | 7.19 | 0.06 | 1675 | 88 | 2 | FF |
| 41 | S5Q10 | 14 | 9.37 | 0.68 | 0.034 | 7.7 | 7.58 | 0.04 | 1685.976 | 87 | 2.75 | FF |
| 42 | S5Q11 | 14 | 9.77 | 0.95 | 0.047 | 8.8 | 7.35 | 0.15 | 1729.573 | 88 | 2.5 | FF |
| 43 | S5Q12 | 12 | 9.12 | 0.59 | 0.026 | 13.1 | 8.1 | 0.03 | 1728.049 | 87 | 1 | FF |
| 44 | S5Q13 | 16 | 7.12 | 1.04 | 0.035 | 16 | 7.55 | 0.03 | 1740.244 | 79 | 1.25 | FF |
| 45 | S6Q1 | 16 | 2.37 | 0.92 | 0.046 | 13 | 7.01 | 0.12 | 1840.244 | 84 | 1 | FF |
| 46 | S6Q2 | 13 | 7.75 | 0.84 | 0.042 | 11.12 | 7.51 | 0.11 | 1878.354 | 86 | 1 | FF |
| 47 | S6Q3 | 11 | 9.37 | 0.37 | 0.018 | 10 | 8.3 | 0.02 | 1937.5 | 84 | 2.75 | FF |
| 48 | S6Q4 | 15 | 9 | 0.77 | 0.038 | 14 | 7.82 | 0.21 | 1971.037 | 87 | 1.25 | FF |
| 49 | S6Q5 | 11 | 6.81 | 0.72 | 0.036 | 7 | 7.77 | 0.2 | 2004.573 | 84 | 3 | FF |
| 50 | S6Q6 | 11 | 10.47 | 0.58 | 0.026 | 10 | 7.86 | 0.11 | 2029.268 | 83 | 2.75 | FF |
| 51 | S6Q7 | 15 | 9.5 | 0.72 | 0.036 | 12 | 7.18 | 0.02 | 2068.293 | 87 | 3 | FF |
| 52 | S6Q8 | 17 | 4.87 | 0.61 | 0.03 | 15 | 7.03 | 0.35 | 2120.732 | 84 | 2.5 | FF |
| 53 | S6Q9 | 17 | 9.13 | 0.73 | 0.035 | 10 | 7.62 | 0.01 | 2088.72 | 86 | 2.25 | FF |
| 54 | S1Q1 | 17 | 7.3 | 0.87 | 0.043 | 5.7 | 7.45 | 0.12 | 1227.134 | 88 | 2.5 | FF |
| 55 | FS1Q1 | 21 | 8.1 | 0.98 | 0.054 | 9.2 | 7.32 | 0.12 | 1004.268 | 87 | 2 | FA |
| 56 | FS1Q2 | 21 | 9.01 | 1.32 | 0.051 | 18 | 7.8 | 0.05 | 1021.341 | 71 | 2 | FA |
| 57 | FS1Q3 | 14 | 8.8 | 0.31 | 0.065 | 9 | 7.9 | 0.07 | 1039.634 | 51 | 2 | FA |
| 58 | FS1Q4 | 12 | 9.25 | 0.72 | 0.061 | 8 | 7.81 | 0.35 | 1070.427 | 89 | 2 | FA |
| 59 | FS1Q5 | 14 | 10.2 | 1.4 | 0.018 | 9 | 7.2 | 0.18 | 1098.171 | 57 | 1.5 | FA |
| 60 | FS1Q6 | 14 | 11.12 | 0.82 | 0.022 | 12 | 7.28 | 0.21 | 1110.061 | 59 | 2.5 | FA |
| 61 | FS2Q1 | 16 | 7.25 | 0.91 | 0.062 | 12.15 | 7.12 | 0.15 | 1148.78 | 83 | 2.5 | FA |
| 62 | FS2Q2 | 13 | 11.75 | 0.74 | 0.027 | 8 | 7.66 | 0.05 | 1157.317 | 77 | 2 | FA |
| 63 | FS2Q3 | 14 | 4.09 | 1.52 | 0.036 | 11 | 7.82 | 0.04 | 1179.268 | 84 | 2.75 | FA |
| 64 | FS2Q4 | 12 | 3.12 | 0.82 | 0.041 | 18 | 8.18 | 0.12 | 1205.183 | 86 | 2.75 | FA |
| 65 | FS2Q5 | 14 | 7.5 | 1.54 | 0.031 | 11 | 7.93 | 0.1 | 1241.463 | 89 | 2.75 | FA |
| 66 | FS2Q6 | 11 | 11.25 | 0.77 | 0.038 | 12.1 | 8.21 | 0.08 | 1218.902 | 79 | 1.25 | FA |
| 67 | FS2Q7 | 13 | 2.75 | 0.94 | 0.023 | 8.9 | 7.29 | 0.03 | 1168.293 | 89 | 2.5 | FA |
| 68 | FS2Q8 | 14 | 4.55 | 0.51 | 0.025 | 17.19 | 7.65 | 0.06 | 1156.402 | 77 | 1.25 | FA |
| 69 | FS2Q9 | 16 | 5.62 | 0.74 | 0.037 | 15 | 7.15 | 0.11 | 1101.22 | 89 | 2.75 | FA |
| 70 | FS2Q10 | 12 | 6.25 | 0.68 | 0.034 | 7.8 | 7.35 | 0.14 | 1070.427 | 83 | 2.75 | FA |
| 71 | FS2Q11 | 16 | 5.5 | 0.98 | 0.049 | 12.14 | 6.46 | 0.1 | 1044.207 | 83 | 2.75 | FA |
| 72 | FS3Q1 | 12 | 10 | 0.81 | 0.04 | 8.13 | 7.18 | 0.3 | 1115.549 | 88 | 2 | FA |
| 73 | FS3Q2 | 10 | 10 | 0.81 | 0.04 | 8.13 | 7.18 | 0.3 | 1088.11 | 77 | 2 | FA |
| 74 | FS3Q3 | 10 | 11 | 0.49 | 0.024 | 8 | 7.43 | 0.03 | 1067.683 | 88 | 1.5 | FA |
| 75 | FS3Q4 | 9 | 10.75 | 1.02 | 0.026 | 13.2 | 6.78 | 0.21 | 1021.646 | 84 | 2.25 | FA |
| 76 | FS3Q5 | 7 | 10.62 | 0.46 | 0.035 | 11 | 7.37 | 0.16 | 1117.988 | 88 | 1.5 | FA |
| 77 | FS3Q6 | 6 | 10.15 | 0.48 | 0.022 | 7.99 | 7.92 | 0.29 | 1148.476 | 86 | 2 | FA |
| 78 | FS3Q7 | 9 | 11 | 0.7 | 0.031 | 9.7 | 7.89 | 0.13 | 1167.073 | 82 | 1.5 | FA |
| 79 | FS3Q8 | 3 | 9.75 | 0.32 | 0.03 | 5.67 | 8.09 | 0.06 | 1195.122 | 88 | 1 | FA |
| 80 | FS3Q9 | 3 | 10.5 | 0.4 | 0.037 | 8 | 7.46 | 0.11 | 1217.073 | 83 | 3 | FA |
| 81 | FS3Q10 | 3 | 10.22 | 0.45 | 0.016 | 10.37 | 6.88 | 0.12 | 1245.732 | 80 | 1 | FA |
| 82 | FS4Q1 | 15 | 10.75 | 0.9 | 0.04 | 9.1 | 7.39 | 0.111 | 1227.134 | 87 | 1.5 | FA |
| 83 | FS4Q2 | 17 | 10.87 | 0.94 | 0.053 | 10 | 7.69 | 0.2 | 1261.89 | 88 | 1 | FA |
| 84 | FS4Q3 | 17 | 11.62 | 0.85 | 0.025 | 18 | 7.91 | 0.04 | 1293.293 | 88 | 1.25 | FA |
| 85 | FS4Q4 | 15 | 11.37 | 0.94 | 0.033 | 14 | 7.08 | 0.05 | 1313.72 | 78 | 1 | FA |
| 86 | FS4Q5 | 17 | 10.37 | 0.63 | 0.077 | 9.9 | 7.56 | 0.03 | 1349.695 | 87 | 1 | FA |
| 87 | FS4Q6 | 12 | 10 | 0.54 | 0.027 | 10.2 | 7.15 | 0.25 | 1372.561 | 86 | 1.25 | FA |
| 88 | FS4Q7 | 11 | 6.5 | 0.77 | 0.038 | 14 | 7.55 | 0.31 | 1406.098 | 76 | 1 | FA |
| 89 | FS4Q8 | 8 | 10.22 | 0.78 | 0.022 | 10.37 | 6.88 | 0.12 | 1443.598 | 79 | 1.25 | FA |
| 90 | FS4Q9 | 12 | 10.75 | 0.85 | 0.042 | 11 | 7.44 | 0.08 | 1478.354 | 86 | 2.25 | FA |
| 91 | FS4Q10 | 11 | 10.25 | 0.74 | 0.021 | 13 | 6.77 | 0.18 | 1460.061 | 88 | 2.25 | FA |
| 92 | FS5Q1 | 15 | 10.87 | 0.82 | 0.041 | 15 | 7.21 | 0.02 | 1522.561 | 86 | 1.5 | FA |
| 93 | FS5Q2 | 14 | 10.25 | 0.67 | 0.032 | 9 | 7.52 | 0.03 | 1546.951 | 86 | 1.5 | FA |
| 94 | FS5Q3 | 12 | 10.11 | 0.7 | 0.053 | 8 | 7.32 | 0.06 | 1574.39 | 87 | 1.5 | FA |
| 95 | FS5Q4 | 15 | 12.25 | 1.04 | 0.024 | 8 | 8.24 | 0.14 | 1599.39 | 86 | 1 | FA |
| 96 | FS5Q5 | 14 | 5 | 0.73 | 0.022 | 7.5 | 7.07 | 0.03 | 1643.598 | 87 | 1 | FA |
| 97 | FS5Q6 | 12 | 11.37 | 0.94 | 0.042 | 10 | 6.88 | 0.07 | 1672.256 | 85 | 1.5 | FA |
| 98 | FS5Q7 | 13 | 6.75 | 0.96 | 0.063 | 10 | 7.02 | 0.12 | 1677.744 | 74 | 1.5 | FA |
| 99 | FS5Q8 | 12 | 9.37 | 0.54 | 0.027 | 12 | 7.8 | 0.17 | 1707.012 | 64 | 1 | FA |
| 100 | FS5Q9 | 12 | 10.75 | 0.7 | 0.037 | 8.4 | 7.68 | 0.05 | 1744.512 | 82 | 1.5 | FA |
| 101 | FS5Q10 | 10 | 10.75 | 0.41 | 0.039 | 6.8 | 7.98 | 0.09 | 1750.305 | 89 | 2 | FA |
| 102 | FS6Q1 | 21 | 5 | 1.06 | 0.06 | 12.12 | 7.09 | 0.21 | 1090.549 | 75 | 1.25 | FA |
| 103 | FS6Q2 | 20 | 4.59 | 0.85 | 0.042 | 18 | 7.36 | 0.08 | 1098.171 | 87 | 1.25 | FA |
| 104 | FS6Q3 | 19 | 4.37 | 0.71 | 0.035 | 14 | 7.19 | 0.05 | 1121.951 | 87 | 1.25 | FA |
| 105 | FS6Q4 | 22 | 11.62 | 0.54 | 0.076 | 12.31 | 7.38 | 0.04 | 1139.634 | 83 | 1 | FA |
| 106 | FS6Q5 | 28 | 2.37 | 1.37 | 0.068 | 8.6 | 7.38 | 0.15 | 1175.61 | 86 | 1 | FA |
References
- Iglesias, V.; Yospin, G.I.; Whitlock, C. Reconstruction of fire regimes through integrated paleoecological proxy data and ecological modeling. Front. Plant Sci. 2015, 5, 785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. BioScience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Mouillot, F.; Field, C.B. Fire history and the global carbon budget: A 1 × 1 fire history reconstruction for the 20th century. Glob. Chang. Biol. 2005, 11, 398–420. [Google Scholar] [CrossRef]
- Andersen, A.N. Burning issues in savanna ecology and management. In Fire in Tropical Savannas: The Kapalga Experiment; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–14. [Google Scholar]
- Argañaraz, J.P.; Pizarro, G.G.; Zak, M.; Landi, M.A.; Bellis, L.M. Human and biophysical drivers of fires in Semiarid Chaco mountains of Central Argentina. Sci. Total Environ. 2015, 520, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Ahmad, S.; Erum, S.; Butt, A. Monitoring forest cover change of Margalla Hills over a period of two decades (1992–2011): A spatiotemporal perspective. J. Ecosyst. Ecography 2015, 6, 174–181. [Google Scholar] [CrossRef]
- Bano, S.; Khan, S.M.; Alam, J.; Alqarawi, A.A.; Abd_Allah, E.F.; Ahmad, Z.; Rahman, I.U.; Ahmad, H.; Aldubise, A.; Hashem, A. Eco-Floristic studies of native plants of the Beer Hills along the Indus River in the districts Haripur and Abbottabad, Pakistan. Saudi J. Biol. Sci. 2018, 25, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.A. Mega-fires, tipping points and ecosystem services: Managing forests and woodlands in an uncertain future. For. Ecol. Manag. 2013, 294, 250–261. [Google Scholar] [CrossRef]
- Kent, M. Vegetation Description and Data Analysis: A Practical Approach; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Hussain, F.; Sher, Z.; Lal Badshah, K.A.; Aziz, S.; Resham, S. Floristic and vegetation diversity of gadoon hills outer himalayas district Swabi, Pakistan. Pak. J. Bot 2023, 55, 257–276. [Google Scholar] [CrossRef]
- Rahman, A.U.; Khan, S.M.; Khan, S.; Hussain, A.; Rahman, I.U.; Iqbal, Z.; Ijaz, F. Ecological assessment of plant communities and associated edaphic and topographic variables in the Peochar Valley of the Hindu Kush mountains. Mt. Res. Dev. 2016, 36, 332–341. [Google Scholar] [CrossRef]
- Turak, E.; Brazill-Boast, J.; Cooney, T.; Drielsma, M.; DelaCruz, J.; Dunkerley, G.; Fernandez, M.; Ferrier, S.; Gill, M.; Jones, H. Using the essential biodiversity variables framework to measure biodiversity change at national scale. Biol. Conserv. 2017, 213, 264–271. [Google Scholar] [CrossRef]
- ur Rahman, A.; Khan, S.M.; Ahmad, Z.; Alamri, S.; Hashem, M.; Ilyas, M.; Aksoy, A.; Dülgeroğlu, C.; Khan, G.; Ali, S. Impact of multiple environmental factors on species abundance in various forest layers using an integrative modeling approach. Glob. Ecol. Conserv. 2021, 29, e01712. [Google Scholar] [CrossRef]
- Townsend, A.R.; Asner, G.P.; Cleveland, C.C. The biogeochemical heterogeneity of tropical forests. Trends Ecol. Evol. 2008, 23, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Becknell, J.M.; Powers, J.S. Stand age and soils as drivers of plant functional traits and aboveground biomass in secondary tropical dry forest. Can. J. For. Res. 2014, 44, 604–613. [Google Scholar] [CrossRef]
- Ameztegui, A.; Coll, L.; Brotons, L.; Ninot, J.M. Land-use legacies rather than climate change are driving the recent upward shift of the mountain tree line in the P yrenees. Glob. Ecol. Biogeogr. 2016, 25, 263–273. [Google Scholar] [CrossRef]
- Ahmad, Z.; Khan, S.M.; Abd_Allah, E.F.; Alqarawi, A.A.; Hashem, A. Weed species composition and distribution pattern in the maize crop under the influence of edaphic factors and farming practices: A case study from Mardan, Pakistan. Saudi J. Biol. Sci. 2016, 23, 741–748. [Google Scholar] [CrossRef]
- Daubenmire, R. Plant Communities: A Textbook of Plant Synecology; Harper & Row: New York, NY, USA, 1968. [Google Scholar]
- Braun-Blanquet, J. Plant Sociology: The Study of Plant Communities; McGraw-Hill Book Co., Inc.: New York, NY, USA, 1932. [Google Scholar]
- Marandi, A.; Polikarpus, M.; Jõeleht, A. A new approach for describing the relationship between electrical conductivity and major anion concentration in natural waters. Appl. Geochem. 2013, 38, 103–109. [Google Scholar] [CrossRef]
- Tfaily, M.M.; Chu, R.K.; Toyoda, J.; Tolić, N.; Robinson, E.W.; Paša-Tolić, L.; Hess, N.J. Sequential extraction protocol for organic matter from soils and sediments using high resolution mass spectrometry. Anal. Chim. Acta 2017, 972, 54–61. [Google Scholar] [CrossRef]
- DeBano, L.F. The effect of fire on soil properties. In Proceedings of the Proceedings Management and Productivity of Western-Montane—Forest Soils, Boise, ID, USA, 10–12 April 1990; pp. 151–155. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Gray, D.M.; Dighton, J. Biochemistry. Mineralization of forest litter nutrients by heat and combustion. Soil Biol. Biochem. 2006, 38, 1469–1477. [Google Scholar] [CrossRef]
- Agbeshie, A.A.; Abugre, S.; Atta-Darkwa, T.; Awuah, R. A review of the effects of forest fire on soil properties. J. For. Res. 2022, 33, 1419–1441. [Google Scholar] [CrossRef]
- Rafaqat, W.; Iqbal, M.; Kanwal, R.; Weiguo, S. Evaluation of Wildfire Occurrences in Pakistan with Global Gridded Soil Properties Derived from Remotely Sensed Data. Remote Sens. 2022, 14, 5503. [Google Scholar] [CrossRef]
- Lewis, W.M., Jr. Effects of fire on nutrient movement in a South Carolina pine forest. Ecology 1974, 55, 1120–1127. [Google Scholar] [CrossRef]
- Stavi, I. Wildfires in Grasslands and Shrublands: A Review of Impacts on Vegetation, Soil, Hydrology, and Geomorphology. Water 2019, 11, 1042. [Google Scholar] [CrossRef]
- Kala, C.P. Environmental and socioeconomic impacts of forest fires: A call for multilateral cooperation and management interventions. Nat. Hazards Res. 2023, 3, 286–294. [Google Scholar] [CrossRef]
- Hou, X.; Orth, R. Observational evidence of wildfire-promoting soil moisture anomalies. Sci. Rep. 2020, 10, 11008. [Google Scholar]
- McLauchlan, K.K.; Higuera, P.E.; Miesel, J.; Rogers, B.M.; Schweitzer, J.; Shuman, J.K.; Tepley, A.J.; Varner, J.M.; Veblen, T.T.; Adalsteinsson, S.A.; et al. Fire as a fundamental ecological process: Research advances and frontiers. J. Ecol. 2020, 108, 2047–2069. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.M.; Siddiq, Z.; Ahmad, Z.; Ahmad, K.S.; Abdullah, A.; Hashem, A.; Al-Arjani, A.B.; Abd_Allah, E.F. Carbon sequestration potential of reserve forests present in the protected Margalla Hills National Park. J. King Saud Univ. Sci. 2022, 34, 101978. [Google Scholar] [CrossRef]
- Memoli, V.; Panico, S.C.; Santorufo, L.; Barile, R.; Di Natale, G.; Di Nunzio, A.; Toscanesi, M.; Trifuoggi, M.; De Marco, A.; Maisto, G. Do wildfires cause changes in soil quality in the short term? Int. J. Environ. Res. Public Health 2020, 17, 5343. [Google Scholar] [CrossRef]
- Akhtar, N.; Muhammad, R.; Saeed, K.; Khan, M.F.; Shah, M.; Zeb, J.; Ahmad, S.; Afridi, A.J.; Hussain, A. Distribution of wild mammalian fauna of Mahaban and Malka Valley District Buner. Pak. J. Zool. 2018, 50, 1–3. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.M.; Ahmad, Z.; Abdullah, A.; Kazi, N.; Nawaz, I.; Almutairi, K.F.; Avila-Quezada, G.D.; Abd_Allah, E.F. Relative Humidity, Soil Phosphorus, and Stand Structure Diversity Determine Aboveground Biomass along the Elevation Gradient in Various Forest Ecosystems of Pakistan. Sustainability 2023, 15, 7523. [Google Scholar] [CrossRef]
- Mumshad, M.; Ahmad, I.; Khan, S.M.; Rehman, K.; Islam, M.; Sakhi, S.; Khan, S.U.; Afridi, S.G.; Shams, S.; Azam, S.; et al. Phyto-ecological studies and distribution pattern of plant species and communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. PLoS ONE 2021, 16, e0257493. [Google Scholar] [CrossRef]
- Habib, S.; Badshah, L.; Ahmad, I.; Ahmad, W.; Abdullah; Khan, S.M. Ecological Assessment of the Native Flora of Matta Kharari Village, Swat, Khyber Pakhtunkhwa, Pakistan. Proc. Pakistan Acad. Sci. 2023, 60, 273–282. [Google Scholar]
- Rana, M.; Mulk, K.S.; Ali, S.; Khalid, A.; Ahmad, Z. Carbon credit, trading, green economy, and clean development mechanisms. In Agroforestry for Carbon and Ecosystem Management; Jhariya, M.K., Meena, R.S., Banerjee, A., Kumar, S., Raj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, Chapter 11; pp. 147–159. [Google Scholar]
- Khalid, A.; Khan, S.M.; Ali, S.; Rana, M.; Abdullah; Ahmad, Z. Carbon fraction and pools in plants and soil. In Agroforestry for Carbon and Ecosystem Management; Jhariya, M.K., Meena, R.S., Banerjee, A., Kumar, S., Raj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, Chapter 10; pp. 135–146. [Google Scholar]
- Chaney, R.; Slonim, S.; Slonim, S. Determination of calcium carbonate content in soils. In Geotechnical Properties, Behavior, and Performance of Calcareous Soils; ASTM International: West Conshohocken, PA, USA, 1982. [Google Scholar]
- Haq, Z.U.; Khan, S.M.; Iqbal, J.; Razzaq, A.; Iqbal, M. Phyto-medicinal studies in district Lower Dir HinduKush Range Khyber Pakhtunkhwa, Pakistan. Pak. J. Weed Sci. Res. 2019, 25, 235. [Google Scholar]
- Menhinick, E.F. A comparison of some species-individuals diversity indices applied to samples of field insects. Ecology 1964, 45, 859–861. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. Ecological Diversity; John Wiley & Sons: New York, NY, USA, 1975. [Google Scholar]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Fleming, G.M.; Diffendorfer, J.E.; Zedler, P.H. The relative importance of disturbance and exotic-plant abundance in California coastal sage scrub. Ecol. Appl. 2009, 19, 2210–2227. [Google Scholar] [CrossRef]
- McIntyre, S.; Lavorel, S.; Landsberg, J.; Forbes, T. Disturbance response in vegetation–towards a global perspective on functional traits. J. Veg. Sci. 1999, 10, 621–630. [Google Scholar] [CrossRef]
- Belaoussoff, S.; Kevan, P.G.; Murphy, S.; Swanton, C. Assessing tillage disturbance on assemblages of ground beetles (Coleoptera: Carabidae) by using a range of ecological indices. Biodivers. Conserv. 2003, 12, 851–882. [Google Scholar] [CrossRef]
- Biswas, S.R.; Mallik, A.U. Disturbance effects on species diversity and functional diversity in riparian and upland plant communities. Ecology 2010, 91, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, C.; Wang, W.; Zhou, H.; Xue, Y.; Xu, J.; Xue, P.; Yan, H. Effects of different grazing disturbances on the plant diversity and ecological functions of alpine grassland ecosystem on the qinghai-Tibetan plateau. Front. Plant Sci. 2021, 12, 765070. [Google Scholar] [CrossRef]
- Ullah, H.; Khan, S.M.; Jaremko, M.; Jahangir, S.; Ullah, Z.; Ali, I.; Ahmad, Z.; Badshah, H. Vegetation assessments under the influence of environmental variables from the Yakhtangay Hill of the Hindu-Himalayan range, North Western Pakistan. Sci. Rep. 2022, 12, 20973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Biswas, A. The effects of forest fire on soil organic matter and nutrients in boreal forests of North America: A review. In Adaptive Soil Management: From Theory to Practices; Springer: Singapore, 2017; pp. 465–476. [Google Scholar]
- Close, D.C.; Davidson, N.J.; Swanborough, P.W.; Corkrey, R. Does low-intensity surface fire increase water-and nutrient-availability to overstorey Eucalyptus gomphocephala? Plant Soil 2011, 349, 203–214. [Google Scholar] [CrossRef]
- Certini, G.; Nocentini, C.; Knicker, H.; Arfaioli, P.; Rumpel, C. Wildfire effects on soil organic matter quantity and quality in two fire-prone Mediterranean pine forests. Geoderma 2011, 167, 148–155. [Google Scholar] [CrossRef]
- Sánchez Meador, A.; Springer, J.D.; Huffman, D.W.; Bowker, M.A.; Crouse, J.E. Soil functional responses to ecological restoration treatments in frequent-fire forests of the western United States: A systematic review. Restor. Ecol. 2017, 25, 497–508. [Google Scholar] [CrossRef]
- Ferrer, I.; Thurman, E.M.; Zweigenbaum, J.A.; Murphy, S.F.; Webster, J.P.; Rosario-Ortiz, F.L. Wildfires: Identification of a new suite of aromatic polycarboxylic acids in ash and surface water. Sci. Total Environ. 2021, 770, 144661. [Google Scholar] [CrossRef]
- Kutiel, P.; Naveh, Z. The effect of fire on nutrients in a pine forest soil. Plant Soil 1987, 104, 269–274. [Google Scholar] [CrossRef]
- Kutiel, P.; Naveh, Z. Soil properties beneath Pinus halepensis and Quercus calliprinos trees on burned and unburned mixed forest on Mt. Carmel, Israel. For. Ecol. Manag. 1987, 20, 11–24. [Google Scholar] [CrossRef]
- Fraterrigo, J.M.; Rembelski, M.K. Frequent fire reduces the magnitude of positive interactions between an invasive grass and soil microbes in temperate forests. Ecosystems 2021, 24, 1738–1755. [Google Scholar] [CrossRef]
- Hahn, G.E.; Coates, T.A.; Aust, W.M. Soil chemistry following single-entry, dormant season prescribed fires in the Ridge and Valley Province of Virginia, USA. Commun. Soil Sci. Plant Anal. 2021, 52, 2065–2073. [Google Scholar] [CrossRef]
- Hopkins, J.R.; Huffman, J.M.; Platt, W.J.; Sikes, B.A. Frequent fire slows microbial decomposition of newly deposited fine fuels in a pyrophilic ecosystem. Oecologia 2020, 193, 631–643. [Google Scholar] [CrossRef]
- Muqaddas, B.; Zhou, X.; Lewis, T.; Wild, C.; Chen, C. Long-term frequent prescribed fire decreases surface soil carbon and nitrogen pools in a wet sclerophyll forest of Southeast Queensland, Australia. Sci. Total Environ. 2015, 536, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, L.; Wang, X.; Wei, X.; Gao, H.; Zhang, Y.; Cheng, J. Effects of wildfire and topography on soil nutrients in a semiarid restored grassland. Plant Soil 2018, 428, 123–136. [Google Scholar] [CrossRef]
- Ul Haq, A.Z.; Khan, S.M. The indispensable bond between Mazri Palm (Nannorrhops ritchiana) and the Indian Porcupine (Hystrix indica) leads them towards extinction! Biodivers. Conserv. 2019, 28, 3387–3388. [Google Scholar]
- Pausas, J.G.; Austin, M.P. Patterns of plant species richness in relation to different environments: An appraisal. J. Veg. Sci. 2001, 12, 153–166. [Google Scholar] [CrossRef]
- Abdullah, A.; Khan, S.M.; Pieroni, A.; Haq, A.; Haq, Z.U.; Ahmad, Z.; Sakhi, S.; Hashem, A.; Al-Arjani, A.-B.F.; Alqarawi, A.A.; et al. A Comprehensive Appraisal of the Wild Food Plants and Food System of Tribal Cultures in the Hindu Kush Mountain Range: A Way Forward for Balancing Human Nutrition and Food Security. Sustainability 2021, 13, 5258. [Google Scholar] [CrossRef]
- Neary, D.G.; Ryan, K.C.; DeBano, L.F. Wildland Fire in Ecosystems: Effects of Fire on Soils and Water; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Ogden, UT, USA, 2005; p. 42.
- Akburak, S.; Son, Y.; Makineci, E.; Çakir, M. Impacts of low-intensity prescribed fire on microbial and chemical soil properties in a Quercus frainetto forest. J. For. Res. 2018, 29, 687–696. [Google Scholar] [CrossRef]
- Francos, M.; Stefanuto, E.; Úbeda, X.; Pereira, P. Long-term impact of prescribed fire on soil chemical properties in a wildland-urban interface. Northeastern Iberian Peninsula. Sci. Total Environ. 2019, 689, 305–311. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Albert-Belda, E.; Gomez-Munoz, B.; Moreno, J.M. High fire frequency reduces soil fertility underneath woody plant canopies of Mediterranean ecosystems. Sci. Total Environ. 2021, 752, 141877. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Farguell, J.; Úbeda, X. Long-term dynamics of soil chemical properties after a prescribed fire in a Mediterranean forest (Montgrí Massif, Catalonia, Spain). Sci. Total Environ. 2016, 572, 1329–1335. [Google Scholar] [CrossRef]
- Downing, T.A.; Imo, M.; Kimanzi, J.; Otinga, A.N. Effects of wildland fire on the tropical alpine moorlands of Mount Kenya. Catena 2017, 149, 300–308. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Huerta, S.; Calvo, L. Soil-vegetation relationships in Mediterranean forests after fire. For. Ecosyst. 2021, 8, 18. [Google Scholar] [CrossRef]
- Fultz, L.M.; Moore-Kucera, J.; Dathe, J.; Davinic, M.; Perry, G.; Wester, D.; Schwilk, D.W.; Rideout-Hanzak, S. Forest wildfire and grassland prescribed fire effects on soil biogeochemical processes and microbial communities: Two case studies in the semi-arid Southwest. Appl. Soil Ecol. 2016, 99, 118–128. [Google Scholar] [CrossRef]
- Fernández-García, V.; Marcos, E.; Fernández-Guisuraga, J.M.; Taboada, A.; Suárez-Seoane, S.; Calvo, L. Impact of burn severity on soil properties in a Pinus pinaster ecosystem immediately after fire. Int. J. Wildland Fire 2019, 28, 354–364. [Google Scholar] [CrossRef]
- Korchef, A.; Touaibi, M. Effect of pH and temperature on calcium carbonate precipitation by CO2 removal from iron-rich water. Water Environ. J. 2020, 34, 331–341. [Google Scholar] [CrossRef]
- Moeslund, J.E.; Arge, L.; Bøcher, P.K.; Dalgaard, T.; Svenning, J.C. Topography as a driver of local terrestrial vascular plant diversity patterns. Nord. J. Bot. 2013, 31, 129–144. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Givnish, T.J. Altitudinal gradients in tropical forest composition, structure, and diversity in the Sierra de Manantlán. J. Ecol. 1998, 86, 999–1020. [Google Scholar]
- Bennie, J.; Hill, M.O.; Baxter, R.; Huntley, B. Influence of slope and aspect on long-term vegetation change in British chalk grasslands. J. Ecol. 2006, 94, 355–368. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species composition and community structure of western Himalayan moist temperate forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Måren, I.E.; Karki, S.; Prajapati, C.; Yadav, R.K.; Shrestha, B.B. Facing north or south: Does slope aspect impact forest stand characteristics and soil properties in a semiarid trans-Himalayan valley? J. Arid Environ. 2015, 121, 112–123. [Google Scholar] [CrossRef]
- Khan, S.M.; Page, S.; Ahmad, H.; Zahidullah; Shaheen, H.; Ahamd, M.; Harper, D. Phyto-climatic gradient of vegetation and habitat specificity in the high elevation western himalayas. Pak. J. Bot. 2013, 45, 223–230. [Google Scholar]
- Kharkwal, G.; Mehrotra, P.; Rawat, Y.; Pangtey, Y. Phytodiversity and growth form in relation to altitudinal gradient in the Central Himalayan (Kumaun) region of India. Curr. Sci. 2005, 89, 873–878. [Google Scholar]
- Hussain, M.; Khan, S.; Abd_Allah, E.; Ul Haq, Z.; Alshahrani, T.; Alqarawi, A.; Ur Rahman, I.; Iqbal, M.; Ahmad, H. Assessment of plant communities and identification of indicator species of an Ecotonal Forest zone at Durand Line, District Kurram, Pakistan. Appl. Ecol. Environ. Res. 2019, 17, 6375–6396. [Google Scholar] [CrossRef]
- Chawla, A.; Rajkumar, S.; Singh, K.; Lal, B.; Singh, R.; Thukral, A. Plant species diversity along an altitudinal gradient of Bhabha Valley in western Himalaya. J. Mt. Sci. 2008, 5, 157–177. [Google Scholar] [CrossRef]
- Shaheen, H.; Sarwar, R.; Firdous, S.S.; Dar, M.; Ullah, Z.; Khan, S.M. Distribution and structure of conifers with special emphasis on Taxus Baccata in moist temperate forests of Kashmir Himalayas. Pak. J. Bot 2015, 47, 71–76. [Google Scholar]
- Suliman, M.; Khan, S.M.; Ali, S.; Abdullah, A.; Rahman, A.U.; Ullah, H.; Dost, M. Role of forest’s woody vegetation in the climate change mitigation through carbon sequestration in northern Pakistan. In Agroforestry for Carbon and Ecosystem Management; Jhariya, M.K., Meena, R.S., Banerjee, A., Kumar, S., Raj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, Chapter 14; pp. 191–204. [Google Scholar]
- Ali, S.; Khan, S.M.; Abdullah, A.; Ahmad, Z. Dryland agroforestry; mitigating role in reducing air pollution and climate change impacts. In Agroforestry for Carbon and Ecosystem Management; Jhariya, M.K., Meena, R.S., Banerjee, A., Kumar, S., Raj, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; Volume 1, Chapter 20; pp. 271–282. [Google Scholar]


| Summary of Shannon Diversity Indices of Fire Free Areas | ||||||||
|---|---|---|---|---|---|---|---|---|
| Species | chao | chao.se | jack1 | jack1.se | jack2 | boot | boot.se | n |
| 173 | 207.255 | 13.37985 | 217.1509 | 9.611166 | 233.0838 | 194.7548 | 5.553475 | 53 |
| Summary of Shannon Diversity Indices of fire-affected areas | ||||||||
| Species | chao | chao.se | jack1 | jack1.se | jack2 | boot | boot.se | n |
| 122 | 137.5431 | 7.86368 | 148.4808 | 5.981573 | 152.7606 | 135.8537 | 4.166071 | 52 |
| Fit Measurements of SEM of Edaphic Factors on Species Richness of Fire-Free Areas | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Chisq | Pvalue | NFI | CFI | RMSEA | GFI | AGFI | SRMR | RMR | AIC |
| 4.165 | 0.384 | 0.931 | 0.997 | 0.028 | 0.959 | 0.946 | 0.063 | 0.062 | 408.022 |
| Fit measurements of SEM of edaphic factors on species richness of fire-affected areas. | |||||||||
| Chisq | Pvalue | NFI | CFI | RMSEA | GFI | AGFI | SRMR | RMR | AIC |
| 1.64 | 0.65 | 0.978 | 0.976 | 0.001 | 0.973 | 0.963 | 0.032 | 0.031 | 1135.254 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Israr, A.; Khan, S.M.; Abdullah, A.; Ejaz, U.; Jehangir, S.; Ahmad, Z.; Hashem, A.; Avila-Quezada, G.D.; Abd_Allah, E.F. Fire-Induced Vegetation Dynamics: An In-Depth Discourse on Revealing Ecological Transformations of the Mahaban and Surrounding Forests. Fire 2024, 7, 27. https://doi.org/10.3390/fire7010027
Israr A, Khan SM, Abdullah A, Ejaz U, Jehangir S, Ahmad Z, Hashem A, Avila-Quezada GD, Abd_Allah EF. Fire-Induced Vegetation Dynamics: An In-Depth Discourse on Revealing Ecological Transformations of the Mahaban and Surrounding Forests. Fire. 2024; 7(1):27. https://doi.org/10.3390/fire7010027
Chicago/Turabian StyleIsrar, Azra, Shujaul Mulk Khan, Abdullah Abdullah, Ujala Ejaz, Sadia Jehangir, Zeeshan Ahmad, Abeer Hashem, Graciela Dolores Avila-Quezada, and Elsayed Fathi Abd_Allah. 2024. "Fire-Induced Vegetation Dynamics: An In-Depth Discourse on Revealing Ecological Transformations of the Mahaban and Surrounding Forests" Fire 7, no. 1: 27. https://doi.org/10.3390/fire7010027
APA StyleIsrar, A., Khan, S. M., Abdullah, A., Ejaz, U., Jehangir, S., Ahmad, Z., Hashem, A., Avila-Quezada, G. D., & Abd_Allah, E. F. (2024). Fire-Induced Vegetation Dynamics: An In-Depth Discourse on Revealing Ecological Transformations of the Mahaban and Surrounding Forests. Fire, 7(1), 27. https://doi.org/10.3390/fire7010027








