Pharmaceutically Active Compound (PhAC) Degradation by Means of Cold Plasma Jet Treatment
Abstract
1. Introduction
2. Pharmaceutical Compounds
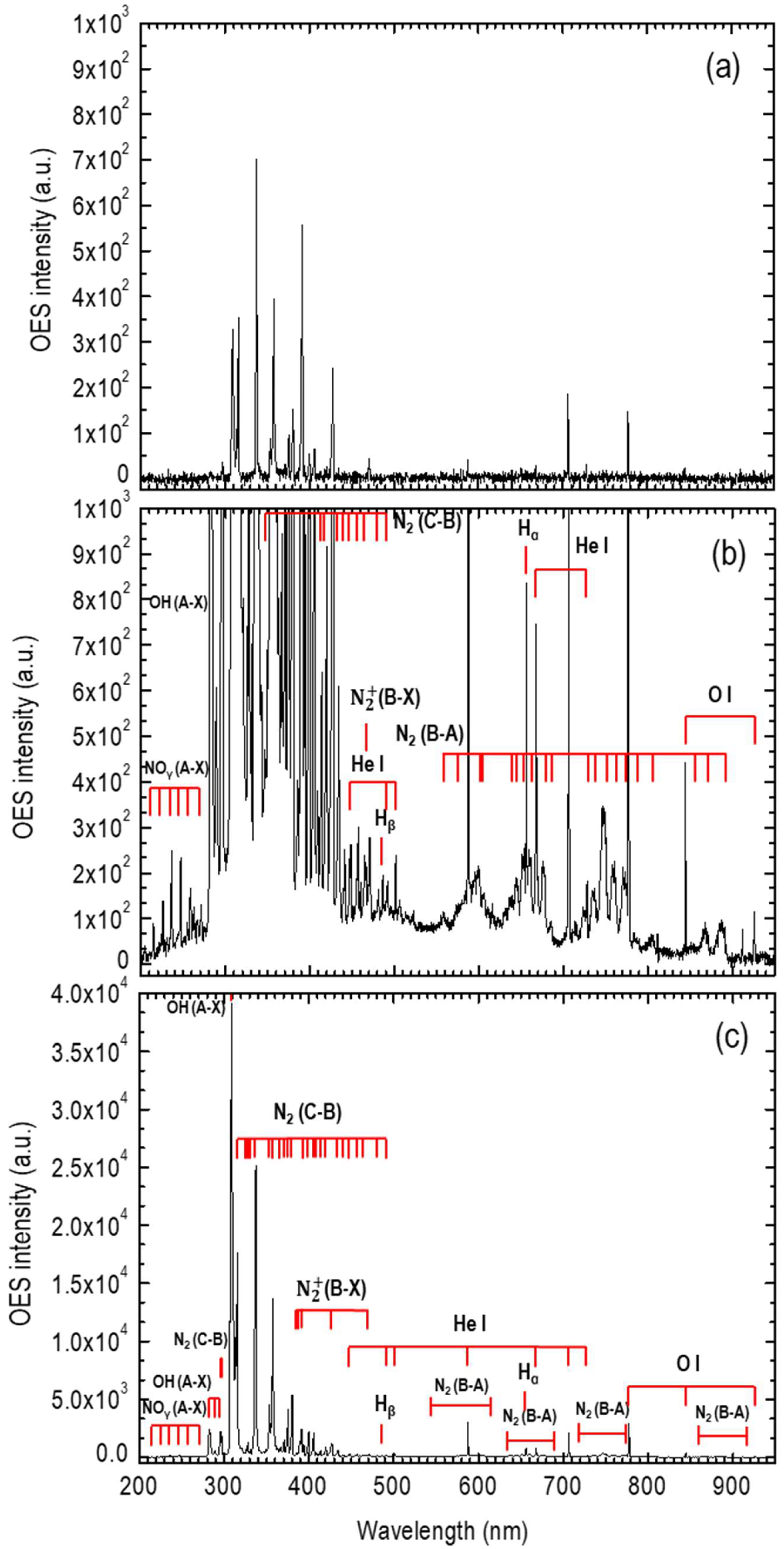
3. Plasma Setup
4. LC-MS/MS Analysis
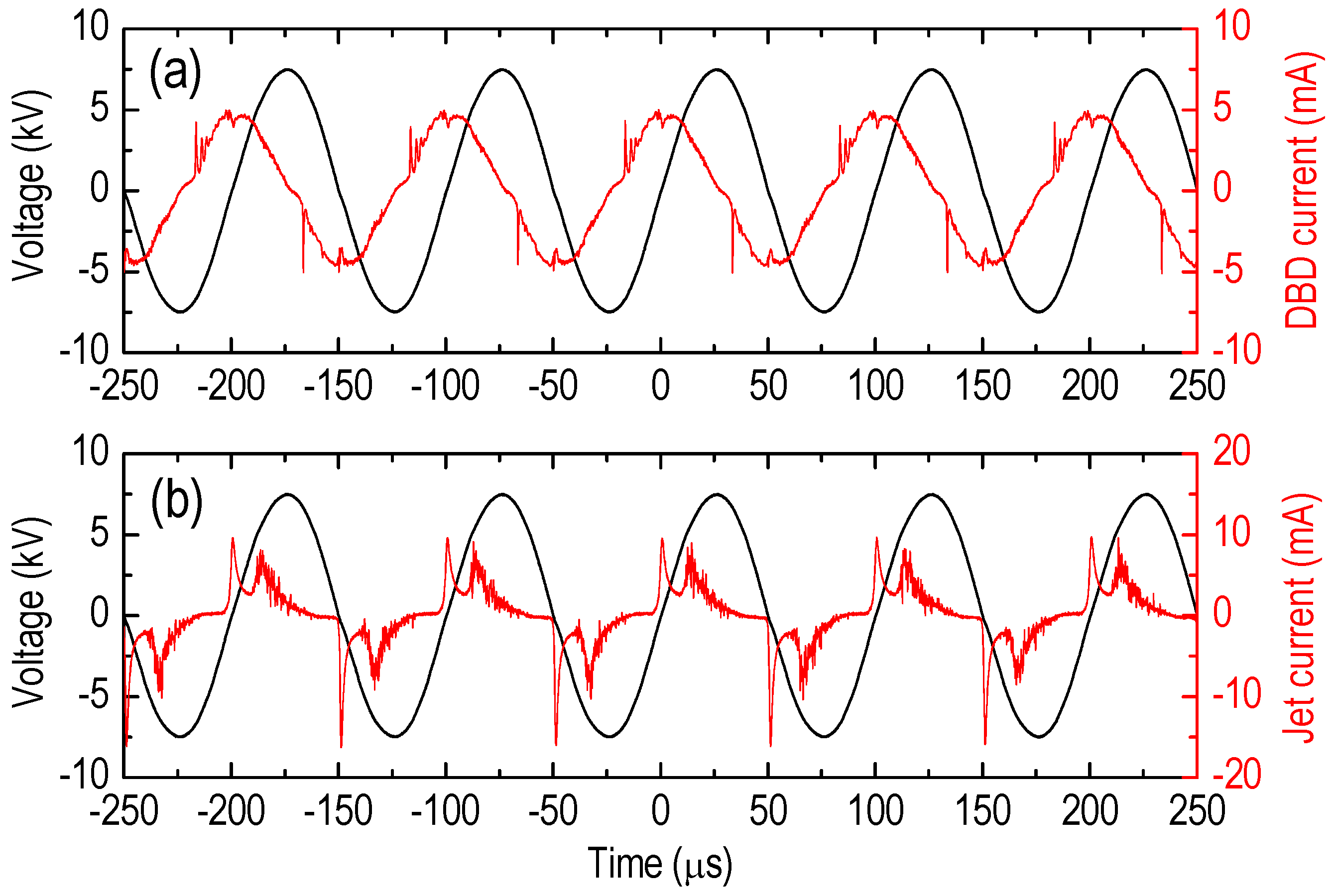
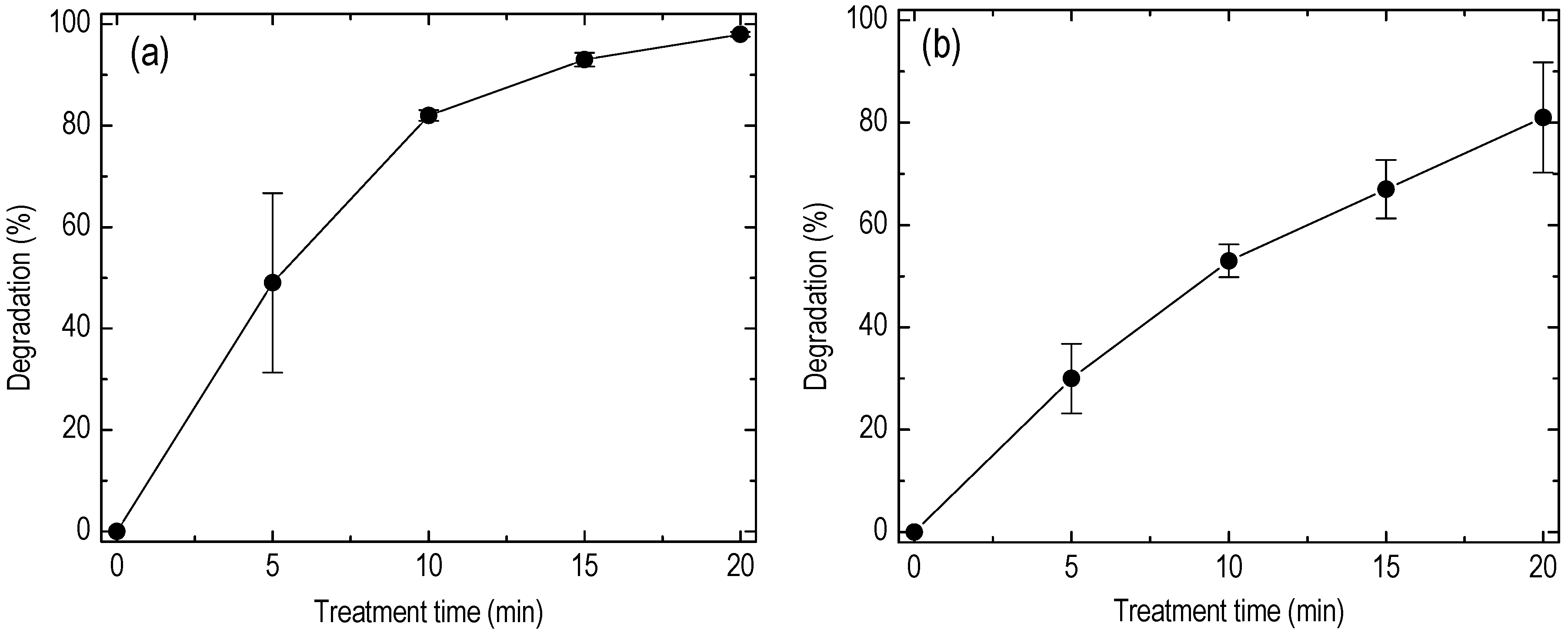
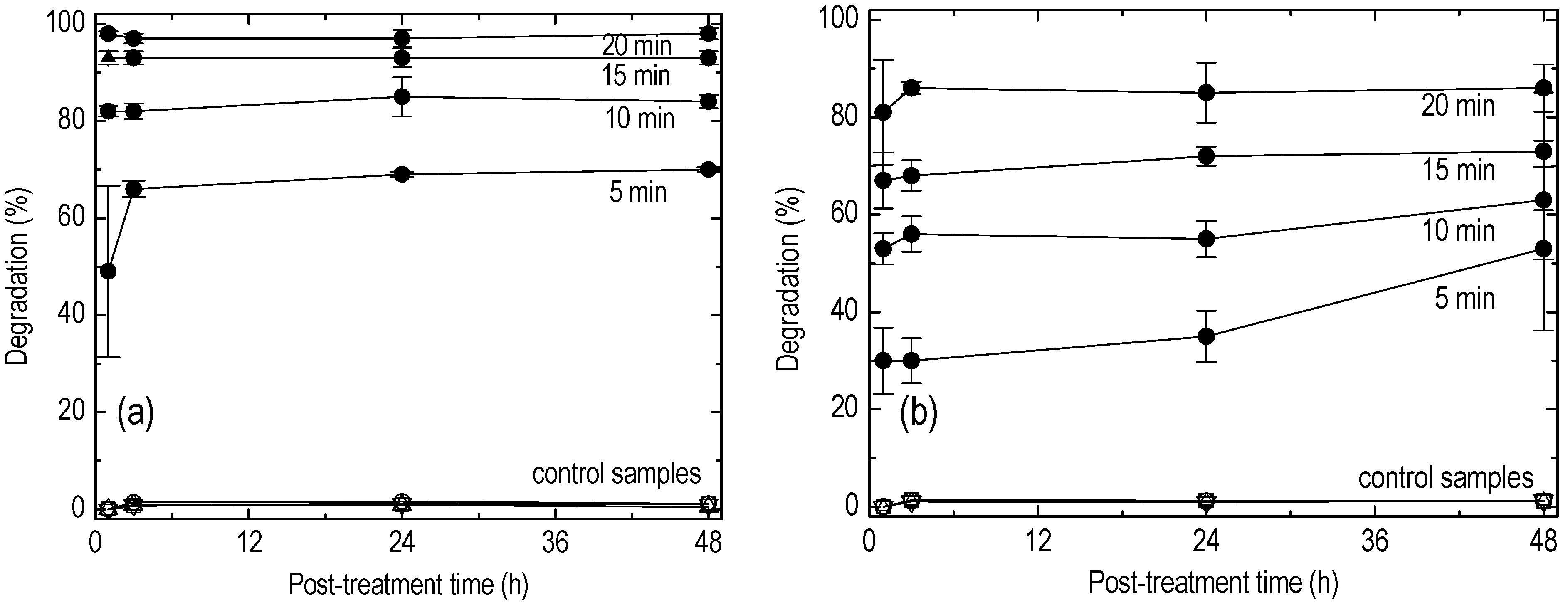
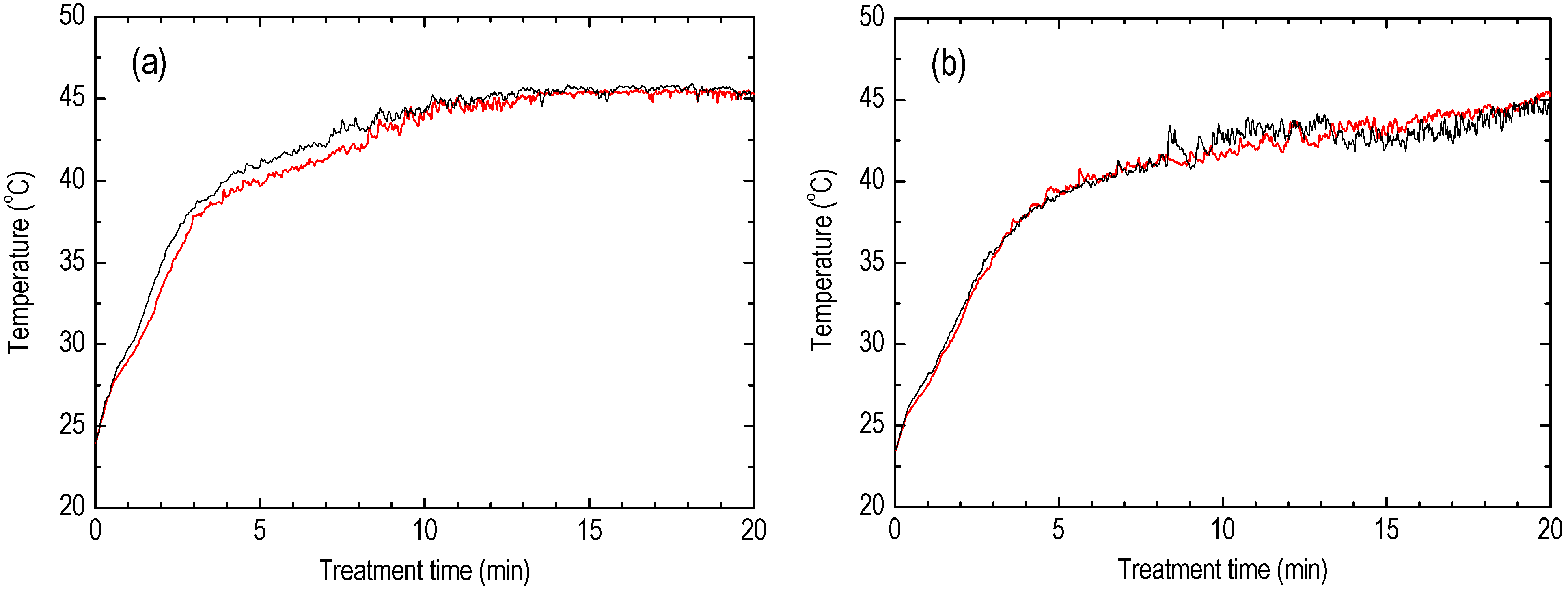
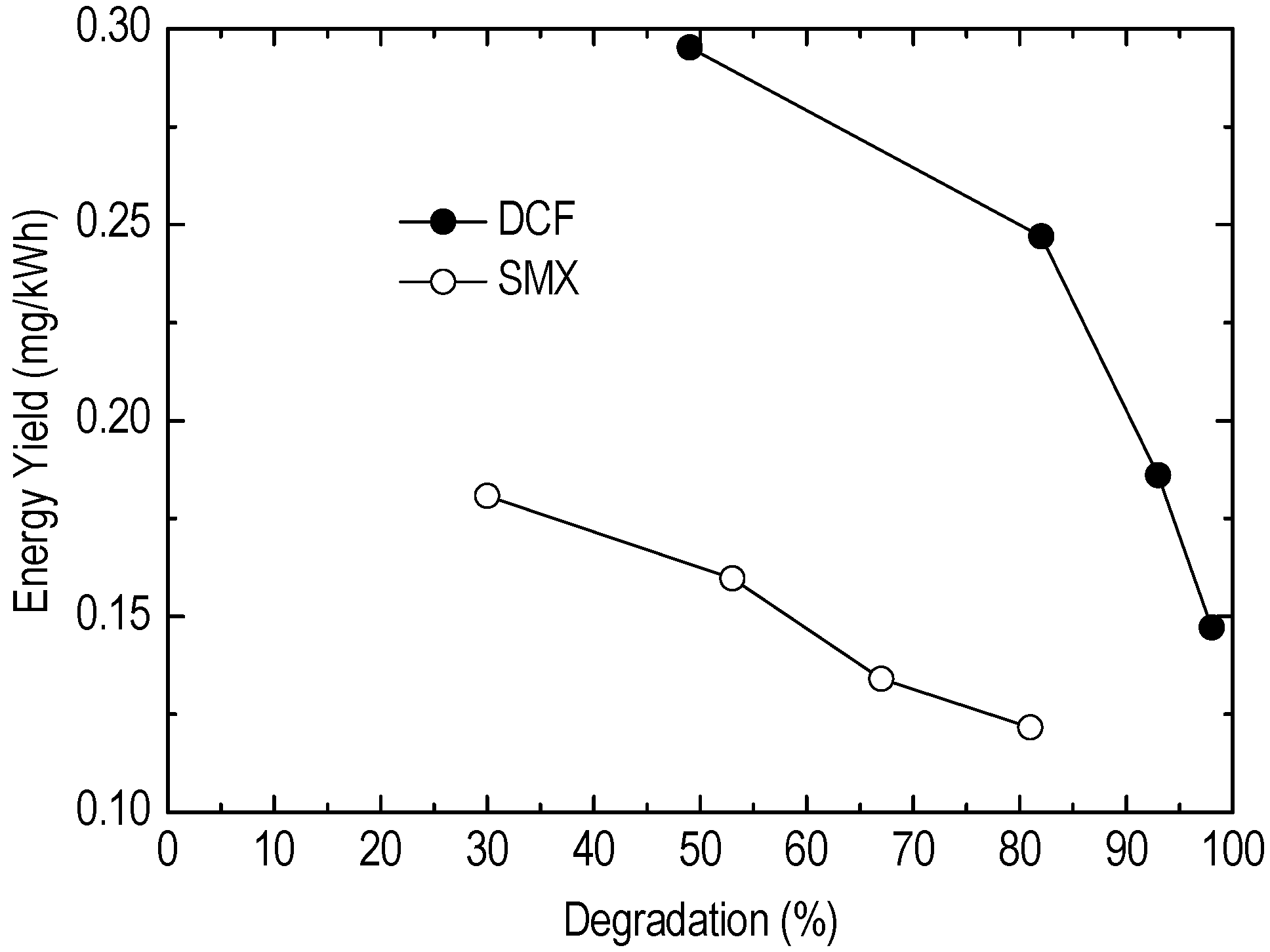
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Species | Wavelength (nm) | Vibrational Transition v′ − v″ (Δv) |
|---|---|---|
| 215.49 | 1-0 (+1) | |
| 226.94 | 0-0 (0) | |
| 237.02 | 0-1 (−1) | |
| 247.87 | 0-2 (−2) | |
| 259.57 | 0-3 (−3) | |
| 272.22 | 0-4 (−4) | |
| 282.90 | 1-0 (+1) | |
| 289.27 | 2-1 (+1) | |
| 296.24 | 3-2 (+1) | |
| 308.9 | 0-0 (0) | |
| 296.20 | 3-1 (+2) | |
| 297.68 | 2-0 (+2) | |
| 315.93 | 1-0 (+1) | |
| 326.81 | 4-4 (0) | |
| 328.53 | 3-3 (0) | |
| 330.90 | 2-2 (0) | |
| 337.17 | 0-0 (0) | |
| 350.05 | 2-3 (−1) | |
| 353.67 | 1-2 (−1) | |
| 367.19 | 3-5 (−2) | |
| 371.05 | 2-4 (−2) | |
| 375.54 | 1-3 (−2) | |
| 380.49 | 0-2 (−2) | |
| 394.3 | 2-5 (−3) | |
| 399.84 | 1-4 (−3) | |
| 405.94 | 0-3 (−3) | |
| 409.48 | 4-8 (−4) | |
| 414.18 | 3-7 (−4) | |
| 420.05 | 2-6 (−4) | |
| 426.97 | 1-5 (−4) | |
| 434.36 | 0-4 (-4) | |
| 441.67 | 3-8 (−5) | |
| 449.02 | 2-7 (−5) | |
| 457.43 | 1-6 (−5) | |
| 464.94 | 4-10 (−6) | |
| 481.47 | 2-8 (−6) | |
| 491.68 | 1-7 (−6) | |
| 559.29 | 6-1 (+5) | |
| 575.52 | 12-8 (+4) | |
| 601.36 | 7-3 (+4) | |
| 606.97 | 6-2 (+4) | |
| 639.47 | 9-6 (+3) | |
| 646.85 | 8-5 (+3) | |
| 654.48 | 7-4 (+3) | |
| 662.36 | 6-3 (+3) | |
| 678.86 | 4-1 (+3) | |
| 687.50 | 3-0 (+3) | |
| 727.33 | 6-4 (+2) | |
| 738.66 | 5-3 (+2) | |
| 750.39 | 4-2 (+2) | |
| 762.62 | 3-1 (+2) | |
| 775.32 | 2-0 (+2) | |
| 789.64 | 7-6 (+1) | |
| 804.74 | 6-5 (+1) | |
| 854.18 | 3-2 (+1) | |
| 872.23 | 2-1 (+1) | |
| 891.19 | 1-0 (+1) | |
| 385.79 | 2-2 (0) | |
| 388.43 | 1-1 (0) | |
| 391.44 | 0-0 (0) | |
| 427.81 | 0-1 (−1) | |
| 470.92 | 0-2 (−2) | |
| 486.1 | ||
| 656.3 | ||
| 447.15 | ||
| 492.19 | ||
| 501.57 | ||
| 587.56 | ||
| 667.82 | ||
| 706.57 | ||
| 728.13 | ||
| O I | 777 | |
| 844.6 | ||
| 926 |
References
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical Residues in Environmental Waters and Wastewater: Current State of Knowledge and Future Research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Occurrence and Removal of PPCPs in Municipal and Hospital Wastewaters in Greece. J. Hazard. Mater. 2010, 179, 804–817. [Google Scholar] [CrossRef] [PubMed]
- Kanteraki, A.E.; Isari, E.A.; Zafeiropoulos, I.; Cangemi, S.; Bountla, A.; Kalavrouziotis, I.K. Structural Analysis and Characterization of Biosolids. A Case Study of Biosolids from Wastewater Treatment Plants in Western Greece. Sci. Total Environ. 2024, 908, 168425. [Google Scholar] [CrossRef] [PubMed]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.; GarcíaJares, C.; Rodríguez, I.; Gómez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2024, 38, 2918–2926. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef]
- De Oliveira, M.; Frihling, B.E.F.; Velasques, J.; Magalhães Filho, F.J.C.; Cavalheri, P.S.; Migliolo, L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci. Total Environ. 2020, 705, 135568. [Google Scholar] [CrossRef]
- Sui, Q.; Zhao, W.; Cao, X.; Lu, S.; Qiu, Z.; Gu, X.; Yu, G. Pharmaceuticals and personal care products in the leachates from a typical landfll reservoir of municipal solid waste in Shanghai, China: Occurrence and removal by a full-scale membrane reactor. J. Hazard. Mater. 2015, 323, 99–108. [Google Scholar] [CrossRef]
- Han, E.J.; Lee, D.S. Signifcance of metabolites in the environmental risk assessment of pharmaceuticals consumed by human. Sci. Total Environ. 2017, 592, 600–607. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Gago-Ferrero, P.; Borova, V.L.; Pavlidou, A.; Hatzianestis, I.; Thomaidis, N.S. Occurrence and Spatial Distribution of 158 Pharmaceuticals, Drugs of Abuse and Related Metabolites in Offshore Seawater. Sci. Total Environ. 2016, 541, 1097–1105. [Google Scholar] [CrossRef]
- Pal, R.; Megharaj, M.; Kirkbride, K.P.; Naidu, R. Illicit Drugs and the Environment—A Review. Sci. Total Environ. 2013, 463–464, 1079–1092. [Google Scholar] [CrossRef]
- McEneff, G.; Barron, L.; Kelleher, B.; Paull, B.; Quinn, B.A. Year-Long Study of the Spatial Occurrence and Relative Distribution of Pharmaceutical Residues in Sewage Effluent, Receiving Marine Waters and Marine Bivalves. Sci. Total Environ. 2014, 476–477, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Thiele-Bruhn, S. Pharmaceutical Antibiotic Compounds in Soils—A Review. J. Plant Nutr. Soil Sci. 2003, 166, 145–167. [Google Scholar] [CrossRef]
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental Impact of Estrogens on Human, Animal and Plant Life: A Critical Review. Environ. Int. 2017, 99, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Kyriacou, M.C.; Georgiadou, E.C.; Papamarkou, R.; Hapeshi, E.; Karaolia, P.; Michael, C.; Fotopoulos, V.; Fatta-Kassinos, D. Uptake and Bioaccumulation of Three Widely Prescribed Pharmaceutically Active Compounds in Tomato Fruits and Mediated Effects on Fruit Quality Attributes. Sci. Total Environ. 2019, 647, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Camacho-Muñoz, D.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Pharmaceutical Compounds in Wastewater and Sludge from Wastewater Treatment Plants: Removal and Ecotoxicological Impact of Wastewater Discharges and Sludge Disposal. J. Hazard. Mater. 2012, 239–240, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.; van Driezum, I.; Ohana, D.; Lynch, G.; Berendsen, B.; Wuijts, S.; van der Hoek, J.P.; de Roda Husman, A.M. The Effective Design of Sampling Campaigns for Emerging Chemical and Microbial Contaminants in Drinking Water and Its Resources Based on Literature Mining. Sci. Total Environ. 2020, 742, 140546. [Google Scholar] [CrossRef] [PubMed]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment Technologies for Emerging Contaminants in Wastewater Treatment Plants: A Review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245e3260. [Google Scholar] [CrossRef]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design parameter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef]
- Prochaska, C.; Zouboulis, A.A. Mini-Review of Urban Wastewater Treatment in Greece: History, Development and Future Challenges. Sustainability 2020, 12, 6133. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of Advanced Oxidation Processes for Water and Wastewater Treatment—A Critical Review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Haritash, A.K. Review of Advanced Oxidation Processes (AOPs) for Treatment of Pharmaceutical Wastewater. Adv. Environ. Res. 2020, 9, 1–17. [Google Scholar]
- Sanito, R.C.; You, S.-J.; Wang, Y.-F. Degradation of Contaminants in Plasma Technology: An Overview. J. Hazard. Mater. 2022, 424, 127390. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zheng, J.; Qiu, S.; Wu, M.; Zhang, Q.; Yan, Z.; Xue, Q. Review on Electrical Discharge Plasma Technology for Wastewater Remediation. Chem. Eng. J. 2014, 236, 348–368. [Google Scholar] [CrossRef]
- Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of Pharmaceutical Compounds in Water by Non-Thermal Plasma Treatment. Water Res. 2015, 81, 124–136. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Svarnas, P.; Klapa, M.I.; Tsakiroglou, C.D. Dielectric Barrier Discharge Plasma Used as a Means for the Remediation of Soils Contaminated by Non-Aqueous Phase Liquids. Chem. Eng. J. 2015, 270, 428–436. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Gkelios, A.; Klapa, M.I.; Kaltsonoudis, C.; Svarnas, P.; Tsakiroglou, C.D. Parametric Analysis of the Operation of a Non-Thermal Plasma Reactor for the Remediation of NAPL-Polluted Soils. Chem. Eng. J. 2016, 301, 353–361. [Google Scholar] [CrossRef]
- Svarnas, P.; Giannakopoulos, E.; Kalavrouziotis, I.; Krontiras, C.; Georga, S.; Pasolari, R.S.; Papadopoulos, P.K.; Apostolou, I.; Chrysochoou, D. Sanitary Effect of FE-DBD Cold Plasma in Ambient Air on Sewage Biosolids. Sci. Total Environ. 2020, 705, 135940. [Google Scholar] [CrossRef]
- Giannakopoulos, E.; Svarnas, P.; Dimitriadou, S.; Kalavrouziotis, I.; Papadopoulos, P.K.; Georga, S.; Krontiras, C. Emerging Sanitary Engineering of Biosolids: Elimination of Salmonella, Escherichia Coli, and Coliforms by Means of Atmospheric Pressure Air Cold Plasma. J. Hazard. Toxic Radioact. Waste 2021, 25, 06021001. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Removal of 2,4-Dichlorophenoxyacetic Acid in Aqueous Solution by Pulsed Corona Discharge Treatment: Effect of Different Water Constituents, Degradation Pathway and Toxicity Assay. Chemosphere 2017, 184, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Jin, X.; Hu, S.; Lan, Y.; Xi, W.; Han, W.; Cheng, C. Study on the Effective Removal of Chlorpyrifos from Water by Dielectric Barrier Discharge (DBD) Plasma: The Influence of Reactive Species and Different Water Components. Chem. Eng. J. 2023, 473, 144755. [Google Scholar] [CrossRef]
- Lonappan, L.; Brar, S.K.; Das, R.K.; Verma, M.; Surampalli, R.Y. Diclofenac and Its Transformation Products: Environmental Occurrence and Toxicity—A Review. Environ. Int. 2016, 96, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, C.; Frimmel, F. Short-Term Tests with a Pilot Sewage Plant and Biofilm Reactors for the Biological Degradation of the Pharmaceutical Compounds Clofibric Acid, Ibuprofen, and Diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Goutam Mukherjee, A.; Ramesh Wanjari, U.; Eladl, M.A.; El-Sherbiny, M.; Elsherbini, D.M.A.; Sukumar, A.; Kannampuzha, S.; Ravichandran, M.; Renu, K.; Vellingiri, B.; et al. Mixed Contaminants: Occurrence, Interactions, Toxicity, Detection, and Remediation. Molecules 2022, 27, 2577. [Google Scholar] [CrossRef]
- Jiang, B.; Li, A.; Cui, D.; Cai, R.; Ma, F.; Wang, Y. Biodegradation and Metabolic Pathway of Sulfamethoxazole by Pseudomonas Psychrophila HA-4, a Newly Isolated Cold-Adapted Sulfamethoxazole-Degrading Bacterium. Appl. Microbiol. Biotechnol. 2014, 98, 4671–4681. [Google Scholar] [CrossRef]
- Bonvin, F.; Omlin, J.; Rutler, R.; Schweizer, W.B.; Alaimo, P.J.; Strathmann, T.J.; McNeill, K.; Kohn, T. Direct Photolysis of Human Metabolites of the Antibiotic Sulfamethoxazole: Evidence for Abiotic Back-Transformation. Environ. Sci. Technol. 2013, 47, 6746–6755. [Google Scholar] [CrossRef]
- Rodríguez-Nava, O.; Ramírez-Saad, H.; Loera, O.; González, I. Evaluation of the Simultaneous Removal of Recalcitrant Drugs (Bezafibrate, Gemfibrozil, Indomethacin and Sulfamethoxazole) and Biodegradable Organic Matter from Synthetic Wastewater by Electro-Oxidation Coupled with a Biological System. Environ. Technol. 2016, 37, 2964–2974. [Google Scholar] [CrossRef]
- Katsoyiannis, A.; Zouboulis, A.; Samara, C. Persistent Organic Pollutants (POPs) in the Conventional Activated Sludge Treatment Process: Model Predictions against Experimental Values. Chemosphere 2006, 65, 1634–1641. [Google Scholar] [CrossRef]
- Kanteraki, A.E.; Isari, E.A.; Svarnas, P.; Kalavrouziotis, I.K. Biosolids: The Trojan Horse or the Beautiful Helen for Soil Fertilization? Sci. Total Environ. 2022, 839, 156270. [Google Scholar] [CrossRef] [PubMed]
- Pizzichetti, R.; Reynolds, K.; Pablos, C.; Casado, C.; Moore, E.; Stanley, S.; Marugán, J. Removal of Diclofenac by UV-B and UV-C Light-Emitting Diodes (LEDs) Driven Advanced Oxidation Processes (AOPs): Wavelength Dependence, Kinetic Modelling and Energy Consumption. Chem. Eng. J. 2023, 471, 144520. [Google Scholar] [CrossRef]
- Mirzaei, A.; Eddah, M.; Roualdes, S.; Ma, D.; Chaker, M. Multiple-Homojunction Gradient Nitrogen Doped TiO2 for Photocatalytic Degradation of Sulfamethoxazole, Degradation Mechanism, and Toxicity Assessment. Chem. Eng. J. 2021, 422, 130507. [Google Scholar] [CrossRef]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in Their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Wang, J. Fenton-like Degradation of Sulfamethoxazole in Cu0/Zn0-Air System over a Broad PH Range: Performance, Kinetics and Mechanism. Chem. Eng. J. 2021, 403, 126320. [Google Scholar] [CrossRef]
- Banaschik, R.; Lukes, P.; Jablonowski, H.; Hammer, M.U.; Weltmann, K.-D.; Kolb, J.F. Potential of Pulsed Corona Discharges Generated in Water for the Degradation of Persistent Pharmaceutical Residues. Water Res. 2015, 84, 127–135. [Google Scholar] [CrossRef]
- Liu, Q.; Ouyang, W.; Yang, X.; He, Y.; Wu, Z.; Ostrikov, K. Plasma-Microbubble Treatment and Sustainable Agriculture Application of Diclofenac-Contaminated Wastewater. Chemosphere 2023, 334, 138998. [Google Scholar] [CrossRef]
- Dobrin, D.; Bradu, C.; Magureanu, M.; Mandache, N.B.; Parvulescu, V.I. Degradation of Diclofenac in Water Using a Pulsed Corona Discharge. Chem. Eng. J. 2013, 234, 389–396. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, J.; Guo, H.; Puyang, C.; Han, J.; Li, Y.; Ruan, Y. Mechanism and Process of Sulfamethoxazole Decomposition with Persulfate Activated by Pulse Dielectric Barrier Discharge Plasma. Sep. Purif. Technol. 2022, 287, 120540. [Google Scholar] [CrossRef]
- Kumar, A.; Škoro, N.; Gernjak, W.; Jovanović, O.; Petrović, A.; Živković, S.; Lumbaque, E.C.; Farré, M.J.; Puač, N. Degradation of Diclofenac and 4-Chlorobenzoic Acid in Aqueous Solution by Cold Atmospheric Plasma Source. Sci. Total Environ. 2023, 864, 161194. [Google Scholar] [CrossRef]
- Silva, S.; Rodrigues, J.A.; Coelho, M.R.; Martins, A.; Cardoso, E.; Cardoso, V.V.; Benoliel, M.J.; Almeida, C.M.M. Occurrence of 290 Pharmaceutical Active Compounds in Sewage Sludge from Two Urban Wastewater Treatment Plants and Their Potential Be-291 haviour in Agricultural Soils. Environ. Sci. Water Res. Technol. 2021, 7, 969–982. [Google Scholar] [CrossRef]
- Gazeli, K.; Noël, C.; Clément, F.; Daugé, C.; Svarnas, P.; Belmonte, T. A Study of Helium Atmospheric-Pressure Guided Streamers for Potential Biological Applications. Plasma Sources Sci. Technol. 2013, 22, 025020. [Google Scholar] [CrossRef]
- Athanasopoulos, D.K.; Svarnas, P.; Liapis, C.M.; Papadopoulos, P.K.; Gazeli, K.; Giotis, K.; Vafeas, P.; Vafakos, G.P.; Giannakakis, V.; Gerakis, A. Combination of ICCD Fast Imaging and Image Processing Techniques to Probe Species–Specific Propagation Due to Guided Ionization Waves. Phys. Scr. 2023, 98, 055609. [Google Scholar] [CrossRef]
- Svarnas, P.; Matrali, S.H.; Gazeli, K.; Antimisiaris, S.G. Assessment of Atmospheric-Pressure Guided Streamer (Plasma Bullet) Influence on Liposomes with Different Composition and Physicochemical Properties. Plasma Process. Polym. 2015, 12, 655–665. [Google Scholar] [CrossRef]
- Svarnas, P.; Spiliopoulou, A.; Koutsoukos, P.; Gazeli, K.; Anastassiou, E. Acinetobacter Baumannii Deactivation by Means of DBD-Based Helium Plasma Jet. Plasma 2019, 2, 77–90. [Google Scholar] [CrossRef]
- Athanasopoulos, D.K.; Svarnas, P.; Gerakis, A. Cold Plasma Bullet Influence on the Water Contact Angle of Human Skin Surface. J. Electrostat. 2019, 102, 103378. [Google Scholar] [CrossRef]
- Girard, F.; Badets, V.; Blanc, S.; Gazeli, K.; Marlin, L.; Authier, L.; Svarnas, P.; Sojic, N.; Clément, F.; Arbault, S. Formation of Reactive Nitrogen Species Including Peroxynitrite in Physiological Buffer Exposed to Cold Atmospheric Plasma. RSC Adv. 2016, 6, 78457–78467. [Google Scholar] [CrossRef]
- Svarnas, P.; Poupouzas, M.; Papalexopoulou, K.; Kalaitzopoulou, E.; Skipitari, M.; Papadea, P.; Varemmenou, A.; Giannakopoulos, E.; Georgiou, C.D.; Georga, S.; et al. Water Modification by Cold Plasma Jet with Respect to Physical and Chemical Properties. Appl. Sci. 2022, 12, 11950. [Google Scholar] [CrossRef]
- Bolouki, N.; Hsieh, J.-H.; Li, C.; Yang, Y.-Z. Emission Spectroscopic Characterization of a Helium Atmospheric Pressure Plasma Jet with Various Mixtures of Argon Gas in the Presence and the Absence of De-Ionized Water as a Target. Plasma 2019, 2, 283–293. [Google Scholar] [CrossRef]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra, 2nd ed.; Chapman and Hall: London, UK, 1976. [Google Scholar]
- Kramida, A.; Ralchenko, Y.; Reader, J. NIST ASD Team. NIST Atomic Spectra Database; Version 5.11; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. Available online: https://physics.nist.gov/asd (accessed on 8 July 2024). [CrossRef]
- Wong, K.S.; Chew, N.S.L.; Low, M.; Tan, M.K. Plasma-ActivatedWater: Physicochemical Properties, Generation Techniques, and Applications. Processes 2023, 11, 2213. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Rapid Degradation, Mineralization and Detoxification of Pharmaceutically Active Compounds in Aqueous Solution during Pulsed Corona Discharge Treatment. Water Res. 2017, 121, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Elg, D.T.; Delgado, H.E.; Martin, D.C.; Sankaran, R.M.; Rumbach, P.; Bartels, D.M.; Go, D.B. Recent Advances in Understanding the Role of Solvated Electrons at the Plasma-Liquid Interface of Solution-Based Gas Discharges. Spectrochim. Acta Part B At. Spectrosc. 2021, 186, 106307. [Google Scholar] [CrossRef]
- Nippatlapalli, N.; Ramakrishnan, K.; Philip, L. Enhanced Degradation of Complex Organic Compounds in Wastewater Using Different Novel Continuous Flow Non—Thermal Pulsed Corona Plasma Discharge Reactors. Environ. Res. 2022, 203, 111807. [Google Scholar] [CrossRef] [PubMed]
- Gkika, C.; Petala, A.; Frontistis, Z.; Bampos, G.; Hela, D.; Konstantinou, I.; Mantzavinos, D. Heterogeneous Activation of Persulfate by Lanthanum Strontium Cobaltite for Sulfamethoxazole Degradation. Catal. Today 2021, 361, 130–138. [Google Scholar] [CrossRef]
- Alharbi, S.K.; Kang, J.; Nghiem, L.D.; van de Merwe, J.P.; Leusch, F.D.L.; Price, W.E. Photolysis and UV/H2O2 of Diclofenac, Sulfamethoxazole, Carbamazepine, and Trimethoprim: Identification of Their Major Degradation Products by ESI–LC–MS and Assessment of the Toxicity of Reaction Mixtures. Process Saf. Environ. Prot. 2017, 112, 222–234. [Google Scholar] [CrossRef]
- Reddy, P.M.K.; Subrahmanyam, C. Catalytic Plasma Reactor for Degradation and Mineralization of Pharmaceuticals and Personal Care Products. J. Adv. Oxid. Technol. 2015, 18, 161–166. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, X.; Ji, Y.; Wei, J. Sulfate Radical-Based Oxidation of the Antibiotics Sulfamethoxazole, Sulfisoxazole, Sulfathiazole, and Sulfamethizole: The Role of Five-Membered Heterocyclic Rings. Sci. Total Environ. 2019, 692, 201–208. [Google Scholar] [CrossRef]
- Deng, R.; He, Q.; Yang, D.; Dong, Q.; Wu, J.; Yang, X.; Chen, Y. Enhanced Synergistic Performance of Nano-Fe0-CeO2 Composites for the Degradation of Diclofenac in DBD Plasma. Chem. Eng. J. 2021, 406, 126884. [Google Scholar] [CrossRef]



| Structure | Formula | Molecular Weight (g/mol) | pKa | CAS |
|---|---|---|---|---|
Diclofenac (DCF)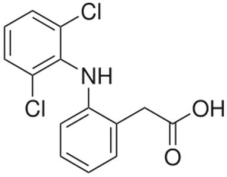 | C14H11C12NO2 | 296.2 | 4.1 | 15307-86-5 |
Sulfamethoxazole (SMX)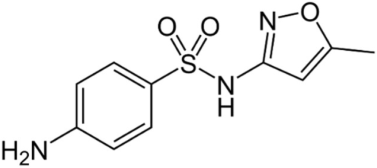 | C10H11N3O3S | 253.3 | 5.6–6.7 | 723-46-6 |
| Compound Name | Precursor Ion (m/z) | Product Ion (m/z) | Collision Energy (V) | Retention Time (min) | Repeatability (RSD %) |
|---|---|---|---|---|---|
| DCF | 296.00 | 214.20 (quantifier) | −12 | 9.192 | 5.55 |
| 249.90 | −31 | 9.191 | 6.07 | ||
| 156.07 | −15 | 6.071 | 4.87 | ||
| SMX | 254.06 | 108.04 | −23 | 6.074 | 6.19 |
| 91.90 (quantifier) | −27 | 6.073 | 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanteraki, A.; Isari, E.A.; Grilla, E.; Giotis, K.; Kalavrouziotis, I.; Svarnas, P. Pharmaceutically Active Compound (PhAC) Degradation by Means of Cold Plasma Jet Treatment. Plasma 2024, 7, 733-748. https://doi.org/10.3390/plasma7030038
Kanteraki A, Isari EA, Grilla E, Giotis K, Kalavrouziotis I, Svarnas P. Pharmaceutically Active Compound (PhAC) Degradation by Means of Cold Plasma Jet Treatment. Plasma. 2024; 7(3):733-748. https://doi.org/10.3390/plasma7030038
Chicago/Turabian StyleKanteraki, Alkistis, Ekavi Aikaterini Isari, Eleni Grilla, Konstantinos Giotis, Ioannis Kalavrouziotis, and Panagiotis Svarnas. 2024. "Pharmaceutically Active Compound (PhAC) Degradation by Means of Cold Plasma Jet Treatment" Plasma 7, no. 3: 733-748. https://doi.org/10.3390/plasma7030038
APA StyleKanteraki, A., Isari, E. A., Grilla, E., Giotis, K., Kalavrouziotis, I., & Svarnas, P. (2024). Pharmaceutically Active Compound (PhAC) Degradation by Means of Cold Plasma Jet Treatment. Plasma, 7(3), 733-748. https://doi.org/10.3390/plasma7030038










