Do Soil Chemical Changes Contribute to the Dominance of Blady Grass (Imperata cylindrica) in Surface Fire-Affected Forests?
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Fire History on Foliar Stoichiometry of Imperata cylindrica
2.2. Effects of Soil Fire History and Fertilization on Survival and Growth of Imperata cylindrica
2.3. Statistical Analyses
3. Results
3.1. Soil Properties and Foliar Chemistry under the Different Fire Regimes
3.2. Growth and Survival of Imperata cylindrica Seedlings in Response to Soil Fire History and Resource Amendment
4. Discussion
4.1. Patterns of Variation in Foliar N:P Ratios in the Field
4.2. Performance of Imperata cylindrica Seedlings in Response to Site Fire History and Amendments
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whelan, R.J. The Ecology of Fire; Cambridge University Press: Melbourne, Australia, 1995. [Google Scholar]
- Noble, I.R.; Slatyer, R.O. Concepts and models of succession in vascular plant communities subject to recurrent fire. In Fire and the Australian Biota; Gill, A.M., Groves, R.H., Noble, I.R., Eds.; Australian Academy of Science: Canberra, Australia, 1981; pp. 311–337. [Google Scholar]
- Pausas, J.G.; Vallejo, V.R. The role of fire in European Mediterranean Ecosystems. In Remote Sensing of Large Wildfires in the European Mediterranean Basin; Chuvieco, E., Ed.; Springer: Berlin, Germany, 1999; pp. 3–16. [Google Scholar]
- Pausas, J.G.; Keeley, J.E. A burning story: The role of fire in the history of life. Bioscience 2009, 59, 593–601. [Google Scholar] [CrossRef]
- Gill, A.M.; Groves, R.H.; Noble, I.R. Fire and the Australian biota; Australian Academy of Science: Canberra, Australia, 1981. [Google Scholar]
- Dantas, V.D.L.; Hirota, M.; Oliveira, R.S.; Pausas, J.G. Disturbance maintains alternative biome states. Ecol. Lett. 2016, 19, 12–19. [Google Scholar] [CrossRef]
- Chambers, D.P.; Attiwill, P.M. The ash-bed effect in Eucalyptus regnans forest: Chemical, physical and microbiological changes in soil after heating or partial sterilisation. Aust. J. Bot. 1994, 42, 739–749. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bülow-Olsen, A.; Just, J.; Liddle, M.J. Growth and flowering history of Xanthorrhoea johnsonii Lee (Liliaceae) in Toohey Forest Queensland. Bot. J. Linn. Soc. 1982, 84, 195–207. [Google Scholar] [CrossRef]
- Keeley, J.E. Role of fire in seed germination of woody taxa in California chaparral. Ecology 1987, 68, 434–443. [Google Scholar] [CrossRef]
- Keeley, J.E.; Pausas, J.G.; Rundel, P.W.; Bond, W.J.; Bradstock, R.A. Fire as an evolutionary pressure shaping plant traits. Trends Plant Sci. 2011, 16, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Ahlgren, I.F.; Ahlgren, C.E. Ecological effects of forest fires. Bot. Rev. 1960, 26, 483–533. [Google Scholar] [CrossRef]
- Keeley, J.E. Fire and invasive species in Mediterranean-climate ecosystems of California. In Proceedings of the Invasive Species Workshop: The Role of Fire in the Control and Spread of Invasive Species; Fire Conference 2000; The First National Congress on Fire Ecology, Prevention, and Management: Tallahassee, FL, USA, 2000. [Google Scholar]
- Working Group I Contribution to The IPCC Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Climate Change 2013. The Physical Science Basis; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Enright, N.J.; Fontaine, J.B.; Bowman, D.M.; Bradstock, R.A.; Williams, R.J. Interval squeeze: Altered fire regimes and demographic responses interact to threaten woody species persistence as climate changes. Front. Ecol. Environ. 2015, 13, 265–272. [Google Scholar] [CrossRef]
- Dozier, H.; Gaffney, J.F.; McDonald, S.K.; Johnson, E.R.; Shilling, D.G. Cogongrass in the United States: History, ecology, impacts, and management. Weed Technol. 1998, 12, 737–743. [Google Scholar] [CrossRef]
- Pendleton, R.L. Cogon Grass, Imperata cylindrica, in the Western Hemisphere. Agron. J. 1948, 40, 1047–1049. [Google Scholar] [CrossRef]
- MacDonald, G.E. Cogongrass (Imperata cylindrica)—Biology, ecology, and management. CRC Crit. Rev. Plant Sci. 2004, 23, 367–380. [Google Scholar] [CrossRef]
- Woods, P. Effects of logging, drought, and fire on structure and composition of tropical forests in Sabah, Malaysia. Biotropica 1989, 21, 290. [Google Scholar] [CrossRef]
- Bryson, C.T.; Carter, R. Cogongrass, Imperata cylindrica, in the United States. Weed Technol. 1993, 7, 1005–1009. [Google Scholar] [CrossRef]
- Kushwaha, S.P.S.; Ramakrishnan, P.S.; Tripathi, R.S. Population dynamics of Imperata cylindrica (L.) Beauv. var. major related to slash and burn agriculture (jhum) in North Eastern India. Proc. Indian Acad. Sci. Plant Sci. 1983, 92, 313–321. [Google Scholar]
- Holzmueller, E.J.; Jose, S. Response of the invasive grass Imperata cylindrica to disturbance in the southeastern forests, USA. Forests 2012, 3, 853–863. [Google Scholar] [CrossRef]
- Yager, L.Y.; Miller, D.L.; Jones, J. Susceptibility of longleaf pine forest associations in South Mississippi to invasion by cogongrass [Imperata cylindrica (L.) Beauv.]. Nat. Areas J. 2010, 30, 226–232. [Google Scholar] [CrossRef]
- Platt, W.J.; Gottschalk, R.M. Effects of exotic grasses on potential fine fuel loads in the groundcover of south Florida slash pine savannas. Int. J. Wildl. Fire 2001, 10, 155–159. [Google Scholar] [CrossRef]
- Peet, N.B.; Watkinson, A.R.; Bell, D.J.; Sharma, U.R. The conservation management of Imperata cylindrica grassland in Nepal with fire and cutting: An experimental approach. J. Appl. Ecol. 1999, 36, 374–387. [Google Scholar] [CrossRef]
- Brook, R.M. Review of literature on Imperata cylindrica (L.) Raeuschel with particular reference to South East Asia. Trop. Pest Manag. 1989, 35, 12–25. [Google Scholar] [CrossRef]
- Garrity, D.P.; Soekardi, M.; Van Noordwijk, M.; De La Cruz, R.; Pathak, P.S.; Gunasena, H.P.M.; Van So, N.; Huijun, G.; Majid, N.M. The Imperata grasslands of tropical Asia: Area, distribution, and typology. Agrofor. Syst. 1997, 36, 3–29. [Google Scholar] [CrossRef]
- Yassir, I.; Van Der Kamp, J.; Buurman, P. Secondary succession after fire in Imperata grasslands of East Kalimantan, Indonesia. Agric. Ecosyst. Environ. 2010, 137, 172–182. [Google Scholar] [CrossRef]
- Brewer, S. Declines in plant species richness and endemic plant species in longleaf pine savannas invaded by Imperata cylindrica. Biol. Invasions 2008, 10, 1257–1264. [Google Scholar] [CrossRef]
- Tothill, J.C.; Mott, J.J.; Gillard, P. Pasture weeds of the tropics and subtropics with special reference to Australia. In Biology and Ecology of Weeds; Holzner, W., Numata, N., Eds.; Springer: Dordrecht, The Netherlands, 1982; pp. 403–427. [Google Scholar]
- Brewer, J.S.; Cralle, S.P. Phosphorus addition reduces invasion of a longleaf pine savanna (Southeastern USA) by a non-indigenous grass (Imperata cylindrica). Plant Ecol. 2009, 167, 237–245. [Google Scholar] [CrossRef]
- King, S.E.; Grace, J.B. The effects of gap size and disturbance type on invasion of wet pine savanna by cogongrass, Imperata cylindrica (Poaceae). Am. J. Bot. 2000, 87, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.W.; Grime, J.P. An experimental study of plant community invasibility. Ecology 1996, 77, 776–790. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Atkins, L. Effect of disturbance and nutrient addition on native and introduced annuals in plant communities in the Western Australian wheatbelt. Aust. J. Ecol. 1988, 13, 171–179. [Google Scholar] [CrossRef]
- Butler, O.M.; Elser, J.J.; Lewis, T.; Mackey, B.; Chen, C. The phosphorus-rich signature of fire in the soil–plant system: A global meta-analysis. Ecol. Lett. 2018, 21, 335–344. [Google Scholar] [CrossRef]
- Butler, O.M.; Lewis, T.; Chen, C. Fire alters soil labile stoichiometry and litter nutrients in Australian eucalypt forests. Int. J. Wildl. Fire 2017, 26, 783–788. [Google Scholar] [CrossRef]
- Butler, O.M.; Elser, J.J.; Lewis, T.; Maunsell, S.C.; Rashti, M.R.; Chen, C. The multi-element stoichiometry of wet eucalypt forest is transformed by recent, frequent fire. Plant Soil. 2020, 447, 447–461. [Google Scholar] [CrossRef]
- Raison, R.J.; Khanna, P.K.; Woods, P.V. Mechanisms of element transfer to the atmosphere during vegetation fires. Can. J. For. Res. 1985, 15, 1–5. [Google Scholar] [CrossRef]
- Urbanski, S.P.; Hao, W.M.; Baker, S. Chemical composition of wildland fire emissions. In Developments in Environmental Science; Bytnerowicz, A., Arbaugh, M.J., Riebau, A.R., Andersen, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 79–107. [Google Scholar]
- García-Oliva, F.; Merino, A.; Fonturbel, M.T.; Omil, B.; Fernández, C.; Vega, J.A. Severe wildfire hinders renewal of soil P pools by thermal mineralization of organic P in forest soil: Analysis by sequential extraction and 31P NMR spectroscopy. Geoderma 2018, 309, 32–40. [Google Scholar] [CrossRef]
- Schaller, J.; Tischer, A.; Struyf, E.; Bremer, M.; Belmonte, D.U.; Potthast, K. Fire enhances phosphorus availability in topsoils depending on binding properties. Ecology 2015, 96, 1598–1606. [Google Scholar] [CrossRef]
- Chen, C.; Hou, E.; Condron, L.; Bacon, G.; Esfandbod, M.; Olley, J.; Turner, B. Soil phosphorus fractionation and nutrient dynamics along the Cooloola coastal dune chronosequence, southern Queensland, Australia. Geoderma 2015, 257–258, 4–13. [Google Scholar] [CrossRef]
- Walker, T.W.; Syers, J.K. The fate of phosphorus during pedogenesis. Geoderma 1976, 15, 1–19. [Google Scholar] [CrossRef]
- Turner, B.L.; Laliberté, E. Soil development and nutrient availability along a 2 million-year coastal dune chronosequence under species-rich Mediterranean shrubland in southwestern Australia. Ecosystems 2015, 18, 287–309. [Google Scholar] [CrossRef]
- Pathak, K.; Nath, A.J.; Sileshi, G.W.; Lal, R.; Das, A.K. Annual burning enhances biomass production and nutrient cycling in degraded Imperata grasslands. Land Degrad. Dev. 2017, 28, 1763–1771. [Google Scholar] [CrossRef]
- Saxena, K.G.; Ramakrishnan, P.S. Growth and allocation strategies of some perennial weeds of slash and burn agriculture (Jhum) in northeastern India. Can. J. Bot. 1983, 61, 1300–1306. [Google Scholar] [CrossRef]
- Santoso, D.; Adiningsih, S.; Mutert, E.; Fairhurst, T.; Van Noordwijk, M. Soil fertility management for reclamation of Imperata grasslands by smallholder agroforestry. Agrofor. Syst. 1996, 36, 181–202. [Google Scholar] [CrossRef]
- Butler, O.M. Forest Stoichiometry Shift in Response to Fire in South-East Queensland, Australia. Honours Thesis, Griffith University, Brisbane, Australia, 2014. [Google Scholar]
- Butler, O.M.; Rashti, M.R.; Lewis, T.; Elser, J.J.; Chen, C. High-frequency fire alters soil and plant chemistry but does not lead to nitrogen-limited growth of Eucalyptus pilularis seedlings. Plant Soil 2018, 432, 191–205. [Google Scholar] [CrossRef]
- Butler, O.M.; Lewis, T.; Chen, C. Prescribed fire alters foliar stoichiometry and nutrient resorption in the understorey of a subtropical eucalypt forest. Plant Soil 2017, 410, 181–191. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chem. ACTA 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Saunders, W.M.H.; Williams, E.G. Observations on the determination of total organic phosphorus in soils. J. Soil Sci. 1955, 6, 254–267. [Google Scholar] [CrossRef]
- Chen, C.R.; Xu, Z.H.; Keay, P.; Zhang, S.L. Total soluble nitrogen in forest soils as determined by persulfate oxidation and by high temperature catalytic oxidation. Aust. J. Soil Res. 2005, 43, 515–523. [Google Scholar] [CrossRef]
- Sparling, G.; Vojvodić-Vuković, M.; Schipper, L. Hot-water, soluble C as a simple measure of labile soil organic matter: The relationship with microbial biomass C. Soil Biol. Biochem. 1998, 30, 1469–1472. [Google Scholar] [CrossRef]
- Tutua, S.S.; Xu, Z.; Chen, C.; Blumfield, T.J. Hot water extractable phosphorus pools as indicators of soil P responses to harvest residue management in an exotic pine plantation of subtropical Australia. J. Soils Sediments 2013, 13, 1573–1578. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice-Hall: Hoboken, NJ, USA, 1958. [Google Scholar]
- De Groot, W.J.; Wang, Y. Calibrating the fine fuel moisture code for grass ignition potential in Sumatra, Indonesia. Int. J. Wildl. Fire 2005, 14, 161–168. [Google Scholar] [CrossRef]
- Pickford, S.; Suharti, M.; Wibowo, A. A note on fuelbeds and fire behaviour in Alang-Alang (Imperata cylindrica). Int. J. Wildl. Fire 1992, 2, 41–46. [Google Scholar] [CrossRef]
- Gusewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Güsewell, S.; Koerselman, W.; Verhoeven, J.T.A. Biomass N:P ratios as indicators of nutrient limitation for plant populations in wetlands. Ecol. Appl. 2012, 13, 372–384. [Google Scholar] [CrossRef]
- Koerselman, W.; Meuleman, A.F.M. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J. App. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Van De Vijver, C.A.D.M.; Poot, P.; Prins, H.H.T. Causes of increased nutrient concentrations in post-fire regrowth in an east African savanna. Plant Soil 1999, 214, 173–185. [Google Scholar] [CrossRef]
- Dijkstra, F.A.; Adams, M.A. Fire eases imbalances of nitrogen and phosphorus in woody plants. Ecosystems 2015, 18, 769–779. [Google Scholar] [CrossRef]
- Patterson, D.T.; Flint, E.P.; Dickens, R. Effects of temperature, photoperiod, and population source on the growth of Cogongrass (Imperata cylindrica). Weed Sci. 1980, 28, 505–509. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Bryson, C.T.; Krutz, L.J.; Ervin, G.N.; Reddy, K.N.; Byrd, J.D. Ecotype variability and edaphic characteristics for Cogongrass (Imperata cylindrica) populations in Mississippi. Invasive Plant Sci. Manag. 2010, 3, 199–207. [Google Scholar] [CrossRef]
- Bui, E.N.; Henderson, B.L. C:N:P stoichiometry in Australian soils with respect to vegetation and environmental factors. Plant Soil 2013, 373, 553–568. [Google Scholar] [CrossRef]
- Rossel, R.A.V.; Bui, E.N. A new detailed map of total phosphorus stocks in Australian soil. Sci. Total Environ. 2016, 542, 1040–1049. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef]
- Wilcut, J.W.; Dute, R.R.; Truelove, B.; Davis, D.E. Factors limiting the distribution of Cogongrass, Imperata cylindrica, and Torpedograss, Panicum repens. Weed Sci. 1988, 36, 577–582. [Google Scholar] [CrossRef]
- Daneshgar, P.; Jose, S. Imperata cylindrica, an alien invasive grass, maintains control over nitrogen availability in an establishing pine forest. Plant Soil 2009, 320, 209–218. [Google Scholar] [CrossRef]
- Collins, A.R. Implications of Plant Diversity and Soil Chemical Properties for Cogongrass (Imperata cylindrica) Invasion in Northwest Florida. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2005. [Google Scholar]
- Adams, M.A.; Attiwill, P.M. Role of Acacia spp. in nutrient balance and cycling in regenerating Eucalyptus regnans F. Muell. forests. I. Temporal changes in biomass and nutrient content. Aust. J. Bot. 1984, 32, 205–215. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Blair, G.J.; Pualillin, P.; Samosir, S. Effect of fertilizers on the yield and botanical composition of pastures in South Sulawesi, Indonesia. Agron. J. 1978, 70, 559–562. [Google Scholar] [CrossRef]
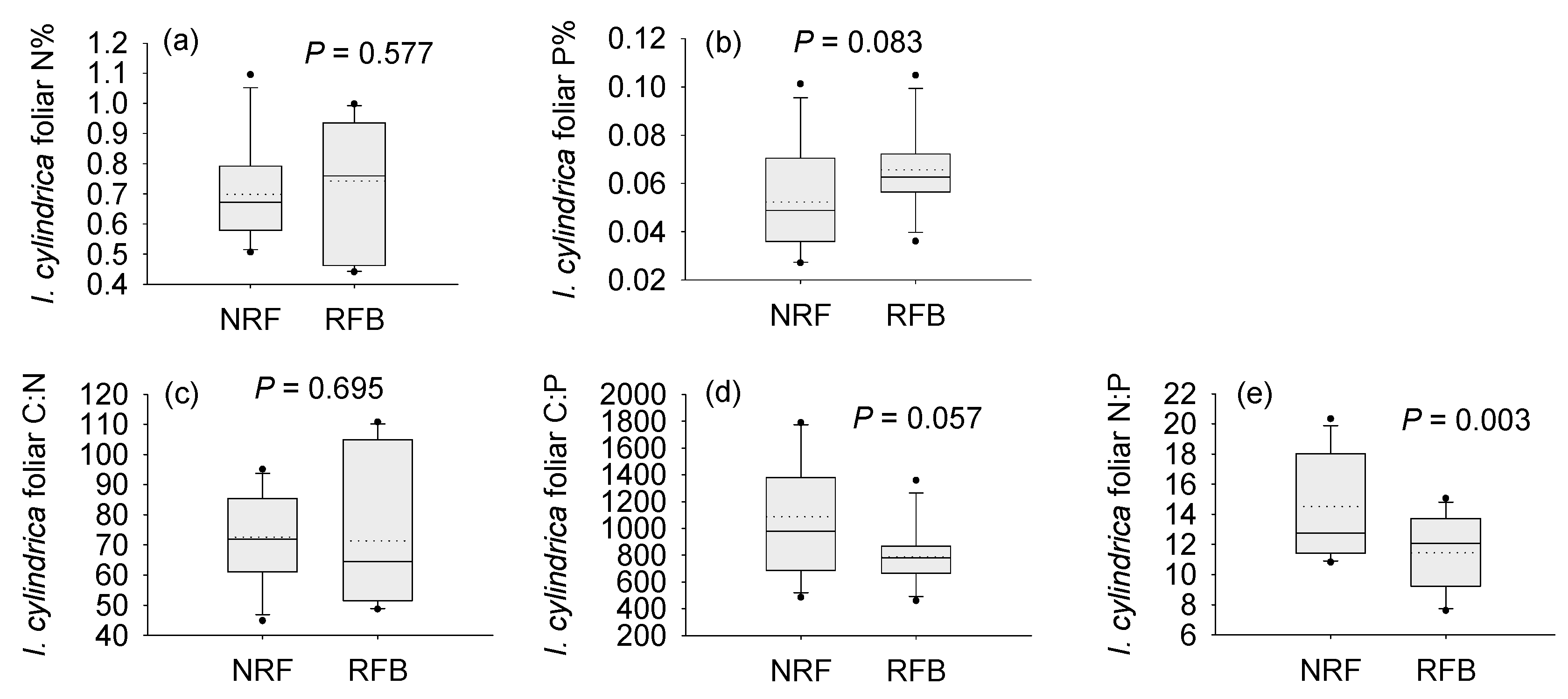
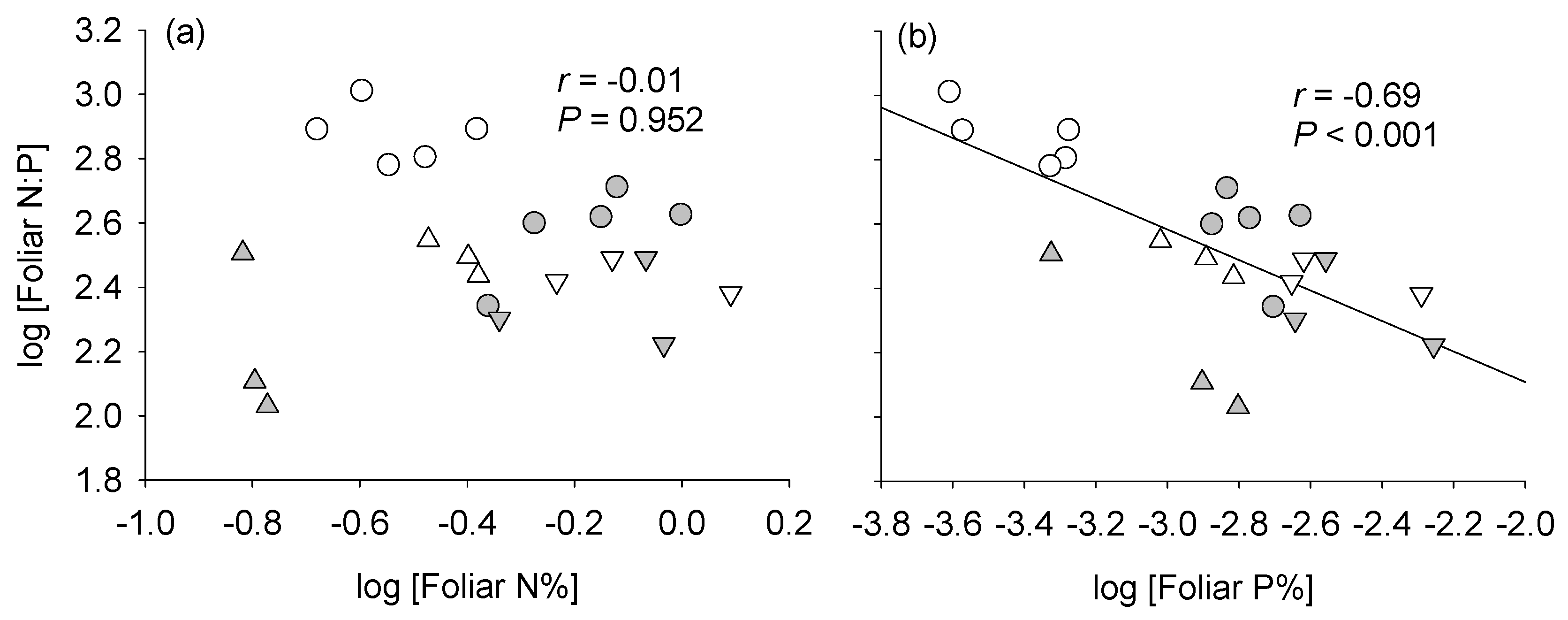
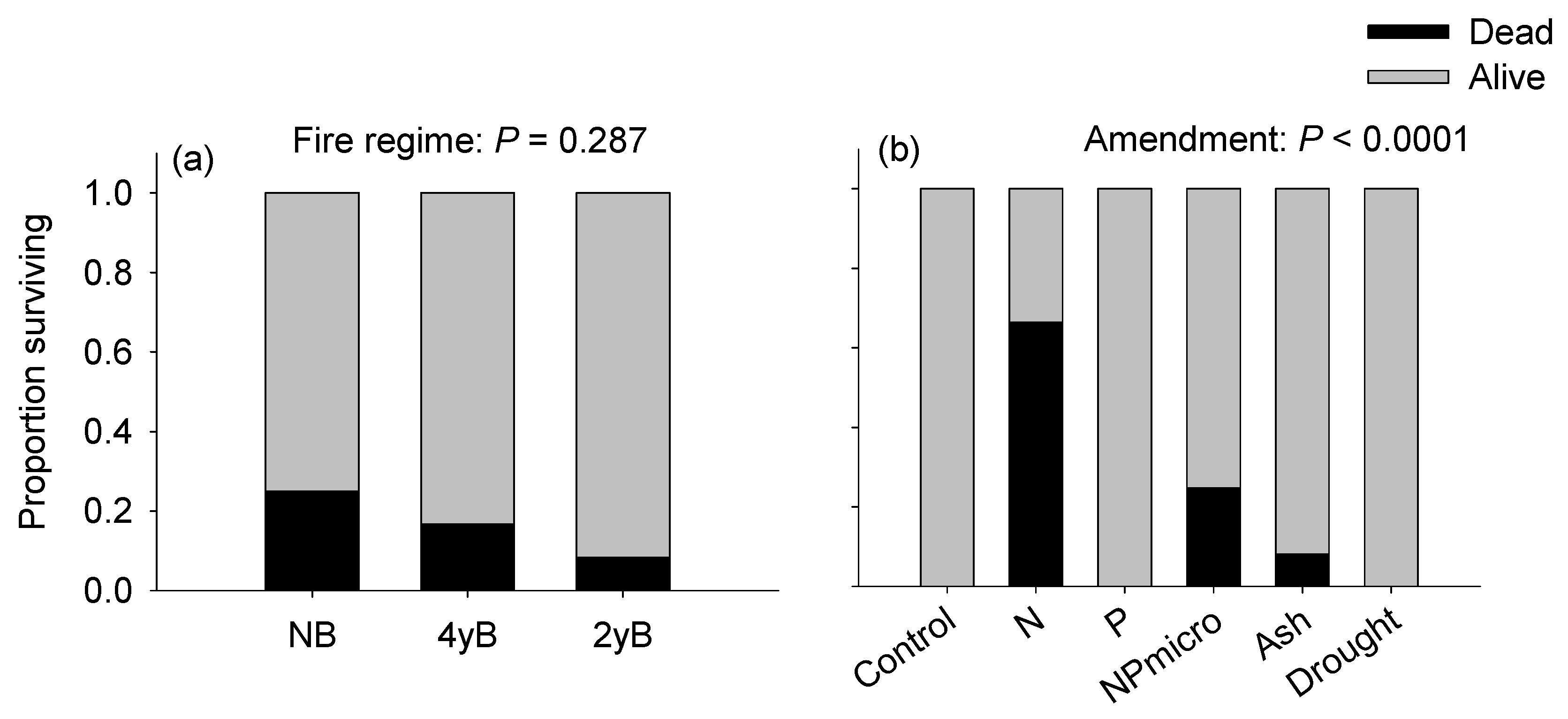

| Site | Vegetation | MAP †† (mm) | MAT (°C) | Fire Regime Contrast(s) |
|---|---|---|---|---|
| Toohey Forest (27°32′24′′ S, 153°3′0′′ E) | Open eucalypt forest. Key species include Eucalyptus acmenoides, Corymbia citriodora, Acacia leiocalyx, and Lophostemon confertus. | 1030 | 20.5 | NRF: No burning since at least 1998 (date of most recent fire unknown); RFB: Low intensity prescribed surface fire in August 2013, July 2005, and August 1998. |
| WRSMCE † A (27°43′12′′ S, 152°51′36′′ E) | Shrubby-to-tall eucalypt forest. Key species include C. citriodora, E. crebra, and L. confertus. | 995 | 23.4 | NRF: Low intensity prescribed surface fire in June 1999; RFB: Low intensity prescribed surface fire in September 2009, April 2008, May 2003, and 1995. |
| WRSMCE B(27°41′60′′ S, 152°50′60′′ E) | Shrubby-to-tall eucalypt forest. Key species include E. crebra, E. siderophloia, and C. henryi. | 995 | 23.4 | NRF: No burning since at least 1998 (date of most recent fire unknown); RFB: Moderate to high intensity wildfire in October 2012, and previous medium intensity wildfires in August 2004 and ca. December 2000. |
| Peachester(26°52′12′′ S, 152°50′60′′ E) | Wet sclerophyll forest. Key species include E. pilularis, E. microcorys, C. intermedia, and L. confertus. | 1684 | 23.3 | NB: No burning since 1969; 4yB: low-to-medium intensity (<2500 kW m−2) prescribed surface fire, every four years (on average) since 1972; 2yB: low-to-medium intensity (<2500 kW m−2) prescribed surface fire, every two years (on average) since 1972. |
| Resource Amendment Levels † | Treatment Details | N |
|---|---|---|
| Control | Plants grown for 219 days; soil moisture at 50% WHC; no soil amendments. | 12 |
| +Nitrogen | Plants grown for 219 days; soil moisture at 50% WHC; 100 mg N kg soil−1 added (as NH4NO3) to pots on days 0 and 96 (220 mg N added in total). | 12 |
| +Phosphorus | Plants grown for 219 days; soil moisture at 50% WHC; 100 mg P kg soil−1 added (as NaH2PO4) to pots on days 0 and 96 of growth period (220 mg P added in total). | 12 |
| NPmicro † | Plants grown for 219 days; soil moisture at 50% WHC; 100 mg N, 100 mg P, 25 mg K, 12.5 mg Ca, 12.5 mg Mg, 5.45 mg Fe, 3.95 mg Mn, 2.0 mg Zn, 0.5 mg Cu, 0.45 mg Mo, 0.1 mg B, 37.3 mg Na, 81.9 mg Cl, and 5.7 mg S per kg soil−1 added to pots on days 0 and 96. | 12 |
| Ash | Plants grown for 219 days; soil moisture at 50% WHC; 1.5 g of laboratory-produced Imperata cylindrica ash applied to soil surface of pots on day 0. | 12 |
| Drought | Plants grown for 219 days; soil moisture at 50% WHC from days 0–96 and 35% WHC from days 96–219 of the growth period; no soil amendments. | 12 |
| Soil Property ††† | Toohey Forest | WRSMCE A | WRSMCE B | Peachester State Forest | |||||
|---|---|---|---|---|---|---|---|---|---|
| NRF | RFB | NRF | RFB | NRF | RFB | NB | 4yB | 2yB | |
| Bulk density (g cm−3) | 1.04 (0.83–1.26) | 1.03 (0.89–1.18) | 1.15 (1.05–1.24) | 1.00 (0.74–1.26) | 1.21 (1.03–1.39) | 1.21 (1.05–1.37) | 0.81 (0.63–1.00) | 0.83 (0.64–1.02) | 0.91 (0.66–1.17) |
| pH | 4.88 (4.58–5.17) | 4.65 (4.48–4.83) | 5.85 (5.75–5.95) | 5.65 (5.36–5.94) | 5.25 (5.17–5.32) | 5.58 (5.29–5.86) | 3.77 (3.69–3.85) | 3.89 (3.66–4.11) | 4.21 (3.75–4.66) |
| EC (µS cm−1) | 38.0 (23.6–52.3) | 33.9 (25.2–42.5) | 19.0 (15.8–22.1) | 16.7 (15.3–18.1) | 20.9 (18.1–23.8) | 29.2 (20.7–37.7) | 52.1 (41.4–62.8) | 52.4 (42.0–62.8) | 38.5 (33.2–43.8) |
| Total C (%) | 2.31 (1.60–3.01) | 2.64 (2.16–3.12) | 1.70 (1.24–2.17) | 1.63 (1.44–1.82) | 1.50 (1.22–1.78) | 1.72 (1.40–2.04) | 4.28 (2.93–5.63) | 6.53 (4.86–8.20) | 3.07 (−0.3–6.45) |
| Total N (%) | 0.080 (0.05–0.11) | 0.075 (0.06–0.09) | 0.066 (0.05–0.08) | 0.057 (0.04–0.07) | 0.058 (0.04–0.08) | 0.072 (0.06–0.09) | 0.176 (0.12–0.23) | 0.259 (0.17–0.34) | 0.101 (0.01–0.19) |
| Total P (mg kg−1) | 73.7 (59–88) | 86.4 (70–103) | 168.2 (150–186) | 110.8 (104–118) | 120.5 (111–130) | 148.0 (130–166) | 81.1 (60–102) | 123.0 (60–186) | 81.6 (14–149) |
| Total Pi (mg kg−1) | 6.8 (6–8) | 9.2 (6–13) | 41.0 (33–49) | 27.0 (22–32) | 25.6 (21–30) | 36.8 (28–46) | 22.2 (14–30) | 33.6 (21–46) | 20.4 (2–38) |
| Total Po (mg kg−1) | 66.9 (53–81) | 77.1 (57–97) | 127.2 (106–148) | 83.8 (81–87) | 94.9 (85–105) | 111.2 (91–131) | 58.9 (42–75) | 89.4 (37–142) | 61.2 (11–112) |
| Labile OC (mg kg−1) | 690 (550–831) | 578 (459–698) | 433 (333–533) | 354 (287–420) | 339 (284–393) | 406 (302–511) | 1134 (724–1543) | 1323 (878–1768) | 758 (441–1075) |
| Labile N (mg kg−1) | 54.5 (43.7–65.3) | 44.0 (35.0–53.0) | 54.0 (42.9–65.1) | 37.1 (28.5–45.6) | 30.2 (28.4–32.1) | 48.4 (39.1–57.7) | 97.6 (60.1–135) | 84.3 (44.2–125) | 62.7 (36.1–89.3) |
| Labile P (mg kg−1) | 1.05 (0.87–1.24) | 1.61 (0.65–2.57) | 1.05 (0.59–1.51) | 1.22 (0.91–1.53) | 1.05 (0.76–1.35) | 1.71 (1.31–2.12) | 0.31 (−0.3–0.86) | 0.20 (0.02–0.38) | 0.88 (0.69–1.08) |
| Total N:P ratio †††† | 10.6 (7.7–14.7) | 8.7 (6.1–12.3) | 3.9 (2.9–5.2) | 5.1 (3.8–6.7) | 4.7 (3.5–6.2) | 4.8 (3.4–6.8) | 21.56 (15.2–30.5) | 21.47 (17.8–25.9) | 12.18 (8.6–17.3) |
| Labile N:P ratio | 51.6 (43.3–61.5) | 29.3 (15.8–54.5) | 53.7 (41.0–70.3) | 30.4 (24.9–37.2) | 29.3 (21.7–39.5) | 28.4 (19.7–40.8) | 517 (57–4697) | 457 (195–1067) | 69.4 (51–95) |
| Species | Foliar N (%) | Foliar P (%) | Foliar C:N | Foliar C:P | Foliar N:P | |
|---|---|---|---|---|---|---|
| Toohey Forest | Imperata cylindrica | 0.71 (0.60–0.83) | 0.048 (0.036–0.060) | 69.7 (59–82) | 1068 (822–1387) | 15.3 (13–18) |
| Acacia disparrima | 2.15 (1.93–2.37) | 0.084 (0.063–0.104) | 25.0 (22–28) | 670 (518–867) | 26.8 (22–32) | |
| Acacia leiocalyx | 2.03 (1.78–2.27) | 0.053 (0.037–0.070) | 25.7 (22–30) | 1048 (758–1447) | 40.7 (33–51) | |
| Alphitonia excelsa | 1.64 (1.45–1.83) | 0.074 (0.063–0.085) | 32.1 (28–36) | 720 (615–842) | 22.4 (21–24) | |
| Lomandra confertifolia | 1.01 (0.80–1.21) | 0.049 (0.038–0.060) | 50.2 (41–62) | 1036 (824–1301) | 20.7 (19–22) | |
| Lophostemon confertus | 1.29 (1.08–1.50) | 0.063 (0.045–0.081) | 41.1 (34–49) | 858 (639–1153) | 20.9 (17–25) | |
| WRSMCE A | Imperata cylindrica | 0.56 (0.43–0.68) | 0.053 (0.043–0.062) | 88.7 (71–111) | 933 (758–1148) | 10.5 (8–13) |
| Acacia concurrens | 2.06 (1.68–2.44) | 0.061 (0.050–0.072) | 26.9 (22–33) | 909 (737–1119) | 33.8 (30–39) | |
| Alphitonia excelsa | 1.53 (1.27–1.79) | 0.106 (0.090–0.122) | 33.5 (28–40) | 481 (416–557) | 14.4 (11–19) | |
| Jacksonia scoparia | 1.65 (1.45–1.85) | 0.056 (0.040–0.071) | 31.6 (28–36) | 966 (694–1344) | 30.6 (24–39) | |
| WRSMCE B | Imperata cylindrica | 0.91 (0.75–1.04) | 0.083 (0.067–0.100) | 54.3 (47–63) | 590 (489–710) | 10.9 (10–12) |
| Acacia concurrens | 1.81 (1.60–2.03) | 0.063 (0.050–0.076) | 29.9 (27–34) | 871 (724–1048) | 29.2 (27–32) | |
| Alphitonia excelsa | 1.71 (1.50–1.92) | 0.115 (0.079–0.152) | 29.9 (26–34) | 464 (354–607) | 15.5 (12–20) | |
| Lophostemon confertus | 1.21 (1.11–1.31) | 0.068 (0.050–0.087) | 42.4 (39–46) | 767 (582–1010) | 18.1 (15–22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, O.M.; Lewis, T.; Chen, C. Do Soil Chemical Changes Contribute to the Dominance of Blady Grass (Imperata cylindrica) in Surface Fire-Affected Forests? Fire 2021, 4, 23. https://doi.org/10.3390/fire4020023
Butler OM, Lewis T, Chen C. Do Soil Chemical Changes Contribute to the Dominance of Blady Grass (Imperata cylindrica) in Surface Fire-Affected Forests? Fire. 2021; 4(2):23. https://doi.org/10.3390/fire4020023
Chicago/Turabian StyleButler, Orpheus M., Tom Lewis, and Chengrong Chen. 2021. "Do Soil Chemical Changes Contribute to the Dominance of Blady Grass (Imperata cylindrica) in Surface Fire-Affected Forests?" Fire 4, no. 2: 23. https://doi.org/10.3390/fire4020023
APA StyleButler, O. M., Lewis, T., & Chen, C. (2021). Do Soil Chemical Changes Contribute to the Dominance of Blady Grass (Imperata cylindrica) in Surface Fire-Affected Forests? Fire, 4(2), 23. https://doi.org/10.3390/fire4020023






