Grass Canopy Architecture Influences Temperature Exposure at Soil Surface
Abstract
1. Introduction
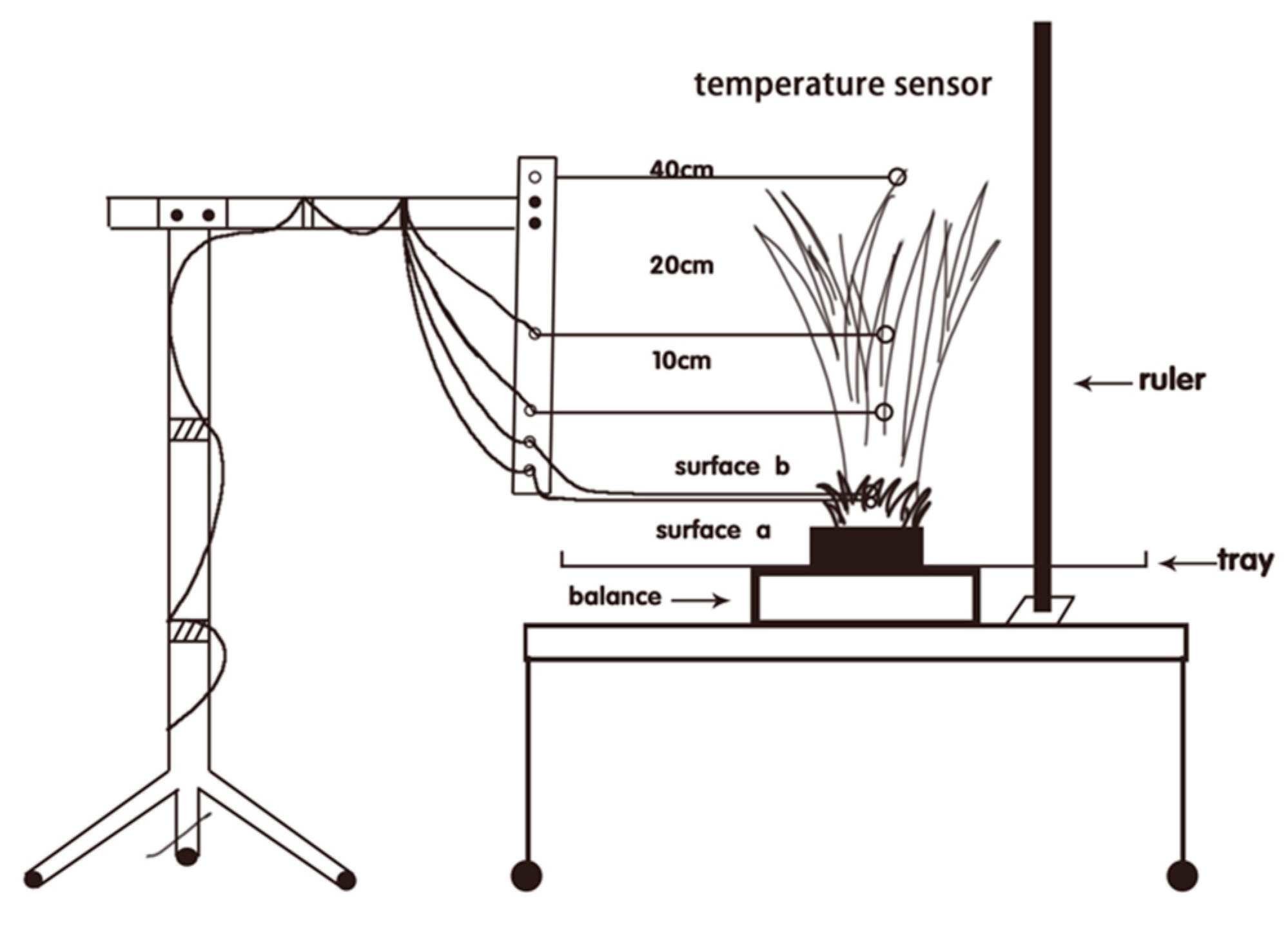
2. Materials and Methods
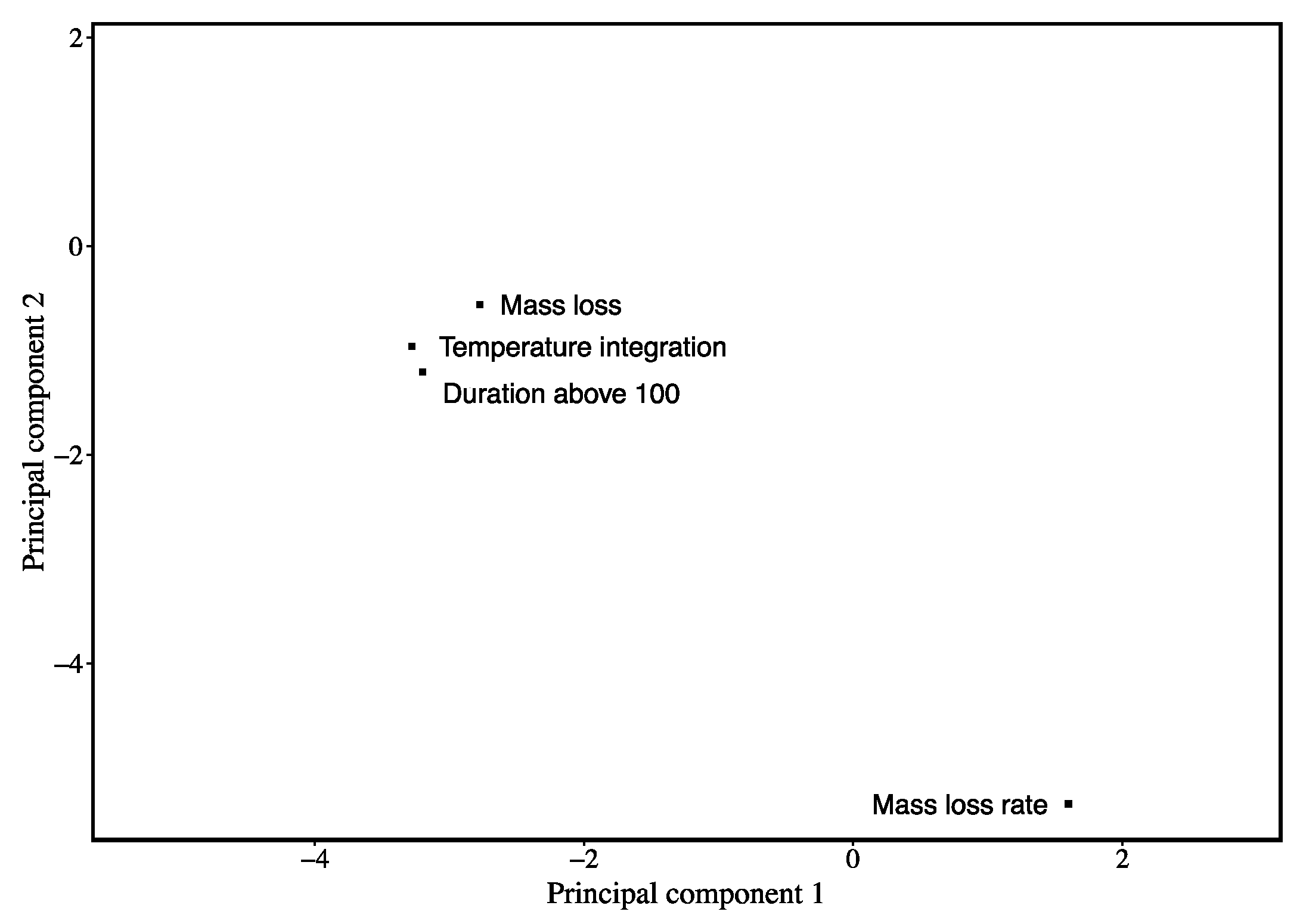
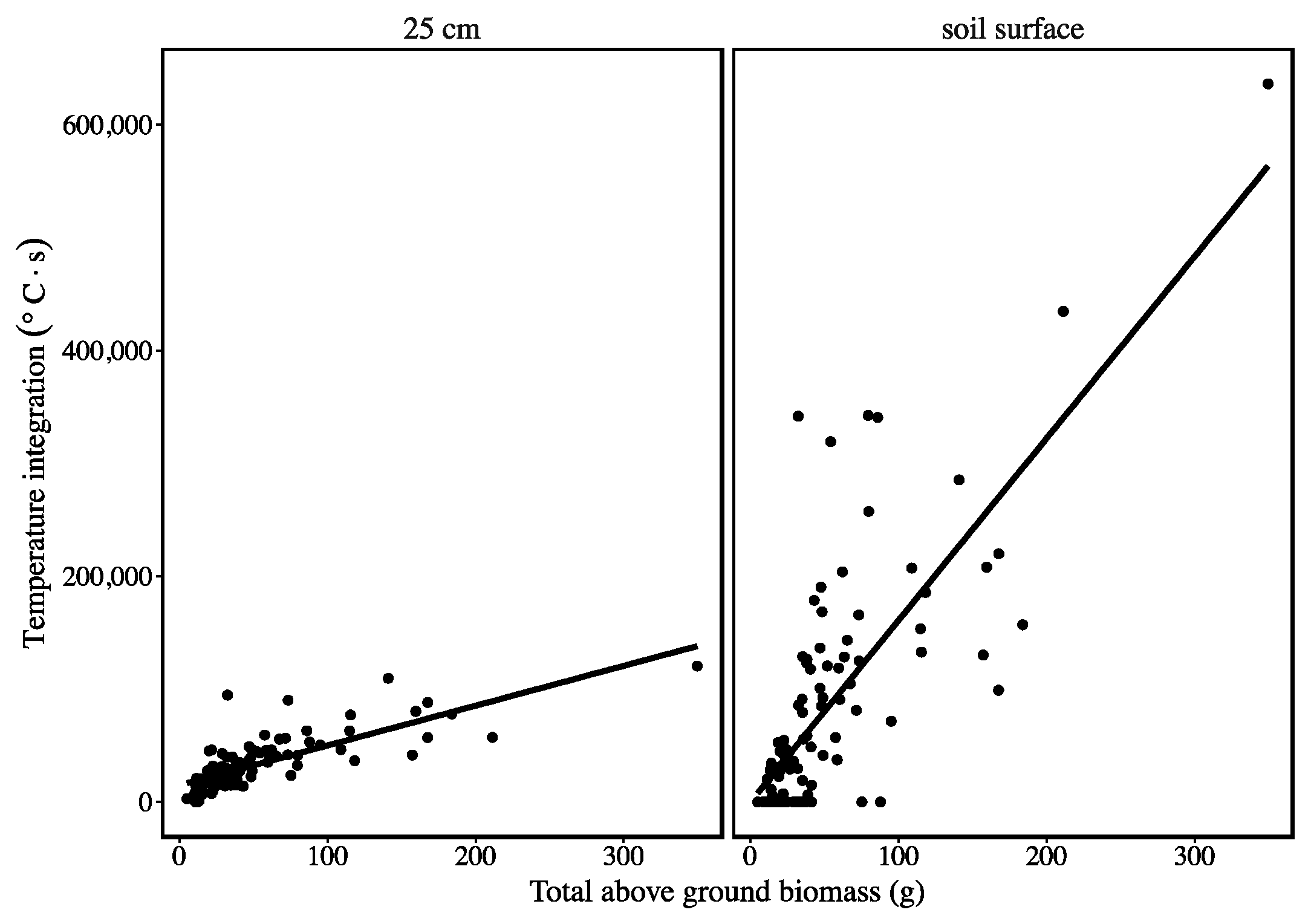
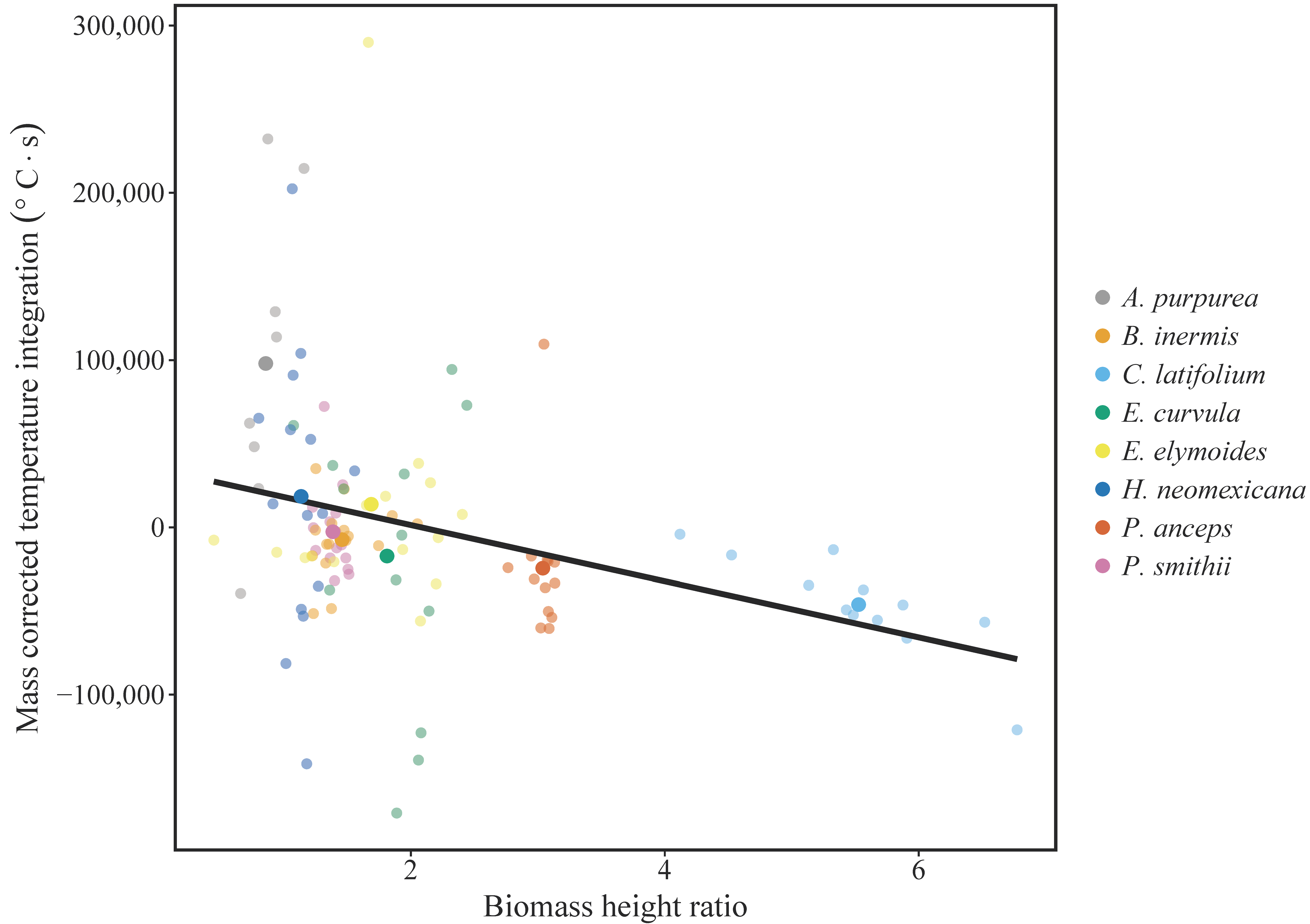
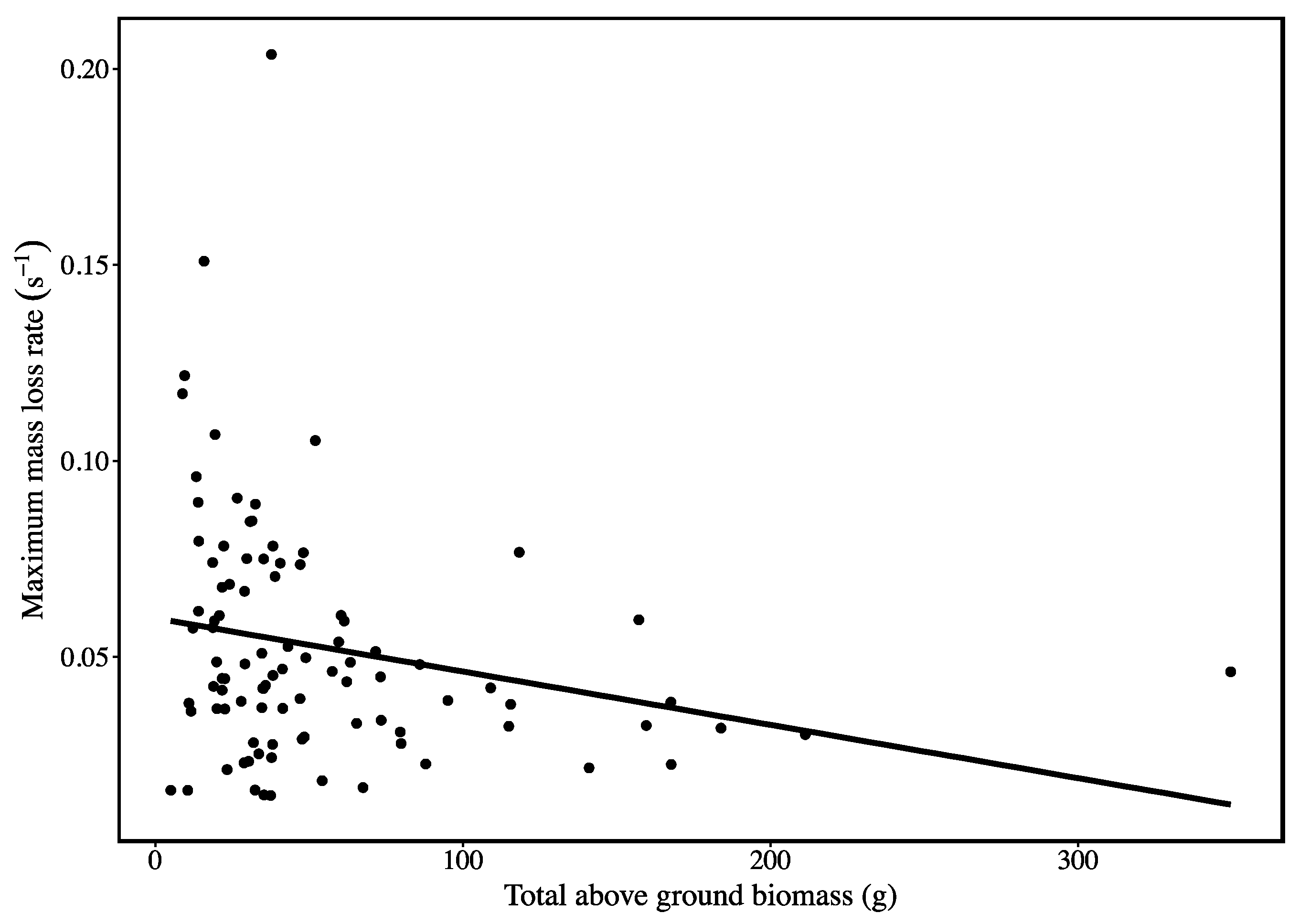
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savadogo, P.; Zida, D.; Sawadogo, L.; Tiveau, D.; Tigabu, M.; Oden, P.C. Fuel and Fire Characteristics in Savanna—Woodland of West Africa in Relation to Grazing and Dominant Grass Type. Int. J. Remote Sens. 2007, 16, 531–539. [Google Scholar] [CrossRef]
- Stocks, B.J.; Van Wilgen, B.W.; Trollope, W.S.W.; McRae, D.J.; Mason, J.A.; Weirich, F.; Potgieter, A.L.F. Fuels and Fire Behavior Dynamics on Large-Scale Savanna Fires in Kruger National Park, South Africa. J. Geophys. Res. 1996, 101, 23541–23550. [Google Scholar] [CrossRef]
- Raison, R.J. Modification of the Soil Environment by Vegetation Fires, with Particular Reference to Nitrogen Transformations: A Review. Plant Soil 1979, 51, 73–108. [Google Scholar] [CrossRef]
- Neary, D.G.; Klopatek, C.C.; Debano, L.F.; Ffolliott, P.F. Fire Effects on Belowground Sustainability: A Review and Synthesis. For. Ecol. Manag. 1999, 122, 51–71. [Google Scholar] [CrossRef]
- Shackleton, C.M.; Scholes, R.J. Impact of Fire Frequency on Woody Community Structure and Soil Nutrients in the Kruger National Park. Koedoe 2000, 43, 75–81. [Google Scholar] [CrossRef]
- Zida, D.; Sawadogo, L.; Tigabu, M.; Tiveau, D.; Ode, P.C. Dynamics of Sapling Population in Savanna Woodlands of Burkina Faso Subjected to Grazing, Early Fire and Selective Tree Cutting for a Decade. For. Ecol. Manag. 2007, 243, 102–115. [Google Scholar] [CrossRef]
- D’Odorico, P.; Laio, F.; Ridolfi, L. A Probabilistic Analysis of Fire-Induced Tree-Grass Coexistence in Savannas. Am. Nat. 2006, 167, E79–E87. [Google Scholar] [CrossRef]
- Rothermel, R.C. A Mathematical Model for Predicting Fire Spread in Wildland Fuels; USDA Forest Service Research Paper INT No. INT-115, 40; Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1972. [Google Scholar]
- Schwilk, D.W. Flammability Is a Niche Construction Trait: Canopy Architecture Affects Fire Intensity. Am. Nat. 2003, 162, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Jolly, W.M. Sensitivity of a Surface Fire Spread Model and Associated Fire Behavior Fuel Models to Changes in Live Fuel Moisture. Int. J. Wildl. Fire 2007, 16, 503–509. [Google Scholar] [CrossRef]
- De Lillis, M.; Llusia, J.; Alessio, G.A.; Pen, J. Implications of Foliar Terpene Content and Hydration on Leaf Flammability of Quercus Ilex and Pinus Halepensis. Plant Biol. 2008, 10, 123–128. [Google Scholar] [CrossRef]
- Scarff, F.; Westoby, M. The Influence of Tissue Phosphate on Plant Flammability: A Kinetic Study. Polym. Degrad. Stab. 2008, 93, 1930–1934. [Google Scholar] [CrossRef]
- Paula, S.B.; Pausas, J.G.C.; Lloret, F.A. Fuel Loading and Flammability in the Mediterranean Basin Woody Species with Different Post-Fire Regenerative Strategies. Int. J. Wildl. Fire 2010, 19, 783–794. [Google Scholar] [CrossRef]
- Baeza, M.J.; Santana, V.M.; Pausas, J.G.; Vallejo, V.R. Successional Trends in Standing Dead Biomass in Mediterranean Basin Species. J. Veg. Sci. 2011, 22, 467–474. [Google Scholar] [CrossRef]
- Page, W.G.; Jenkins, M.J.; Runyon, J.B. Mountain Pine Beetle Attack Alters the Chemistry and Flammability of Lodgepole Pine Foliage. Can. J. For. Res. 2012, 42, 1631–1647. [Google Scholar] [CrossRef]
- Scarff, F.; Gray, B.; Westoby, M. Exploring Phosphate Effects on Leaf Flammability Using a Physical Chemistry Model. Int. J. Wildl. Fire 2012, 21, 1042–1051. [Google Scholar] [CrossRef]
- Setterfield, S.A.; Rossiter, N.A.; Hutley, L.B.; Douglas, M.M.; Williams, R.J. Turning up the Heat: The Impacts of Andropogon gayanus (Gamba Grass) Invasion on Fire Behaviour in Northern Australian Savannas. Divers. Distrib. 2010, 16, 854–861. [Google Scholar] [CrossRef]
- Rossiter, N.A.; Setterfield, S.A.; Douglas, M.M.; Hutley, L.B. Testing the Grass-Fire Cycle: Alien Grass Invasion in the Tropical Savannas of Northern Australia. Divers. Distrib. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Hughes, F.; Vitousek, P.M.; Tunison, T. Alien Grass Invasion and Fire in the Seasonal Submontane Zone of Hawai. Ecology 1991, 72, 743–746. [Google Scholar] [CrossRef]
- Ripley, B.; Donald, G.; Osborne, C.P.; Abraham, T.; Martin, T. Experimental Investigation of Fire Ecology in the C3 and C4 Subspecies of Alloteropsis semialata. J. Ecol. 2010, 98, 1196–1203. [Google Scholar] [CrossRef]
- Simpson, K.J.; Ripley, B.S.; Christin, P.A.; Belcher, C.M.; Lehmann, C.E.R.; Thomas, G.H.; Osborne, C.P. Determinants of Flammability in Savanna Grass Species. J. Ecol. 2016, 104, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Swezy, D.M.; Agee, J.K. Prescribed-Fire Effect on Fine Root and Tree Mortality in Old-Growth Ponderosa Pine. Can. J. For. Res. 1991, 21, 626–634. [Google Scholar] [CrossRef]
- Monsanto, P.G.; Agee, J.K. Long-Term Post-Wildfire Dynamics of Coarse Woody Debris after Salvage Logging and Implications for Soil Heating in Dry Forests of the Eastern. For. Ecol. Manag. 2008, 255, 3952–3961. [Google Scholar] [CrossRef]
- Sieg, C.H.; McMillin, J.D.; Fowler, J.F.; Allen, K.K.; Negron, J.F.; Wadleigh, L.L.; Anhold, J.A.; Gibson, K.E. Best Predictors for Postflre Mortality of Ponderosa Pine Trees in the Intermountain West. For. Sci. 2006, 52, 718–728. [Google Scholar]
- Ryan, K.C.; Peterson, D.L.; Reinhardt, E.D. Modeling Long-Term Fire-Caused Mortality of Douglas-Fir. For. Sci. 1988, 34, 190–199. [Google Scholar]
- Stephens, S.L.; Finney, M.A. Prescribed Fire Mortality of Sierra Nevada Mixed Conifer Tree Species: Effects of Crown Damage and Forest Floor Combustion. For. Ecol. Manag. 2002, 162, 261–271. [Google Scholar] [CrossRef]
- Andreas, B.; Nardini, A.; Mayr, S. Post-Fire Effects in Xylem Hydraulics of Picea abies, Pinus sylvestris and Fagus sylvatica. New Phytol. 2018, 217, 1484–1493. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A.; Tyree, M.T. Moving beyond the Cambium Necrosis Hypothesis of Post-Fire Tree Mortality: Cavitation and Deformation of Xylem in Forest Fires. New Phytol. 2012, 194, 254–263. [Google Scholar] [CrossRef] [PubMed]
- West, A.G.; Nel, J.A.; Bond, W.J.; Midgley, J.J. Experimental Evidence for Heat Plume-Induced Cavitation and Xylem Deformation as a Mechanism of Rapid Post-Fire Tree Mortality. New Phytol. 2016, 211, 828–838. [Google Scholar] [CrossRef] [PubMed]
- Hély, C.; Flannigan, M.D.; Bergeron, Y. Modeling Tree Mortality Following Wildfire in the Southeastern Canadian Mixed-Wood Boreal Forest. For. Sci. 2003, 49, 566–576. [Google Scholar]
- Alexander, M.E.; Cruz, M.G. Interdependencies between Flame Length and Fireline Intensity in Predicting Crown Fire Initiation and Crown Scorch Height. Int. J. Wildl. Fire 2012, 21, 95–113. [Google Scholar] [CrossRef]
- Ryan, K.C.; Frandsen, W.H. Basal Injury from Smoldering Sires in Mature Pinus Ponderosa Laws. Int. J. Wildl. Fire 1991, 1, 107–118. [Google Scholar] [CrossRef]
- Bova, A.S.; Dickinson, M.B. Linking Surface-Fire Behavior, Stem Heating, and Tissue Necrosis. Can. J. For. Res. 2005, 35, 814–822. [Google Scholar] [CrossRef]
- Busse, M.D.; Hubbert, K.R.; Fiddler, G.O.; Shestak, C.J.; Powers, R.F. Lethal Soil Temperatures during Burning of Masticated Forest Residues. Int. J. Wildl. Fire 2005, 14, 267–276. [Google Scholar] [CrossRef]
- Schwilk, D.W. Dimensions of Plant Flammability. New Phytol. 2015, 206, 486–488. [Google Scholar] [CrossRef] [PubMed]
- Pausas, J.G.; Keeley, J.E.; Schwilk, D.W. Flammability as an Ecological and Evolutionary Driver. J. Ecol. 2017, 105, 289–297. [Google Scholar] [CrossRef]
- De Magalhães, R.M.Q.; Schwilk, D.W. Leaf Traits and Litter Flammability: Evidence for Non-Additive Mixture Effects in a Temperate Forest. J. Ecol. 2012, 100, 1153–1163. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Elvira, A.; van Kempen, L.; van Logtestijn, R.S.P.; Aptroot, A.; Cornelissen, J.H.C. Flammability across the Gymnosperm Phylogeny: The Importance of Litter Particle Size. New Phytol. 2015, 206, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Engber, E.A.; Varner, J.M. Patterns of Flammability of the California Oaks: The Role of Leaf Traits. Can. J. For. Res. 2012, 42, 1965–1975. [Google Scholar] [CrossRef]
- Cruz, M.G.; Alexander, M.E.; Wakimoto, R.H. Development and Testing of Models for Predicting Crown Fire Rate of Spread in Conifer Forest Stands. Can. J. For. Res. 2005, 35, 1626–1639. [Google Scholar] [CrossRef]
- Cassandra, V.A.; van Logtestijn, R.; Cornwell, W.; Cornelissen, H. Species Composition and Fire: Non-Additive Mixture Effects on Ground Fuel Flammability. Front. Plant Sci. 2012, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Gordon, D.; Gutierrez, M.; Lee, D.; Molina, D.; Schroeder, R.; Sapsis, D.; Stephens, S.; Chambers, M. Assessing the Flammability of Domestic and Wildland Vegetation. In Proceedings of the 12th Conference on Fire and Forest Meteorology, Jekyll Island, GA, USA, 26–28 October 1993. [Google Scholar]
- Fill, J.M.; Moule, B.M.; Varner, J.M.; Mousseau, T.A. Flammability of the Keystone Savanna Bunchgrass Aristida Stricta. Plant Ecol. 2016, 217, 331–342. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: https://www.R-project.org/ (accessed on 30 November 2017).
- Bowman, D.M.J.S.; Wilson, B.A. Fuel Characteristics of Coastal Monsoon Forests, Northern Territory, Australia. J. Biogeogr. 1988, 15, 807–817. [Google Scholar] [CrossRef]
- Britton, C.M.; Dodd, J.D.; Weichert, A.T. Energy Values of Plant Species and Litter of an Andropogon-Paspalum Grassland. J. Biogeogr. 1976, 3, 389–395. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Modelling for Nested Data. In Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; pp. 127–128. [Google Scholar]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar]
- Singmann, H.; Bolker, B.; Westfall, J.; Aust, F. Afex: Analysis of Factorial Experiments. Version 0.19-1. 2018. Available online: https://CRAN.R-project.org/package=afex (accessed on 8 March 2018).
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Luke, S.G. Evaluating Significance in Linear Mixed-Effects Models in R. Behav. Res. Methods 2017, 49, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Odion, D.C.; Davis, F.W. Fire, Soil Heating, and the Formation of Vegetation Patterns in Chaparral. Ecol. Monogr. 2000, 70, 149–169. [Google Scholar] [CrossRef]
- Reinhardt, E.D. Using FOFEM 5.0 to Estimate Tree Mortality, Fuel Consumption, Smoke Production and Soil Heating from Wildland Fire. In Proceedings of the 2nd International Wildland Fire Ecology and Fire Management Congress and 5th Symposium on Fire and Forest Meteorology, Orlando, FL, USA, 16–20 November 2003; pp. 16–20. [Google Scholar]
- Sharrow, S.H.; Wright, H.A. Effects of Fire, Ash, and Litter on Soil Nitrate, Temperature, Moisture and Tobosagrass Production in the Rolling Plains. Soc. Range Manag. 1977, 30, 266–270. [Google Scholar] [CrossRef]
- Hulbert, L.C. Causes of Fire Effects in Tallgrass Prairie. Ecology 1988, 69, 46–58. [Google Scholar] [CrossRef]
- Wright, H.A. A Method to Determine Heat-Caused Mortality in Bunchgrasses. Ecology 1970, 51, 582–587. [Google Scholar] [CrossRef]
- Catry, F.X.; Rego, F.; Moreira, F.; Fernandes, P.M.; Pausas, J.G. Post-Fire Tree Mortality in Mixed Forests of Central Portugal. For. Ecol. Manag. 2010, 260, 1184–1192. [Google Scholar] [CrossRef]
- Clarke, P.J.; Lawes, M.J.; Midgley, J.J.; Lamont, B.B.; Ojeda, F.; Burrows, G.E.; Enright, N.J.; Knox, K.J.E. Resprouting as a Key Functional Trait: How Buds, Protection and Resources Drive Persistence after Fire. New Phytol. 2013, 197, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Lawes, M.J.; Adie, H.; Russell-Smith, J.; Murphy, B.; Midgley, J.J. How Do Small Savanna Trees Avoid Stem Mortality by Fire? The Roles of Stem Diameter, Height and Bark Thickness. Ecosphere 2011, 2, art42. [Google Scholar] [CrossRef]
- Bowman, D.M.J.S.; Haverkamp, C.; Rann, K.D.; Prior, L.D. Differential Demographic Filtering by Surface Fires: How Fuel Type and Fuel Load Affect Sapling Mortality of an Obligate Seeder Savanna Tree. J. Ecol. 2017, 106, 1–13. [Google Scholar] [CrossRef]
- Stephens, S.L.; Moghaddas, J.J. Experimental Fuel Treatment Impacts on Forest Structure, Potential Fire Behavior, and Predicted Tree Mortality in a California Mixed Conifer Forest. For. Ecol. Manag. 2005, 215, 21–36. [Google Scholar] [CrossRef]
- Balfour, D.A.; Midgley, J.J. Fire Induced Stem Death in an African Acacia Is Not Caused by Canopy Scorching. Austral Ecol. 2006, 31, 892–896. [Google Scholar] [CrossRef]
- Michaletz, S.T. Xylem Dysfunction in Fires: Towards a Hydraulic Theory of Plant Responses to Multiple Disturbance Stressors. New Phytol. 2018, 217, 1391–1393. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, M.B. Heat Transfer and Vascular Cambium Necrosis in the Boles of Trees during Surface Fires. In Forest Fire Research & Wildland Fire Safety; Viegas, D.X., Ed.; Millpress: Rotterdam, The Netherlands, 2002. [Google Scholar]
- Grootemaat, S.; Wright, I.J.; Van Bodegom, P.M.; Cornelissen, J.H.C.; Cornwell, W.K. Burn or Rot: Leaf Traits Explain Why Flammability and Decomposability Are Decoupled across Species. Funct. Ecol. 2015, 29, 1486–1497. [Google Scholar] [CrossRef]
- Matthews, S. A Process-Based Model of Fine Fuel Moisture. Int. J. Wildl. Fire 2006, 15, 155–168. [Google Scholar] [CrossRef]
- Wotton, B.M. A Grass Moisture Model for the Canadian Forest Fire Danger Rating System. In Proceedings of the 8th Symposium on Fire and Forest Meteorology, Kalispell, MT, USA, 13–15 October 2009. [Google Scholar]
- Kidnie, S.; Cruz, M.G.; Gould, J.S.; Nichols, D.; Bessell, R.; Slijepcevic, A. Effects of Curing on Grassfires: II. Effect of Grass Senescence on the Rate of Fire Spread. Int. J. Wildl. Fire 2015, 24, 828–837. [Google Scholar] [CrossRef]
- Whittaker, R.H.; Gilbert, L.E.; Connell, J.H. Analysis of Two-Phase Pattern in a Mesquite Grassland, Texas. J. Ecol. 1979, 67, 935–952. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Coughenour, M.B. Savanna Tree Influence on Understory Vegetation and Soil Nutrients in Northwestern Kenya. J. Veg. Sci. 1990, 1, 325–332. [Google Scholar] [CrossRef]
- Collins, B.; Wein, G. Stem Elongation Response to Neighbour Shade in Sprawling and Upright Polygonum Species. Ann. Bot. 2000, 86, 739–744. [Google Scholar] [CrossRef]
- Sasidharan, R.; Chinnappa, C.C.; Voesenek, L.A.C.J.; Pierik, R. The Regulation of Cell Wall Extensibility during Shade Avoidance: A Study Using Two Contrasting Ecotypes. Plant Physiol. 2008, 148, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Scholes, R.J.; Archer, S.R. Tree-Grass Interactions in Savannas. Annu. Rev. Ecol. Systemat. 1997, 28, 517–544. [Google Scholar] [CrossRef]
- Van Langevelde, F.; van de Vijver, C.A.D.M.; Kumar, L.; van de Koppel, J.; de Ridder, N.; van Andel, J.; Skidmore, A.K.; Hearne, J.W.; Stroosnijder, L.; Bond, W.J.; et al. Effects of Fire and Herbivory on the Stability of Savanna Ecosystems. Ecology 2003, 84, 337–350. [Google Scholar] [CrossRef]
- Menaut, J.C.; Gignoux, J.; Prado, C.; Clobert, J. Tree Community Dynamics in a Humid Savanna of the Cote-d’Ivoire: Modelling the Effects of Fire and Competition with Grass and Neighbours. J. Biogeogr. 1990, 17, 471–481. [Google Scholar] [CrossRef]
- Beckage, B.; Platt, W.J.; Gross, L.J. Vegetation, Fire, and Feedbacks: A Disturbance-Mediated Model of Savannas. Am. Nat. 2009, 174, 805–818. [Google Scholar] [CrossRef] [PubMed]
| Biomass-Height Ratio | Biomass Density (g cm−3) | Biomass-Height Ratio: Biomass Density * | Relative Humidity (%) | ||
|---|---|---|---|---|---|
| Temperature integration at 0 cm (°C·s) | Estimate | −28,600 | 12,500 | −24,000 | 11,500 |
| p value | 0.038 | 0.194 | 0.081 | 0.071 | |
| Temperature integration at 25 cm (°C·s) | Estimate | −1910 | 2260 | 2880 | −2790 |
| p value | 0.577 | 0.311 | 0.317 | 0.296 | |
| Max mass-loss rate (s−1) | Estimate | 0.008 | −0.002 | −0.002 | 0.008 |
| p value | 0.325 | 0.655 | 0.287 | 0.546 |
| Species | Shade Tolerance | Total Mass (g) | Biomass-Height Ratio | Biomass Density (g cm-3) | Surface Temperature Integration (°C·s) | Canopy Temperature Integration (°C·s) | Max Mass-Loss Rate (s−1) |
|---|---|---|---|---|---|---|---|
| A. purpurea | Intolerant | 54.06 ± 21.39 | 0.86 ± 0.16 | 0.0040 ± 0.0016 | 184,750 ± 118,090 | 33,770 ± 9303.3 | 0.03 ± 0.01 |
| B. inermis | Intolerant | 25.94 ± 9.62 | 1.46 ± 0.24 | 0.0019 ± 0.0008 | 34,145 ± 21,426 | 26,127 ± 10,399 | 0.05 ± 0.02 |
| C. latifolium | Tolerant | 29.50 ± 16.50 | 5.46 ± 0.64 | 0.0007 ± 0.0003 | 9434 ± 14,482 | 19,072 ± 10,061 | 0.06 ± 0.02 |
| E. curvula | Intolerant | 127.75 ± 82.42 | 1.78 ± 0.42 | 0.0019 ± 0.0012 | 196,100 ± 155,930 | 57,213 ± 24,476 | 0.05 ± 0.02 |
| E. elymoides | Intolerant | 33.37 ± 23.09 | 1.69 ± 0.55 | 0.0032 ± 0.0013 | 67,199 ± 90,330 | 31,029 ± 24,214 | 0.05 ± 0.03 |
| H. neomexicana | Intolerant | 74.06 ± 40.20 | 1.14 ± 0.17 | 0.0038 ± 0.0019 | 137,490 ± 91,345 | 54,096 ± 26,103 | 0.05 ± 0.02 |
| P. anceps | Tolerant | 23.88 ± 11.35 | 3.04 ± 0.10 | 0.0007 ± 0.0004 | 13,742 ± 49,547 | 12,561 ± 9814.1 | 0.07 ± 0.06 |
| P. smithii | Intolerant | 23.35 ± 10.62 | 1.39 ± 0.10 | 0.0026 ± 0.0011 | 34,626 ± 38,135 | 22,540 ± 11,357 | 0.06 ± 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Schwilk, D.W. Grass Canopy Architecture Influences Temperature Exposure at Soil Surface. Fire 2018, 1, 35. https://doi.org/10.3390/fire1030035
Gao X, Schwilk DW. Grass Canopy Architecture Influences Temperature Exposure at Soil Surface. Fire. 2018; 1(3):35. https://doi.org/10.3390/fire1030035
Chicago/Turabian StyleGao, Xiulin, and Dylan W. Schwilk. 2018. "Grass Canopy Architecture Influences Temperature Exposure at Soil Surface" Fire 1, no. 3: 35. https://doi.org/10.3390/fire1030035
APA StyleGao, X., & Schwilk, D. W. (2018). Grass Canopy Architecture Influences Temperature Exposure at Soil Surface. Fire, 1(3), 35. https://doi.org/10.3390/fire1030035





