Effect of Prosthetic Material and Support Type on Stress Distribution of Fixed Partial Dentures: A Finite Element Study
Abstract
1. Introduction
2. Materials and Methods
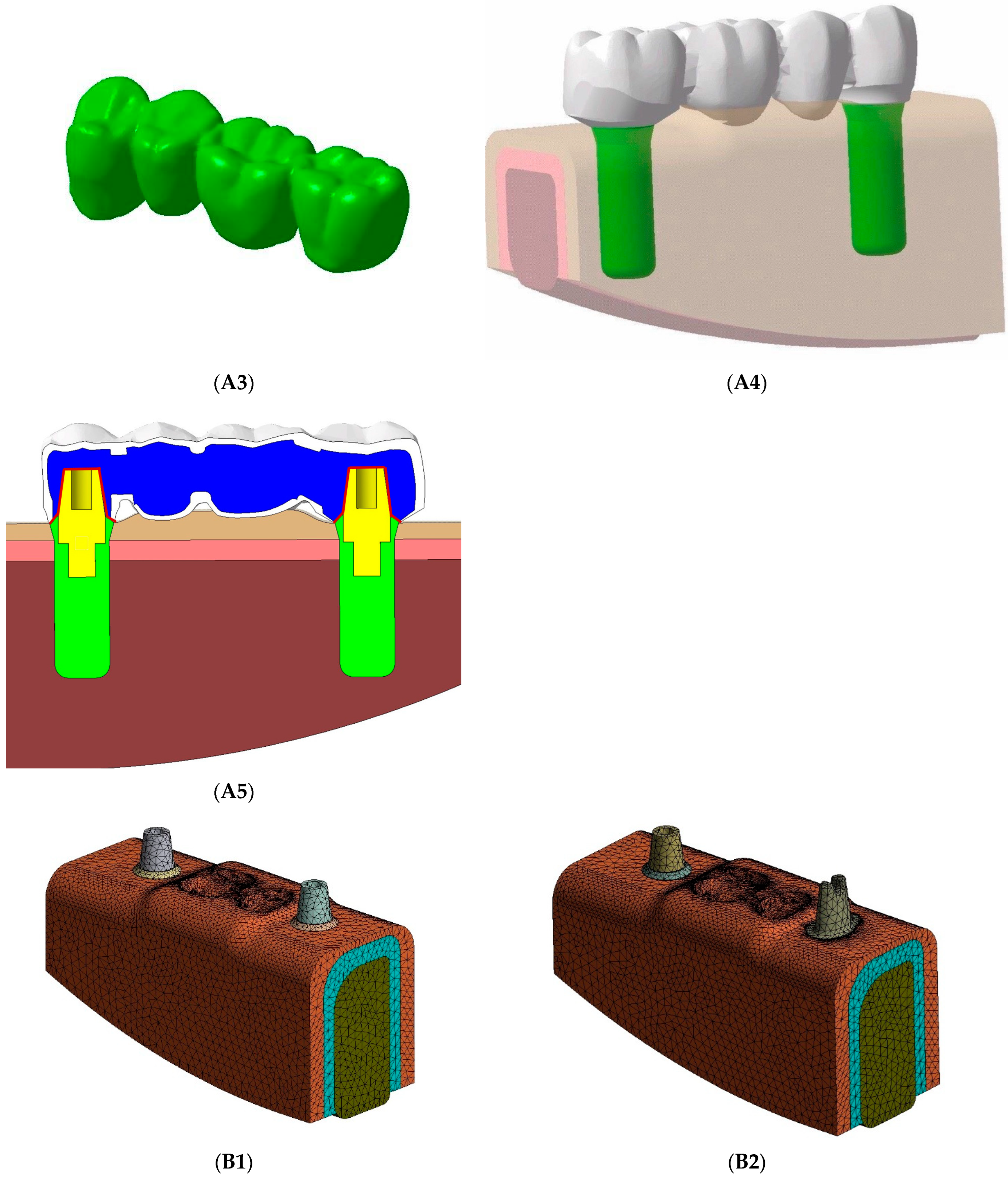
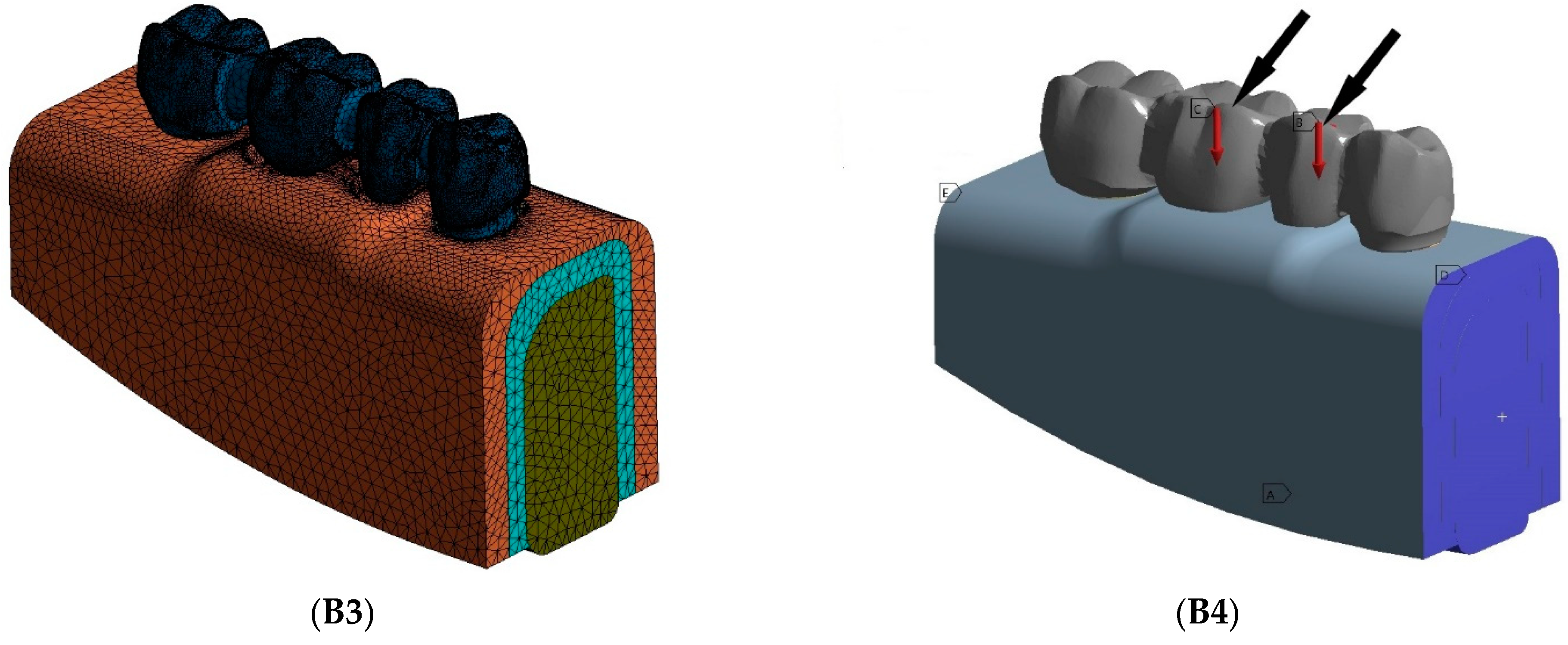
2.1. FE Model Design and Model Generation
2.2. Material Propreties and Boundary Conditions
2.3. Verification and Validation of the FE Models
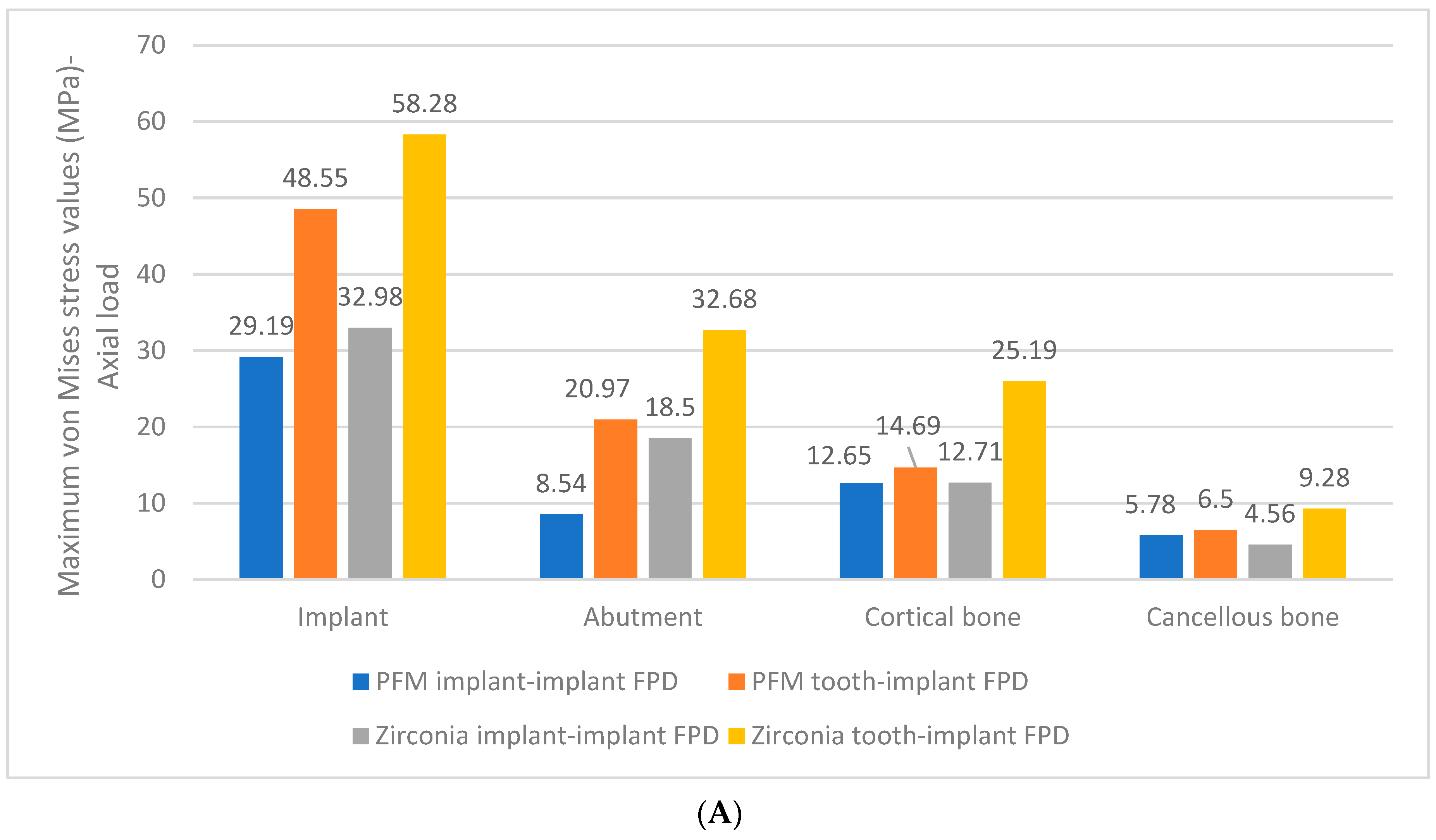
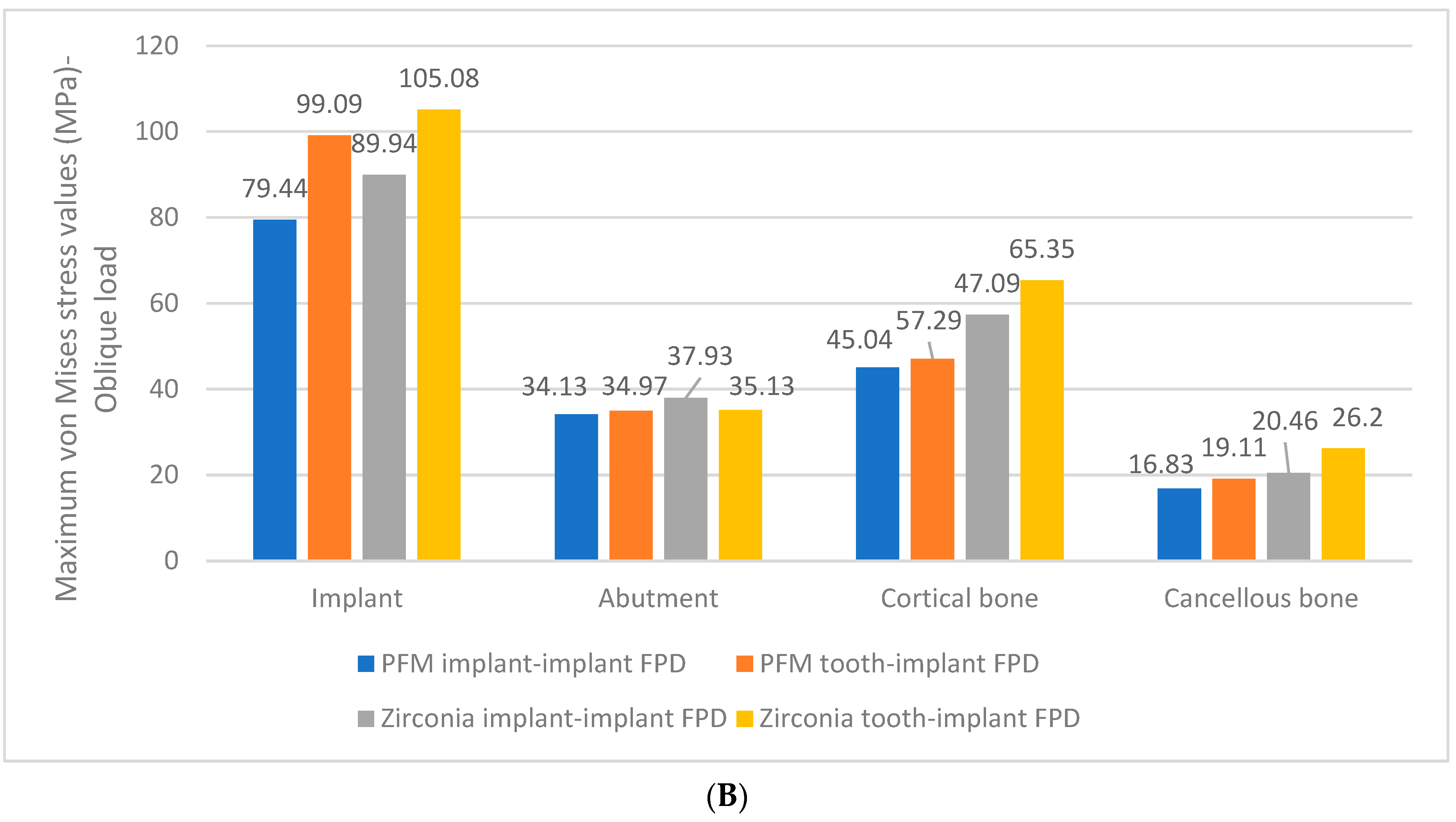
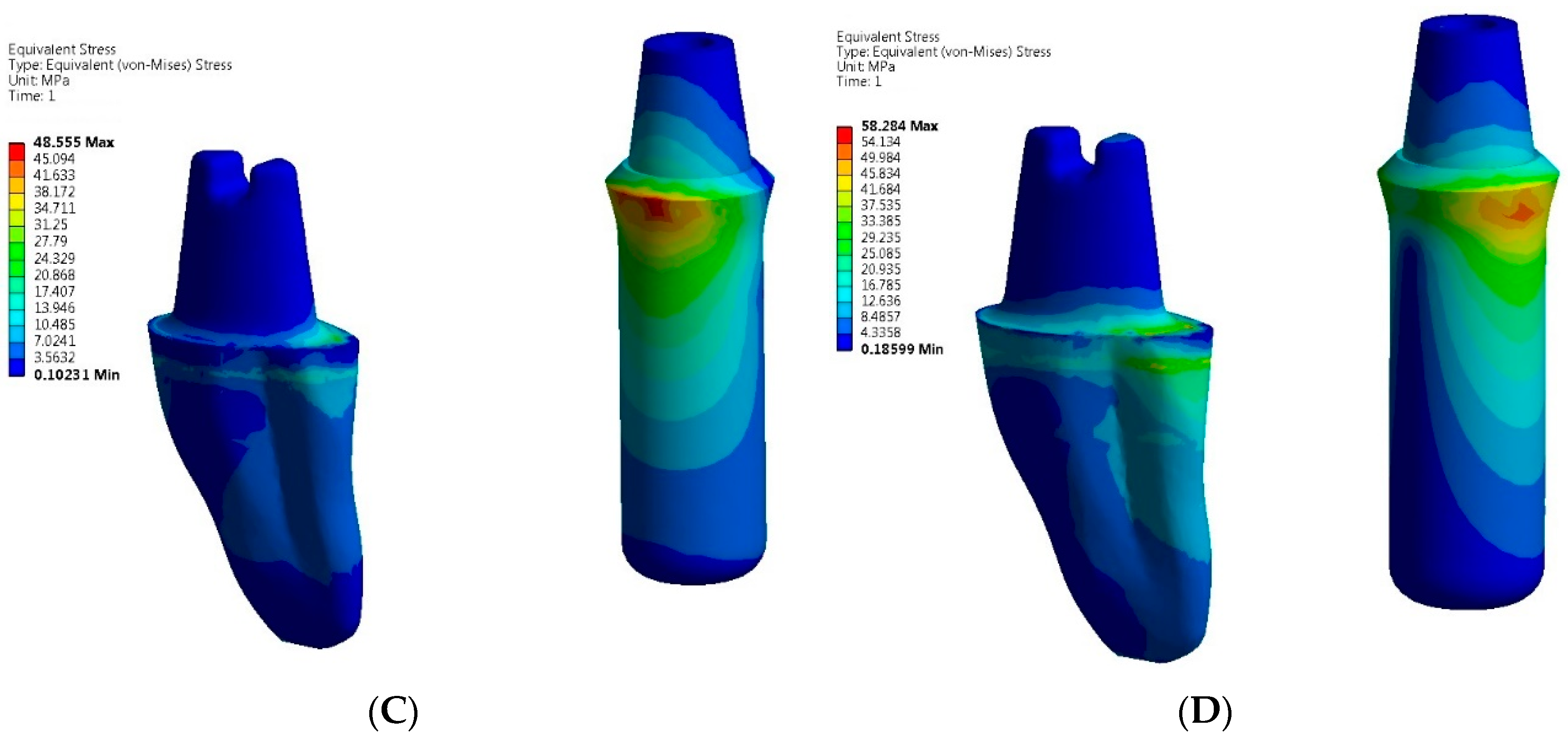
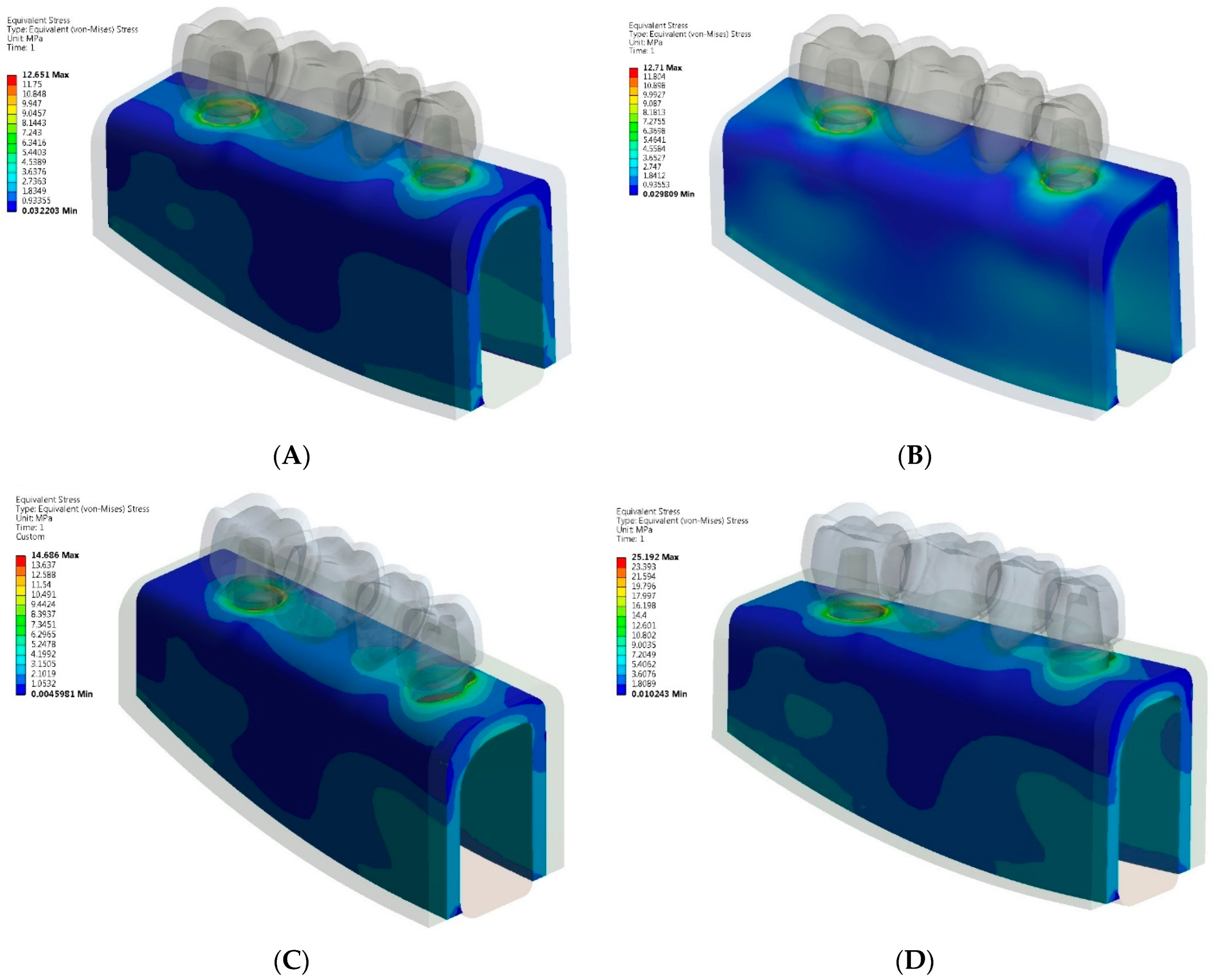
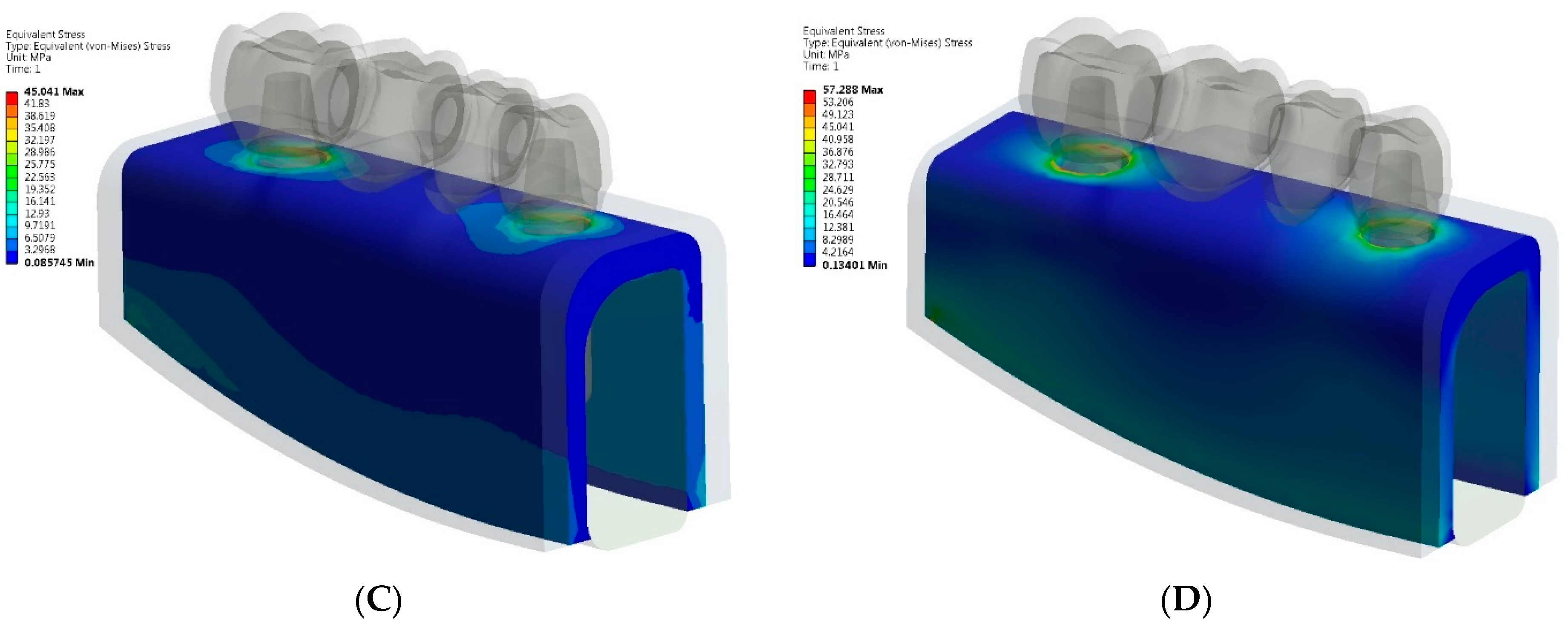
3. Results
4. Discussion
5. Conclusions
- The greatest von Mises stress values were higher in zirconia implant and tooth-implant FPDs compared to PFM FPDs under both axial and oblique loading scenarios.
- The zirconia tooth-implant-supported FPD demonstrated the highest recorded stress values within the implant-abutment system.
- Peak stresses were localized at the neck of the implants in both zirconia implant and tooth-implant supported FPDs, with the highest stress values observed at these sites.
- Comparable maximum stress levels were noted in the cortical bone for both PFM and zirconia implant-supported FPDs. However, the zirconia tooth-implant-supported FPD model demonstrated nearly double the stress value in the cortical bone relative to the PFM tooth-implant-supported FPD model.
- Greater maximum von Mises stresses were identified in the cortical bone as opposed to the cancellous bone, irrespective of the prosthetic material used.
- Based on the stress distribution patterns, PFM FPDs are identified as the preferred option for multiple-unit tooth-implant-supported FPDs.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.E.; Heimisdottir, K. Dental implants–Are they better than natural teeth? Eur. J. Oral Sci. 2018, 126, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Kaiser, D.A.; Jones, J.D. Guidelines for splinting implants. J. Prosthet. Dent. 2000, 84, 210–214. [Google Scholar] [CrossRef] [PubMed]
- von Stein-Lausnitz, M.; Nickenig, H.J.; Wolfart, S.; Neumann, K.; von Stein-Lausnitz, A.; Spies, B.C.; Beuer, F. Survival rates and complication behaviour of tooth implant-supported, fixed dental prostheses: A systematic review and meta-analysis. J. Dent. 2019, 88, 103167. [Google Scholar] [CrossRef] [PubMed]
- Rungsiyakull, C.; Chen, J.; Rungsiyakull, P.; Li, W.; Swain, M.; Li, Q. Bone’s responses to different designs of implant-supported fixed partial dentures. Biomech. Model Mechanobiol. 2015, 14, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Pjetursson, B.E.; Tan, K.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. II. Combined tooth-implant-supported FPDs. Clin. Oral Implants Res. 2004, 15, 643–653. [Google Scholar] [CrossRef]
- Gunne, J.; Astrand, P.; Lindh, T.; Borg, K.; Olsson, M. Tooth-implant and implant supported fixed partial dentures: A 10-year report. Int. J. Prosthodont. 1999, 12, 216–221. [Google Scholar]
- Olsson, M.; Gunne, J.; Astrand, P.; Borg, K. Bridges supported by free-standing implants versus bridges supported by tooth and implant. Clin. Oral Implant. Res. 1995, 6, 114–121. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of survival and complication rates of tooth-supported fixed dental prostheses (FDPs) and implant-supported FDPs and single crowns (SCs). Clin. Oral Implant. Res. 2007, 18, 97–113. [Google Scholar] [CrossRef]
- Vieira, F.R.; Bitencourt, S.B.; Rosa, C.D.D.R.D.; Vieira, A.B.; Santos, D.M.D.; Goiato, M.C. Influence of Different Restoring Materials on Stress Distribution in Prosthesis on Implants: A Review of Finite Element Studies. Eur. J. Dent. 2023, 17, 1–6. [Google Scholar] [CrossRef]
- Arena, A.; Prete, F.; Rambaldi, E.; Bignozzi, M.C.; Monaco, C.; Fiore, A.D.; Chevalier, J. Nanostructured Zirconia-Based Ceramics and Composites in Dentistry: A State-of-the-Art Review. Nanomaterials 2019, 9, 1393. [Google Scholar] [CrossRef]
- Sailer, I.; Strasding, M.; Valente, M.; Zwahlen, M.; Liu, S.; Pjetursson, B.E. A systematic review of the survival and complication rates of zirconia-ceramic and metal-ceramic multiple-unit fixed dental prostheses. Clin. Oral Implant. Res. 2018, 29, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.; Caldari, M.; Scotti, R. Clinical Evaluation of Zirconia-Based Restorations on Implants: A Retrospective Cohort Study from the AIOP Clinical Research Group. Int. J. Prosthodont. 2016, 28, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Rexhepi, I.; Santilli, M.; D’Addazio, G.; Tafuri, G.; Manciocchi, E.; Caputi, S.; Sinjari, B. Clinical applications and mechanical properties of CAD-CAM materials in restorative and prosthetic dentistry: A systematic review. J. Funct. Biomater. 2023, 14, 431. [Google Scholar] [CrossRef]
- Muller, D.; Rues, S. In vitro chipping behaviour of all-ceramic crowns with a zirconia framework and feldspathic veneering: Comparison of CAD/CAM-produced veneer with manually layered veneer. J. Oral Rehabil. 2013, 40, 519–525. [Google Scholar]
- Ishigaki, S.; Nakano, T.; Yamada, S.; Nakamura, T.; Takashima, F. Biomechanical stress in bone surrounding an implant under simulated chewing. Clin. Oral Implant. Res. 2003, 14, 97–102. [Google Scholar] [CrossRef]
- Miyata, T.; Kobayashi, Y.; Araki, H.; Ohto, T.; Shin, K. The influence of controlled occlusal overload on peri-implant tissue. Part 3: A histologic study in monkeys. Int. J. Oral Maxillofac. Implant. 2000, 15, 425–431. [Google Scholar]
- Ciftçi, Y.; Canay, S. Stress distribution on the metal framework of the implant-supported fixed prosthesis using different veneering materials. Int. J. Prosthodont. 2001, 14, 406–411. [Google Scholar]
- Gungor, M.B.; Yilmaz, H. Evaluation of stress distributions occurring on zirconia and titanium implant-supported prosthesis: A three-dimensional finite element analysis. J. Prosthet. Dent. 2016, 116, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Sotto-Maior, B.S.; Senna, P.M.; da Silva, W.J.; Rocha, E.P.; Del Bel Cury, A.A. Influence of crown-to-implant ratio, retention system, restorative material, and occlusal loading on stress concentrations in single short implants. Int. J. Oral Maxillofac. Implant. 2012, 27, e13–e18. [Google Scholar]
- Meriç, G.; Erkmen, E.; Kurt, A.; Eser, A. Influence of prosthesis type and material on the stress distribution in bone around implants: A 3-dimensional finite element analysis. J. Dent. Sci. 2011, 6, 25–32. [Google Scholar] [CrossRef]
- Arinc, H. Effects of Prosthetic Material and Framework Design on Stress Distribution in Dental Implants and Peripheral Bone: A Three-Dimensional Finite Element Analysis. Med. Sci. Monit. 2018, 24, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Toussi, C.A.; Ezatpour, H.R.; Haddania, J.; Shiri, J.G. Effect of using different metal and ceramic materials as restorations on stress distribution around dental implants: A comparative finite element study. Mater. Res. Express 2018, 5, 11. [Google Scholar]
- Jain, H.; Kalra, T.; Kumar, M.; Bansal, A.; Jain, D. Three-Dimensional Finite Element Analysis to Evaluate Stress Distribution in Tooth and Implant Supported Fixed Partial Denture–An In Vitro Study. Dent. J. Adv. Stud. 2020, 8, 84–91. [Google Scholar] [CrossRef]
- Tsouknidas, A.; Giannopoulos, D.; Savvakis, S.; Michailidis, N.; Evdokia, L.; Fytanidis, D.; Pissiotis, A.; Michalakis, K. The influence of bone’s quality on the biomechanical behavior of a tooth-implant fixed partial denture. A 3D Finite Element Analysis (FEA). Int. J. Oral Maxillofac. Implant. 2016, 31, e143–e154. [Google Scholar] [CrossRef]
- Rand, A.; Kohorst, P.; Greuling, A.; Borchers, L.; Stiesch, M. Stress distribution in all-ceramic posterior 4-unit fixed dental prosthesis supported in different ways: Finite element analysis. Implant Dent. 2016, 25, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Rosenstiel, S.F.; Land, M.F.; Fujimoto, J. Contemporary Fixed Prosthodontics, 3rd ed.; Mosby: Maryland Heights, MO, USA, 2001. [Google Scholar]
- Stegaroiu, R.; Kusakari, H.; Nishiyama, S.; Miyakawa, O. Influence of prosthesis material on stress distribution in bone and implant: A 3- dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 1998, 13, 781–790. [Google Scholar]
- Tada, S.; Stegaroiu, R.; Kitamurs, E.; Miyakawa, O.; Kusakari, H. Influence of implant design and bone quality on stress/strain distribution in bone around implants: A 3-dimensional finite element analysis. Int. J. Oral Maxillofac. Implant. 2003, 18, 357–368. [Google Scholar]
- Huang, L.S.; Huang, Y.C.; Yuan, C.; Ding, S.J.; Yan, M. Biomechanical evaluation of bridge span with three implant abatment designs and two connectors for tooth-implant supported prosthesis: A finite element analysis. J. Dent. Sci. 2023, 18, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Swain, M.V.; Li, Q.; Ironside, J.; Steven, G.P. Fibre reinforced composite dental bridge. Part II: Numerical investigation. Biomaterials 2004, 25, 4995–5001. [Google Scholar] [CrossRef]
- Lin, C.h.L.; Wang, J.C.h.; Chang, W.J. Biomechanical interactions in tooth–implant-supported fixed partial dentures with variations in the number of splinted teeth and connector type: A finite element analysis. Clin. Oral Implant. Res. 2008, 19, 107–117. [Google Scholar] [CrossRef]
- Alberto, L.H.J.; Kalluri, L.; Esquivel-Upshaw, J.F.; Duan, Y. Three-dimensional finite element analysis of different connector designs for all-ceramic implant-supported fixed dental prostheses. Ceramics 2022, 5, 34–43. [Google Scholar] [CrossRef]
- Ebadian, B.; Fathi, A.; Tabatabaei, S. Stress distribution in 5-unit fixed partial dentures with a pier abutment and Rigid and Nonrigid Connectors with Two Different Occlusal Schemes: A Three-Dimensional Finite Element Analysis. Int. J. Dent. 2023, 3347197. [Google Scholar] [CrossRef]
- Jae-Hyun, L.; Jang, H.Y.; Lee, S.Y. Finite Element Analysis of Dental Implants with Zirconia Crown Restorations: Conventional Cement-Retained vs. Cementless Screw-Retained. Materials 2021, 14, 2666. [Google Scholar] [CrossRef] [PubMed]
- Degirmenci, K.; Kocak-Buyukdere, A.; Ekici, B. Evaluation of reliability of zirconia materials to be used in implant-retained restoration on the atrophic bone of the posterior maxilla: A finite element study. J. Adv. Prosthodont. 2019, 11, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, K.; Lang, N.P.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (FPDs) after an observation period of at least 5 years. I. Implant-supported FPDs. Clin. Oral Implant. Res. 2004, 15, 625–642. [Google Scholar] [CrossRef]
- Weber, H.P.; Zimering, Y. Survival and complication rates of fixed partial dentures supported by a combination of teeth and implants. J. Evid. Based Dent. Pract. 2010, 10, 58–60. [Google Scholar] [CrossRef]
- Gomes, E.A.; Assunção, W.G.; Tabata, L.F.; Barão, V.A.; Delben, J.A.; de Sousa, E.A. Effect of passive fit absence in the prosthesis/implant/retaining screw system: A two-dimensional finite element analysis. J. Craniofacial Surg. 2009, 20, 2000–2005. [Google Scholar] [CrossRef]
- Menicucci, G.; Mossolov, A.; Mozzati, M.; Lorenzetti, M.; Preti, G. Tooth-implant connection: Some biomechanical aspects based on finite element analyses. Clin. Oral Implant. Res. 2002, 13, 334–341. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, C.; Wang, Z.; Zeng, T.; Wang, Y. Optimization of stress distribution of bone-implant interface. Biomater. Adv. 2023, 147, 213342. [Google Scholar] [CrossRef]
- Alemayehu, D.B.; Jeng, Y.R. Three-Dimensional Finite Element Investigation into Effects of Implant Thread Design and Loading Rate on Stress Distribution in Dental Implants and Anisotropic Bone. Materials 2021, 14, 6974. [Google Scholar] [CrossRef]




| Materials | Young’s Modulus (GPa) | Poisson’s Ratio |
|---|---|---|
| Titanium | 110 | 0.35 |
| Ni-Cr alloy | 204 | 0.30 |
| Corticale bone | 13.7 | 0.30 |
| Cancellous bone | 1.37 | 0.30 |
| Dentin | 18.6 | 0.31 |
| Periodontal ligament | 69 | 0.45 |
| Feldspathic porcelain | 0.069 | 0.35 |
| Monolithic zirconia | 220 | 0.30 |
| Resin cement | 8.3 | 0.30 |
| Mucosa | 0.34 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eric, J.; Bjelovic, L.; Radovic, I.; Krunic, J.; Milic-Lemic, A.; Gupta, N.; Ali, K. Effect of Prosthetic Material and Support Type on Stress Distribution of Fixed Partial Dentures: A Finite Element Study. Ceramics 2024, 7, 1204-1217. https://doi.org/10.3390/ceramics7030079
Eric J, Bjelovic L, Radovic I, Krunic J, Milic-Lemic A, Gupta N, Ali K. Effect of Prosthetic Material and Support Type on Stress Distribution of Fixed Partial Dentures: A Finite Element Study. Ceramics. 2024; 7(3):1204-1217. https://doi.org/10.3390/ceramics7030079
Chicago/Turabian StyleEric, Jelena, Ljiljana Bjelovic, Igor Radovic, Jelena Krunic, Aleksandra Milic-Lemic, Nidhi Gupta, and Kamran Ali. 2024. "Effect of Prosthetic Material and Support Type on Stress Distribution of Fixed Partial Dentures: A Finite Element Study" Ceramics 7, no. 3: 1204-1217. https://doi.org/10.3390/ceramics7030079
APA StyleEric, J., Bjelovic, L., Radovic, I., Krunic, J., Milic-Lemic, A., Gupta, N., & Ali, K. (2024). Effect of Prosthetic Material and Support Type on Stress Distribution of Fixed Partial Dentures: A Finite Element Study. Ceramics, 7(3), 1204-1217. https://doi.org/10.3390/ceramics7030079






