1. Introduction
Cordierite Mg
2Al
4Si
5O
18 (or 2MgO·2Al
2O
3·5SiO
2) is one of the main compounds of the ternary system MgO-SiO
2-Al
2O
3. Although there are different ways to synthesize cordierite, the most common is by reactive sintering, at a temperature of around 1400 °C, of mixtures of raw materials containing the required oxides, such as talc, clay and alumina [
1]. Besides their interesting dielectric properties [
2], these materials have received a great deal of attention due to their very low coefficients of thermal expansion (<3 × 10
−6 K
−1) and thermal conductivity (2–3 W m
−1 K
−1) [
3,
4]. They are thus widely used in high-temperature applications, such as catalyst substrates and filtration [
5,
6,
7,
8]. For the automotive industry, most of the catalytic converters are produced by the extrusion of a cordierite honeycomb substrate coated with a “washcoat”, consisting of precious metals (Pt, Pd, …) [
9,
10].
Considering the huge number of motor vehicles produced in the world, recycling of end-of-life converters is of growing interest from economic and environmental points of view. Studies are carried out on the recovery of the precious metals of the washcoat by pyrometallurgy and hydrometallurgy. Furthermore, new approaches are considered to obtain zero waste by recycling the ceramic substrate [
11]. On the other hand, a non-negligible quantity of non-conform fired cordierite substrates (without washcoat) are produced by the industry. The reuse of these production wastes for the manufacture of high-added value products is, therefore, of high interest and would be a first step to demonstrate the recyclability of the cordierite.
However, the use, as raw material, of a powder of milled fired substrates, and, therefore, mineralogically containing cordierite as nearly single phase, fundamentally changes the sintering mechanism. Indeed, the absence of physicochemical transformations and reactions between the different raw materials of a mixture (e.g., talc, kaolin, …) consequently leads to sintering essentially based on diffusion mechanisms in the solid state [
12,
13]. In this case, the suitability of the powder edifice for consolidation and densification is strongly conditioned to the use of a sub-micron size powder and a high sintering temperature. Using dilatometry curves, Camerucci et al. evidenced the solid-state sintering of cordierite between 850 °C and 1350 °C. Above 1350 °C, shrinkage is accelerated by the occurrence of a liquid phase. Their study also highlights the influence of particle size distribution on the densification. Firstly, using a monomodal distribution, the finer the particle size, the greater the densification. Secondly, using a bimodal distribution, with an optimum ratio, allows an increase in the degree of densification [
12]. However, obtaining a powder from downgraded parts requires several grinding steps, and the energy cost and process duration increase sharply with the desired fineness. An alternative solution is the use of a sintering additive to lower the sintering temperature. Ogiwara et al. studied the influence of the addition of 3%wt of Li
2O–Bi
2O
3. They succeeded in achieving a densification of 97% at 1050 °C for 2 h. However, the mechanical properties were degraded, for example, flexural strength going from 243 MPa to 120 MPa [
13]. Even if it is considered that extensive densification is not useful for the intended application, sufficient consolidation to ensure mechanical strength is essential. It is, therefore, of great interest to find an alternative to conventional sintering to produce consolidated and geometrically stable parts at the lowest possible energy cost.
For several years now, geopolymer materials have been attracting growing interest from scientists and industrialists alike. Without the need for energy-intensive heat treatments, the geopolymerization process makes it possible to form silicate inorganic materials with good performance in terms of mechanical properties, chemical resistance and durability [
14,
15,
16]. Geopolymers are obtained by reaction between alkaline silicate, or more rarely acidic reagent, and amorphous aluminosilicate powders, at room or low temperatures. The resulting solid has a three-dimensional amorphous structure that contains mostly covalent bonds. A large body of research focuses on the formulation of geopolymers for construction applications [
17,
18]. However, more and more studies are also focusing on the development of geopolymers for applications that usually use ceramic materials [
19,
20].
Unfortunately, one of the downsides of geopolymers is their poor mechanical properties at high temperatures. During a rise in temperature, dehydration and dihydroxylation of the inorganic network occur, possibly followed by its crystallization and other phenomena. These transformations induce strong dimensional variations and cracking [
21,
22]. To overcome this issue, the geopolymer paste can be loaded with mineral filler to produce a “composite” material [
19,
23,
24]. By doing so, the geopolymer allows a “cold” consolidation of the powder edifice and the reaction between the geopolymer and the filler during a rise in temperature can possibly stabilize the structure of the material and enhance its final properties.
The type of alkali and the alkali/Al ratio have a strong influence on the properties of the geopolymer materials after curing but also at high temperatures. Bernal et al. studied the influence of alkali over the evolution of shrinkage with temperature until 1000 °C on samples cured at ambient temperature for 7 days and 5 years. For alkali/Al = 1, Na-based geopolymers show a first stage of shrinkage of 7.5% at 250 °C related to dehydration and dihydroxylation, and a second shrinkage around 750 °C related to viscous sintering. The final shrinkage is 23%. For K-geopolymers, the first shrinkage is lower (5%) and the viscous sintering begins at 900 °C; the final shrinkage is 10%. Finally, Rb- and Cs-geopolymers show the same behavior with a smoother shrinkage for the removal of water, and no sintering is observable. The final shrinkage is around 7.5%. After 5 years of curing, Na-geopolymers show the same behavior but the shrinkage related to sintering is more drastic and the final shrinkage is 27.5%. Finally, K-geopolymers show the same behavior as the Rb-geopolymers, and the curing time has no effect over the evolution of shrinkage with temperature [
25]. The potassium-based geopolymers show better resistance to high temperatures compared to sodium-based geopolymer pastes [
23]. A study was undertaken by Kohout et al. [
26] about the effect of the K/Al molar ratio on the thermomechanical properties of metakaolinite-based geopolymer composites. In their study, a chamotte was used as a filler for the geopolymer matrix. From the structural point of view, it was shown that the pure geopolymer stays amorphous until 800 °C. For higher temperatures, the crystallization of leucite (KAlSi
2O
6) and kalsilite (KAlSiO
4) happens depending on the K/Al ratio; the higher the ratio, the more phases appear at lower temperatures. It was also highlighted that the geopolymer in the composites shows the same transformations as the pure geopolymer. Regarding the mechanical properties, a high K/Al molar ratio tends to bring down the mechanical properties of the samples. For unfired samples, the maximal value of the Young’s modulus is obtained with a ratio near 1.0. Additionally, the modulus tends towards lower values when the sample is fired at temperatures up to 1000 °C. On the other hand, in their work, K. Hemra and P. Aungkavattana demonstrated that using cordierite as filler leads to a strong increase in the compressive strength after heat treatment at 800 °C/2 h compared to the geopolymer alone [
24]. They also showed that no new crystalline compounds are formed during the heat treatment.
Cesium-based geopolymers reinforced with 5–35 wt% cordierite were synthesized by Wei Chen et al. [
27]. The main crystalline phases in the product, obtained after thermal treatment at 1400 °C, are cordierite and cubic pollucite, Cs
2O·Al
2O
3·4SiO
2, which is formed by crystallization of the geopolymer matrix over 700 °C. The cordierite filler does not react with the geopolymer. The shrinkage resulting from the sintering is less than 5% and occurs over 800 °C. The lowest coefficient of thermal expansion results from the highest cordierite load and is 1.89 × 10
−6 K
−1, which is unexpectedly lower than the CTE of pure cordierite. In their work, Chengying Bai et al. [
28] investigated the synthesis by a replica route of cordierite-based geopolymer foams that have potential applications such as porous supports or high-temperature filter components. The geopolymers were prepared by incorporating MgO as an additional magnesium source in metakaolin and fly ash reacted with NaOH solution and sodium silicate solution. Sintering at 1200 °C for 2 h led to porous cordierite-based ceramics. However, an extensive structural characterization showed that the transformation of cordierite after the thermal treatment was incomplete.
Finally, some recent works demonstrate that it is possible to obtain geopolymer composite parts by additive manufacturing. Gasmi et al. studied the production of metakaolin-based geopolymer and composites with feldspar and wollastonite by robocasting [
29]. Additive manufacturing techniques appear to be highly promising for producing substrates or filters with optimized geometries. Kovacev et al. used digital-light processing (DLP) to produce substrates with two designs of diamond unit cells. For the same inlet gas temperature, it was found that the conversion efficiency was higher for the substrates produced by DLP compared to traditional honeycomb substrates [
30].
In this context, we investigate the manufacture of cordierite–geopolymer composites. As previously explained, the first motivations for the choice of cordierite as the mineral filler are its intrinsic properties and the reuse of industrial wastes. Besides this, it is well known that the crystal structure of the cordierite can accept the insertion of potassium atoms [
31,
32]. This can possibly lead to interactions between the potassium silicate-based geopolymer and the cordierite filler during the rise in temperature, and then affect the dimension stability and the final properties of the composite. More precisely, our interest is in the influence of the K/Al ratio and the cordierite fraction on the stability of the dimensions and porosity during heating at 1000 °C, and on the final Young’s modulus and coefficient of thermal expansion. The final goal of this work is to select the most promising compositions for a further study that will focus on their use in additive manufacturing.
2. Materials and Methods
2.1. Materials
The cordierite powder used in this work comes from non-conform (over tolerance limits or presence of flaws) honeycomb substrates for catalytic converters that were supplied by an industrial partner. These substrates are first crushed with a pestle into a coarse powder, which is further wet-milled using a Turbula shaker (Willy A. Bachofen AG, Basel, Switzerland) at a speed of 46 RPM with alumina media, and finally dried to obtain the final powder. The parameters for the milling are the following:
The particle size distribution of the final cordierite powder was characterized by using a Malvern Mastersizer 2000 laser granulometer (Malvern Panalytical, Malvern, UK) (
Table 1). The median size of the particles d
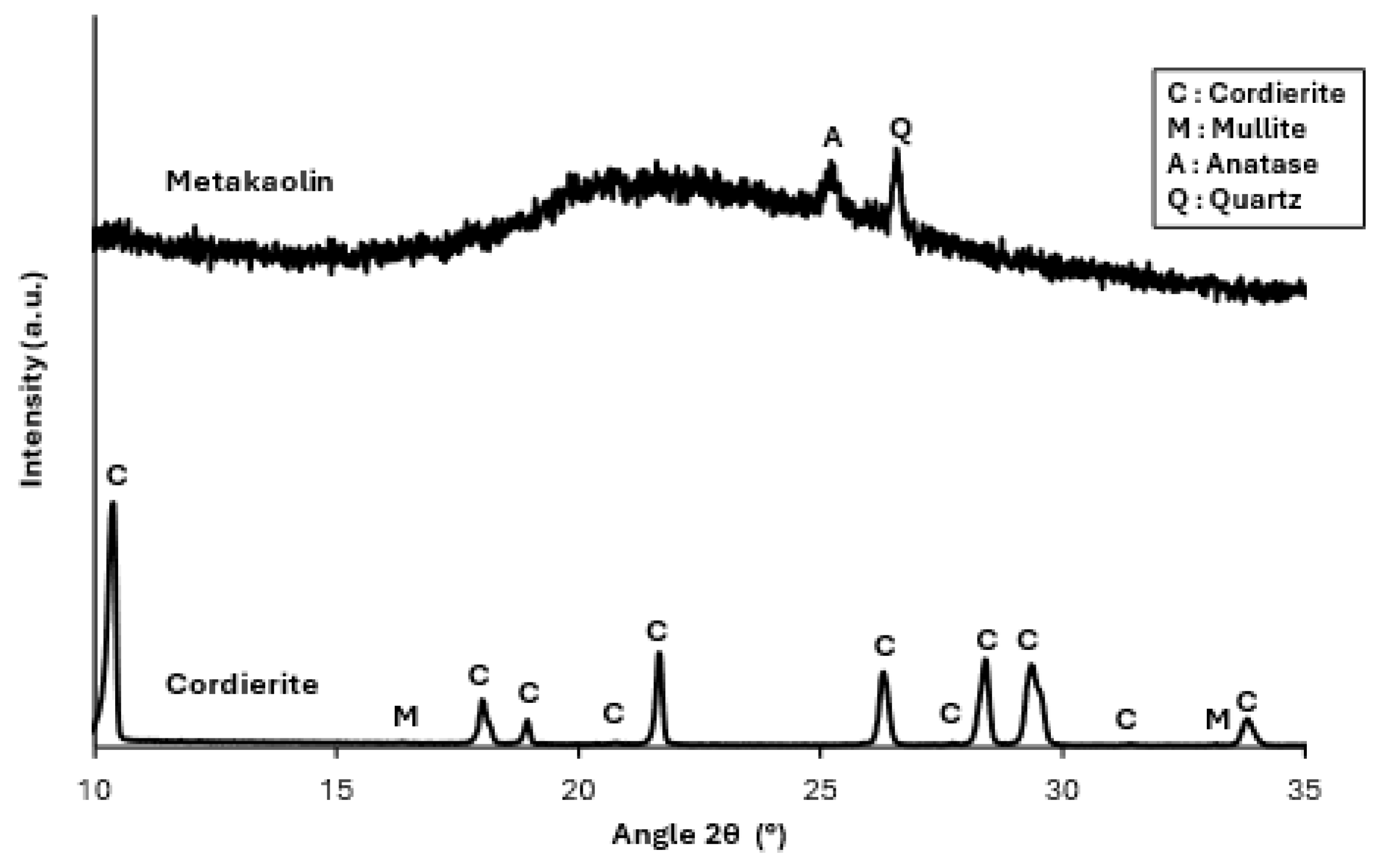
50 is 26.9 µm. An X-ray diffraction (XRD) analysis was performed on the powder using a Panalytical Empyrean diffractometer (Malvern Panalytical, Malvern, UK) with a Cu Kα radiation source. The X-ray pattern shows the diffraction peaks of cordierite (JCPDS #00-013-0294), and a small amount of mullite (JCPDS #00-015-0776) (
Figure 1). An XRF analysis was performed with Horiba XGT 9000 equipment (Horiba, Kyoto, Japan) to ascertain the composition of the powder and compare it to the stoichiometric cordierite (
Table 2).
The metakaolin (Al
2O
3·2SiO
2) used for this work comes from Temcon-Solutions GmbH (Winnweiler, Germany) under the name Tempozz
® M88. Its particle size distribution shows a median particle size d
50 = 4.4 µm (
Table 1). Its composition measured from XRF analysis (
Table 2) indicates a ratio SiO
2/Al
2O
3 = 2.01, which is very close to 2.00, corresponding to pure kaolinite. The XRD analysis (
Figure 1) shows the characteristic background bump of an amorphous material, and two weak intensity peaks that can be attributed to the presence of small amounts of quartz (JCPDS #00-033-1161) and anatase (JCPDS #00-021-1272).
The potassium silicate used in this work comes from Wöllner GmbH (Ludwigshafen, Germany) and is named Geosil
® 14517. The characteristics of the potassium silicate solution are listed in
Table 3.
2.2. Preparation of Geopolymer Composites
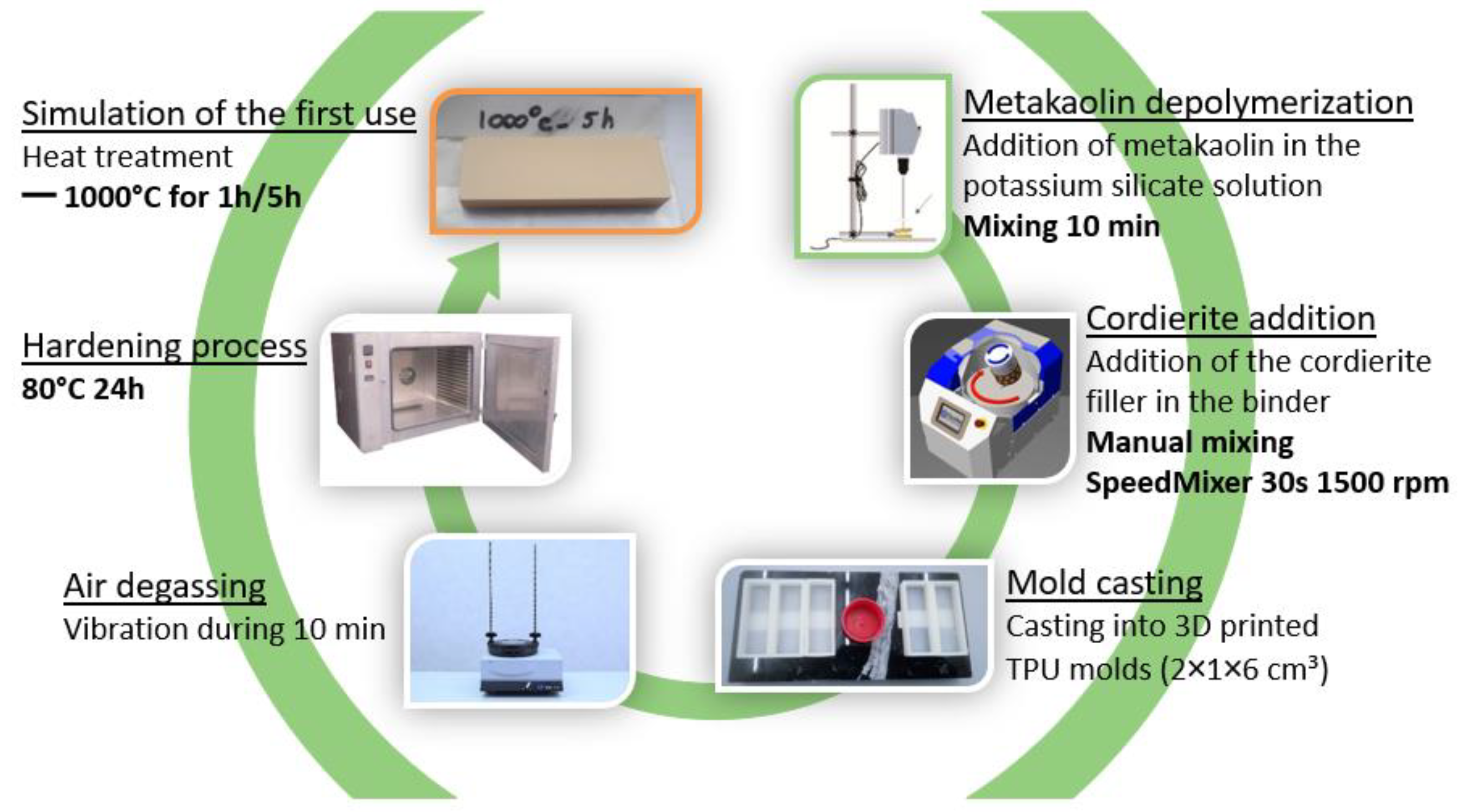
The process used for the synthesis of the geopolymer composites is shown in
Figure 2.
The preparation of the geopolymer binder starts with the potassium silicate solution and the metakaolin. The metakaolin is added into the silicate solution and is mixed with a laboratory mixer for 10 min. During this stage, the depolymerization of the metakaolin takes place and there is formation of oligomers. The mixture is very fluid and easy to mix. For some samples of 100% geopolymer, no cordierite filler is added, and step 2 is skipped.
When the geopolymer is ready, it is added to the cordierite powder and mixed by hand. Depending on the percentage of filler, the mixture is more difficult to mix, this is why a SpeedMixer at 1500 rpm for 30 s is used to obtain good homogeneity of the paste and avoid lumps. The geopolymer paste flows easily under agitation.
The mixture is then cast into 3D-printed TPU molds of dimensions 2 × 1 × 6 cm. The 3D-printed TPU molds are flexible and make it easier to remove the samples. They also limit the adhesion of the geopolymer paste from the walls.
Due to its high viscosity, the geopolymer composite can capture air, which makes large pores. To prevent air in the samples, the filled molds are vibrated on a vibrating table for 10 min.
The samples are sealed in plastic bags with a water recipient to ensure constant humidity and to prevent water from evaporating. Then, the samples are kept in an oven at 80 °C for 24 h. Once the samples are cured, they are unmolded and polished at grade 120. Next, the characterization of the samples is performed.
To investigate the evolution of the properties of the composites during the first use at high temperature, some samples undergo heat treatment with the following conditions:
Heating ramp: 5 °C/min
Plateau: 1000 °C for 5 h
Cooling ramp: 5 °C/min
2.3. Investigated Compositions and Characterization Methods
The purpose of this work is to investigate the influences of (i) the K/Al ratio of the geopolymer binder and (ii) the cordierite fraction on the stability of the dimensions and porosity during heating at 1000 °C, and on the final Young’s modulus and coefficient of thermal expansion.
Table 4 presents the compositions that were prepared using K/Al ratios of 0.75, 1, 1.5 and 2.3, and cordierite weight fractions of 0, 53%, 60% and 65%.
After curing, five samples of each composition are characterized in terms of:
Geometric apparent density da;
Absolute density d using a Micromeritics AccuPyc II 1340 device (Micromeritics, Norcross, GA, USA) (after milling);
Total porosity p calculated from the equation p = 1 − da/d;
Crystalline and amorphous phases by means of a Panalytical Empyrean diffractometer with a Cu kα radiation source with a range of 2θ between 9° and 35°. The detector is a PIXcel1D (RTMS detector) with an active length of 3.3473°. The step size and the counting time are 0.0131° and 8.67 s, respectively
Room temperature Young’s modulus E using the Impulse Excitation Technique (IET) with a GrindoSonic MK3 materials tester (Grindosonic, Heverlee, Belgium).
For each composition, the same characterization was also performed on one sample that underwent heat treatment at 1000 °C for 5 h.
For selected compositions, additional characterization techniques were applied on cured samples to follow the evolution of their properties during heat treatment at 1000 °C, with a soaking time of 1 h and heating ramp of 5 °C/min as follows:
Crystallographic changes by high-temperature diffraction (HT-XRD) on powdered samples by means of a Panalytical Empyrean (HTK-2000N) diffractometer with a Cu Kα radiation source with a range of 2θ between 9° and 35°. The detector is a 1Der detector (RTMS detector) with an active length of 2.1223°. The step size and the counting time are 0.0167° and 10.16 s, respectively
Dimension changes by dilatometry analysis on 5 × 5 × 20 mm3 samples using a Dil 402 Expedis dilatometer (Netzsch, Selb, Germany);
Evolution of the Young’s modulus E using a GrindoSonic MK7 non-destructive materials tester coupled with a Nabertherm furnace HT-1600 °C.
4. Discussion
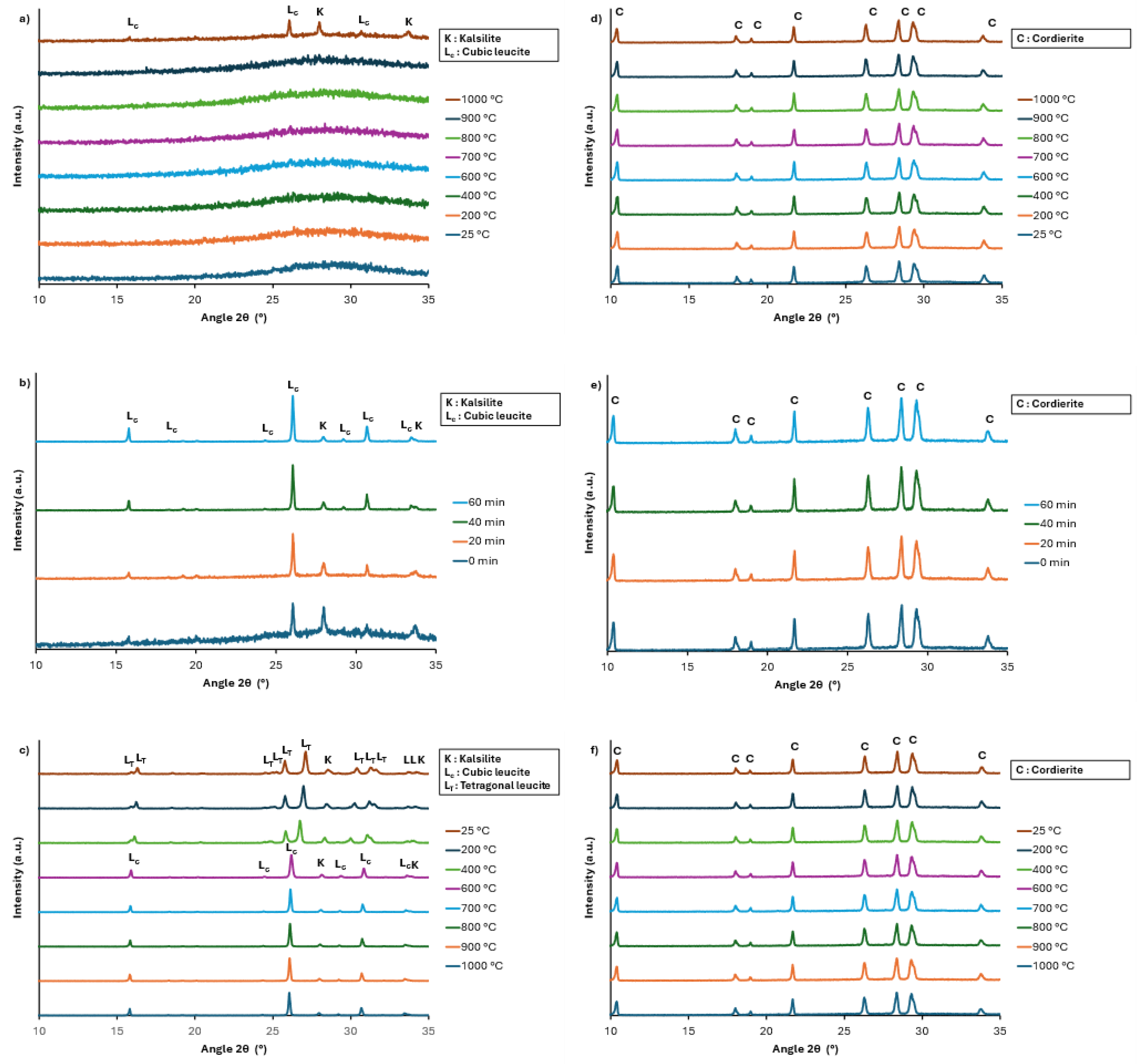
The HT-XRD analysis shows that for the geopolymer with K/Al = 1 (
Figure 8), the crystallization of the leucite and kalsilite occurs during heating between 900 and 1000 °C. During the plateau at 1000 °C for 1 h, the amount of kalsilite decreases and that of leucite increases, thereby indicating the possible reaction (1):
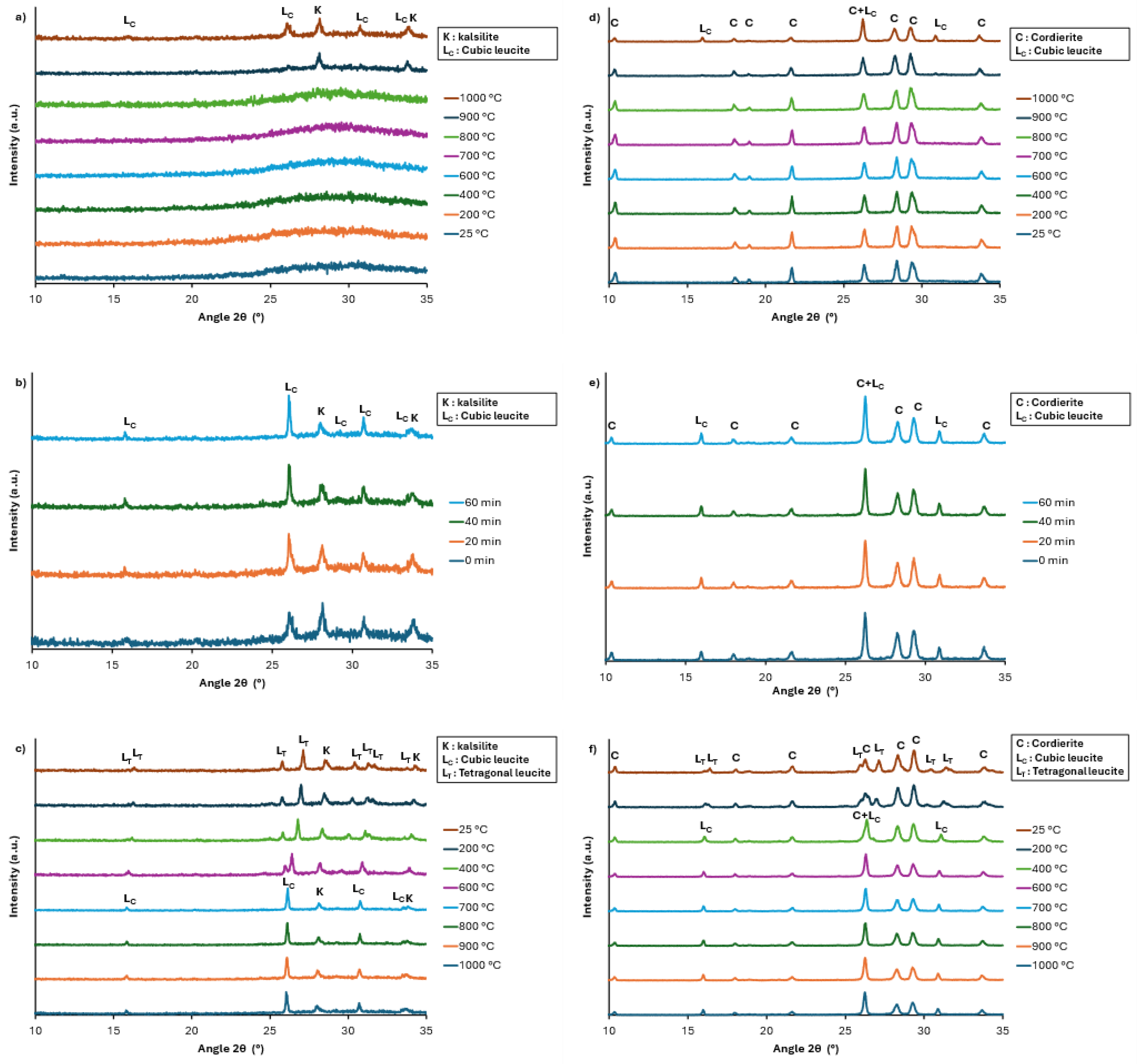
The XRD pattern of the geopolymer heat-treated at 1000 °C for 5 h (
Figure 6) shows leucite as the single crystalline phase. For the composites synthesized with this geopolymer and 60 wt% cordierite, neither leucite nor kalsilite is detected during the HT-XRD analysis. When comparing the HT-XRD analysis to those performed at RT after heat treatment of 5 h (
Figure 5 and
Figure 6), which show the presence of weak peaks of leucite, we can conclude that the addition of cordierite to the geopolymer delays the crystallization of leucite. When K/Al = 2.3, the HT-XRD analysis evidences the crystallization of kalsilite between 800 °C and 900 °C, and leucite between 900 °C and 1000 °C during the heating stage (
Figure 9). As for the geopolymer with K/Al = 1, a transformation of kalsilite to leucite occurs during the plateau of 1 h at 1000 °C. However, due to the higher potassium content, the XRD pattern of this geopolymer heat-treated during 5 h (
Figure 7) still shows the presence of kalsilite. For the composites synthesized with this geopolymer and 60 wt% cordierite, the HT-XRD analysis also shows the crystallization of leucite at 1000 °C but does not allow the presence of kalsilite to be inferred. However, this latter is present on the XRD pattern of the composite heat-treated for 5 h (
Figure 7). When comparing the results of the XRD analysis, according to the K/Al ratio, it is concluded that the addition of cordierite has a stronger influence on the crystallization of the geopolymer when K/Al is low. More specifically, the crystallization does not take place during the heating stage up to 1000 °C when K/Al = 1, in contrast to what happens when K/Al = 2.3. On the other hand, the calculation by Rietveld refinement of the unit cell parameters on the cordierite highlights the expansion of the cell when the temperature goes over 700 °C for the composite with K/Al = 2.3 and 60 wt% cordierite (
Figure 10). This can be explained by the insertion of potassium ions in the channels of the structure of the cordierite [
31,
32]. This mechanism also involves the substitution of Si atoms by Al atoms in the tetrahedral sites, and possibly ends by the formation of the compound K
0.5Mg
2Al
4.5Si
4.5O
18, which has a hexagonal symmetry (indialite-like):
The consequence is a change in the composition of the geopolymer that can modify its behavior at sintering and crystallization. This phenomenon is not observed when K/Al = 1.
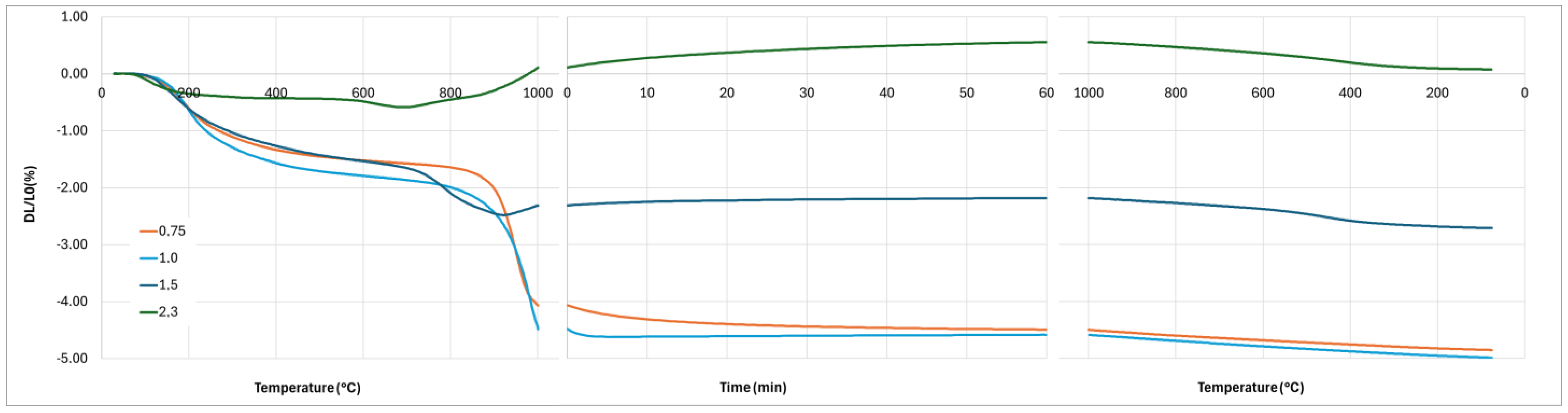
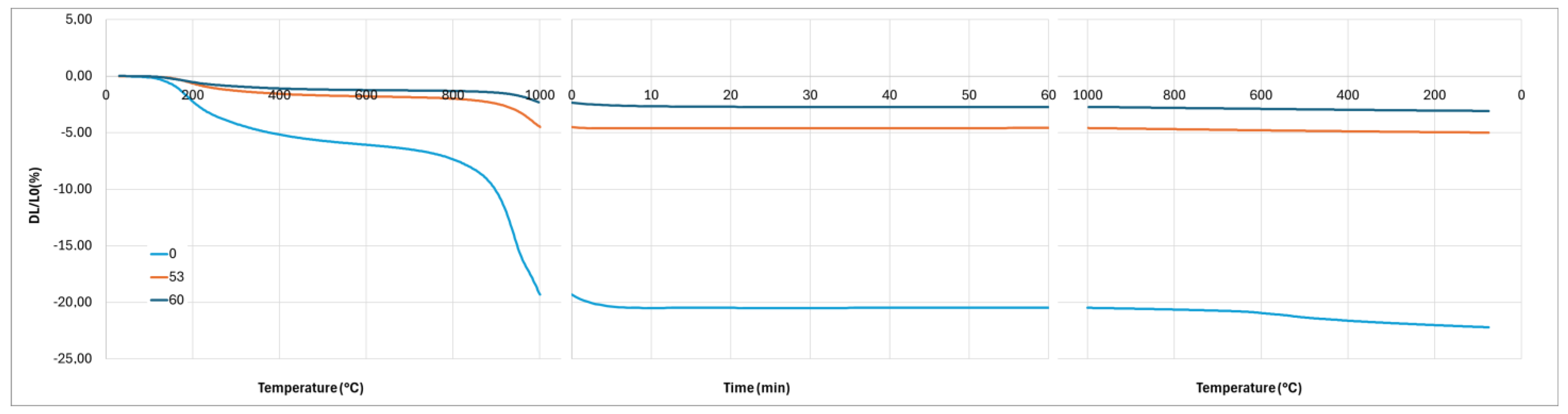
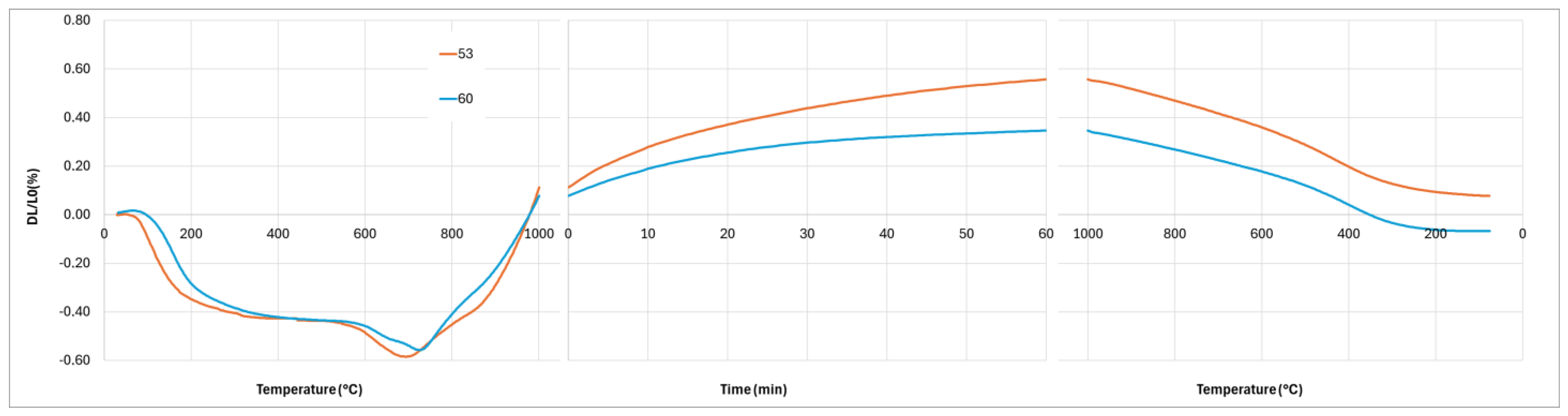
The crystallization of the geopolymer and the interaction between the geopolymer and the cordierite can explain the differences observed on the dilatometry curves (
Figure 11,
Figure 12 and
Figure 13). Firstly, the first stage of shrinkage, from room temperature to 300 °C, is related to dehydration and dihydroxylation [
25]. Secondly, the increase in the amount of K
2O lowers the temperature of the beginning of the second stage of the shrinkage that can be associated with sintering of the material. This can be explained by the amorphous nature of the geopolymer that probably induces a viscous flow sintering mechanism [
34,
35]. On the other hand, the shrinkage due to sintering stops around 700 °C when K/Al = 2.3. This temperature corresponds to the beginning of the expansion of the unit cell of the cordierite, resulting in the diffusion of potassium ions in the structure. This phenomenon, together with the crystallization of kalsilite, lowers the K
2O content in the geopolymer and thereby stops the viscous flow mechanism. Moreover, the transformation of kalsilite to leucite (Equation (1)) leads to a volume expansion due to the significantly lowest absolute density of leucite (2.47 g/cm
3 against 2.60 g/cm
3). The magnitude of this expansion depends on the density of the silica involved in the reaction, and consequently its state. This reaction may explain the dilatation observed on the dilatometric curves when the temperature is over 900 °C and during the plateau at 1000 °C, with dilatation enhanced by the geopolymer fraction (
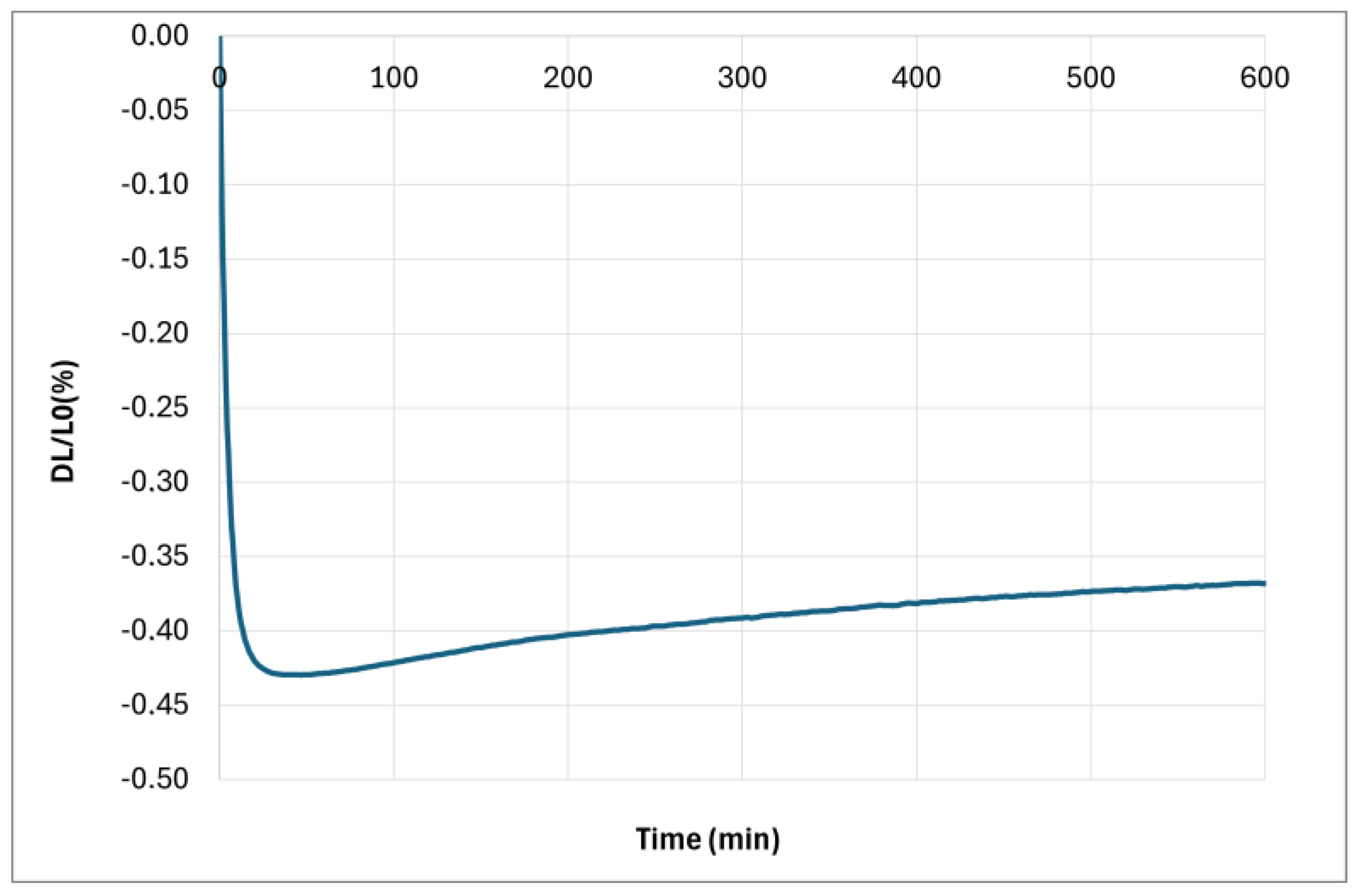
Figure 13). Finally, the presence of a significant amount of leucite after 1 h at 1000 °C in the composite when K/Al = 2.3 explains the non-linear contraction during the cooling by the allotropic transformation of cubic-leucite to tetragonal-leucite. It is noted that this transformation is also present for the composite with K/Al =1.5. By contrast, no diffusion of potassium ions in the cordierite nor crystallization of the geopolymer take place during the heating stage when K/Al = 1, and probably 0.75. So, a significant shrinkage due to sintering is observed between 800 °C and 1000 °C. Sintering still takes place during the plateau of 1 h at 1000 °C as the crystallization of leucite occurs later, as previously explained. To investigate the effect of this crystallization on the dimensions of the composite, a new dilatometry analysis, with a plateau of 10 h at 1000 °C, was performed.
Figure 16 shows the relative variation in dimensions during the plateau. This new analysis confirms the shrinkage observed with the first analysis with a plateau of 1 h, but it also highlights a weak expansion after 1 h. If we attribute this expansion to the transformation of kalsilite to leucite, as explained previously, this leads to the inference of prior crystallization of a low amount of kalsilite.
In terms of absolute density (
Table 5), after curing, the density decreases when the K/Al ratio increases because of the larger amount of potassium silicate used, which has a lower density (1.50 g/cm
3) than the metakaolin (2.70 g/cm
3). For a given K/Al ratio, the absolute density of the composite increases with the amount of cordierite, as the absolute density of cordierite is higher (2.55 g/cm
3) than the geopolymer alone. After heat treatment, the absolute density of the composites increases due to the partial crystallization of the geopolymer in leucite (2.47 g/cm
3) and/or kalsilite (2.60 g/cm
3). Consequently, the variation in absolute density is larger when K/Al increases because of the lowest initial value, but also related to the presence of kalsilite in the heat-treated samples.
The differences in shrinkage, together with the differences in the evolution of the absolute density, explain why the porosity of the composite remains nearly constant when K/Al is low whereas it strongly increases when it is high (
Table 5).
The fractions of leucite, kalsilite and cordierite in the composites play a key role in the coefficient of thermal expansion (CTE) (
Table 10). A high fraction of cordierite and low fractions of leucite and kalsilite are favorable to a low value due to the very high CTEs of the two potassium aluminum silicates—20 × 10
−6 K
−1 for kalsilite, 22 × 10
−6 K
−1 for tetragonal leucite (above 600 °C) and 3 × 10
−6 K
−1 for cubic leucite (under 600 °C) [
33,
36]. Moreover, the CTEs given in the present work are the average values calculated between 1000 °C and RT, so they include the contraction due to the allotropic transformation of leucite. This explains the very high value measured for the pure geopolymer, but also the much lower CTEs of the composite with K/Al = 0.75 and 1 by comparison to those of the composites with K/Al = 1.5 and 2.3. The decrease in the CTE when K/Al increases from 1.5 to 2.3 is probably due to the crystallization of less leucite due to the insertion of a part of the potassium in the structure of the cordierite. This is also supported by the presence of kalsilite in the composite heat-treated during 5 h at 1000 °C, which evidences the non-complete transformation of kalsilite in leucite. One must also consider the effect of the insertion of the potassium ions and the aluminum/silicon substitution in the structure of the cordierite that possibly lowers the CTE when stabilizing the hexagonal symmetry [
32]. However, the measurement of the unit cell parameters of orthorhombic cordierite derived from the HT-XRD patterns seems, by contrast, to highlight an increase in the variation with temperature (
Figure 10). This point must be clarified in future work as the heating conditions could influence the relative importance of the diffusion and stabilization of the potassium ions in the cordierite structure, and their contribution to the crystallization of kalsilite and leucite.
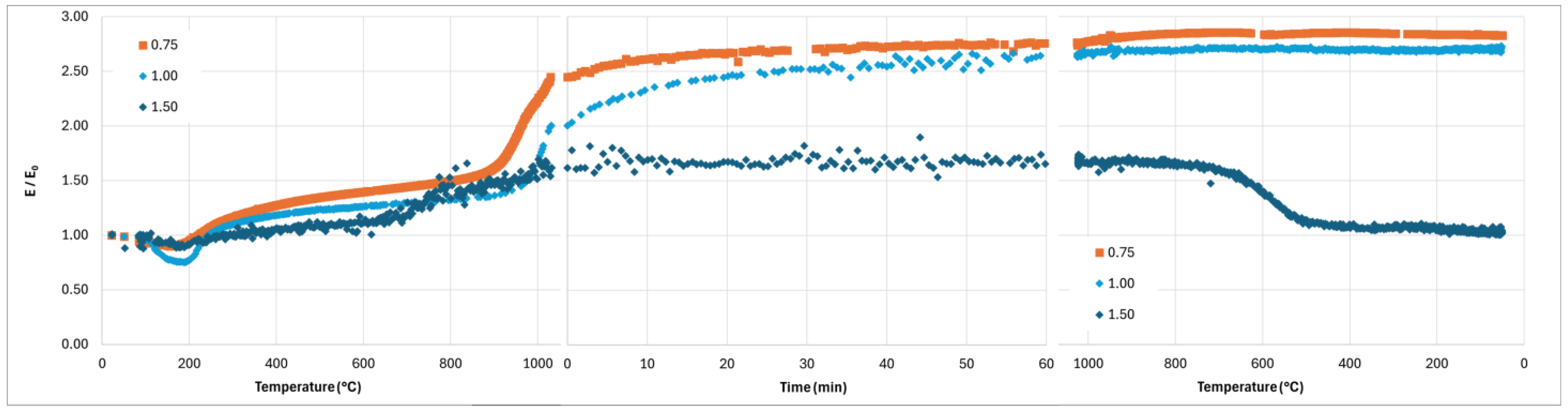
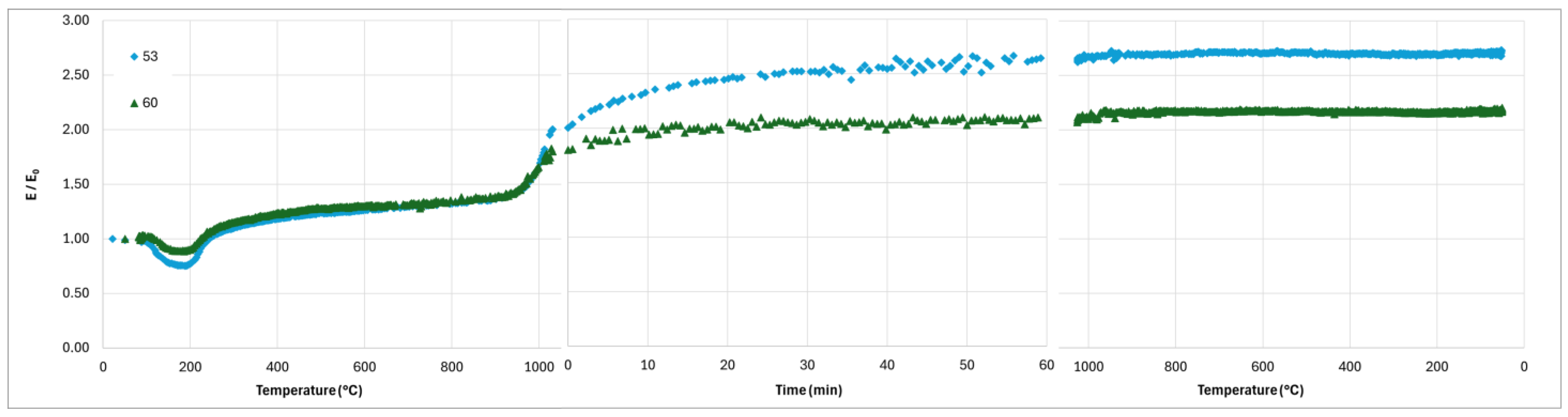
Finally, the strong increase in the Young’s modulus of the composite with K/Al = 0.75 and 1 when the temperature goes over 900–950 °C during the heating stage of the thermal treatment (
Figure 14 and
Figure 15) evidences a consolidation mechanism correlated to the shrinkage due to sintering. The rapid decrease in the Young’s modulus during the cooling stage when K/Al = 1.5 occurs at the same temperature as the contraction observed on the dilatometry curve (
Figure 11). This can be explained by damage due to internal stresses generated by the rapid volume change of 1.2% induced by the allotropic transformation of leucite [
33].