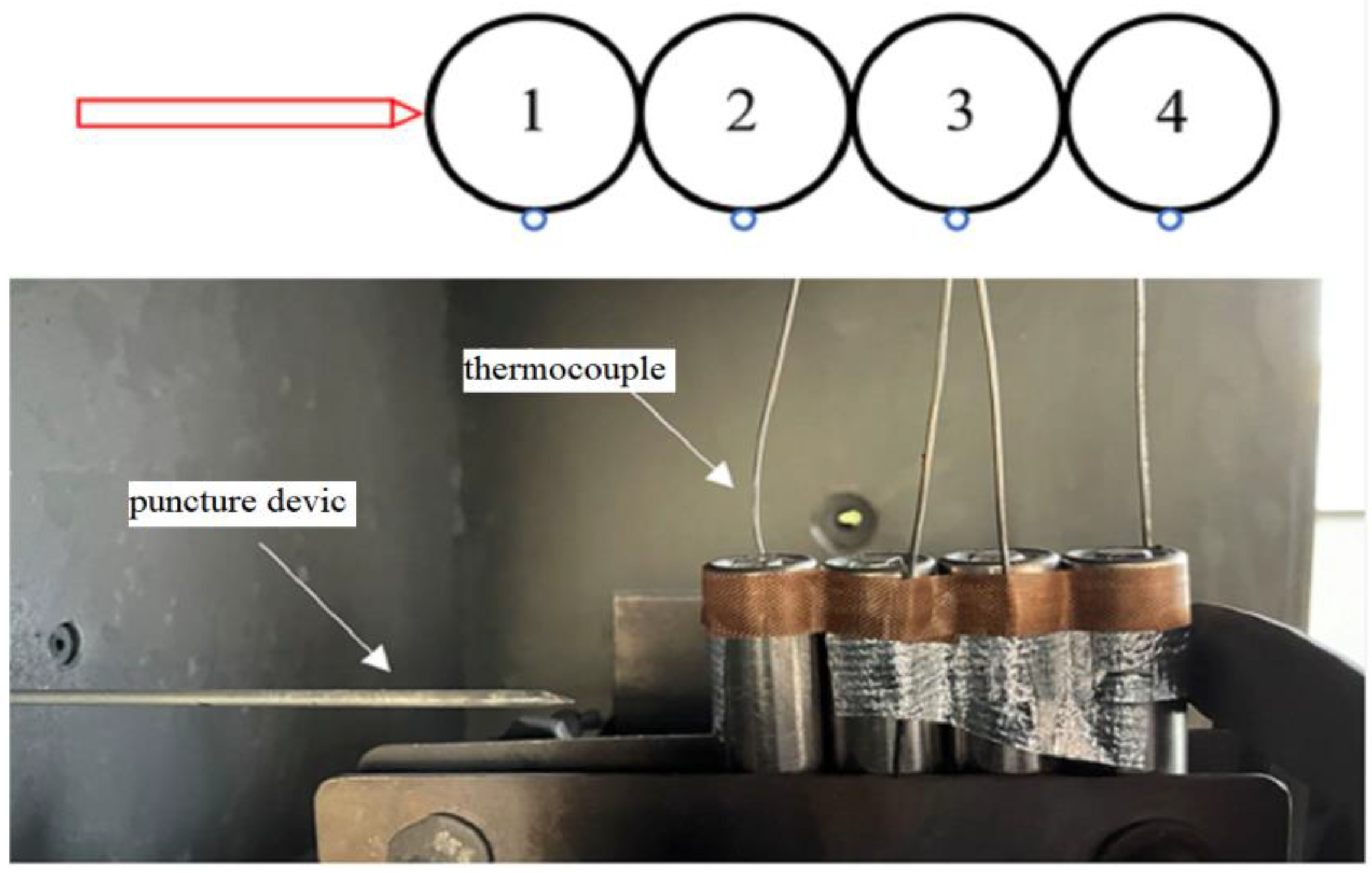
1. Introduction
Lithium-ion batteries have become an important component in new energy vehicles, energy storage power plants, and other electronic devices due to their high energy density, fast charging rate, and long-life cycle [
1,
2]. However, the high energy density of lithium-ion batteries also means that they are a high fire hazard. As the materials of lithium-ion batteries are mostly organically flammable and chemically active, it is easy to induce an internal thermal runaway reaction and release flammable gas to cause combustion or even an explosion when subjected to high temperatures, overcharge and over discharge, puncture, and extrusion [
3]. At present, many scholars, domestic and overseas, have conducted studies on the thermal runaway behavior of lithium-ion batteries.
As for the thermal runaway characteristics of lithium-ion batteries, Ishikawa et al. have revealed the causes of thermal runaway of Li-ion batteries. It will produce a self-discharge phenomenon at high temperatures, and the battery capacity will decline rapidly. Then, the SEI film at the negative electrode of the battery will thermally decompose and generate a large amount of heat and oxygen inside the battery. The heat melting of the diaphragm will lead to a short circuit of the positive and negative electrodes, converting the electrical energy inside the battery into heat energy released. At the same time, the rest of the constituent materials inside the battery also react one after another at high temperatures [
4,
5,
6]. Feng et al. found that 12% of the heat generated by the thermal runaway of a lithium-ion battery can heat the neighboring battery to the thermal runaway critical condition and then form a chain of thermal runaway propagation. The heat transfer between batteries is mainly between the battery shells [
7]. Man et al. found that setting up effective heat dissipation within the battery pack would help stop the dangerous consequences of abnormal battery heating [
8]. Deng Zhibin et al. carried out experiments on the thermal runaway characteristics and propagation of lithium-ion batteries under two different conditions: open system and semi-enclosed system. The results showed that thermal runaway propagation did not occur in the open system but occurred in the semi-enclosed system with metal packaging [
9,
10]. Wu et al. proposed a low-complexity SOC and anode potential prediction method for LIBs using a simplified electrochemical model-based observer under variable load conditions [
11]. Garcia et al. presented a novel simulation approach consisting of coupling fundamental and applicate aspects of lithium-ion battery simulations [
12].
As for lithium-ion battery thermal runaway suppression technology, the Federal Aviation Administration conducted a simulation study for lithium-battery fire-suppression methods. The results showed that a small amount of liquid extinguishing agent could effectively intervene in the propagation of thermal runaway and control the scale of the fire, while non-liquid class extinguishing agents are less effective in suppressing them [
13,
14]. Liu Yujun et al. used a self-built test platform of fire extinguishing to study the effect of traditional fire extinguishing agents to extinguish lithium-ion battery monomer fires. The results showed that water has better applicability in lithium-ion battery fires due to its optimal cooling effect. In contrast, dry powder and carbon dioxide extinguishing agents have a poor extinguishing effect and will reignite after extinguishing [
15]. Huang Qiang et al. studied the fire extinguishing effect of various extinguishing agents in the thermal runaway fire of a lithium iron phosphate battery pack. The results showed that the effect of the medium-pressure fine water mist is the best, and continuous spraying can effectively prevent re-ignition. Perfluorinated hexanone and heptafluoropropane extinguishing agents are less effective, and there is the phenomenon of re-ignition after extinguishing the fire [
16]. Wang et al. showed that heptafluoropropane can effectively extinguish open fires. However, its insufficient cooling effect makes it unable to block the internal reaction of lithium-ion batteries, and it is easy to re-ignite after extinguishing [
17].
In summary, cryogenic cooling is the key to extinguishing lithium-ion battery fires because the fires originate from the continuous exothermic reaction inside the battery. Most of the above-mentioned scholars’ studies focus on the cooling and extinguishing of lithium-ion batteries after thermal runaway; there is still less research on the use of cryogenic cooling means to prevent thermal runaway of lithium-ion batteries. At the same time, some studies have shown that low temperature almost does not cause irreversible damage to the performance of lithium-ion batteries [
18]. Therefore, it is necessary to study the thermal runaway behavior under the effect of cryogenic cooling. Based on this, a study on the thermal runaway suppression effect of ternary lithium-ion batteries under cryogenic cooling is carried out in this paper through a self-built lithium-ion battery puncture test platform. The research content can provide some reference for solving the safety problems of lithium-ion batteries in storage and transportation.
3. Analysis of Battery Thermal Runaway
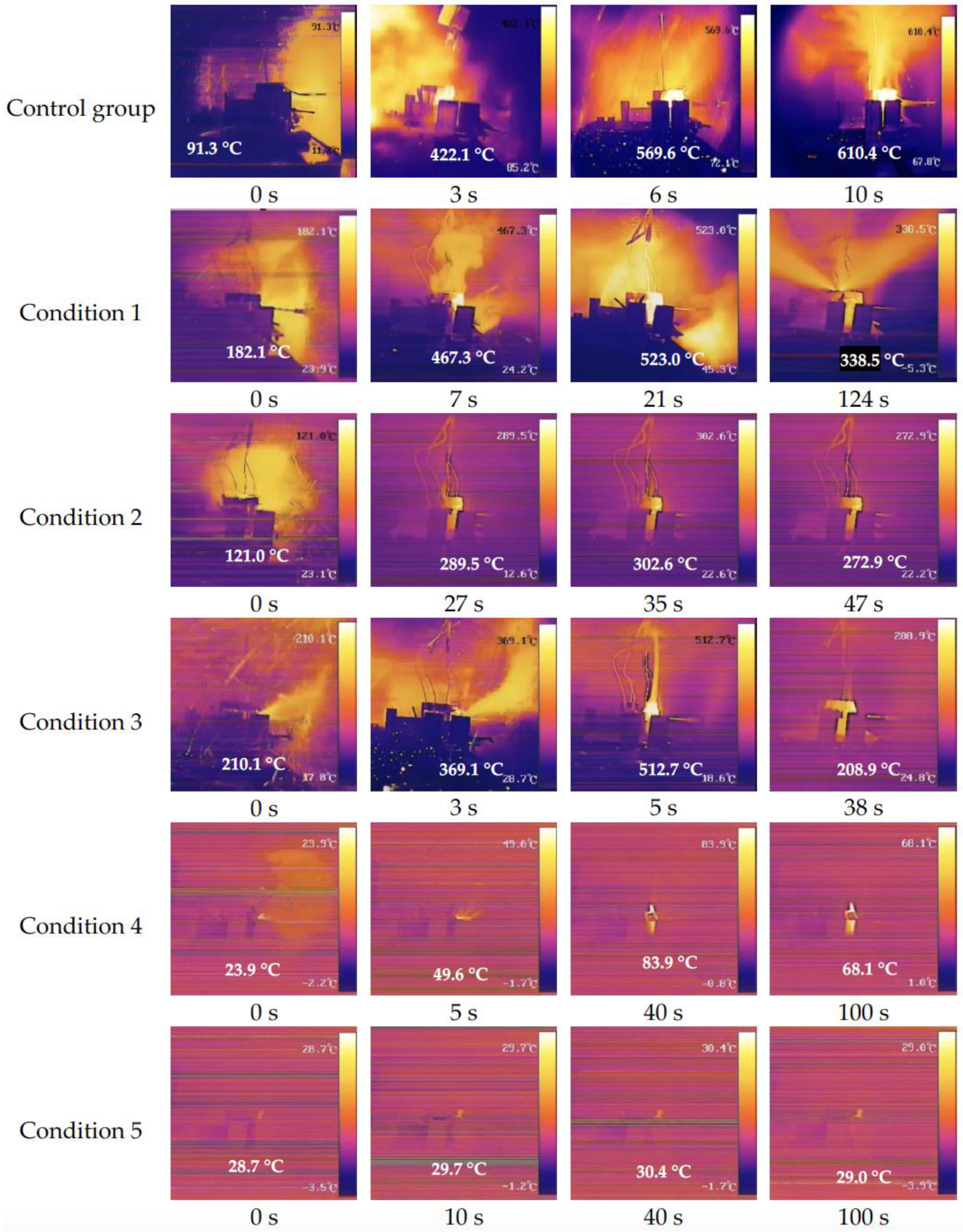
The thermal runaway phenomena of the Li-ion battery pack for the six working conditions are shown in
Figure 2.
The ternary lithium-ion battery pack reacted violently after puncture under the control group conditions (cell temperature of 20 °C), and four batteries burst one after another in a very short period of time, with the highest temperature in the chamber reaching 610 °C. From the first battery thermal runaway to the fourth battery explosion, took a total of less than 10 s.
The No. 1 lithium-ion battery under the condition 1 (cell temperature of 0 °C) emitted a large amount of sparks immediately after it was penetrated by the steel needle; the highest temperature in the chamber instantly reached 180 °C. After the battery No. 1 exploded, battery No. 2 temperature rose rapidly, and the explosion occurred in 7 s; the chamber fire rapidly expanded. Battery No. 3 exploded in 21 s and burning lasted about 12 s after the fire receded. The high temperature in the chamber continued to heat the battery No. 4. The thermal runaway primary explosion occurred in about 124 s and burning continued for about 30 s after the flame was extinguished. Compared with the lithium-ion battery combustion and explosion phenomenon at room temperature, the occurrence time of combustion and explosion of batteries No. 2 to No. 4 have different degrees of lag. This phenomenon proves that low temperatures can reduce the material activity inside the ternary lithium-ion battery, which has the effect of hindering the chain propagation of thermal runaway.
In condition 2 (cell temperature of −10 °C), the thermal runaway phenomenon of Li-ion battery No. 1 after puncture, is similar to that of the control group and condition 1. Battery No. 2 caught fire at 27 s under the heating of battery No. 1; it did not explode but burned steadily in the form of a laminar fire. Battery No. 2 flame was extinguished after burning for about 20 s. The thermal runaway of batteries No. 3 and No. 4 did not occur in the conditions of 2, because the time distance of the battery No. 2 fire breaking-out time to the battery No. 1 thermal runaway was longer. The energy released by the two batteries could not be superimposed, and batteries No. 3 and No. 4 did not get enough energy, so the fire did not occur.
When the temperature of the ternary lithium-ion battery is further reduced, the phenomenon of thermal runaway chain propagation of the whole battery pack will be more suppressed.
Battery No. 1 under condition 3 (cell temperature of −20 °C) also had thermal runaway after puncture, with sparks and jet fire emitted from the puncture site. However, the jet fire turned into a stable laminar fire after about 3 s and burned for about 38 s before extinguishing. The remaining three cells did not experience thermal runaway. From the infrared view, it can be found that although the temperature inside the compartment of battery No. 1 can reach up to 500 °C after thermal runaway, the internal chemical reaction activity of the remaining three batteries was suppressed under the effect of low temperature, and thermal runaway did not occur either.
Under working condition 4 (cell temperature of −30 °C), a small amount of smoke and sparks came out at the puncture of battery No. 1, and a chemical reaction occurred inside the battery, with the temperature continuing to rise. After reaching 80 °C, the temperature began to drop, and the energy released inside the battery was insufficient, so the battery No. 1 failed to have thermal runaway and the rest of the batteries did not show any obvious reaction. Similarly, the thermal runaway did not occur after the puncture of the battery under working condition 5, no smoke and sparks were generated at the puncture of battery No. 1, and only a small amount of electrolyte flowed out.
Summing up the phenomena of the six groups tested, it can be seen that cryogenic cooling can significantly weaken the internal material activity of the ternary lithium-ion battery, which can play a role in inhibiting the thermal runaway of the battery. Among them, battery No. 1 of the control group, under working condition 1, working condition 2, and working condition 3 had thermal runaway immediately after puncture, as shown in
Table 3. In contrast, all four batteries of working conditions 4 and 5 did not have thermal runaway.
4. Results and Discussion
4.1. Cell Temperature Variation
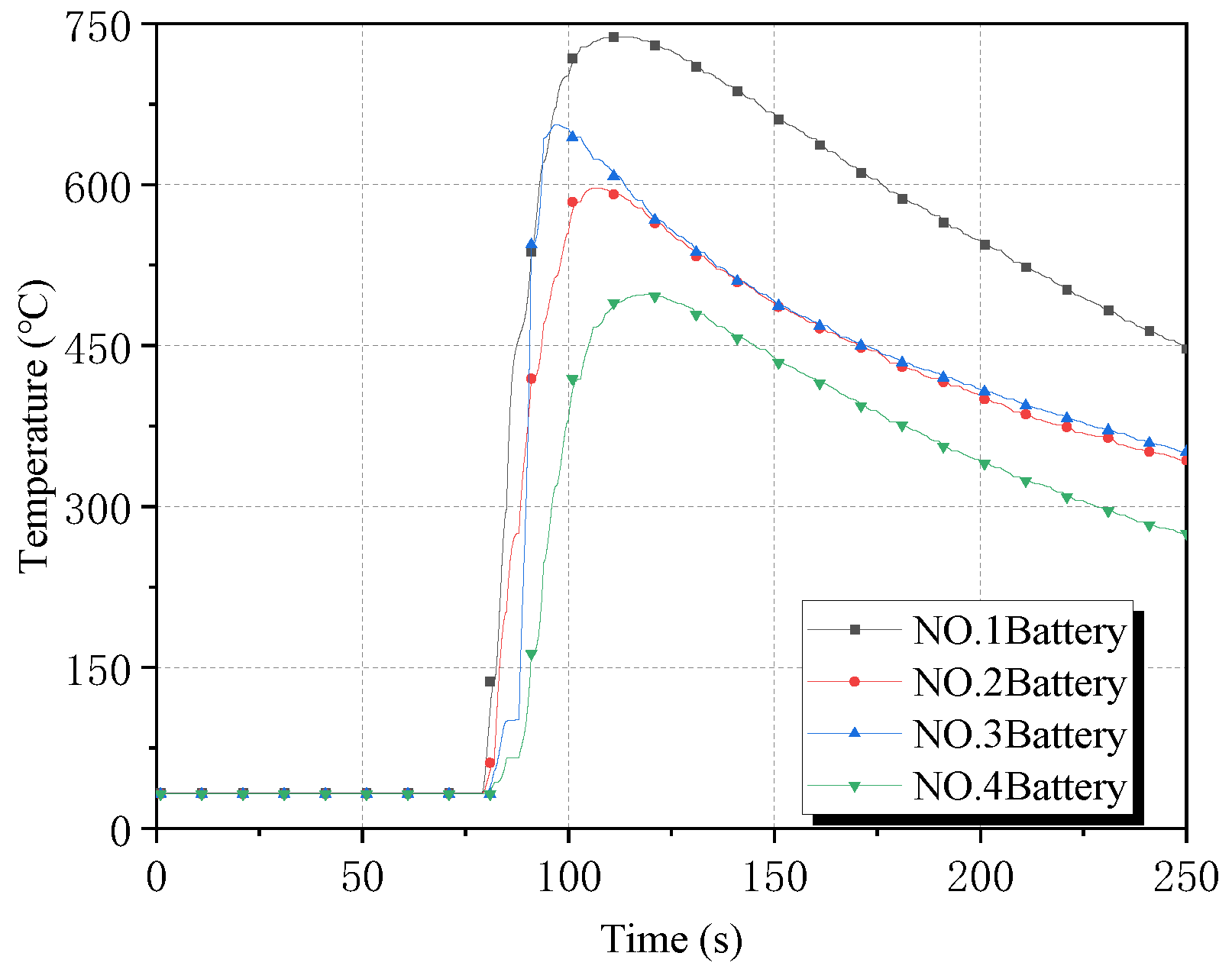
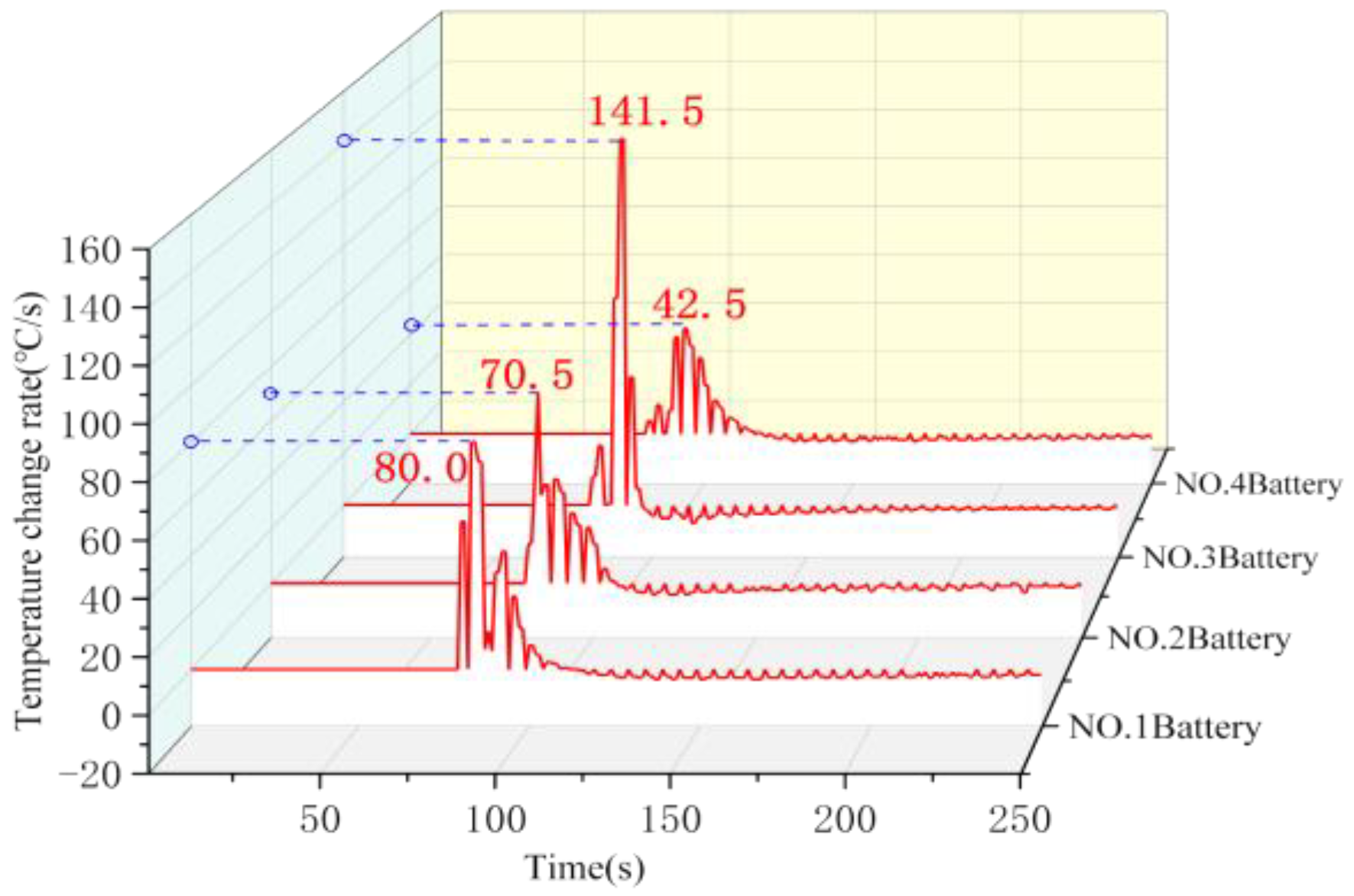
Figure 3 and
Figure 4, respectively, show the surface temperature curve and temperature change rate curve of the four batteries in the control group. Battery No. 1 underwent thermal runaway rapidly after puncture, and the surface temperature rose sharply, and the temperature rising rate reached the first peak value of 50.0 °C/s. The heat released from battery No. 1 then triggered the chain thermal runaway of the battery pack, and battery No. 2 then burst, and the instantaneous temperature rising rate reached 70.5 °C/s. At the same time, the heat release of battery No. 2 further aggravated the thermal runaway reaction rate of battery No. 1, and the temperature rising rate of it rose to the highest value of 80.0 °C/s under the superposition of heat. Battery No. 3 and battery No. 4 burst successively under the simultaneous heating of battery No. 1 and battery No. 2, and the peak-surface temperature rising rate of battery No. 3 reached the highest value of 141.5 °C/s.
In the whole chain of the thermal runaway process, battery No. 1 ignited the earliest, of which the duration of the internal reaction was the longest, and its surface temperature rose to a maximum of 738 °C after battery No. 4 ignited. The temperature maxima of batteries No. 2–4 were 597 °C, 656 °C, and 501 °C, respectively.
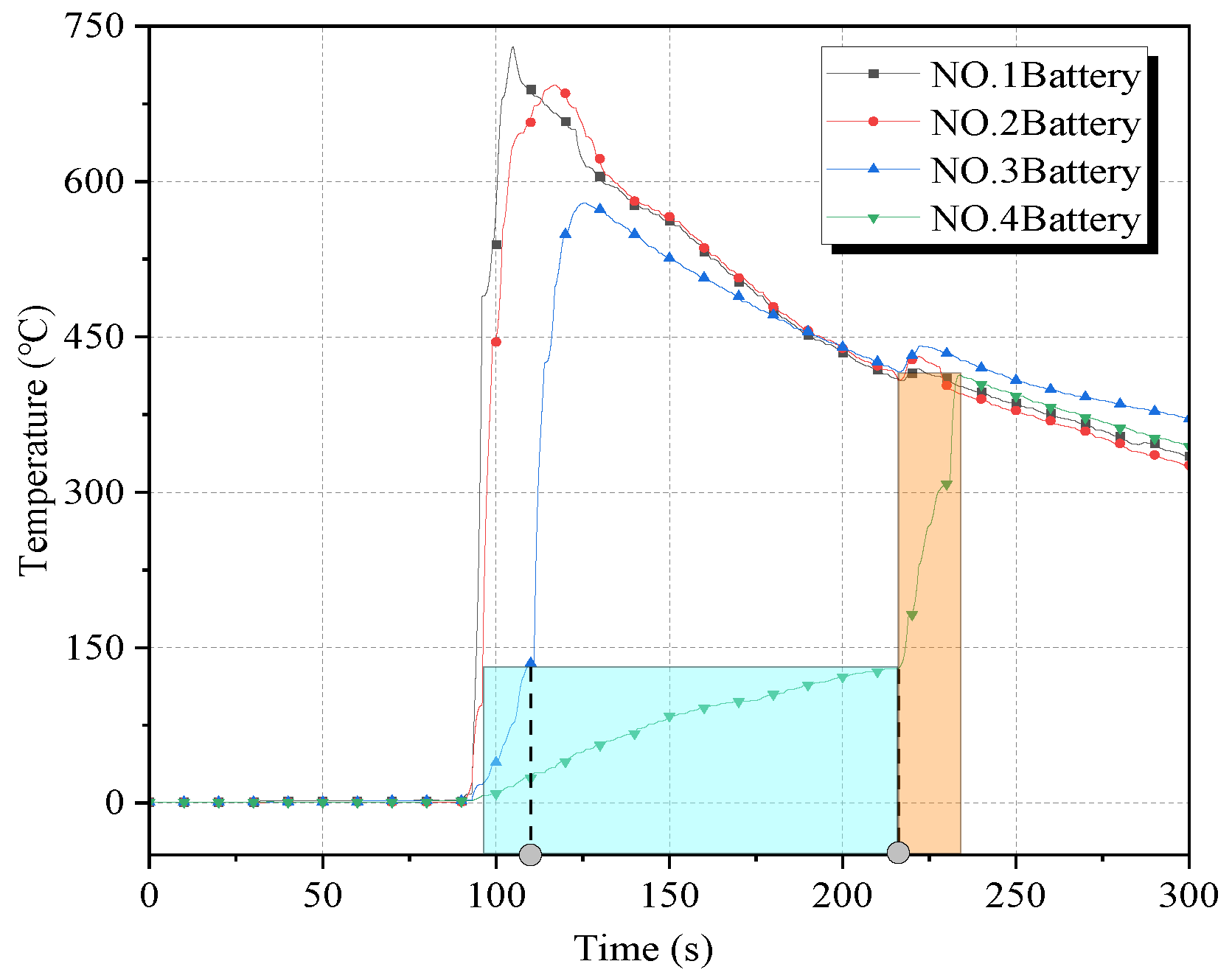
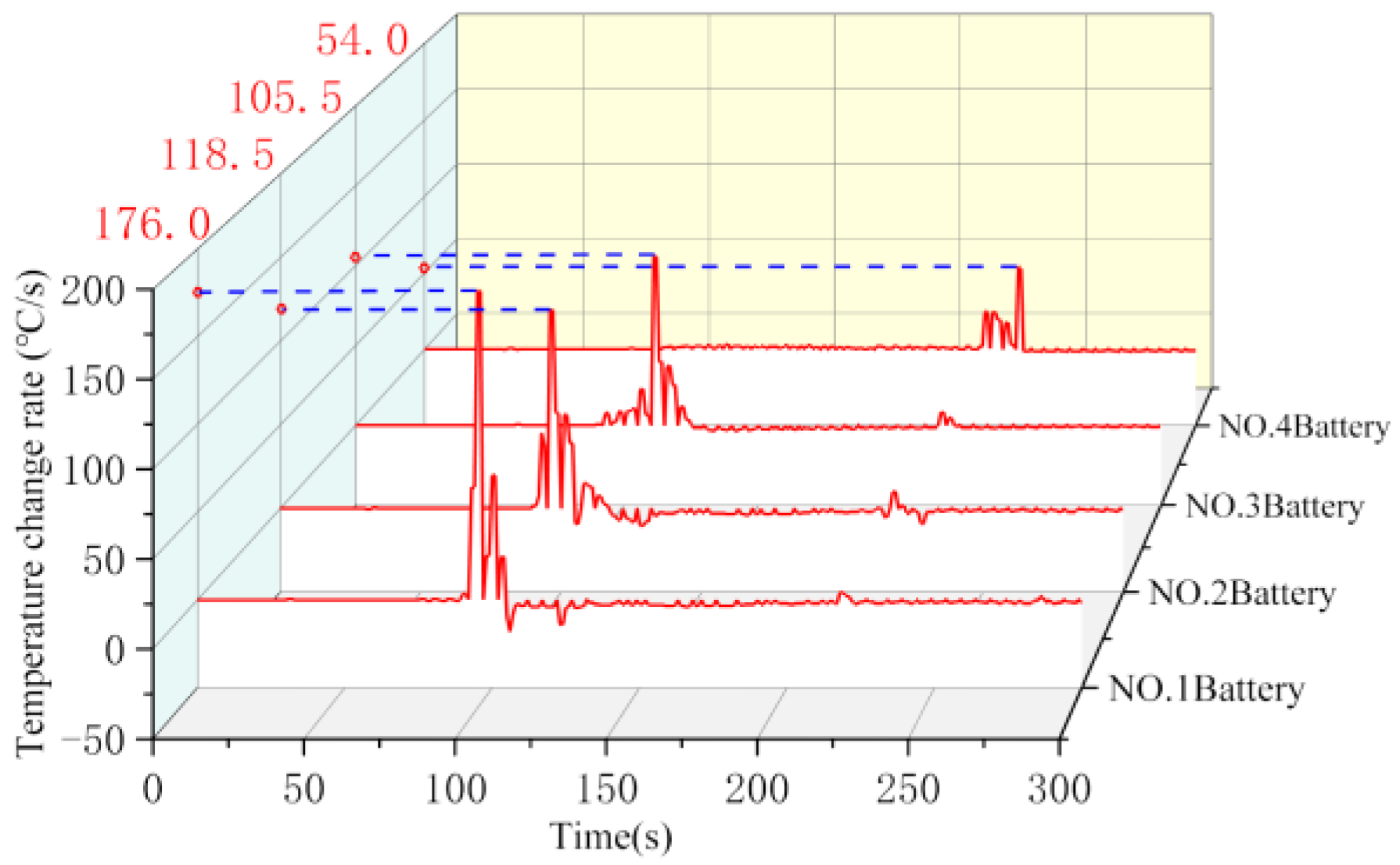
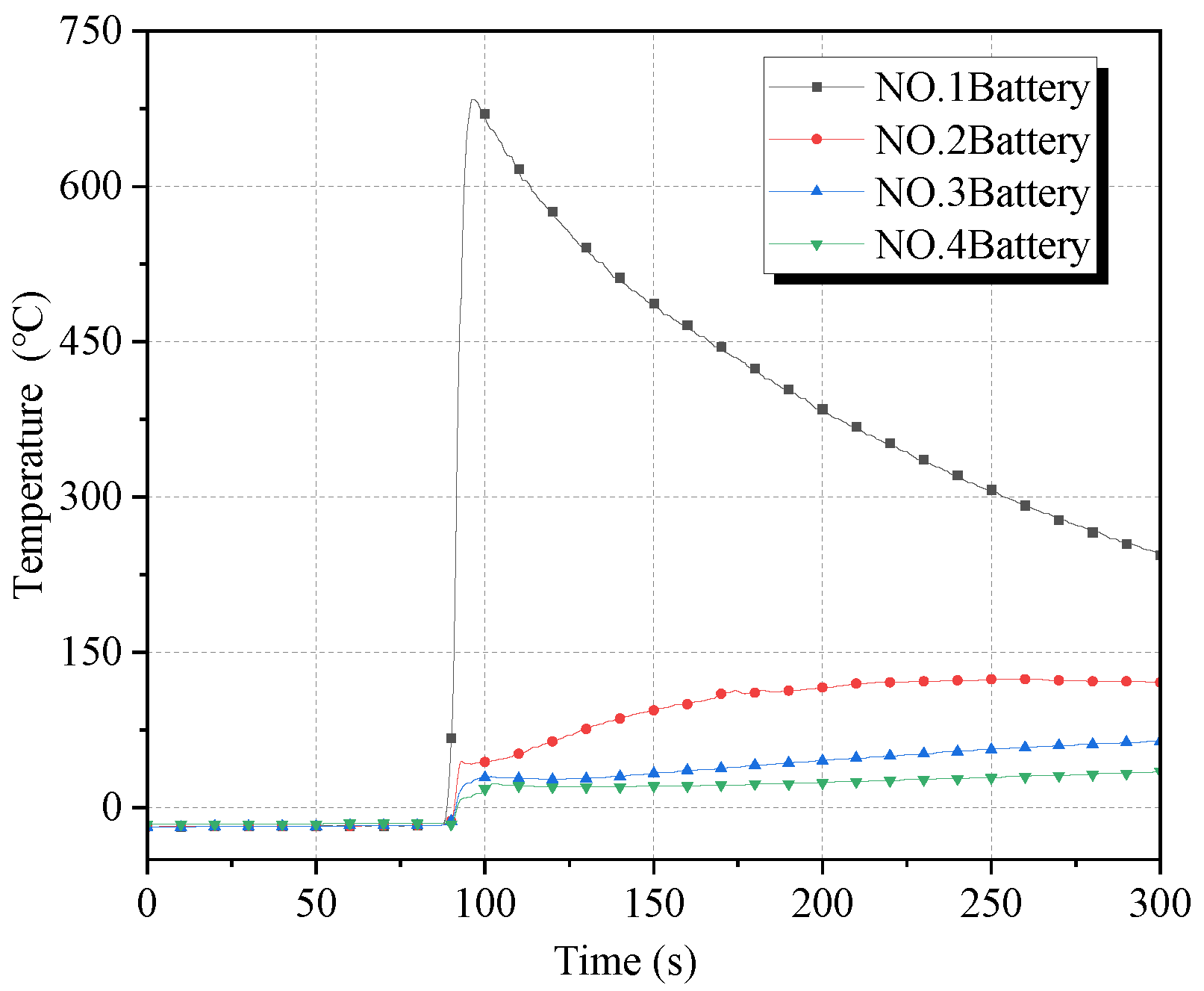
The surface temperature curve and the temperature change rate curve of the four batteries in working condition 1 are given in
Figure 5 and
Figure 6, respectively. Among them, the two black dashed lines in
Figure 5 correspond to the moments when the thermal runaway of batteries No. 3 and No. 4 occurred, respectively. As can be seen from the figure, the temperature of battery No. 1 rises rapidly after puncture, and the temperature rising rate rises to more than 70 °C/s. With the explosion of battery No. 2, the temperature rising rate of battery No. 1 increased to 176.0 °C/s, and the maximum temperature reached 730 °C. Battery No. 2 reached a maximum temperature rising rate of 118.5 °C/s after the explosion, and the maximum temperature reached 693 °C. Because of the effect of cryogenic cooling, the onset time of thermal runaway of No. 3 battery was delayed to a certain extent compared with that of the control group. The peak temperature rising rate at the moment of explosion was the largest, which was 105.5 °C/s, and the maximum temperature after explosion was 572 °C. The temperature of battery No. 4 under the heating effect of the first three batteries rose slowly after about 124 s of heating time (blue area in
Figure 5) before detonation, during which the maximum temperature was 416 °C; the maximum temperature rising rate was 54.0 °C/s. In battery No. 4 detonation, the temperature of 1–3 batteries had dropped to about 400 °C. With the detonation of battery No. 4, the temperature of batteries 1–3 had a small rebound; the temperature fell again after a few seconds.
Comparing condition 1 with the control group, it was found that cryogenic cooling somewhat enhanced the heat release rate of the battery after puncture, but faster energy release meant a shorter combustion duration. Its heating effect on subsequent cells was shorter also, so cryogenic cooling somewhat inhibited the chain propagation of thermal runaway, and the maximum temperature of each cell was significantly reduced compared with that at room temperature.
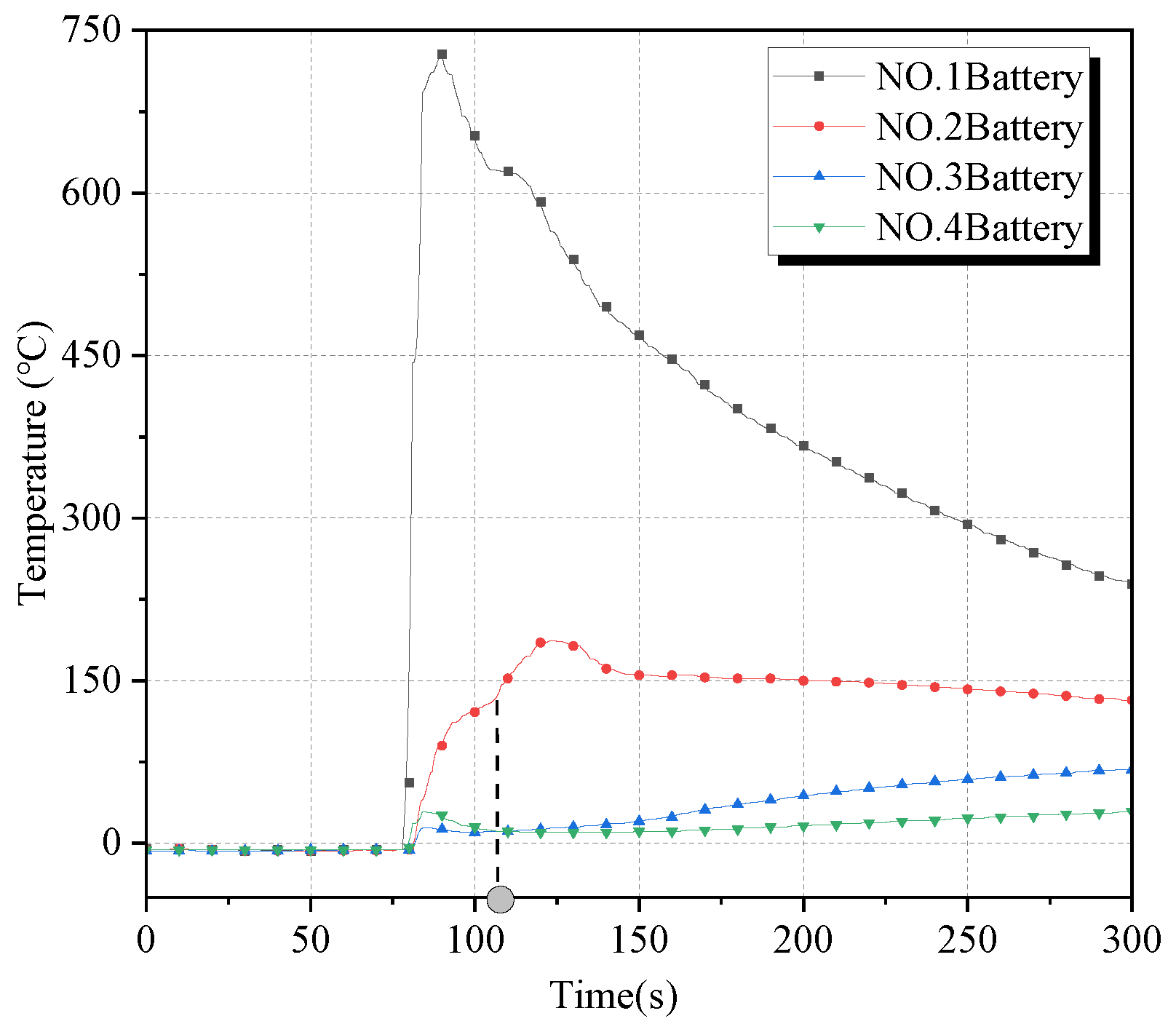
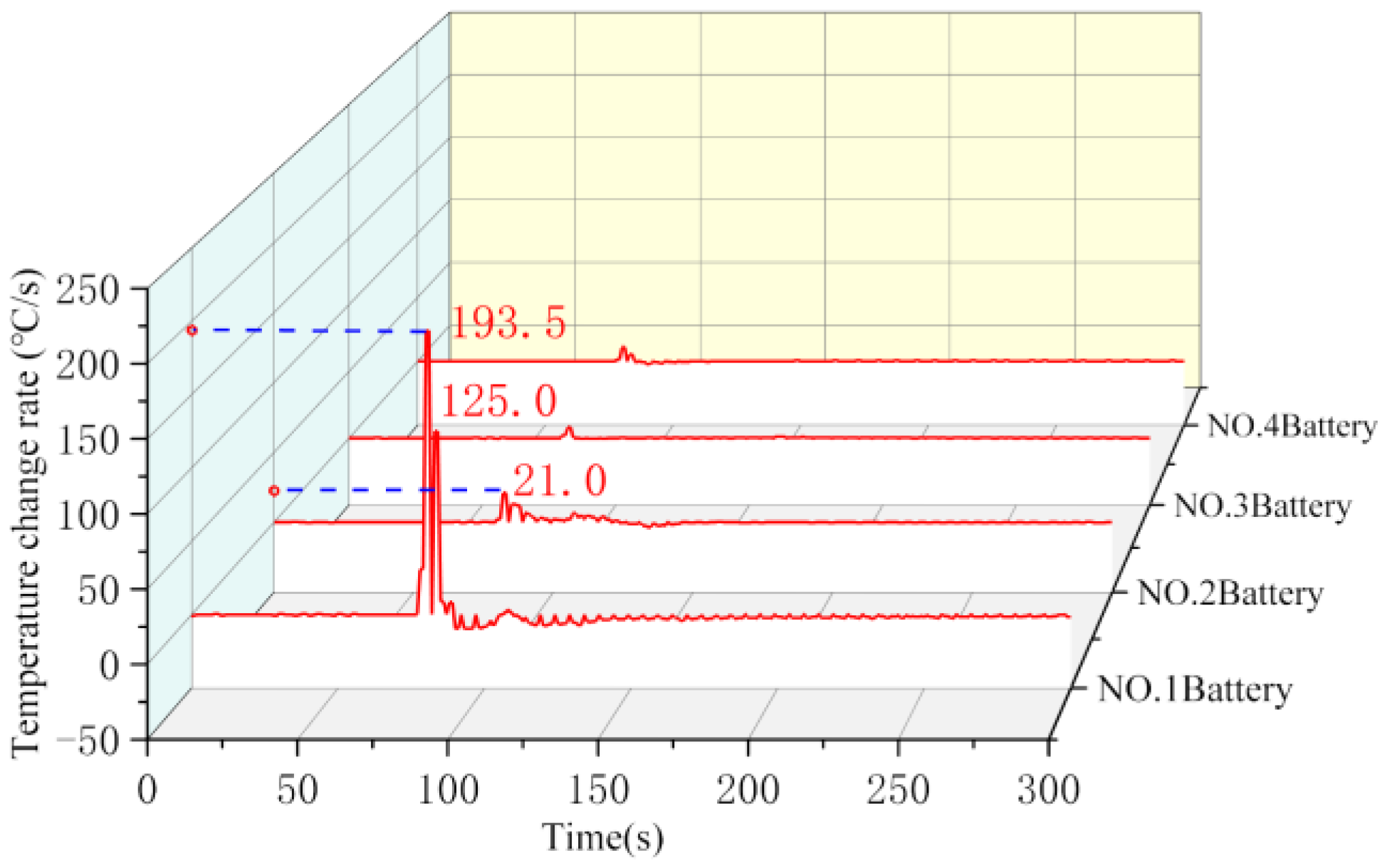
The surface temperature curves and temperature change rate curves of the four batteries in condition 2 are shown in
Figure 7 and
Figure 8. The temperature change pattern of Battery No. 1 was more consistent with condition 1, with the maximum temperature rising rate reaching 193.5 °C/s, and its surface temperature reached the maximum value of 728 °C after 10 s of thermal runaway. At the moment of 27 s, its surface temperature reached 133 °C and then ignited; soon afterward, the temperature continued to rise slowly to a maximum of 186 °C. The surface temperature of batteries No. 3 and No. 4 only had a short rise after the ignition of battery No. 1; the temperature of battery No. 3 rose to a maximum of 75 °C, and that of battery No. 4 did not exceed a maximum of 30 °C.
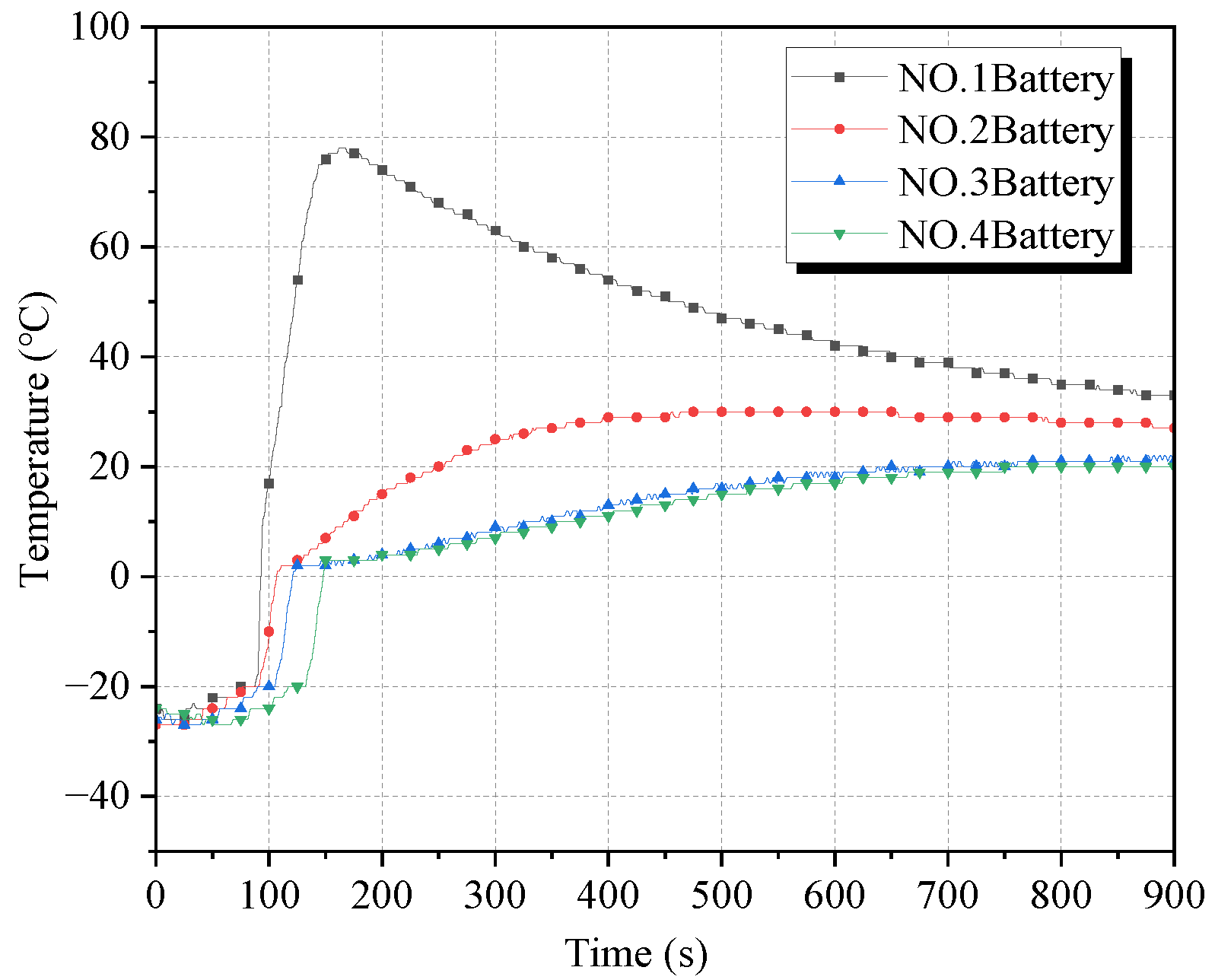
Figure 9 and
Figure 10 give the surface temperature curves and temperature change rate curves of the four batteries in working condition 3, respectively. The suppression effect of cryogenic cooling on the thermal runaway of the battery is further improved under working condition 3. Battery No. 1 did not burst but maintained stable combustion. The maximum temperature rising rate of battery No. 1 reached 212.5 °C/s, and the surface temperature reached the maximum value of 684 °C at 8 s. Batteries No. 2–4 did not have thermal runaway, and the temperature maxima were 124 °C, 69 °C, and 44 °C, respectively.
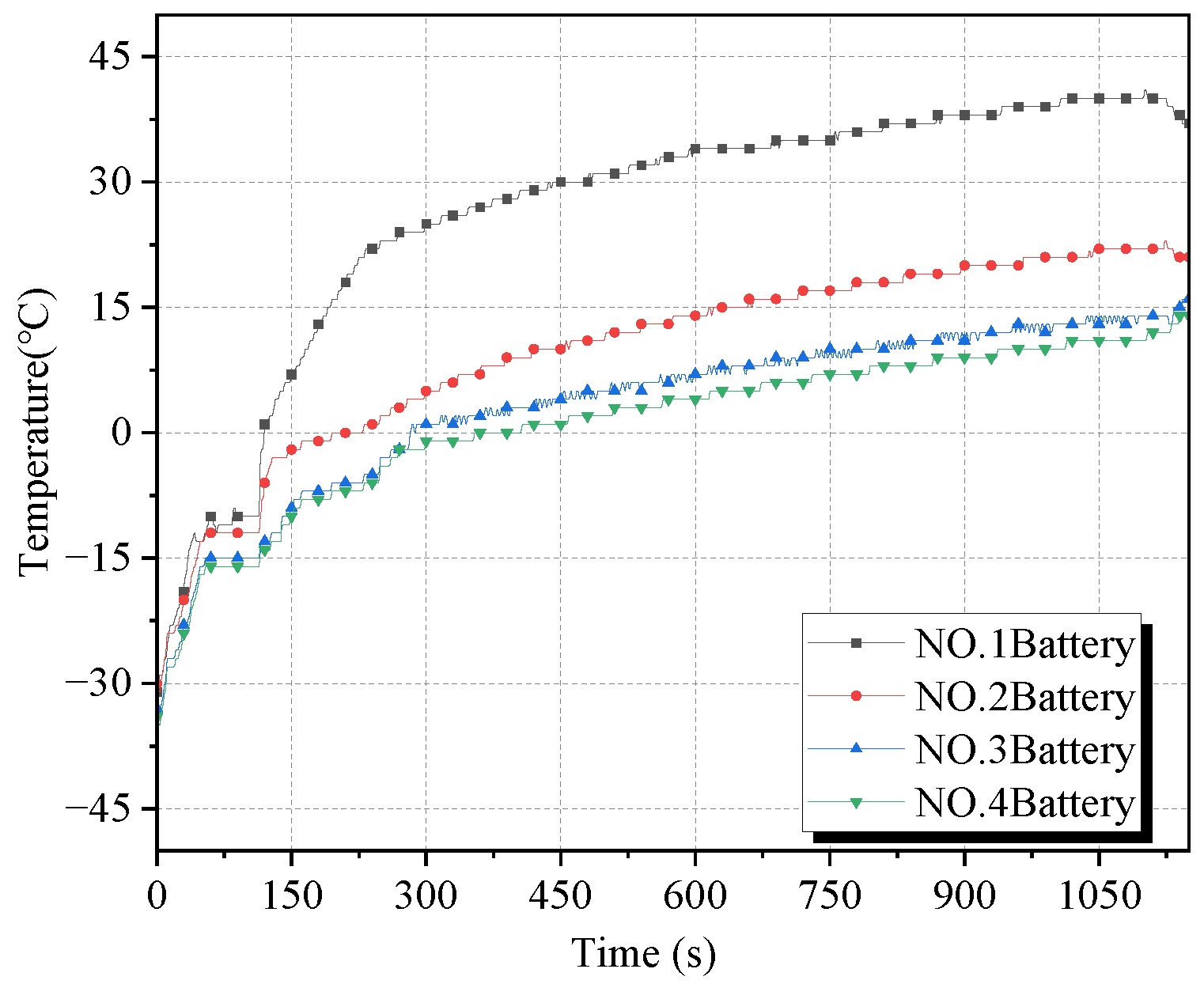
The temperature change curves of the battery surface under working condition 4 and working condition 5 are given in
Figure 11 and
Figure 12, respectively. Since cryogenic cooling drastically reduces the material activity inside the battery, the energy generated by the puncture can only make the internal material regain its activity but cannot cause thermal runaway to occur. The maximum temperature rising rate of battery 1 in working condition 4 and working condition 5 were 7.7 °C/s and 4.0 °C/s, respectively, and the maximum surface temperature was 80 °C and 42 °C respectively. There was only a small increase in the temperature of batteries 2–4 in both conditions, which were in a safe state.
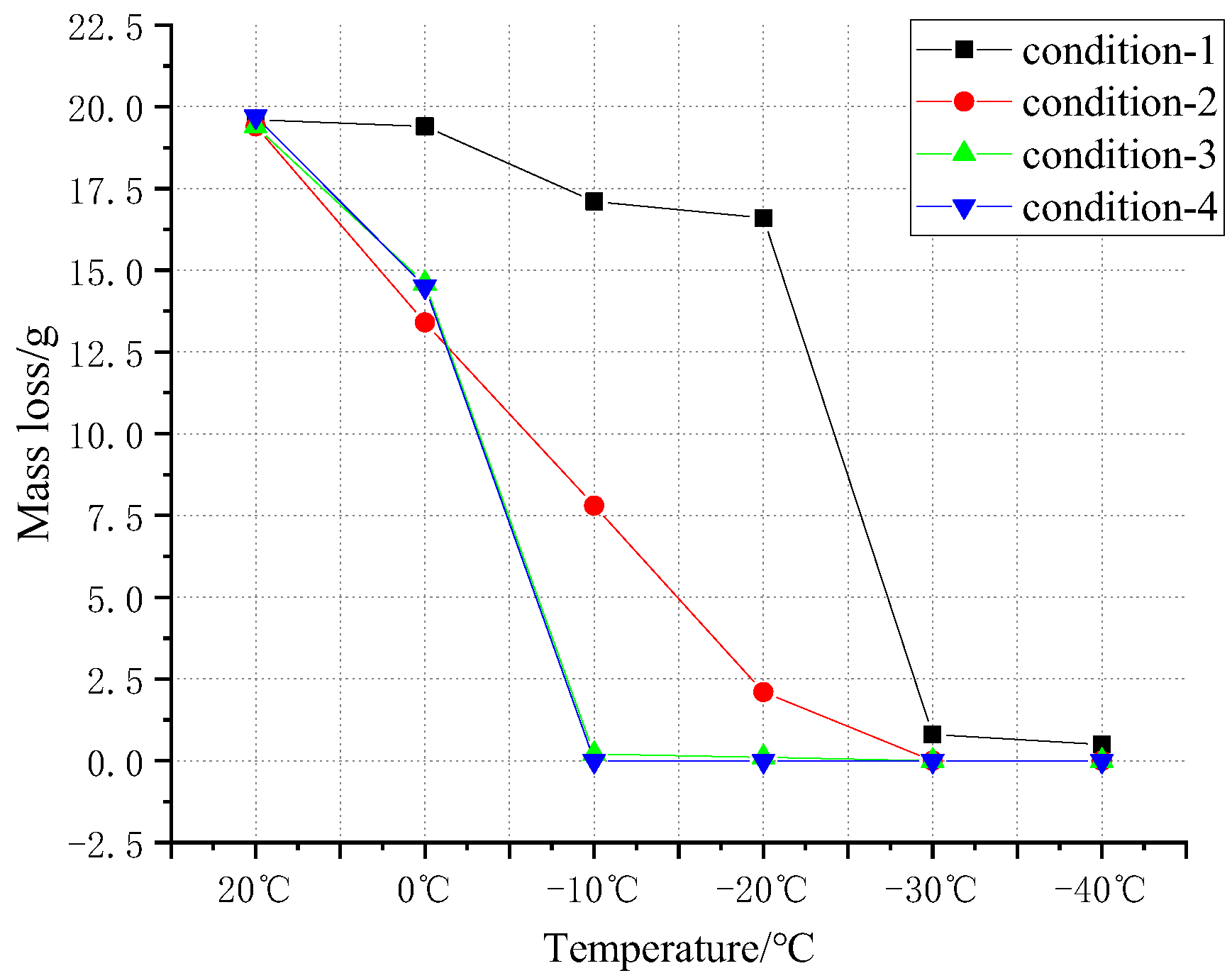
4.2. Battery Mass Change
Table 4 and
Figure 13 give the magnitude of the mass loss of the batteries before and after the six sets of tests. It is obvious from
Figure 13 that the mass loss decreases rapidly with the decrease in temperature. When the temperature drops to −30 °C, the mass loss of other batteries, except for the punctured battery, drops to 0. Specifically, the mass of all cells before the test was 48.1 g. The battery masses of different conditions had different degrees of variation after the test. As can be seen from the table, the control group (20 °C) had the most violent combustion and explosion phenomenon after thermal runaway, and the mass of all four batteries had a significant loss, with a mass loss rate between 40.3% and 41.0%. In condition 1, the mass loss of battery No. 1 in thermal runaway was close to condition zero, while the mass loss rate of batteries No. 2–4 dropped to 30% or less. In the second condition (−10 °C), only battery No. 1 and battery No. 2 burst, and the mass loss rate of battery No. 1 was about 35.6%, which was somewhat lower than the first two conditions. The mass loss rate of battery No. 2 was 16.2%, which was significantly lower than the normal ambient temperature. In condition 3 (−20 °C), only battery No. 1 was burned, while battery No. 2 was heated, and a chemical reaction occurred inside, and gas was released from inside, so there was a mass loss of about 4%. In conditions 4 (−30 °C) and 5 (40 °C), all the batteries had thermal runaway. Only the directly punctured battery had some electrolyte outflow, the rest of the batteries were intact, and the thermal runaway was effectively controlled.
4.3. CO Gas Concentration Changes in the Chamber
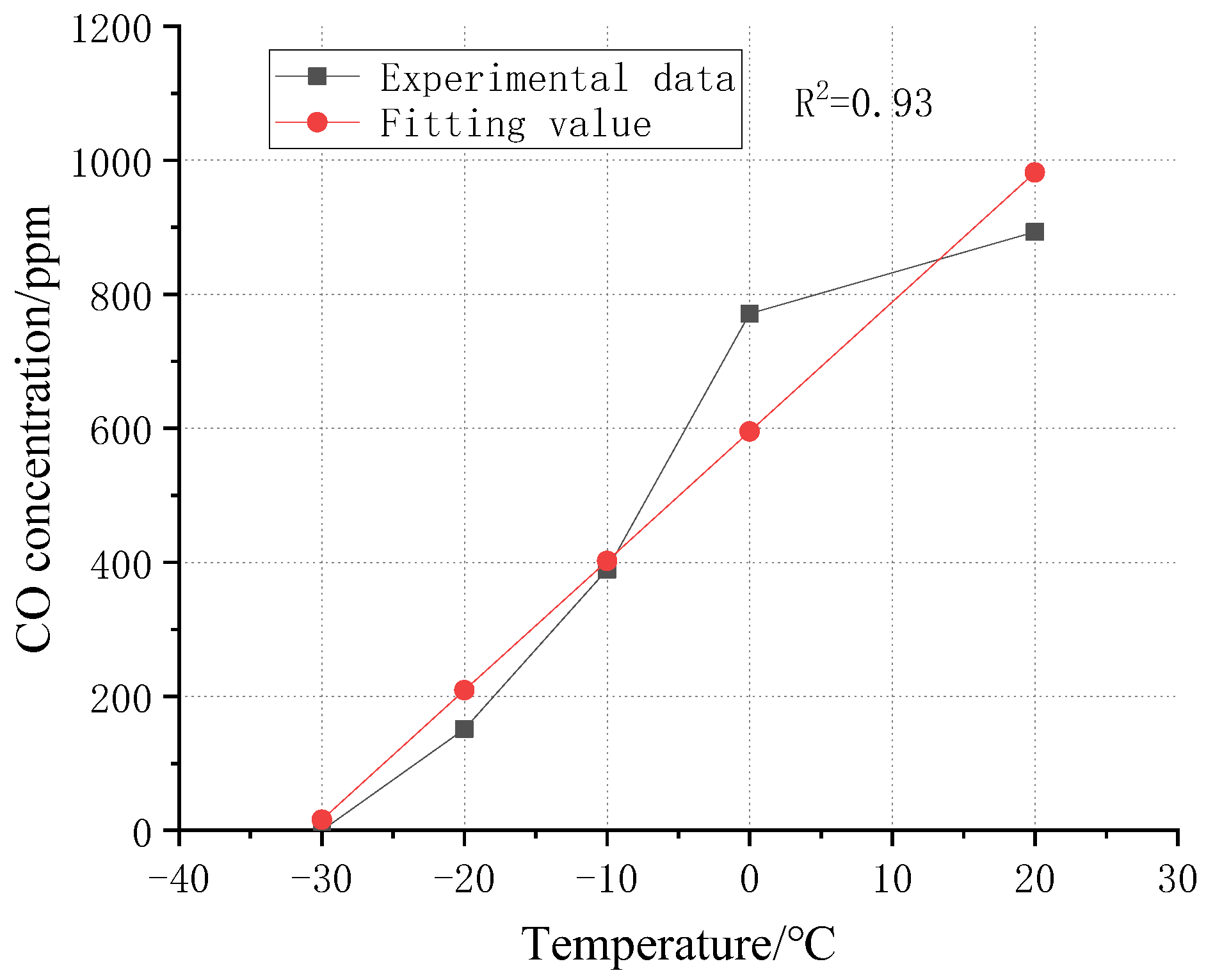
The maximum values of CO gas concentration during the test are given in
Table 5 and
Figure 14. It is obvious from
Figure 14 that CO concentration decreases linearly with temperature. The square difference of R is 0.93, which indicates that the linear correlation is good. When the temperature drops to −30 °C, CO concentration drops to 0.
The four batteries in condition 1 all had thermal runaway and explosion. However, compared with the control group, the thermal runaway of Battery 4 in condition 1 occurred a long time later than Battery 3, about 100 s, which led to the dispersion of the release process of gas products. The thermal runaway of Battery 4 in the control group was only 4 s later than that of Battery 3, which meant that CO accumulated rapidly in a short time. The highest CO concentration in condition 1 was 771 ppm, which was 13.7% lower than the control group. In condition 2, only two batteries had thermal runaway and explosion when punctured, and the gas quantity in the test chamber was significantly reduced. The highest CO concentration was 389 ppm, which was 56.4% lower than that in the control group. In condition 3, only one battery had thermal runaway, and the highest CO concentration was only 151 ppm. Under conditions 4 and 5, no obvious thermal runaway reaction occurred after the battery was punctured. Moreover, only a small amount of gas was released under condition 4. Due to a certain distance between the detection point and the batteries, the detected values of CO concentration were nearly 0 ppm.
5. Conclusions
In this paper, the thermal runaway phenomenon of ternary lithium-ion batteries under the effect of cryogenic cooling is investigated experimentally, and the main conclusions are as follows:
- (1)
Cryogenic cooling will reduce the reaction activity of the materials inside the battery and make the reaction take longer to reach the required temperature. Thus, it serves to weaken the release and propagation of energy after the thermal runaway of the battery.
- (2)
The thermal runaway chain reaction of the battery pack was somewhat hindered in 0 °C working conditions, and the ignition and explosion time of the last battery was delayed 114 s compared with the room temperature condition. When the temperature dropped to −10 °C, only two batteries had thermal runaway, and the thermal runaway propagation chain stopped at the third battery. When the temperature dropped to −30 °C and below, no thermal runaway occurred in all cells after puncture.
- (3)
Cryogenic cooling leads to changes in the relevant characteristic parameters of the lithium battery pack after puncture, among which the cell mass and CO gas concentration in the chamber decreased most significantly. The highest CO concentration in the 0 °C conditions decreased by 13.7%, and the mass loss decreased by 20.7% compared with the room temperature condition. The two decreased by 83.1% and 75.9%, respectively, in the condition of the cryogenic state reduced to −20 °C. When the temperature was further reduced, the cell mass and CO gas concentration did not change significantly since the thermal runaway was suppressed.
Finally, according to the above research, low temperatures can significantly reduce the fire risk of lithium batteries. Taking into consideration both cost-effectiveness and safety factors, we suggest that this technology can be used in transportation vehicles and storage warehouses of lithium batteries.