Characterisation of the Electrical Properties of Wastes Vitrified from Canarian Island Basaltic Quarries: Original Glasses and Glass-Ceramics
Abstract
1. Introduction
2. Materials and Methods
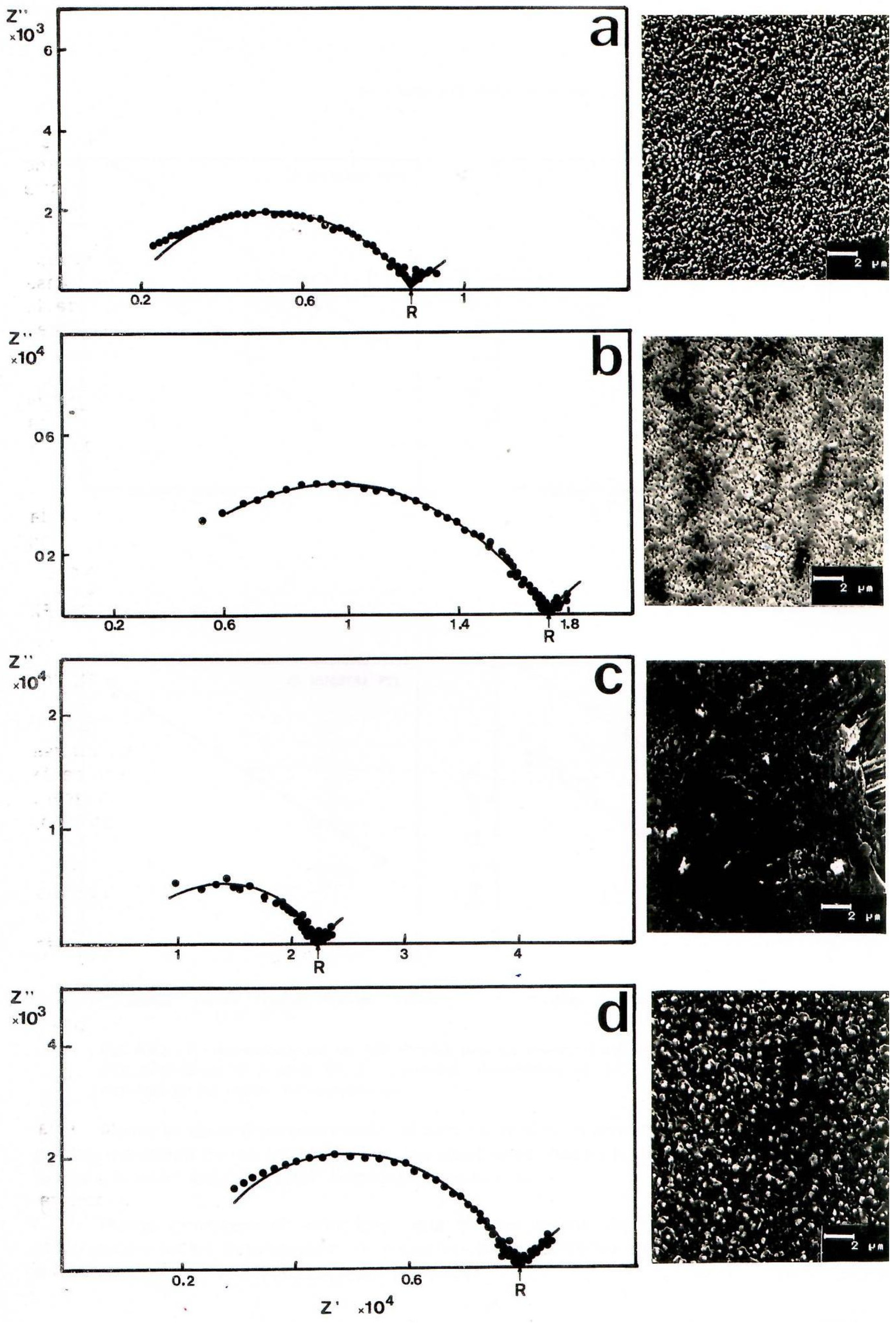
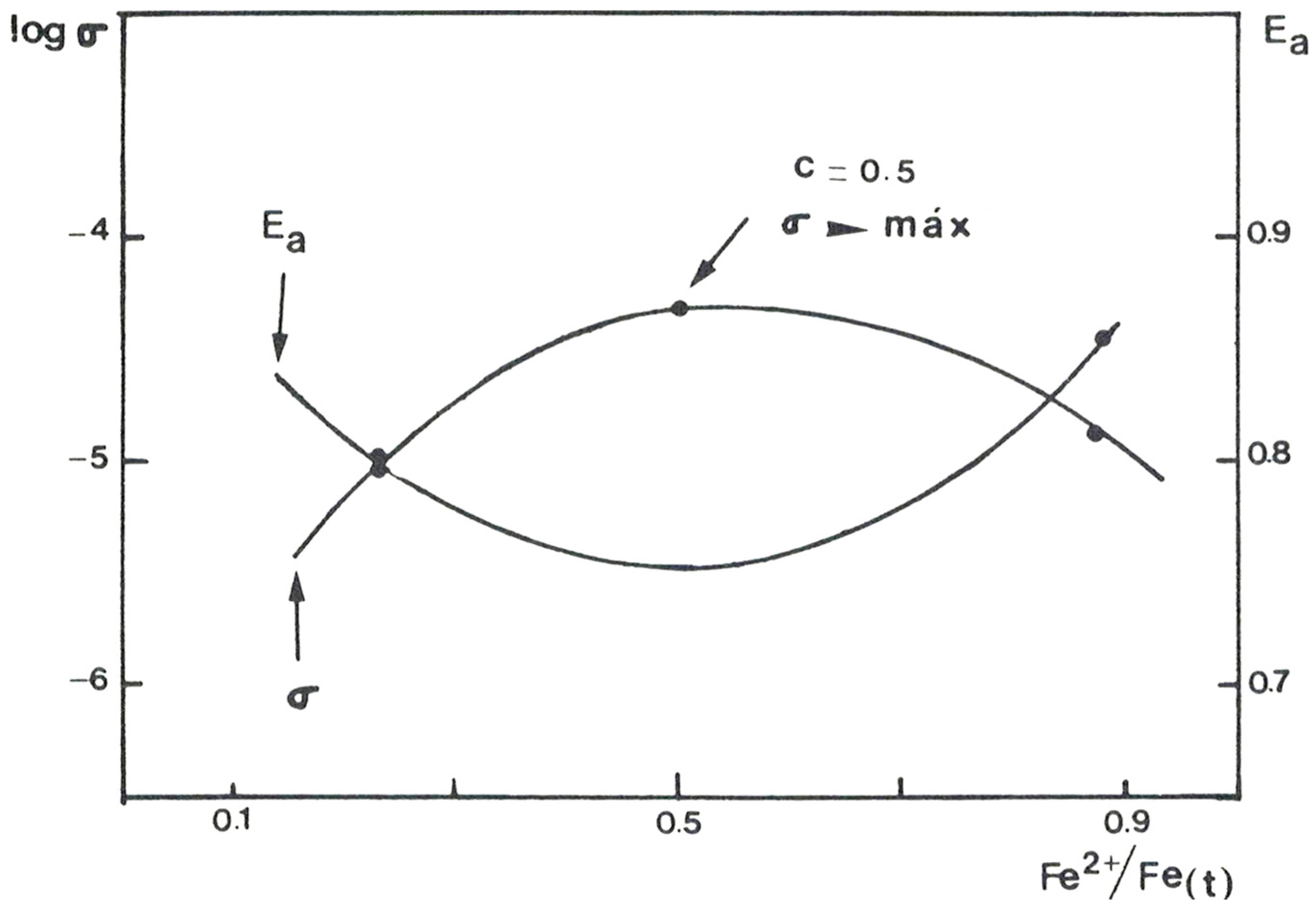
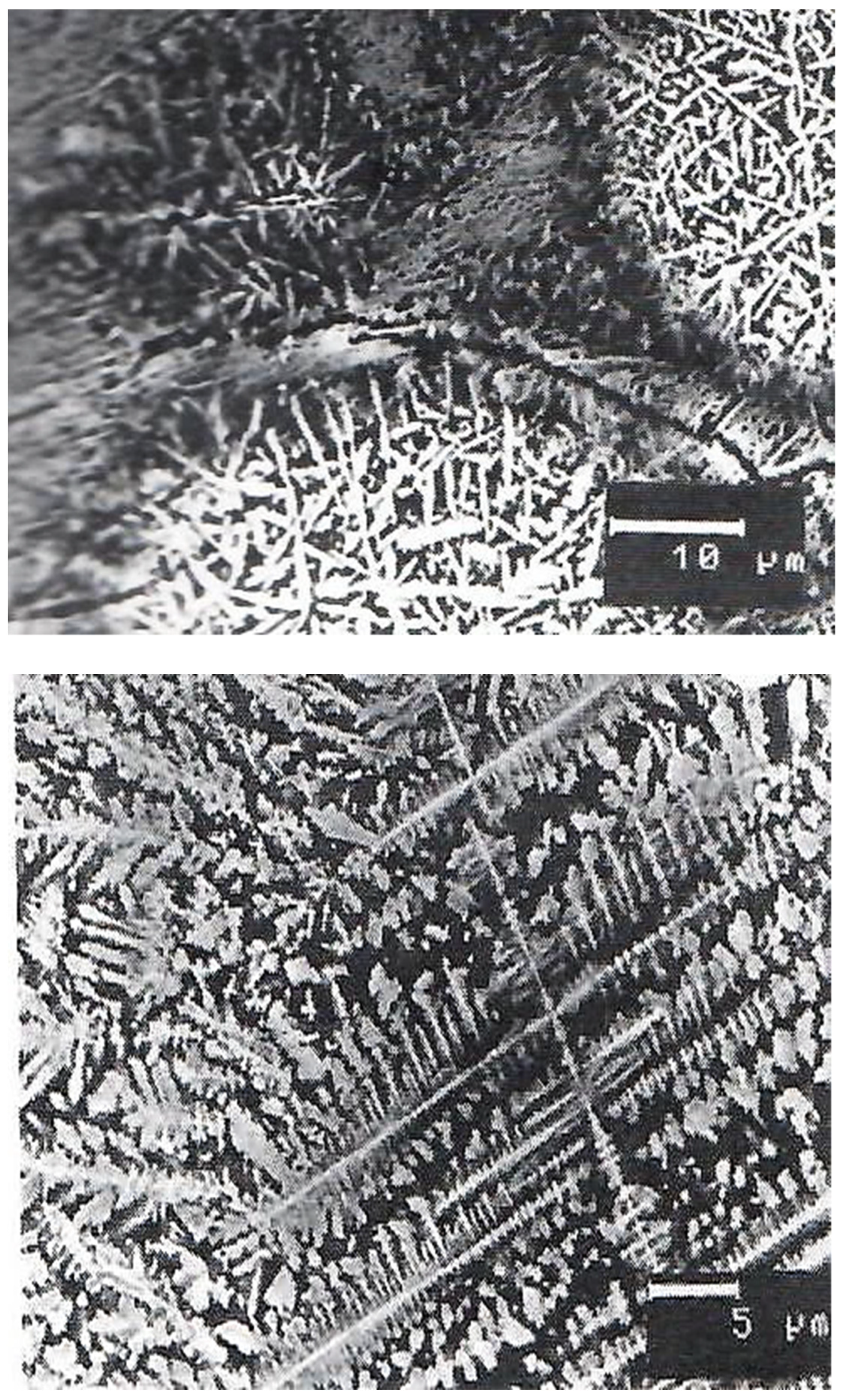
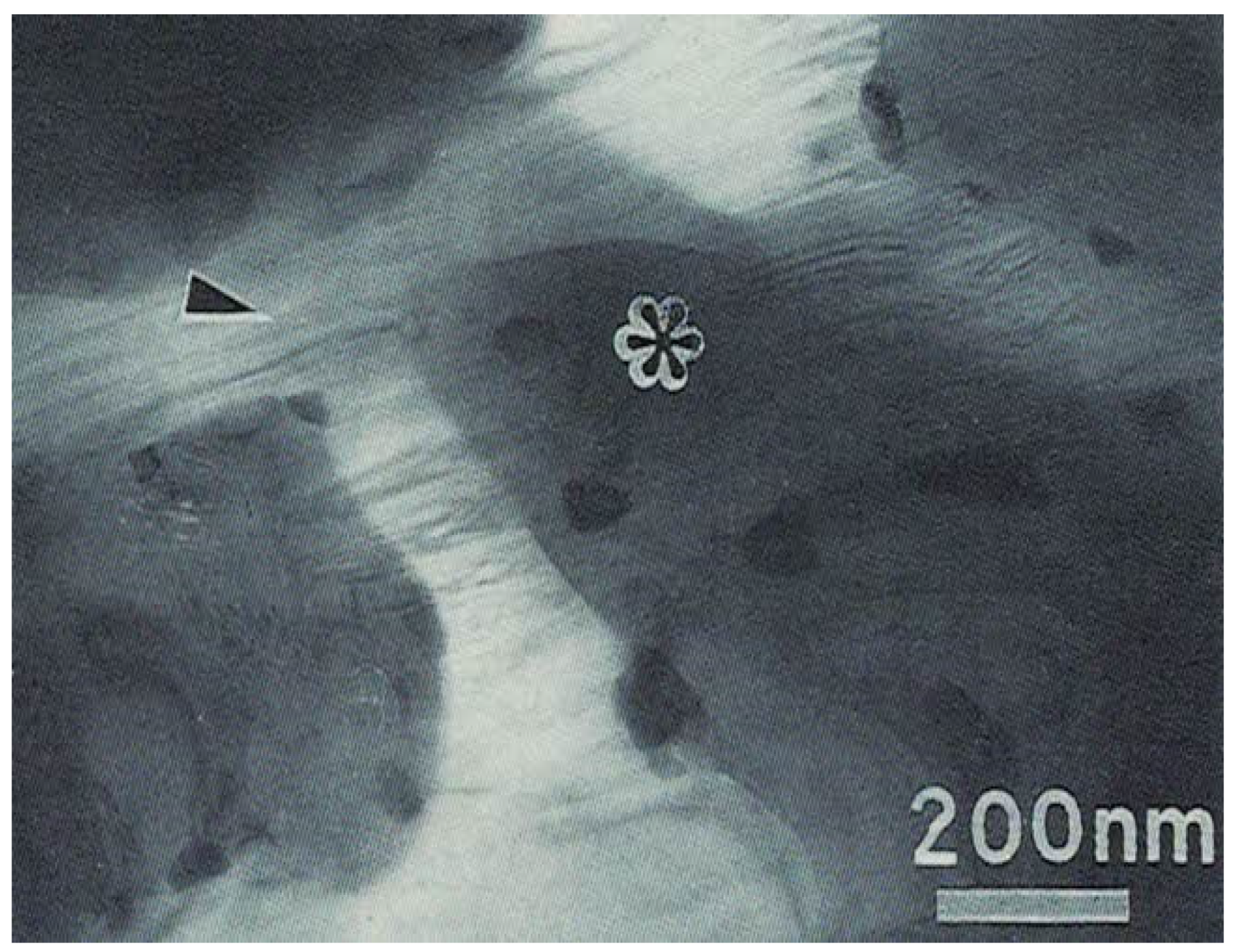
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Locsei, B.P. Molten Silicates and Their Properties; Akademiai Kiado: Budapest, Hungary, 1970. [Google Scholar]
- Lima, L.F.; Zorzi, J.E.; Cruz, R.C.D. Basalt glass-ceramics: A short review. Bol. Soc. Esp. Ceram. Vidr. 2022, 61, 2–12. [Google Scholar] [CrossRef]
- Alper, M.A. Microstructure Developed from Fusion Casting. In Ceramic Microstructures; Fulrath, R.M., Pask, J.A., Eds.; John Wiley and Sons: New York, NY, USA, 1966. [Google Scholar]
- Beall, G.H.; Rittler, H.L. Basalt Glass-ceramics. Am. Cer. Soc. Bull. 1976, 55, 579–582. [Google Scholar]
- De Vicente, I. Estudio de los Mecanismos de Nucleación y Cristalización en Vidrios Obtenidos a Partir de Rocas Basálticas Canarias. Ph.D. Thesis, Universidad Complutense de Madrid, Madrid, Spain, 1992. [Google Scholar]
- Kovacs, G. Glazuri Bazaltice; Editura Presa Universitarâ Românâ: Cluj-Napoca, Romania, 2000. [Google Scholar]
- Suleimenov, S.T.; Abduvaliev, T.; Sharafiev, M.S.; Tropina, L.G. Investigating Daubanisk Tephrite basalts for making stone castings. Glass Ceram. 1966, 23, 192–195. [Google Scholar] [CrossRef]
- Mari, E.A. El desarrollo de las tecnologías de vitrificación para la inmovilización de residuos radiactivos. Bol. Soc. Esp. Cerám. Vidr. 1986, 25, 307–314. [Google Scholar]
- Chick, L.A.; Lokken, R.O.; Thomas, L.E. Basalt glass-ceramics for immobilization of transuranic nuclear waste. Am. Ceram. Soc. Bull. 1983, 62, 83–88. [Google Scholar]
- Lazanas, A.C.; Prodromidis, M.I. Electrochemical Impedance Spectroscopy—A Tutorial. ACS Meas. Sci. Au 2023, 3, 162–193. [Google Scholar] [CrossRef] [PubMed]
- Morse, S.A. Basalt and Phase Diagrams: An Introduction to the Quantitative Use of Phase Diagrams in Igneous Petrology; Springer: Heidelberg, Germany, 1980. [Google Scholar]
- Park, J.S.; Shackelford, J.F.; Rincón, J.M.; Callejas, P.; Vicente-Mingarro, I. Measurement of residual stress in engineering ceramics by X-ray diffraction. A case study of a Spanish basalt glass-ceramic. Bol. Soc. Esp. Ceram. Vidr. 1990, 29, 419–421. [Google Scholar]
- Boukamp, B.A. Equivalent Circuit (EQUIVCRT.PAS); Boukamp, B.A., Ed.; Department of Chemical Technology, Twente University: Enschede, The Netherlands, 1989. [Google Scholar]
- Gil Escrig, L.; Prades, M.; Beltrán, H.; Cordoncillo, E.; Masó, N.; West, A.R. Voltage-dependent bulk resistivity of SrTiO3Mg ceramics. J. Am. Ceram. Soc. 2014, 97, 2815–2824. [Google Scholar] [CrossRef]
- Zhang, B.; Yoshino, T. Effect of temperature, pressure and iron content on the electrical conductivity of orthopyroxene. Contrib. Mineral. Petrol. 2016, 171, 102. [Google Scholar] [CrossRef]
- Jurado-Egea, J.R.; Owen, A.E.; Bandyopadhyay, A.K. Electronic conduction in basalt glass and glass-ceramics—Correlation with magnetite crystallization. J. Mater. Sci. 1987, 22, 3602–3606. [Google Scholar] [CrossRef]
- Schirmer, O.F. O− Bound small polarons in oxide materials. J. Phys. Condens. Matter. 2006, 18, R667. [Google Scholar] [CrossRef]
- De Vicente, I.; Callejas, P.; Rincón, J.M. Microestructura y microanálisis de fases minerales cristalizadas en vidrios obtenidos a partir de rocas basálticas canarias. Bol. Soc. Esp. Mineral. 1991, 14, 95–105. [Google Scholar]
- Romero, M.; Rincón, J.M. Surface and Bulk Crystallization of Glass-Ceramic in the Na2O-CaO-ZnO-PbO-Fe2O3-Al2O3-SiO2 System Derived from a Goethite Waste. J. Am. Ceram. Soc. 1999, 82, 1313–1317. [Google Scholar] [CrossRef]
- Vicente-Mingarro, I.; Callejas, P.; Carda, J.; Sales, M.; Rincón, J.M. TEM/STEM/EDX investigation of glass-ceramics obtained from canarian alkali-basalts. In Electron Microscopy 92 (EUREM 92); Lopez Galindo, A., Rodríguez-García, M.I., Eds.; Universidad de Granada: Granada, Spain, 1992; Volume 2, pp. 405–406. [Google Scholar]
- Keith, H.D.; Padden, F.J. A phenomenological theory of spherulitic crystallization. J. Appl. Phys. 1963, 34, 2409–2421. [Google Scholar] [CrossRef]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes. A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Sanito, R.C.; Bernuy- Zumaeta, M.; You, S.-J.; Wang, Y.-F. A review on vitrification technologies of hazardous waste. J. Environ. Manag. 2022, 316, 115243. [Google Scholar] [CrossRef]
- Karamanov, A.; Paunović, P.; Ranguelov, B.; Ljatifi, E.; Kamusheva, A.; Načevski, G.; Karamanova, E.; Grozdanov, A. Vitrification of hazardous Fe-Ni wastes into glass-ceramic with fine crystalline structure and elevated exploitation characteristics. J. Environ. Chem. Eng. 2017, 5, 432–441. [Google Scholar] [CrossRef]
- Davinge, R.W. Effects of microstructure on the mechanical properties of ceramics. In Fracture Mechanics of Ceramics; Plenum Press: New York, NY, USA, 1974; Volume 2, pp. 447–468. [Google Scholar]
- Kelton, K.F.; Greer, A.L. Chapter 8: Crystallization in glasses. In Pergamon Material Sciences; Elsevier: Amsterdam, The Netherlands, 2010; Volume 15, pp. 279–329. [Google Scholar]
- Holand, W.; Beall, G.H. Glass-Ceramic Technology, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Karamanov, A.; Arrizza, L.; Ergul, S. Sintered material from alkaline basaltic tuffs. J. Eur. Ceram. Soc. 2009, 29, 595–601. [Google Scholar] [CrossRef]
- Khater, G.A.; Gomaa, M.M.; Kang, J.; Yue, Y.; Mahmoud, M.A. Thermal, electrical and physical properties of glasses based on basaltic rocks. Silicon 2020, 12, 645–653. [Google Scholar] [CrossRef]
- Nuernberg, R.B.; Bello, T.S.; Fokin, V.M.; Zanotto, E.D.; Rodrigues, A.C.M. Non-stoichiometric crystallization of Li2SiO3-CaSiO3 glasses: Residual glass composition from ionic conductivity. J. Non-Cryst. Solids 2019, 510, 158–165. [Google Scholar] [CrossRef]
- Yeşilkaya, S.S.; Güven Özdemir, Z.; Yilmaz Canli, N.; Yildiz, A.; Içelli, O.; Okutan, M. Electrical and impedance properties of composites: Volcanic basalt rocks. Compos. Part B 2016, 95, 488–492. [Google Scholar] [CrossRef]

| GLASS | VO-HI06 | VR-HI06 | VO-LG16 | VR-LG16 | VO-TF2 | VR-TF2 | VO-FT1 | VR-FT1 |
|---|---|---|---|---|---|---|---|---|
| SiO2 | 46.01 | 45.62 | 50.28 | 50.17 | 45.81 | 46.05 | 47.54 | 46.45 |
| Al2O3 | 17.04 | 16.34 | 18.29 | 17.69 | 16.91 | 16.95 | 15.75 | 12.52 |
| Fe2O3 | 8.99 | 1.40 | 7.33 | 1.28 | 8.53 | 2.37 | 8.79 | 1.40 |
| FeO | 2.85 | 9.91 | 2.53 | 8.19 | 3.25 | 8.76 | 3.32 | 11.67 |
| MnO | 0.17 | 0.19 | 0.22 | 0.22 | 0.20 | 0.20 | 0.14 | 0.15 |
| MgO | 4.75 | 4.76 | 3.18 | 3.13 | 4.75 | 4.72 | 8.94 | 10.20 |
| CaO | 9.55 | 10.09 | 8.14 | 8.51 | 10.08 | 10.17 | 9.51 | 10.94 |
| Na2O | 3.61 | 4.31 | 4.27 | 5.09 | 4.23 | 4.51 | 1.77 | 2.05 |
| K2O | 1.84 | 1.98 | 1.96 | 1.99 | 1.43 | 1.46 | 0.78 | 0.64 |
| TiO2 | 3.77 | 3.86 | 2.53 | 2.57 | 3.48 | 3.51 | 2.90 | 3.43 |
| P2O5 | 1.07 | 1.10 | 1.00 | 1.03 | 1.02 | 1.03 | 0.40 | 0.44 |
| CO2 | 0.12 | 0.14 | 0.11 | 0.11 | 0.11 | 0.08 | 0.12 | 0.08 |
| SO3 | 0.04 | 0.04 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 |
| LOI | 0.18 | 0.26 | 0.13 | - | 0.17 | 0.14 | - | 0.02 |
| TOTAL | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| IOX * | 2.84 | 0.13 | 2.61 | 0.14 | 2.36 | 0.24 | 2.38 | 0.11 |
| Fe2O3 # | 3.11 | 3.17 | 4.84 | 4.96 | 5.06 | 5.03 | 3.41 | 3.91 |
| FeO # | 8.10 | 8.27 | 4.74 | 4.85 | 6.34 | 6.31 | 8.18 | 9.38 |
| Melting Atmosphere | Sample | T (°C) | Equivalent Circuit | Estimated Parameters |
|---|---|---|---|---|
| OXIDANT | VO-HI06 | 500° | (RQ) | Yo = 2.91 × 10−4; n = 0.74 |
| VO-TF2 | 650° | (RQ)(RW) | Yo = 2.43 × 10−8; n = 0.56 Yo (W) = 6.04 × 10−5 | |
| VO-LG16 | 650° | R(RQ)W | Yo = 5.48 × 10−5; n = 0.30 Yo (W) = 6.62 × 10−5 | |
| REDUCING | VR-LG16 | 550° | R(RQ)(RQ)W | Yo = 7.85 × 10−7; n = 0.81 Yo = 5.10 × 10−5; n = 0.76 Yo (W) = 3.35 × 10−4 |
| REDUCING | GCR-HI06 | 350° | Circuit Type R(RQ)W | R = 1.72 × 103 |
| GCR-TF2 | 350° | Circuit Type R(RQ)W | R = 6.19 × 103 | |
| GCR-FT1 | 350° | Circuit Type R(RQ)W | Yo = 1.30 × 10−8; n = 0.73 | |
| GCR-LG16 | 350° | Circuit Type R(RQ)W | Yo (W) = 4.95 × 10−4 |
| Sample | Fe2+/Fetotal | Ea (eV) | Ea (eV) |
|---|---|---|---|
| VO-HI06 | 0.24 | 0.804 | 0.804 |
| VR-HI06 | 0.87 | 0.867 | 0.867 |
| GCR-HI06 | (*) | 0.674 | 0.674 |
| VO-TF2 | 0.28 | 0.801 | 0.801 |
| VR-TF2 | 0.78 | 0.878 | 0.878 |
| GCR-TF2 | (*) | 0.669 | 0.669 |
| VR-FT1 | 0.89 | 0.810 | 0.810 |
| GCR-FT1 | (*) | 0.643 | 0.643 |
| VO-LG16 | 0.10 | 0.763 | 0.763 |
| VR-LG16 | 0.86 | 0.820 | 0.820 |
| GCR-LG16 | (*) | 0.687 | 0.687 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón, J.M.; Callejas, P.; Almendro-Candel, M.B.; Jordan, M. Characterisation of the Electrical Properties of Wastes Vitrified from Canarian Island Basaltic Quarries: Original Glasses and Glass-Ceramics. Ceramics 2025, 8, 1. https://doi.org/10.3390/ceramics8010001
Rincón JM, Callejas P, Almendro-Candel MB, Jordan M. Characterisation of the Electrical Properties of Wastes Vitrified from Canarian Island Basaltic Quarries: Original Glasses and Glass-Ceramics. Ceramics. 2025; 8(1):1. https://doi.org/10.3390/ceramics8010001
Chicago/Turabian StyleRincón, Jesús Ma., Pío Callejas, María Belén Almendro-Candel, and Manuel Jordan. 2025. "Characterisation of the Electrical Properties of Wastes Vitrified from Canarian Island Basaltic Quarries: Original Glasses and Glass-Ceramics" Ceramics 8, no. 1: 1. https://doi.org/10.3390/ceramics8010001
APA StyleRincón, J. M., Callejas, P., Almendro-Candel, M. B., & Jordan, M. (2025). Characterisation of the Electrical Properties of Wastes Vitrified from Canarian Island Basaltic Quarries: Original Glasses and Glass-Ceramics. Ceramics, 8(1), 1. https://doi.org/10.3390/ceramics8010001







