Erbium Orthoniobate-Tantalates: Structural, Luminescent and Mechanical Properties of ErNbxTa1−xO4 Ceramics and Bactericidal Properties of ErNbO4 Powder
Abstract
1. Introduction
2. Methods
3. Results and Discussion
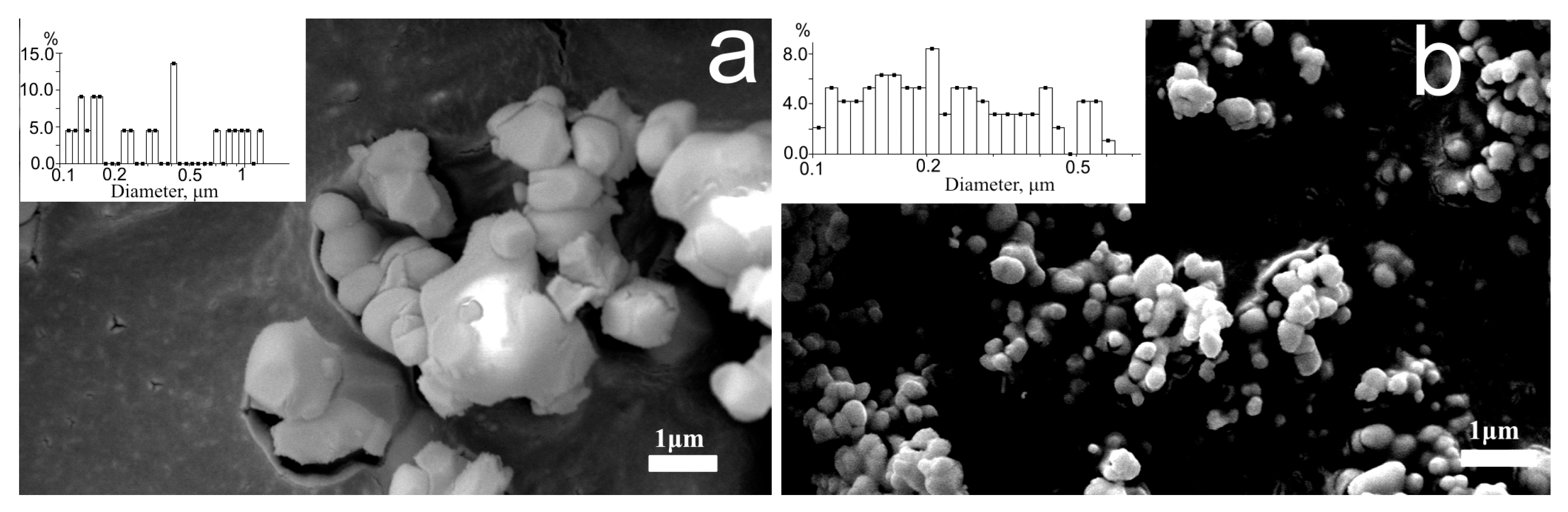
3.1. Dimensional Characteristics and Morphology of Powders
3.2. Structure of Ceramic Solid Solutions
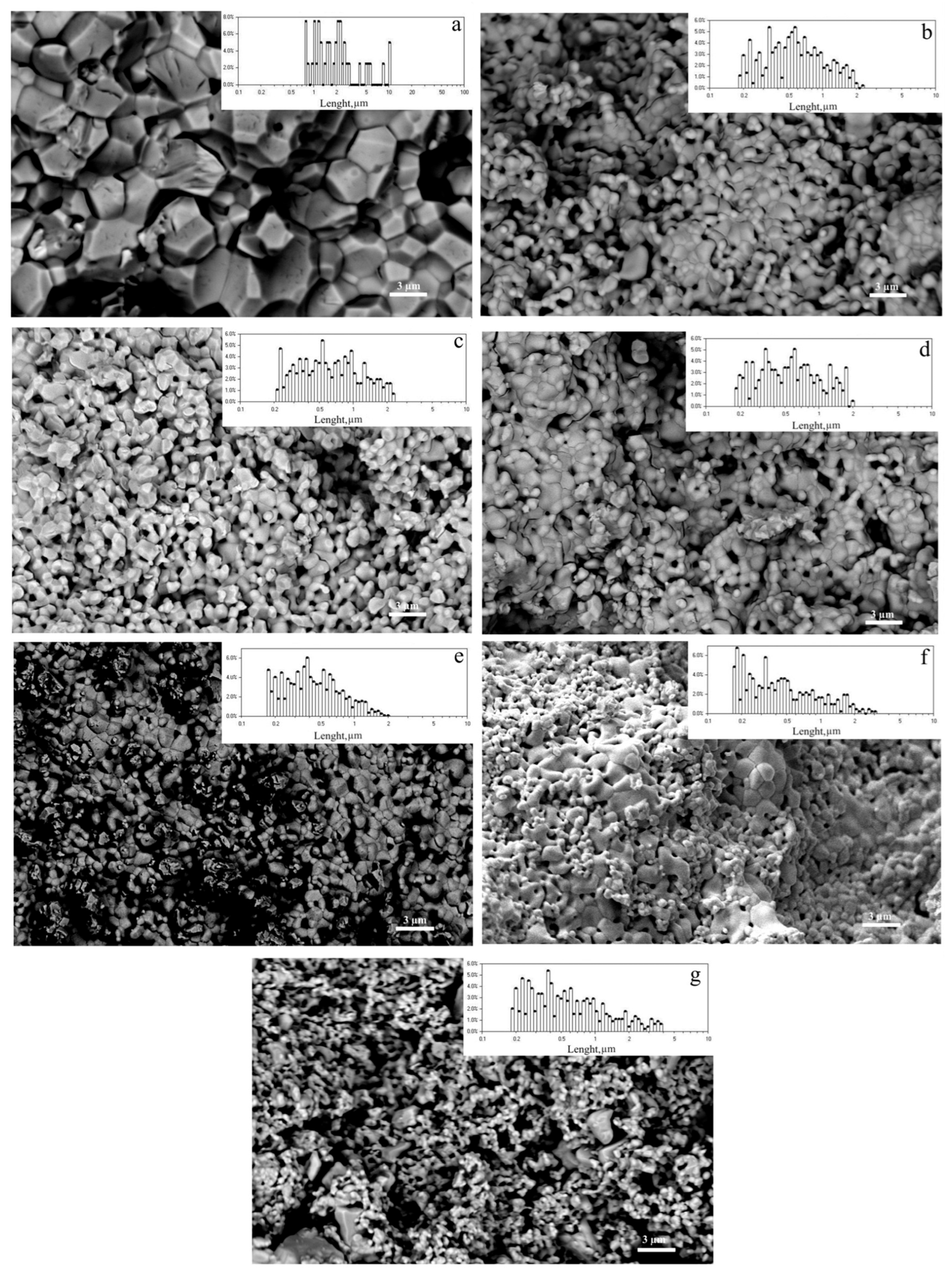
3.3. Morphological and Mechanical Characteristics of Ceramic Solid Solutions ErNbxTa1−xO4 (x = 0; 0.1; 0.3; 0.5; 0.7; 0.9; 1)
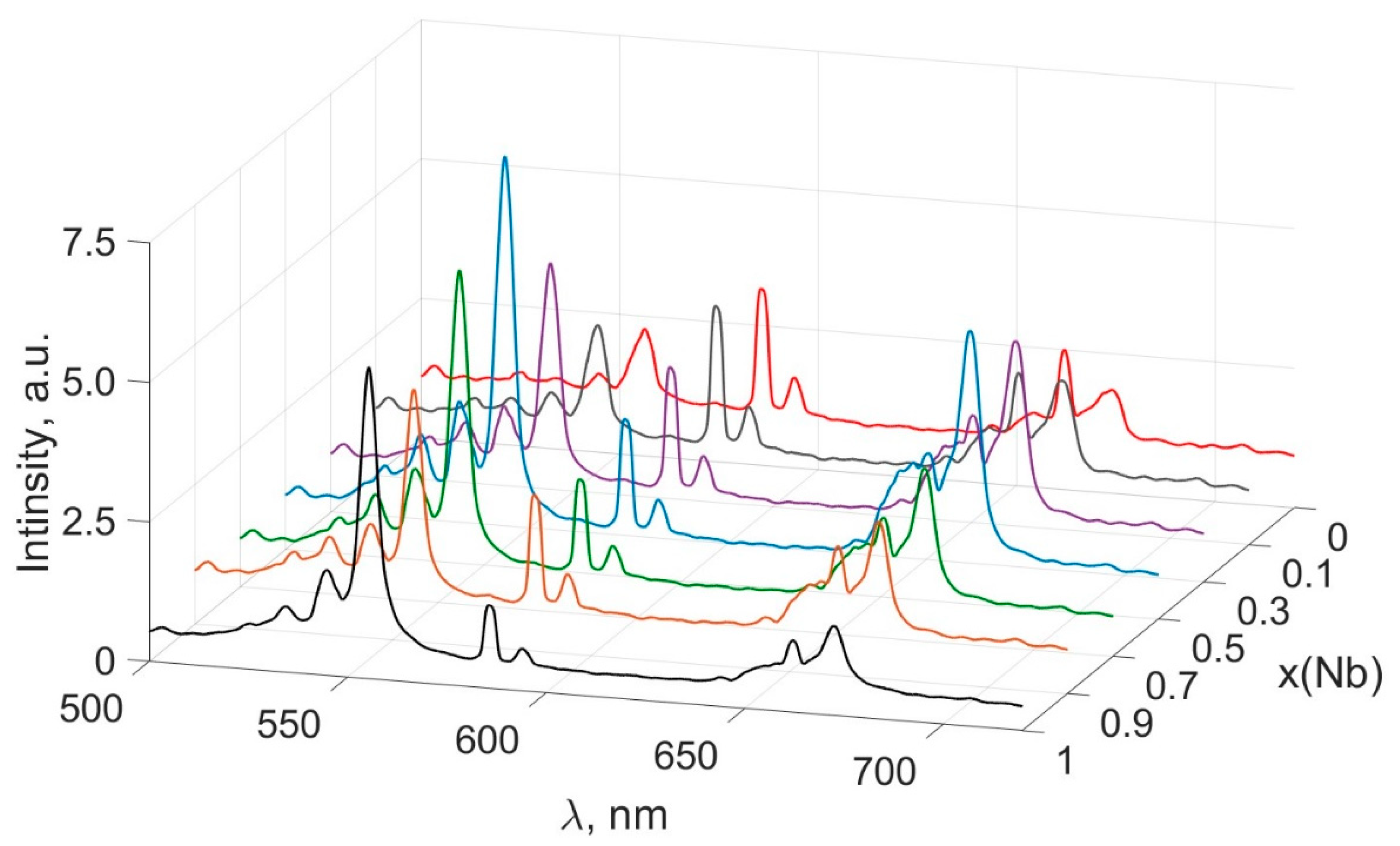
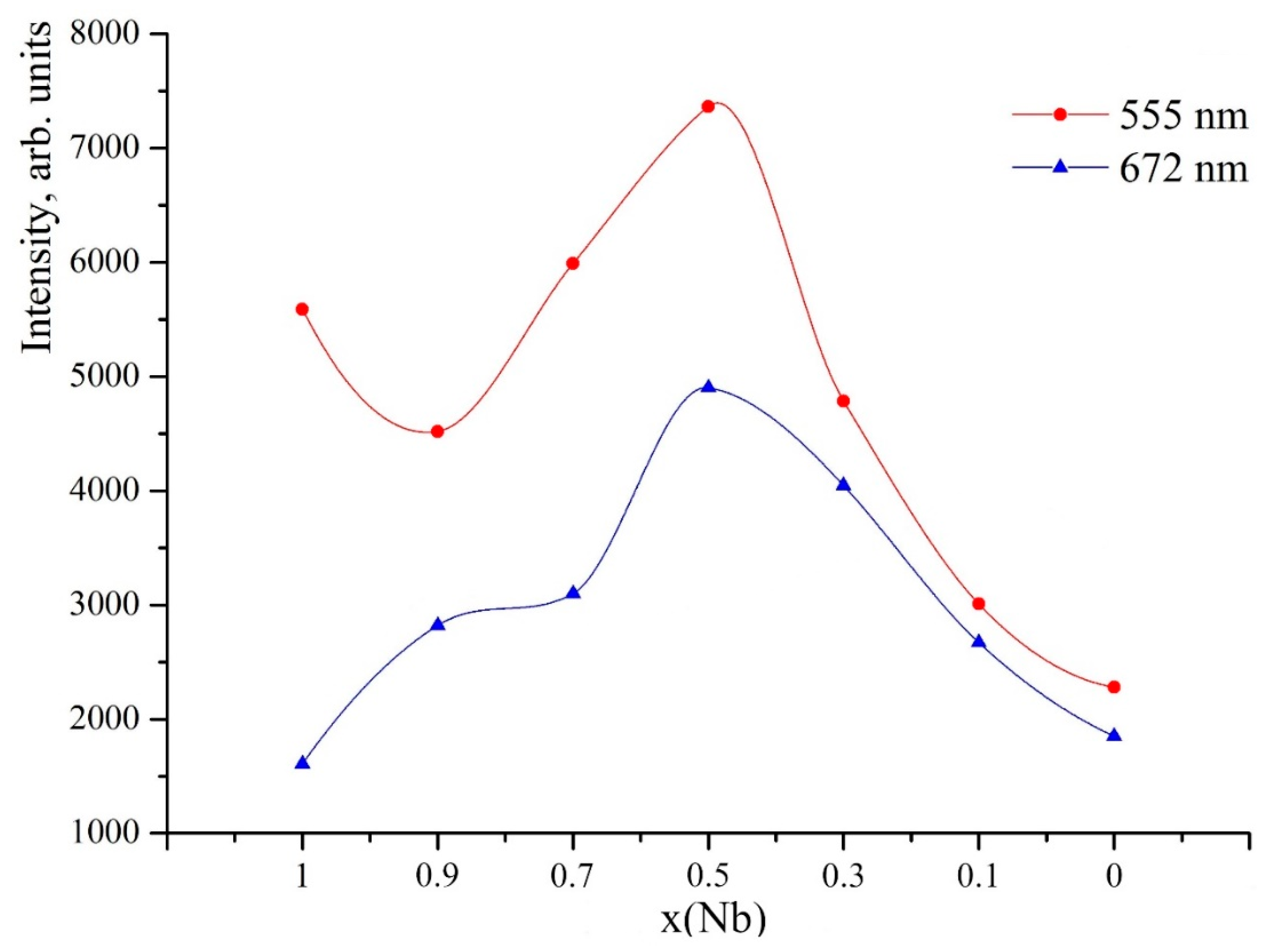
3.4. Study of PL of Ceramic Solid Solutions ErNbxTa1−xO4 (x = 0; 0.1; 0.3; 0.5; 0.7; 0.9; 1)
3.5. Bacteriological Properties of Powder ErNbO4
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ikesue, A. (Ed.) Solid-State Lighting. In Processing of Ceramics Breakthroughs in Optical Materials; Wiley: Hoboken, NJ, USA, 2021; pp. 187–274. [Google Scholar]
- Li, W.; Mi, W.; Chen, L.-J. Advances, challenges and prospects of visible fiber lasers in display technologies. Displays 2024, 82, 102630. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–174. [Google Scholar] [CrossRef]
- Zhang, F. General Introduction to Upconversion Luminescence Materials. In Photon Upconversion Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, P.; Wang, L.; Shi, Q.; Cui, C. Efect of Yb3+ concentration on upconversion luminescence and temperature sensing behavior in Yb3+/Er3+ codoped YNbO4 nanoparticles prepared via molten salt route. Chem. Eng. J. 2016, 297, 26–34. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhong, H.; Xu, S.; Cheng, L.; Sun, J.; Zhang, J.; Li, L.; Chen, B. Up-conversion luminescence, temperature sensing properties and laser-induced heating effect of Er3+/Yb3+co-doped YNbO4 phosphors under 1550 nm excitation. Sci. Rep. 2018, 8, 5736. [Google Scholar] [CrossRef] [PubMed]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Singh, S.; Kachhap, S.; Sharmaa, M.; Singh, S.K. Enhancing the temperature sensing property of a Ca0.79−xBixEr0.01Yb0.2MoO4 phosphor via local symmetry distortion and reduction in non-radiative channels. RSC Adv. 2023, 13, 14991–15000. [Google Scholar] [CrossRef]
- Thakur, H.; Singh, S.; Gathania, A.K.; Singh, S.K.; Singh, R.K. Effect of Li+/K+/Zn2+ doping on the optical and temperature sensing properties of Tm3+ and Yb3+ doped YVO4 phosphor. Ceram. Intern. 2023, 49, 25935–25944. [Google Scholar] [CrossRef]
- Haase, M.; Schafer, H. Upconverting nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, F. NIR luminescent nanomaterials for biomedical imaging. J. Mater. Chem. B 2014, 2, 2422–2443. [Google Scholar] [CrossRef]
- Wei, D.; Qi, J.; Hamblin, M.R.; Wen, X.; Jiang, X.; Yang, H. Near-infrared photoimmunotherapy: Design and potential applications for cancer treatment and beyond. Theranostics 2022, 12, 7108–7131. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infection Globally: Final Report and Reccommendations; Review on Antimicrobial Resistance; Wellcome Trust: London, UK, 2016; p. 80. [Google Scholar]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Tome, J.P.C.; Almeida, A. An insight on bacterial cellular targets of photodynamic inactivation. Future Med. Chem. 2014, 6, 141–164. [Google Scholar] [CrossRef]
- Almeida, A.; Faustino, M.A.F.; Tome, J.P.C. Photodynamic inactivation of bacteria: Finding the effective targets. Future Med. Chem. 2015, 7, 1221–1224. [Google Scholar] [CrossRef]
- Bonnett, R. Photosensitizers of the Porphyrin and Phthalocyanine Series for Photodynamic Therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Liu, J.J.; Zhang, L.C.; Du, X.J.; Cui, F.X.; Du, C.L.; Gao, B.X. PEG Encapsulation of Porphyrins for Cell Imaging and Photodynamic Therapy. Lett. Org. Chem. 2013, 10, 342–347. [Google Scholar] [CrossRef]
- Zhang, J.L.; Deng, L.; Yao, J.Z.; Gu, P.; Yang, F.; Wang, X.X.; Liu, W.; Zhang, Y.Y.; Ke, X.F.; Jing, X.L.; et al. Synthesis and photobiological study of a novel chlorin photosensitizer BCPD-18MA for photodynamic therapy. Bioorg. Med. Chem. 2011, 19, 5520–5528. [Google Scholar] [CrossRef] [PubMed]
- Noemie, T. Tissue distribution and pharmacokinetics of an ATWLPPR-conjugated chlorin-type photosensitizer targeting neuropilin-1 in glioma-bearing nude mice. Photochem. Photobiol. Sci. 2008, 7, 433–441. [Google Scholar] [CrossRef]
- Isakau, H.A. Toward understanding the high PDT efficacy of chlorin e6-polyvinylpyrrolidone formulations: Photophysical and molecular aspects of photosensitizer-polymer interaction in vitro. J. Photochem. Photobiol. B 2008, 92, 165–174. [Google Scholar] [CrossRef]
- Asano, R.; Nagami, A.; Fukumoto, Y.; Yazama, F.; Ito, H.; Sakata, I.; Tai, A. Synthesis and biological evaluation of new chlorin derivatives as potential photosensitizers for photodynamic therapy. Bioorg. Med. Chem. 2013, 21, 2298–2304. [Google Scholar] [CrossRef]
- Maslenikov, I.I.; Reshetov, V.N.; Useinov, A.S. Mapping the elastic modulus of a surface with a NanoScan 3D scanning microscope. Instrum. Exp. Tech. 2015, 58, 711. [Google Scholar] [CrossRef]
- Malygin, G.A. Plasticity and strength of micro- and nanocrystalline materials. Phys. Sol. State. 2007, 49, 1013–1033. [Google Scholar] [CrossRef]
- Anstis, G.R.; Chantikul, P.; Lawn, B.R.; Marshall, D.B. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: I, Direct Crack Measurements. J. Am. Ceram. Soc. 1981, 64, 533–538. [Google Scholar] [CrossRef]
- Chantikul, P.; Anstis, G.R.; Lawn, B.R.; Marshall, D.B. A Critical Evaluation of Indentation Techniques for Measuring Fracture Toughness: II, Strength Method. J. Am. Ceram. Soc. 1981, 64, 539–544. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Trunov, V.K.; Kinzhibalo, L.N.; Efremov, V.A.; Krongauz, V.G. On the M′-structure of “orthotantalates” of the rare-earth elements. Dokl. Akad. Nauk SSSR 1981, 260, 103–106. [Google Scholar]
- Sanderson, R.T. Chemical Bonds and Bond Energy. Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Voloshyna, O.V.; Boiaryntseva, I.A.; Baumer, V.N.; Ivanov, A.I.; Korjik, M.V.; Sidletskiy, O.T. New, dense, and fast scintillators based on rare-earth tantaloniobates. Nucl. Instrum. Meth. A 2014, 764, 227e231. [Google Scholar] [CrossRef]
- Xiao, W.; Yang, Y.; Pi, Z.; Zhang, F. Phase Stability and Mechanical Properties of the Monoclinic, Monoclinic-Prime and Tetragonal REMO4 (M = Ta, Nb) from First-Principles Calculations. Coatings 2022, 12, 73. [Google Scholar] [CrossRef]
- Wu, F.; Wu, P.; Zhou, Y.; Chong, X.; Feng, J. The thermo-mechanical properties and ferroelastic phase transition of RENbO4 (RE = Y, La, Nd, Sm, Gd, Dy, Yb) ceramics. J. Am. Ceram. Soc. 2020, 103, 2727–2740. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, J.; Zhang, P.; Meng, X.; Cao, S.; Wu, J.; Wei, M.; Shi, Y.; Reece, M.J.; Gao, F. Enhanced mechanical and thermal properties of ferroelastic high-entropy rare-earth-niobates. Scr. Mater. 2021, 200, 113912. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Guo, J.; Chong, X.; Feng, J. Mechanical and thermal properties of RETaO4 (RE = Yb, Lu, Sc) ceramics with monoclinic-prime phase. J. Mater. Sci. Technol. 2020, 52, 20–28. [Google Scholar] [CrossRef]
- Suzuki, K.; Kotani, T.; Sato, K. First-principles method justifying the Dieke diagram and beyond. Phys. Rev. Res. 2023, 5, 013111. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hou, Z.P.; Han, F.; Hua, P.R.; Yu, D.Y.; Pun, E.Y.B. Er3+ upconversion fluorescence of ErNbO4 phosphor for optical temperature sensing. IEEE Photon. Technol. Lett. 2014, 26, 1601–1604. [Google Scholar] [CrossRef]
- Zhang, D.L.; Hua, P.R.; Cui, Y.M.; Chen, C.H.; Pun, E.Y.B. Absorption and emission characteristics of Er3NbO7 phosphor: A comparison with ErNbO4 phosphor and Er:LiNbO3 single crystal. J. Lumin. 2007, 127, 453–460. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Cheng, L.; Xu, S.; Sun, J.; Zhang, J.; Zhang, X.; Yang, X.; Chen, B. Concentration-dependent spectroscopic properties and temperature sensing of YNbO4:Er3+ phosphors. RSC Adv. 2017, 7, 23751–23758. [Google Scholar] [CrossRef]
- Singh, S.; Kachhap, S.; Singh, A.K.; Pattnaik, S.; Singh, S.K. Temperature sensing using bulk and nanoparticles of Ca0.79Er0.01Yb0.2MoO4. Methods Appl. Fluoresc. 2022, 10, 044004. [Google Scholar] [CrossRef]
- Hirano, M.; Ishikawa, K. Hydrothermal formation and up-conversion luminescence of Er3+-doped GdNbO4. J. Am. Ceram. Soc. 2017, 100, 2814–2821. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Luminescence phenomena in compounds with fergusonite structure. J. Lumin. 1970, 3, 109–131. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Photoluminescent Efficiency of Phosphors with Electronic Transitions in Localized Centers. J. Electrochem. Soc. 1968, 115, 1067–1076. [Google Scholar] [CrossRef]
- Palatnikov, N.; Smirnov, M.V.; Masloboeva, S.M.; Shcherbina, O.B.; Sidorov, N.V.; Steblevskay, N.I.; Belobeletskaya, M.V. Luminescence properties of Sol–Gel Derived Ceramic GdNbxTa1−xO4 and YNbxTa1−xO4 Solid Solutions. Inorg. Mat. 2020, 56, 437–442. [Google Scholar] [CrossRef]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of gram-negative bacteria by photosensitized Porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef]
- Raetz, C.R.; Ulevitch, R.J.; Wright, S.D.; Sibley, C.H.; Ding, A.; Nathan, C.F. Gram-negative endotoxin an extraordinary lipid with profound effects on eukaryotic transduction. FASEB J. 1991, 5, 2652–2660. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Gasan, T. Photodynamic therapy: A new antimicrobical approach to infectious desease. Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Ergaieg, K.; Chevanne, M.; Cillard, J.; Seux, R. Involvement of both Type I and Type II mechanisms in Gram-positive and Gram-negative bacteria photosensitization by a meso-substituted cationic porphyrin. J. Sol. Energy 2008, 82, 1107–1117. [Google Scholar] [CrossRef]


| Lighting Type | Illumination in the Spectral Range from ~400 to 980 nm, Lux | Energy Illumination in the Spectral Range from 280 to 400 nm, mW/m2 |
|---|---|---|
| Daylight | 2617 ± 209 | 132 ± 13.2 |
| Sample | Analysis Results, wt% | Specific Surface Area, Ssp, m2/g | Average Particle Size of Powders dav, µm | ||
|---|---|---|---|---|---|
| Er | Nb | Ta | |||
| ErNbO4 | 50.40 | 29.62 | - | 1.04 | 0.78 |
| ErNb0.9Ta0.1O4 | 47.68 | 26.37 | 5.75 | 1.39 | 0.64 |
| ErNb0.7Ta0.3O4 | 46.22 | 19.40 | 15.89 | 1.28 | 0.56 |
| ErNb0.5Ta0.5O4 | 44.50 | 13.04 | 24.95 | 1.15 | 0.57 |
| ErNb0.3Ta0.7O4 | 41.65 | 7.53 | 34.07 | 1.54 | 0.42 |
| ErNb0.1Ta0.9O4 | 40.86 | 2.43 | 40.55 | 1.65 | 0.39 |
| ErTaO4 | 41.10 | - | 43.40 | 0.84 | 0.75 |
| Sample Composition | Phase in the Sample | a, Å | b, Å | c, Å | β, ° | V | Rwp, % | Rp, % |
|---|---|---|---|---|---|---|---|---|
| ErNbO4 | 04-006-1916 SPGR: C2/c (15) ErNbO4 | 7.02557(8) | 10.91922(14) | 5.06648(7) | 131.4512(6) | 291.315 | 7.49 | 5.67 |
| ErNb0.9 Ta0.1O4 | 04-006-1916 SPGR: C2/c (15) ErNbO4 | 7.0183(3) | 10.9163(4) | 5.0642(2) | 131.390(2) | 291.077 | 10.19 | 5.96 |
| ErNb0.7 Ta0.3O4 | 04-006-1916 SPGR: C2/c (15) ErNbO4 | 7.0040(3) | 10.9126(4) | 5.0592(2) | 131.1898(19) | 290.992 | 8.68 | 5.32 |
| ErNb0.5 Ta0.5O4 | 04-006-1916 SPGR: C2/c (15) ErNbO4 Phase weight 82% | 6.9911(2) | 10.9080(4) | 5.05474(19) | 131.0188(18) | 290.836 | 6.08 | 4.01 |
| 04-012-5293; ErTaO4 P2/a (13) Phase weight 18% | 5.3035(4) | 5.4538(4) | 5.1333(6) | 96.100(8) | 147.639 | 6.08 | 4.01 | |
| ErNb0.3 Ta0.7O4 | 04-012-5293, ErTaO4 P2/a (13) | 5.2996(3) | 5.4523(3) | 5.0550(3) | 95.276(2) | 145.446 | 10.00 | 6.72 |
| ErNb0.1 Ta0.9O4 | 04-012-5293, ErTaO4 P2/a (13) M | 5.28439(11) | 5.44368(11) | 5.09790(10) | 96.3053(8) | 145.762 | 4.63 | 3.35 |
| ErTaO4 | 04-012-5293, ErTaO4 P2/a (13) M | 5.2827(3) | 5.4402(3) | 5.0981(3) | 96.352(3) | 145.614 | 6.42 | 4.73 |
| SPGR | Distance | Nb Content, x | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 0.9 | 0.7 | 0.5 | 0.3 | 0.1 | 0 | ||
| C2/c (15), Z = 4 | Nb–O(1)a[O(1)b] | 1.76588 | 1.76454 | 1.76171 | 1.75912 | |||
| Nb–O(2)a[O(2)b] | 2.42890 | 2.42703 | 2.42326 | 2.41979 | ||||
| Nb–O(2)c[O(2)d] | 1.82574 | 1.82646 | 1.82990 | 1.83271 | ||||
| P2/a (13), Z = 2 | Ta–O(1)a[O(1)b] | 1.92793 | 1.92689 | 1.91520 | 1.91407 | |||
| Ta–O(1)a[O(1)b] | 2.48018 | 2.45572 | 2.47252 | 2.47237 | ||||
| Ta–O(2)c[O(2)d] | 1.99687 | 1.97556 | 1.98987 | 1.98973 | ||||
| Sample Composition | Microhardness, H, GPa | Young’s Modulus, E, GPa | Crack Resistance, KIC, MPa m0.5 |
|---|---|---|---|
| ErNbO4 | 6.11 ± 0.86 | 297.6 ± 10.8 | 1.44 ± 0.2 |
| ErNb0.9Ta0.1O4 | 2.86 ± 0.31 | 84.8 ± 1.8 | 0.7 ± 0.1 |
| ErNb0.7Ta0.3O4 | 5.68 ± 1.0 | 162.4 ± 6.9 | 1.20 ± 0.1 |
| ErNb0.5Ta0.5O4 | 3.82 ± 0.33 | 137.7 ± 4.3 | 1.10 ± 0.1 |
| ErNb0.3Ta0.7O4 | 5.12 ± 1.1 | 223.1 ± 5.9 | 1.16 ± 0.15 |
| ErNb0.1Ta0.9O4 | 3.4 ± 0.361 | 98.8 ± 2.7 | 0.94 ± 0.1 |
| ErTaO4 | 4.2 ± 0.9 | 139.3 ± 6.8 | 1.06 ± 0.1 |
| Sample | Bacterial Cell Type | Number of Bacterial Cells | |||||
|---|---|---|---|---|---|---|---|
| Initial | in 4 h | in 24 h | |||||
| ×104 cells/mL | % | ×104 cells/mL | % | ×104 cells/mL | % | ||
| 1 | Escherichia coli | 6 ± 0.4 | 100 | 1.2 ± 0.5 | 20.3 | 1.2 ± 0.018 | 20.6 |
| 2 | Bacillus brevis | 6 ± 0.4 | 100 | 1.5 ± 0.19 | 25.0 | 1 ± 0.2 | 16.94 |
| 3 | Micrococcus sp. | 6 ± 0.4 | 100 | 1.25 ± 0.75 | 20.8 | 0.7 ± 0.01 | 11.67 |
| Bacterial Cell Type | Experimental Conditions | Number of Bacterial Cells | |||||
|---|---|---|---|---|---|---|---|
| Initial | in 4 h | in 24 h | |||||
| ×105 cells/mL | % | ×105 cells/mL | % | ×105 cells/mL | % | ||
| Micrococcus sp. | light | 1.41 ± 0.5 | 100 | 0.29 ± 0.18 | 20.6 | 0.16 ± 0.02 | 11.35 |
| dark | 1.41 ± 0.5 | 100 | 0.7 ± 0.3 | 49.65 | 0.7 ± 0.01 | 49.65 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palatnikov, M.; Shcherbina, O.; Fokina, N.; Smirnov, M.; Zelenina, E.; Masloboeva, S.; Manukovskaya, D. Erbium Orthoniobate-Tantalates: Structural, Luminescent and Mechanical Properties of ErNbxTa1−xO4 Ceramics and Bactericidal Properties of ErNbO4 Powder. Ceramics 2025, 8, 130. https://doi.org/10.3390/ceramics8040130
Palatnikov M, Shcherbina O, Fokina N, Smirnov M, Zelenina E, Masloboeva S, Manukovskaya D. Erbium Orthoniobate-Tantalates: Structural, Luminescent and Mechanical Properties of ErNbxTa1−xO4 Ceramics and Bactericidal Properties of ErNbO4 Powder. Ceramics. 2025; 8(4):130. https://doi.org/10.3390/ceramics8040130
Chicago/Turabian StylePalatnikov, Mikhail, Olga Shcherbina, Nadezhda Fokina, Maxim Smirnov, Elena Zelenina, Sofja Masloboeva, and Diana Manukovskaya. 2025. "Erbium Orthoniobate-Tantalates: Structural, Luminescent and Mechanical Properties of ErNbxTa1−xO4 Ceramics and Bactericidal Properties of ErNbO4 Powder" Ceramics 8, no. 4: 130. https://doi.org/10.3390/ceramics8040130
APA StylePalatnikov, M., Shcherbina, O., Fokina, N., Smirnov, M., Zelenina, E., Masloboeva, S., & Manukovskaya, D. (2025). Erbium Orthoniobate-Tantalates: Structural, Luminescent and Mechanical Properties of ErNbxTa1−xO4 Ceramics and Bactericidal Properties of ErNbO4 Powder. Ceramics, 8(4), 130. https://doi.org/10.3390/ceramics8040130










