Hot Modification of Silicomanganese Slag in Its Crystallization and Viscosity Properties for Preparation of Cast Stone
Abstract
1. Introduction
2. Materials and Experiments
2.1. Raw Materials
2.2. Sample Preparation
2.3. Experimental Methods
2.3.1. XRF and XRD Measurement
2.3.2. Non-Isothermal DSC Measurement
2.3.3. Viscosity Measurement
2.3.4. FTIR and XPS Spectra Measurement
2.3.5. Physical Properties Test of Cast Stone
3. Results and Discussion
3.1. Mechanical Properties of Cast Stone
3.2. Analysis of Crystallization Performance of Silicomanganese Slag
3.2.1. Crystallization Temperature
3.2.2. Crystallization Activation Energy
3.2.3. Crystalline Phases
3.3. Analysis of Fluidity Performance of Silicomanganese Slag
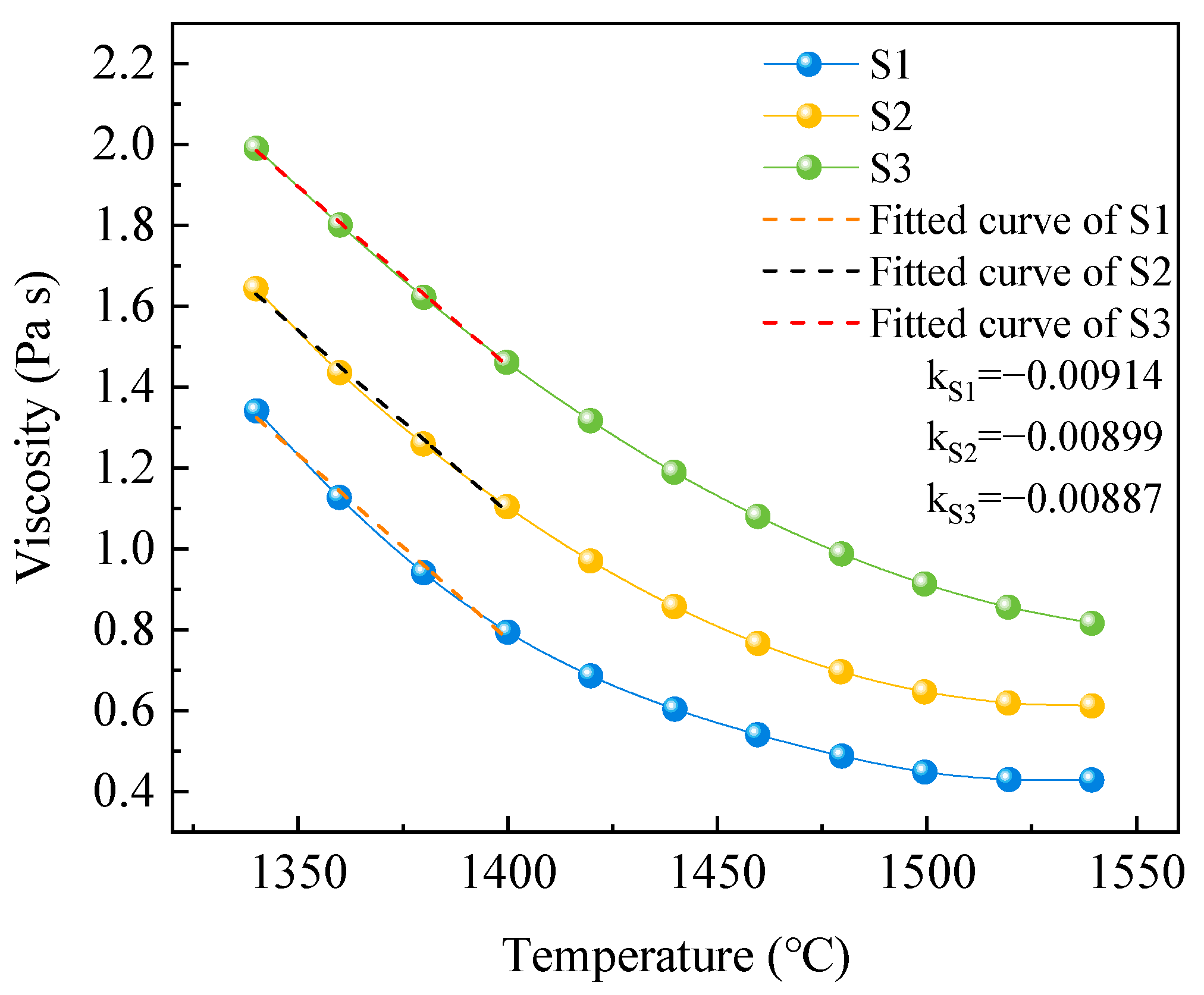
3.3.1. Viscosity of Silicomanganese Slag
3.3.2. Activation Energy for Viscous Flow of Silicomanganese Slag
3.4. The Relationship Between Performance and Structure of Cast Stone
3.4.1. FTIR Spectra Analysis
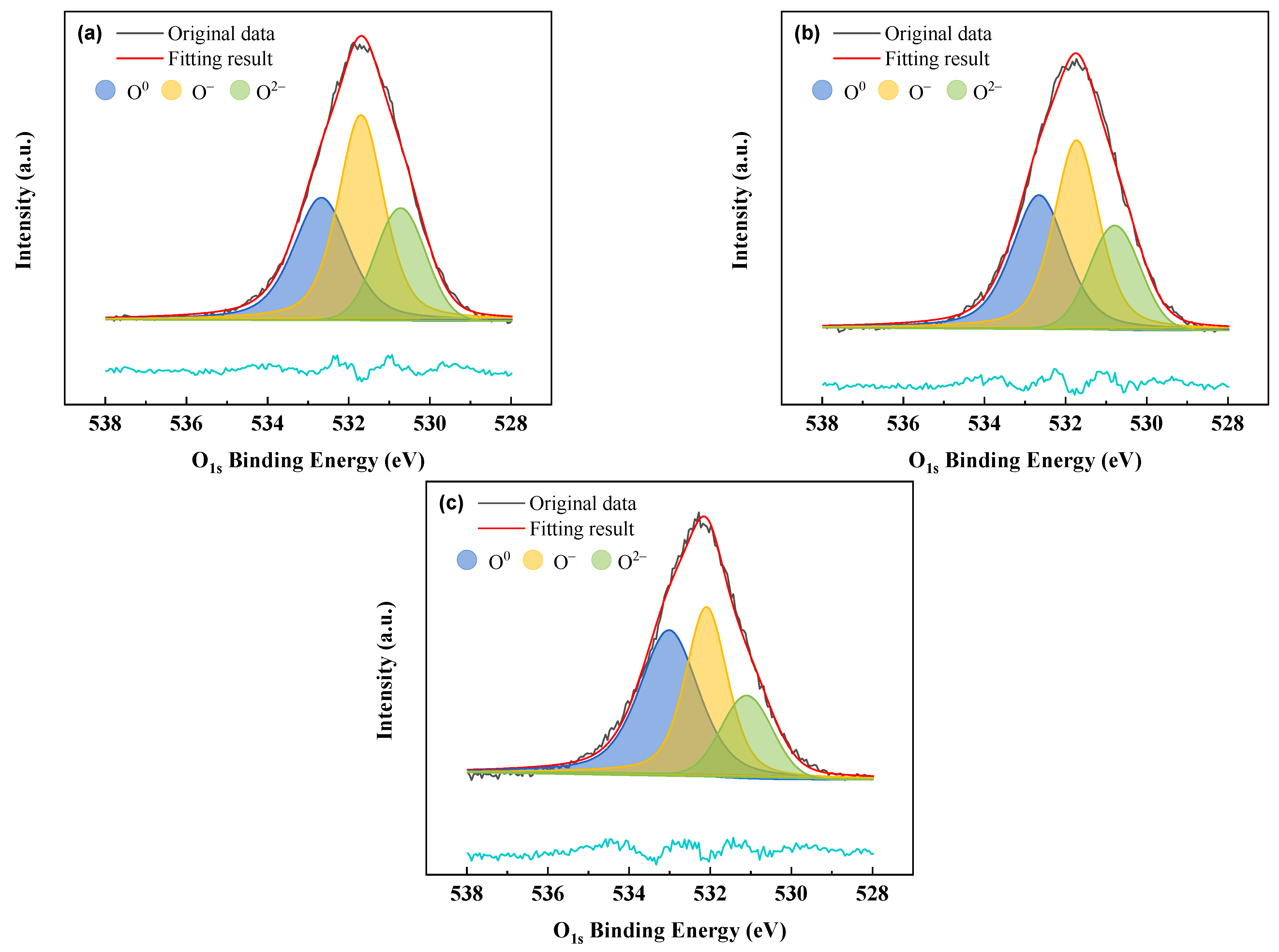
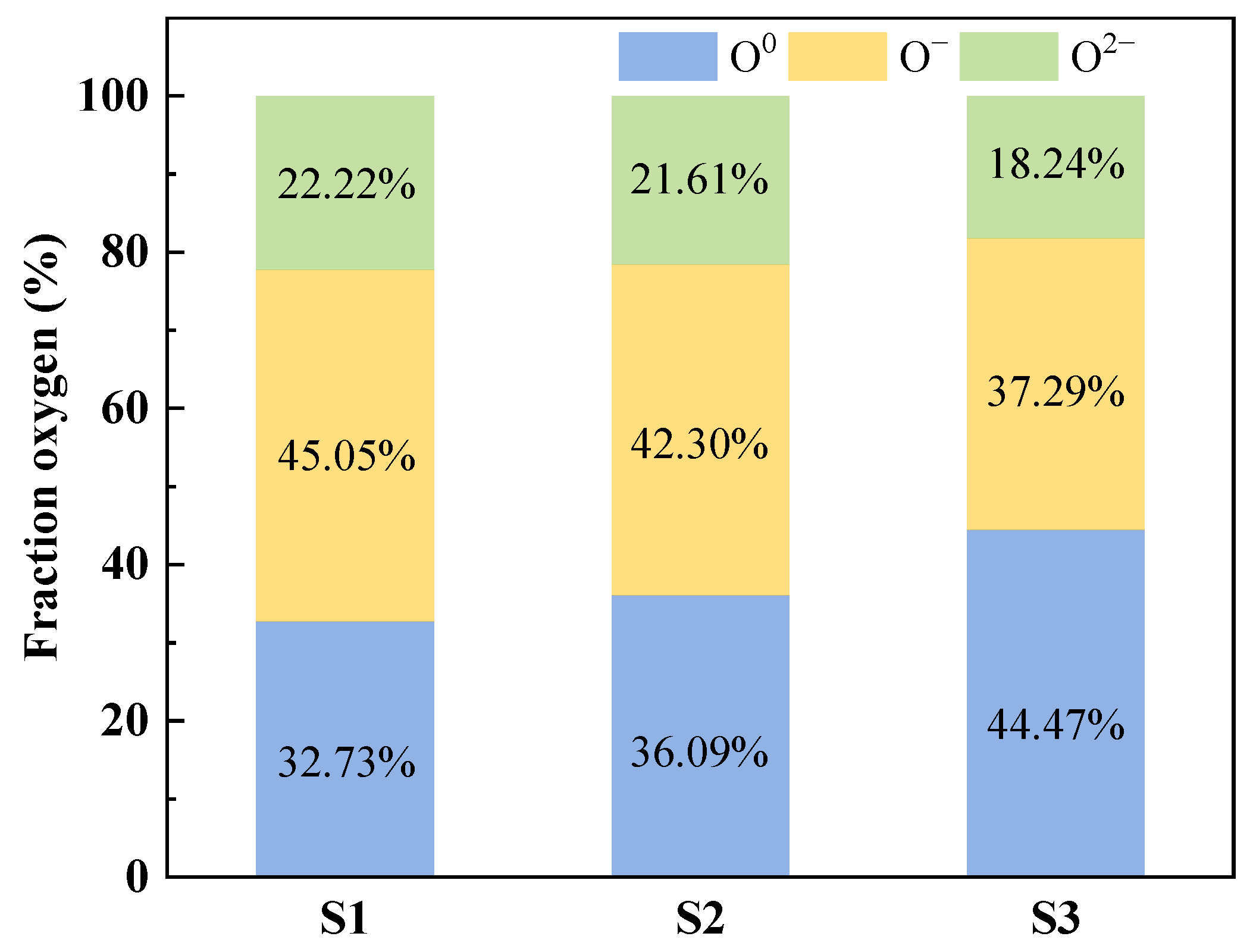
3.4.2. XPS Spectra Analysis
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Wang, H.; Zhu, X.; Qiu, Y.J.; Li, K.; Chen, R.; Liao, Q. A review of waste heat recovery technologies towards molten slag in steel industry. Appl. Energy 2013, 112, 956–966. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Barati, M.; Jahanshahi, S. Granulation and heat recovery from metallurgical slags. J. Sustain. Metall. 2020, 6, 191–206. [Google Scholar] [CrossRef]
- Li, Y.; Dai, W.B. Modifying hot slag and converting it into value-added materials: A review. J. Clean. Prod. 2018, 175, 176–189. [Google Scholar] [CrossRef]
- Cang, D.Q.; Zhang, L.L.; Liu, Y.; Chen, Z.H.; He, B.Y. Secondary resources utilization, problems and countermeasures of the domestic and oversea steel industry. Chin. J. Process Eng. 2022, 22, 1418–1424. [Google Scholar]
- Yang, J.; Li, Y.; Yang, T. Comparative of different modifiers on the crystallization properties of glass–ceramics derived from silicon manganese slag. Chin. J. Eng. 2023, 45, 1918–1927. [Google Scholar]
- Luiza, F.d.L.; Cláudio, A.P.; Janete, E.Z.; Robinson, C.D.C. Effect of iron on the microstructure of basalt glass-ceramics obtained by the petrurgic method. Int. J. Appl. Ceram. 2021, 18, 1950–1959. [Google Scholar]
- Choi, M.; Jung, S. Elucidation of the crystallisation mechanism of iron oxide-devoid BOF slag melt. Ironmak. Steelmak. 2018, 45, 441–446. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y.; Huang, Y. Effects of different crystallization temperatures on temperature difference, crystallization and physical properties of silica manganese slag cast stone. Chin. J. Eng. 2024, 46, 1796–4786. [Google Scholar]
- Chen, K.; Li, Y.; Meng, X.; Meng, L.; Guo, Z. New integrated method to recover the TiO2 component and prepare glass-ceramics from molten itanium-bearing blast furnace slag. Ceram. Int. 2019, 45, 24236–24243. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Chen, K.; Guo, Z. Effects of high gravity on dissolution of solid modifiers in molten slag. Ceram. Int. 2022, 48, 14360–14370. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Meng, L.; Yi, Y.; Guo, Z. Preparation of Glass-Ceramic from Titanium-Bearing Blast Furnace Slag by “Petrurgic” Method. In Energy Technology 2018; Sun, Z., Wang, C., Guillen, D.P., Neelameggham, N.R., Zhang, L., Howarter, J.A., Wang, T., Olivetti, E., Zhang, M., Verhulst, D., et al., Eds.; The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Luo, Y.; Wang, F.; Zhu, H.; Liao, Q.; Xu, Y.; Liu, L. Preparation and characterization of glass-ceramics with granite tailings and titanium-bearing blast furnace slags. J. Non-Cryst. Solids 2022, 582, 121463. [Google Scholar] [CrossRef]
- Dai, W.; Li, Y.; Cang, D. Effects of heat treatment process on the microstructure and properties of BOF slag based glass-ceramics. Chin. J. Eng. 2013, 35, 1507–1512. [Google Scholar]
- Yang, T.; Li, Y.; Zhao, X.; Yang, J. Preparation of densified glass-ceramics with less shrinkage cavities from molten copper slag by Petrurgic method. Ceram. Int. 2023, 49, 20998–21007. [Google Scholar] [CrossRef]
- Liu, X.; Li, B.; Wu, Y. The pretreatment of non-ferrous metallurgical waste slag and its research progress in the preparation of glass-ceramics. J. Clean. Prod. 2023, 404, 136930. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass–ceramics: Their production from wastes—A review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Guo, Y.; Du, Y.; Wang, G.; Xu, Y.; Zhang, H.; Deng, L.; Chen, H.; Zhao, M. The direct experimental observation and solidification mechanism of heavy metals in blast furnace slag glass-ceramics. Int. J. Appl. Ceram. 2024, 21, 796–805. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Fang, J. Effect of crystallization temperature on glass-ceramics derived from tailings waste. J. Alloys Compd. 2020, 838, 155503. [Google Scholar] [CrossRef]
- Liu, W.; Pang, Z.; Zheng, J.; Yin, S.; Zuo, H. The viscosity, crystallization behavior and glass-ceramics preparation of blast furnace slag: A review. Ceram. Int. 2024, 50, 18090–18104. [Google Scholar] [CrossRef]
- Deng, L.; Jia, R.; Yun, F.; Zhang, X.; Li, H.; Zhang, M.; Jia, X.; Ren, D.; Li, B. Influence of Cr2O3 on the viscosity and crystallization behavior of glass ceramics based on blast furnace slag. Mater. Chem. Phys. 2020, 240, 122212. [Google Scholar] [CrossRef]
- Cota, T.G.; Reis, E.L.; Lima, R.M.F.; Cipriano, R.A.S. Incorporation of waste from ferromanganese alloy manufacture and soapstone powder in red ceramic production. Appl. Clay Sci. 2018, 161, 274–281. [Google Scholar] [CrossRef]
- Buruiana, D.L.; Obreja, C.D.; Herbei, E.E.; Ghisman, V. Re-Use of Silico-Manganese Slag. Sustainability 2021, 13, 11771. [Google Scholar] [CrossRef]
- Jin, M.F.; He, F.; Liu, X.Q.; Yang, H.; Xiao, Y.L.; Xie, M.Q.; Xie, J.L. Study on high temperature melt properties and modulation of blast furnace slag. J. Wuhan Univ. Technol. 2023, 45, 38–43. [Google Scholar]
- Wang, Y.C.; Huo, X.G.; Luo, G.P.; Wang, Y.B.; Zhang, F. Influence of Cr2O3 and Fe2O3 on viscidity and melting properties of blast furnace slag. Bull. Chin. Ceram. Soc. 2017, 36, 701–705. [Google Scholar]
- GB/T 18601-2009; Natural Granite Building Slab. General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2009.
- GB/T 4100-2015; Ceramic Tiles. General Administration of Quality Supervision, Inspection, and Quarantine of the People’s Republic of China, Standardization Administration of China: Beijing, China, 2015.
- Shu, Q.; Li, Q.; Medeiros, S.L.S.; Klug, J.L. Development of non-reactive F-free mold fluxes for high aluminum steels: Non-isothermal crystallization kinetics for devitrification. Metall. Mater. Trans. B 2020, 51, 1169–1180. [Google Scholar] [CrossRef]
- Shi, C.; Li, J.; Cho, J.; Jiang, F.; Jung, I. Effect of SiO2 on the crystallization behaviors and in-mold performance of CaF2-CaO-Al2O3 slags for drawing-ingot-type electroslag remelting. Metall. Mater. Trans. B 2015, 46, 2110–2120. [Google Scholar] [CrossRef]
- Shi, C.B.; Cho, J.; Zheng, D.L.; Li, J. Fluoride evaporation and crystallization behavior of CaF2-CaO-Al2O3-(TiO2) slag for electroslag remelting of Ti-containing steels. Int. J. Miner. Metall. Mater. 2016, 23, 627–636. [Google Scholar] [CrossRef]
- Zheng, D.L.; Shi, C.B.; Li, J.; Ju, J. Crystallization kinetics and structure of CaF2–CaO–Al2O3–MgO–TiO2 slag for electroslag remelting. ISIJ Int. 2020, 60, 492–498. [Google Scholar] [CrossRef]
- Shi, C.B.; Seo, M.; Wang, H.; Cho, J.; Kim, S. Crystallization kinetics and mechanism of CaO–Al2O3-based mold flux for casting high-aluminum TRIP steels. Metall. Mater. Trans. B 2015, 46, 345–356. [Google Scholar] [CrossRef]
- Seo, M.; Shi, C.B.; Baek, J.; Cho, J.; Kim, S. Kinetics of isothermal melt crystallization in CaO-SiO2-CaF2-based mold fluxes. Metall. Mater. Trans. B 2015, 46, 2374–2383. [Google Scholar] [CrossRef]
- Bai, Z.; Qiu, G.; Yue, C.; Guo, M.; Zhang, M. Crystallization kinetics of glass-ceramics prepared from high-carbon ferrochromium slag. Ceram. Int. 2016, 42, 19329–19335. [Google Scholar] [CrossRef]
- Sycheva, G.A.; Polyakova, I.G.; Kostyreva, T.G. Volumetric nucleation of crystals catalyzed by Cr2O3 in glass based on furnace slags. Glass. Phys. Chem. 2016, 42, 238–245. [Google Scholar] [CrossRef]
- Shelestak, L.J.; Chavez, R.A.; Mackenzie, J.D. Glasses and glass-ceramics from naturally occurring CaO-MgO-Al2O3-SiO2 materials (I) glass formation and properties. J. Non-Cryst. Solids 1978, 27, 75–81. [Google Scholar] [CrossRef]
- Shi, C.B.; Shin, S.; Zheng, D.L.; Cho, J.; Li, J. Development of low-fluoride slag for electroslag remelting: Role of Li2O on the viscosity and structure of the slag. Metall. Mater. Trans. B 2016, 47, 3343–3349. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Cai, D.; Zhang, J.; Sasaki, Y.; Ostrovski, O. Effects of CaO/SiO2 ratio and Na2O content on melting properties and viscosity of SiO2-CaO-Al2O3-B2O3-Na2O mold fluxes. Metall. Mater. Trans. B 2017, 8, 516–526. [Google Scholar] [CrossRef]
- Kim, J.; Sohn, I. Effect of SiO2/Al2O3 and TiO2/SiO2 ratios on the viscosity and structure of the TiO2–MnO–SiO2–Al2O3 welding flux system. ISIJ Int. 2014, 54, 2050–2058. [Google Scholar] [CrossRef]
- Yu, X.; Wen, G.; Tang, P.; Wang, H. Investigation on viscosity of mould fluxes during continuous casting of aluminium containing TRIP steels. Ironmak. Steelmak. 2009, 36, 623–630. [Google Scholar] [CrossRef]
- Zheng, H.W. Effect of Doping on the Structure and Properties of Li2O-Al2O3-SiO2 System. Ph.D. Thesis, Wuhan University of Technology, Wuhan, China, 2007. [Google Scholar]
- Kim, G.H.; Sohn, I. Effect of Al2O3 on the viscosity and structure of calcium silicate-based melts containing Na2O and CaF2. J. Non-Cryst. Solids 2012, 358, 1530–1537. [Google Scholar] [CrossRef]
- Rezvani, M.; Farahinia, L. Structure and optical band gap study of transparent oxyfluoride glass-ceramics containing CaF2 nanocrystals. Mater. Des. 2015, 88, 252–257. [Google Scholar] [CrossRef]
- Shen, Y.Y.; Liu, X.Y.; Chen, S.J.; Wang, Y.Y.; Yan, W.; Li, J. Unveiling the effect of MnO/SiO2 ratios on the viscosity and structure of mold fluxes for high-Mn cryogenic steels. Ceram. Int. 2023, 49, 29308–29316. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, C.S.; Sohn, I. Viscous behavior of alumina rich calcium-silicate based mold fluxes and its correlation to the melt structure. ISIJ Int. 2013, 53, 170–176. [Google Scholar] [CrossRef]
- Liao, J.; Zhang, Y.; Sridhar, S.; Wang, X.; Zhang, Z. Effect of Al2O3/SiO2 ratio on the viscosity and structure of slags. ISIJ Int. 2012, 52, 753–758. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, Q.; Sridhar, S.; Zhang, M.; Guo, M.; Zhang, Z. Effect of P2O5 and FetO on the viscosity and slag structure in steelmaking slags. Metall. Mater. Trans. B 2015, 46, 758–765. [Google Scholar] [CrossRef]
- Kim, J.R.; Lee, Y.S.; Min, D.J.; Jung, S.M.; Yi, S.H. Influence of MgO and Al2O3 contents on viscosity of blast furnace type slags containing FeO. ISIJ Int. 2004, 44, 1291–1297. [Google Scholar] [CrossRef]
- Ko, K.Y.; Park, J.H. Effect of CaF2 addition on the viscosity and structure of CaO-SiO2-MnO slags. ISIJ Int. 2013, 53, 958–965. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Bancroft, G.M.; Henderson, G.S.; Ho, R.; Dalby, K.N.; Huang, Y.; Yan, Z. Bridging, non-bridging and free (O2−) oxygen in Na2O-SiO2 glasses: An X-ray Photoelectron Spectroscopic (XPS) and Nuclear Magnetic Resonance (NMR) study. J. Non-Cryst. Solids 2011, 357, 170–180. [Google Scholar] [CrossRef]
- Hayashi, M.; Nabeshima, N.; Fukuyama, H.; Nagata, K. Effect of fluorine on silicate network for CaO-CaF2-SiO2 and CaO-CaF2-SiO2-FeOx glasses. ISIJ Int. 2002, 42, 352–358. [Google Scholar] [CrossRef]
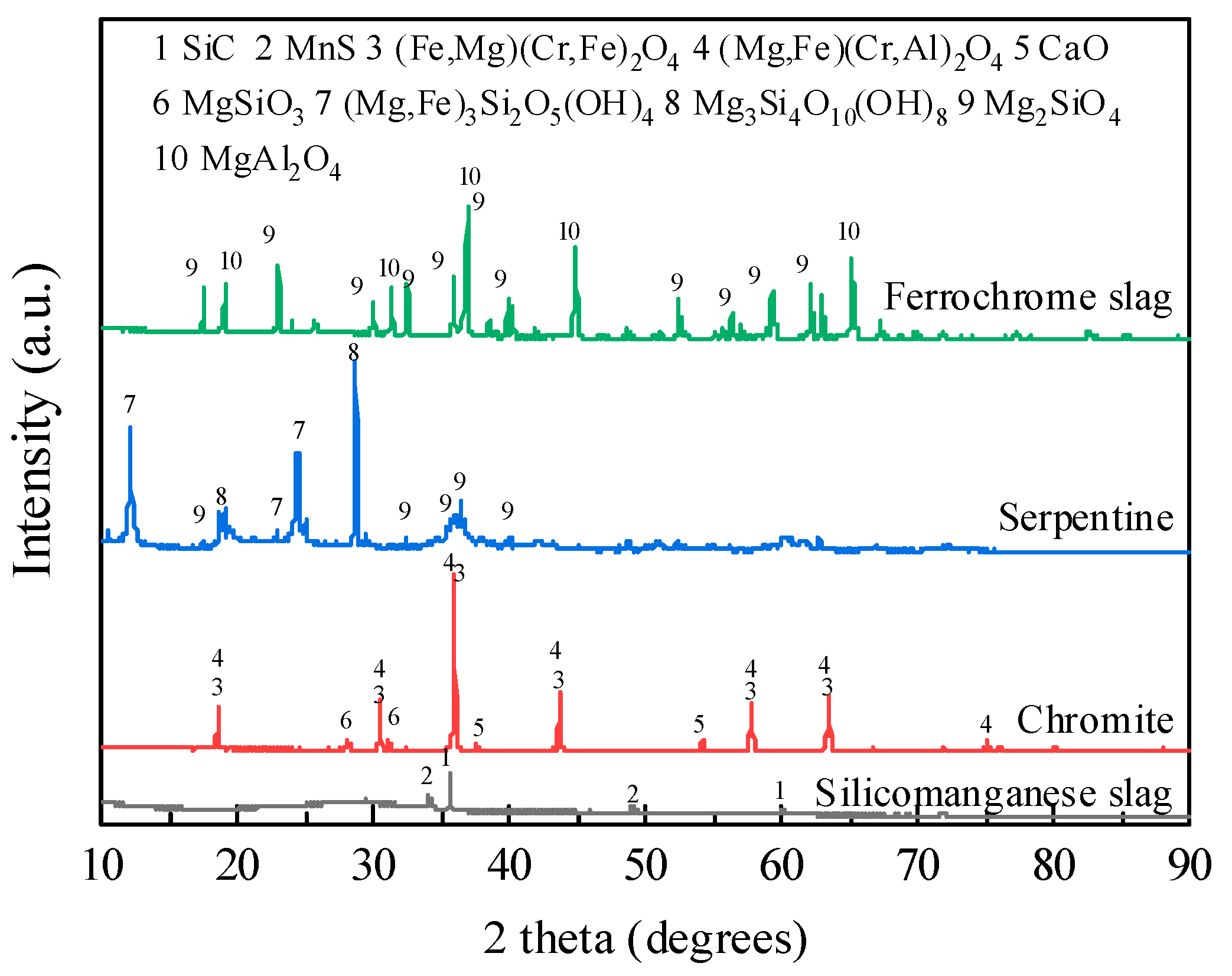
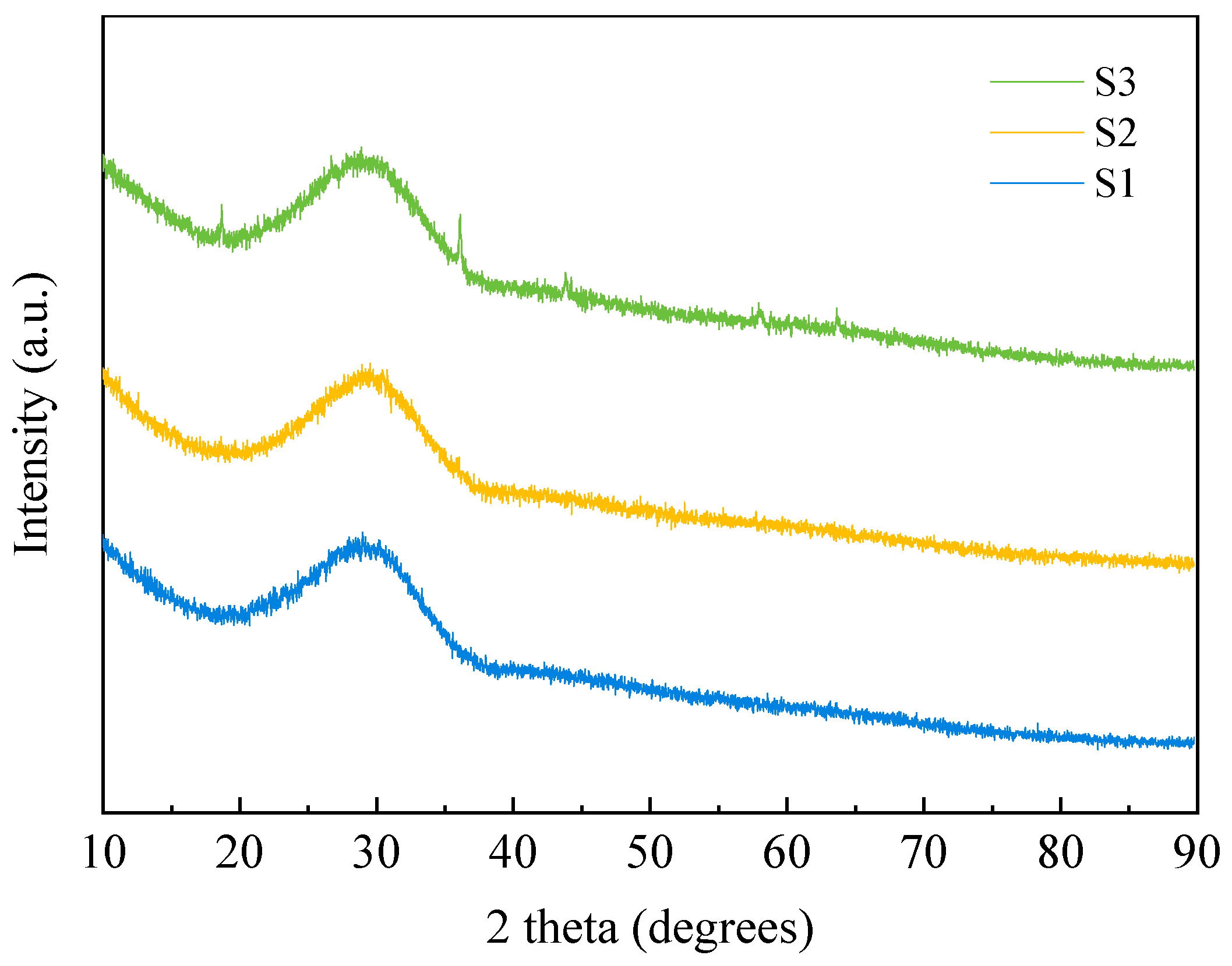
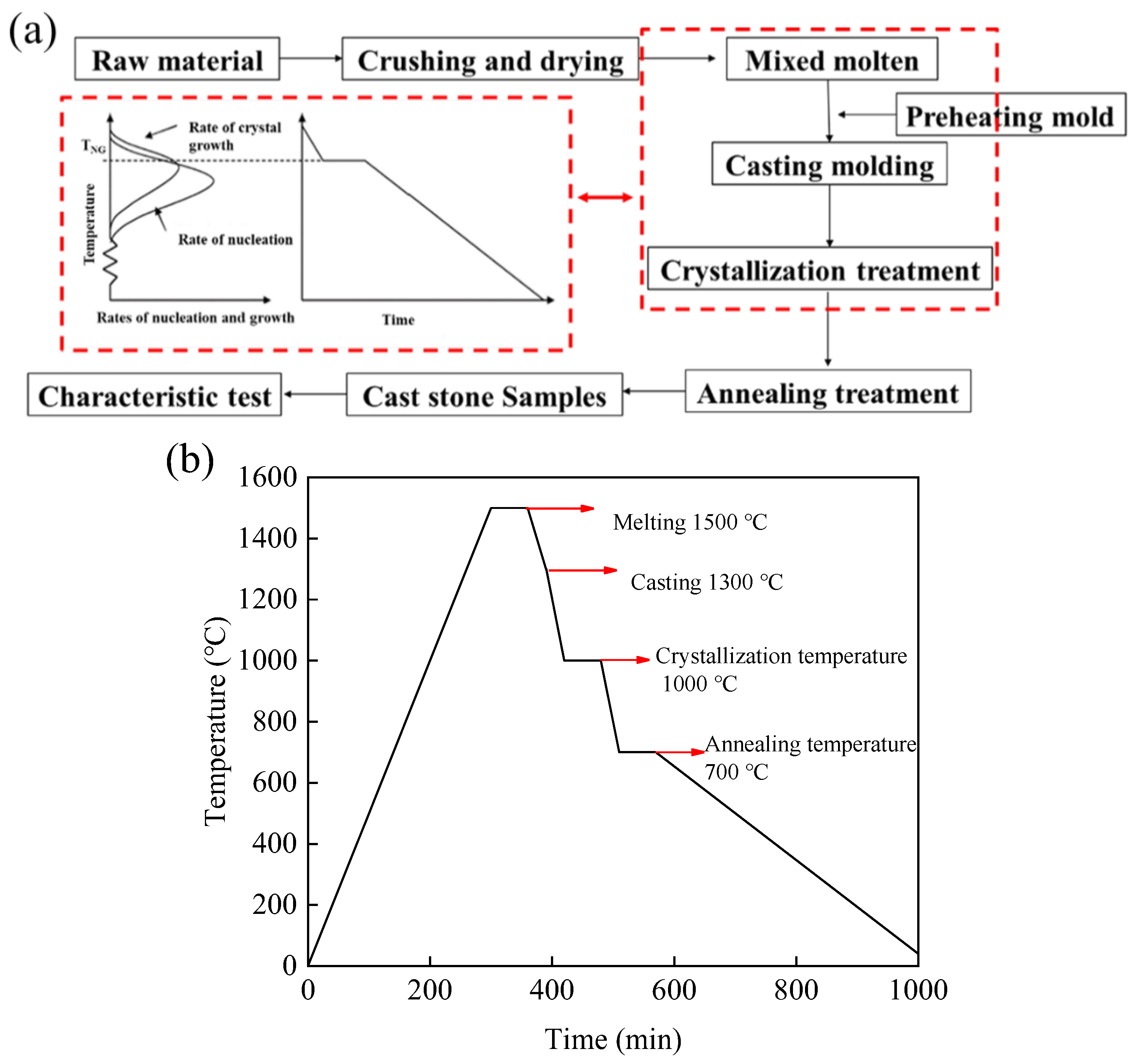


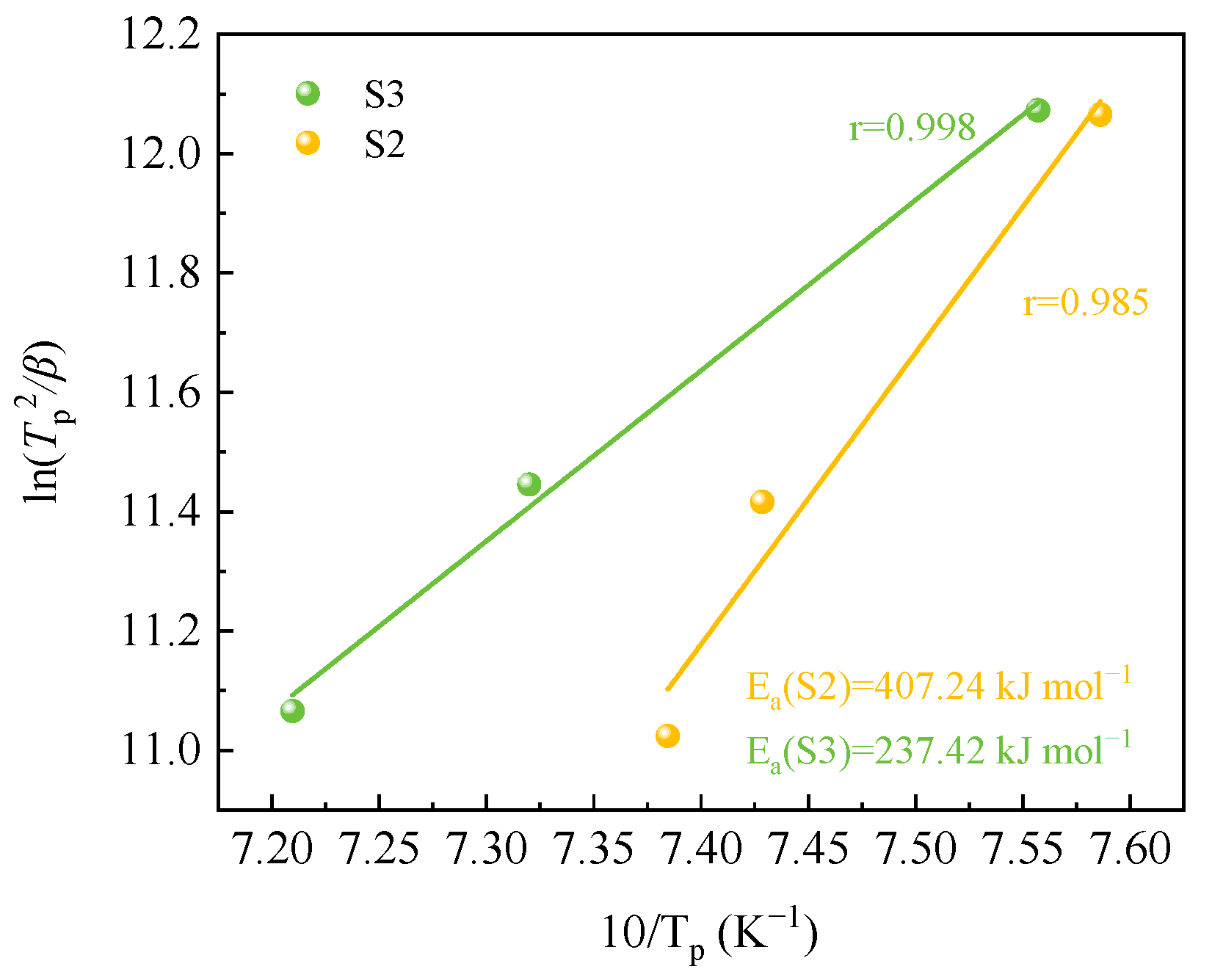



| Raw Material | CaO | SiO2 | Al2O3 | MgO | MnO | Fe2O3 | K2O | Cr2O3 | BaO | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Silicomanganese slag | 27.27 | 38.40 | 13.24 | 3.60 | 7.92 | 2.27 | 1.60 | 0.26 | 2.49 | 97.05 |
| Chromite | 1.04 | 8.30 | 10.25 | 9.84 | 0.56 | 26.40 | 0.06 | 40.50 | — | 96.95 |
| Serpentine | 1.53 | 43.29 | 2.01 | 39.31 | 0.22 | 11.05 | 0.06 | 0.73 | — | 98.20 |
| Ferrochrome slag | 4.90 | 28.80 | 23.20 | 26.10 | 0.60 | 4.23 | 0.12 | 9.73 | — | 97.68 |
| Sample No. | CaO | SiO2 | Al2O3 | MgO | MnO | Fe2O3 | K2O | Cr2O3 | BaO | R (mass%CaO/mass%SiO2) |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | 27.27 | 38.40 | 13.24 | 3.60 | 7.92 | 2.27 | 1.60 | 0.26 | 2.49 | 0.71 |
| S2 | 25.18 | 36.69 | 12.84 | 4.69 | 7.32 | 3.89 | 1.48 | 2.68 | 2.29 | 0.69 |
| S3 | 22.80 | 36.48 | 15.23 | 8.10 | 6.46 | 2.66 | 1.30 | 2.15 | 1.99 | 0.63 |
| Sample/National Standard | Flexural Strength/MPa | Bulk Density/(g/cm−3) | Water Absorption/% |
|---|---|---|---|
| S1 | — | 3.03 | 0.08 |
| S2 | 37.50 | 3.11 | 0.32 |
| S3 | 35.70 | 3.09 | 0.22 |
| Natural granite building slabs (GB/T 18601-2009) [26] | ≥8.3 | ≥2.56 | ≤0.4 |
| Ceramic tiles (GB/T 4100-2015) [27] | ≥35 | — | ≤0.5 |
| Cooling Rate β (°C·min−1) | Tp (°C) | ||
|---|---|---|---|
| S1 | S2 | S3 | |
| 10 | – | 1081 | 1114 |
| 20 | – | 1073 | 1093 |
| 30 | – | 1045 | 1050 |
| Slag No. | S1 | S2 | S3 |
|---|---|---|---|
| Activation energy for viscous flow (kJ mol−1) | 30.38 ± 8.34 | 43.70 ± 5.14 | 74.93 ± 8.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, Y.; Cheng, Z.; Feng, W. Hot Modification of Silicomanganese Slag in Its Crystallization and Viscosity Properties for Preparation of Cast Stone. Ceramics 2025, 8, 22. https://doi.org/10.3390/ceramics8010022
Huang Y, Li Y, Cheng Z, Feng W. Hot Modification of Silicomanganese Slag in Its Crystallization and Viscosity Properties for Preparation of Cast Stone. Ceramics. 2025; 8(1):22. https://doi.org/10.3390/ceramics8010022
Chicago/Turabian StyleHuang, Yi, Yu Li, Zhaoyang Cheng, and Wei Feng. 2025. "Hot Modification of Silicomanganese Slag in Its Crystallization and Viscosity Properties for Preparation of Cast Stone" Ceramics 8, no. 1: 22. https://doi.org/10.3390/ceramics8010022
APA StyleHuang, Y., Li, Y., Cheng, Z., & Feng, W. (2025). Hot Modification of Silicomanganese Slag in Its Crystallization and Viscosity Properties for Preparation of Cast Stone. Ceramics, 8(1), 22. https://doi.org/10.3390/ceramics8010022






