Rheological Behavior of an Algerian Natural Kaolin: Effect of Dispersant
Abstract
1. Introduction
2. Materials and Methods
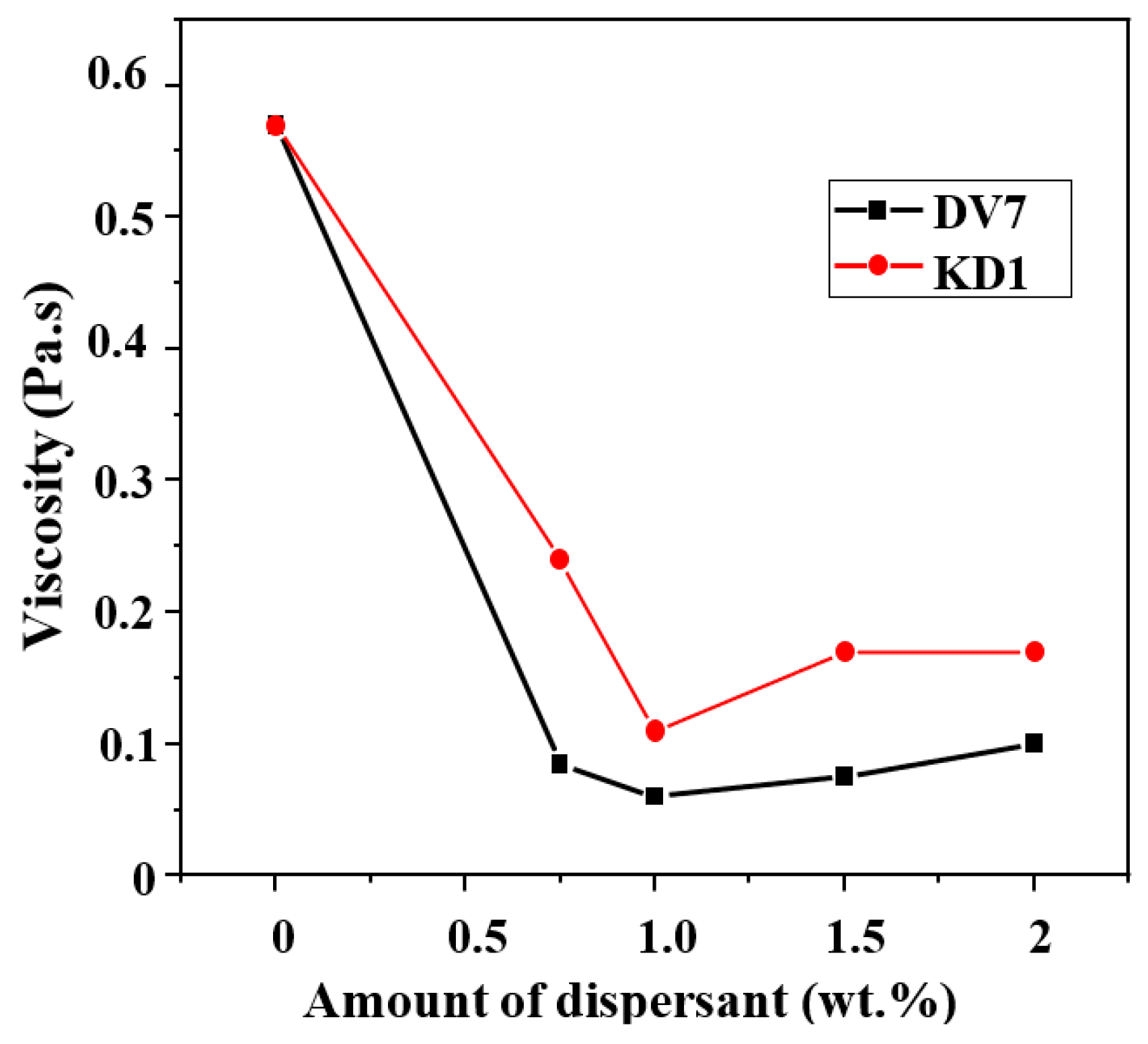
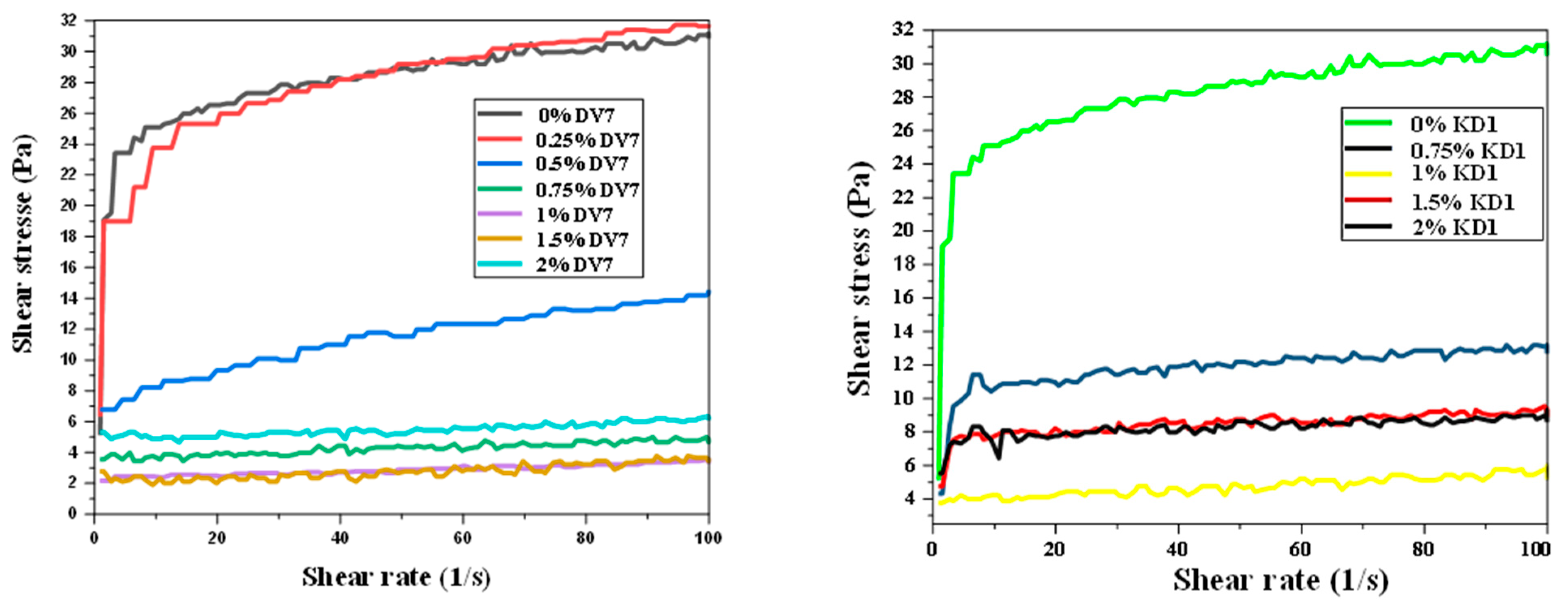
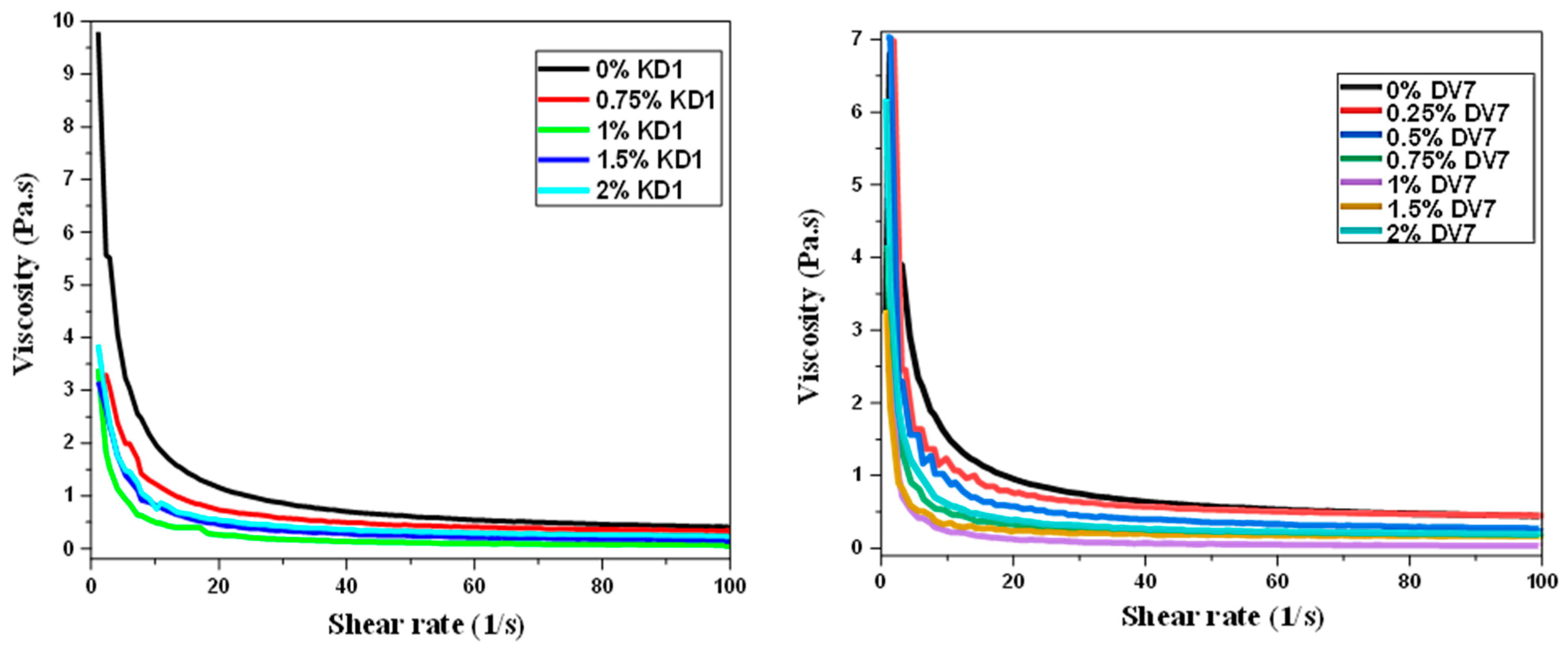
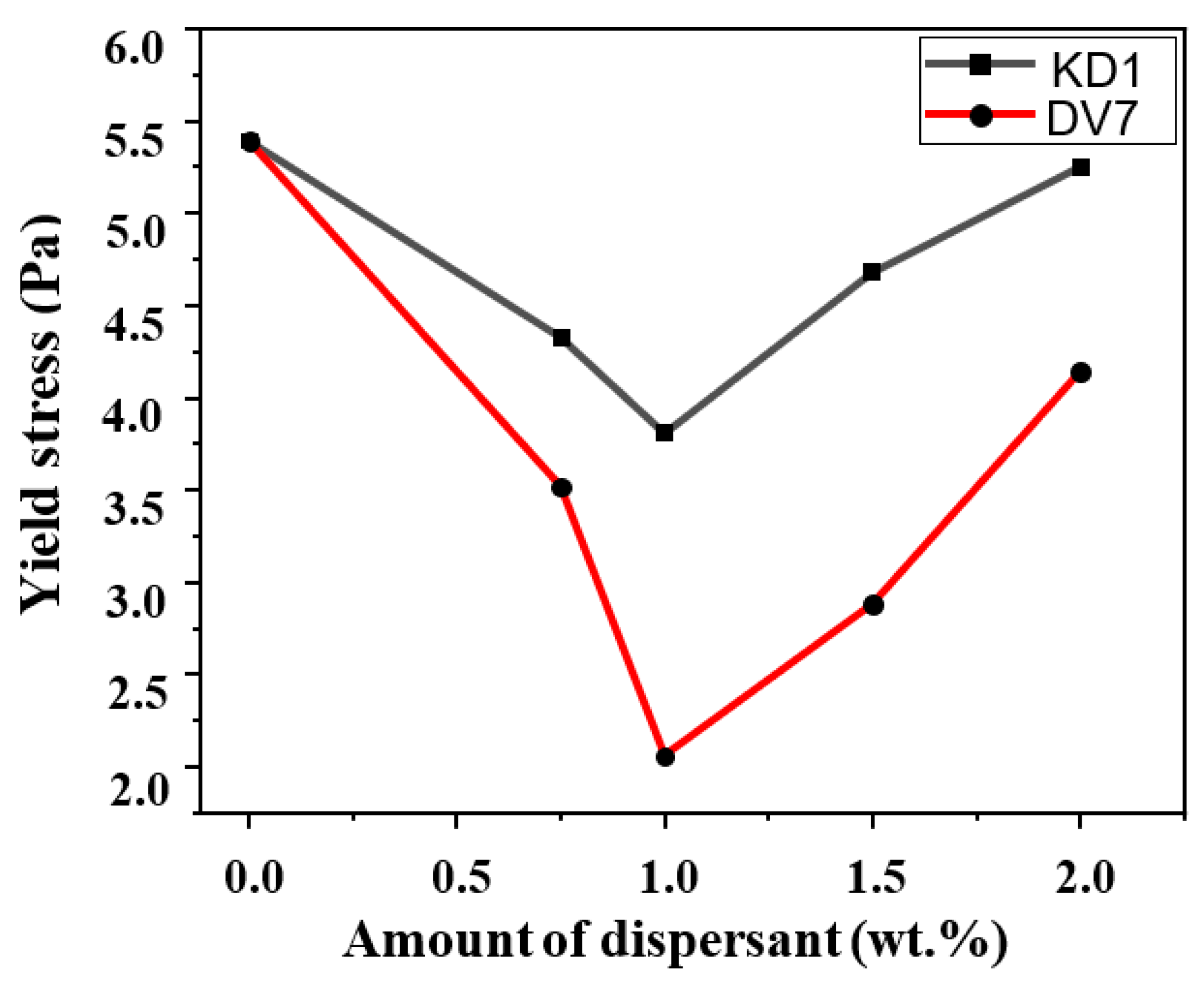
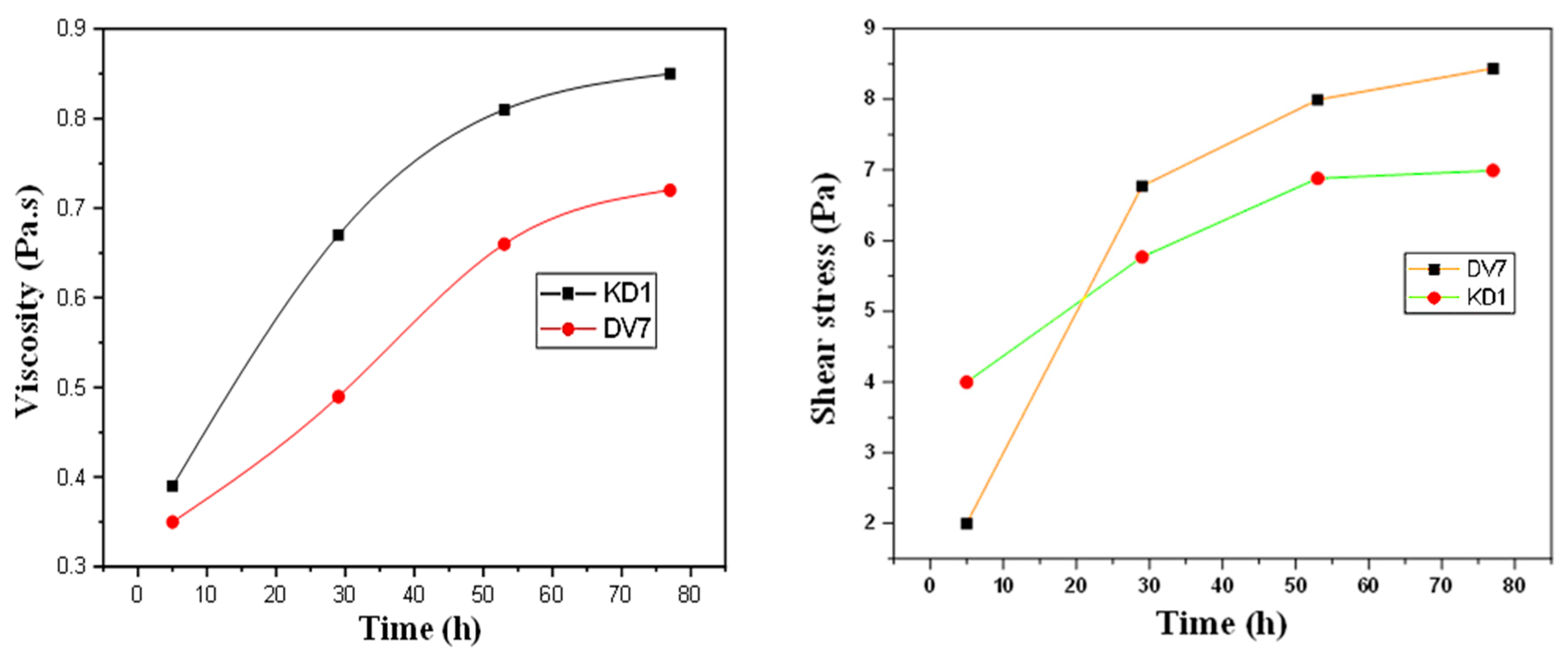
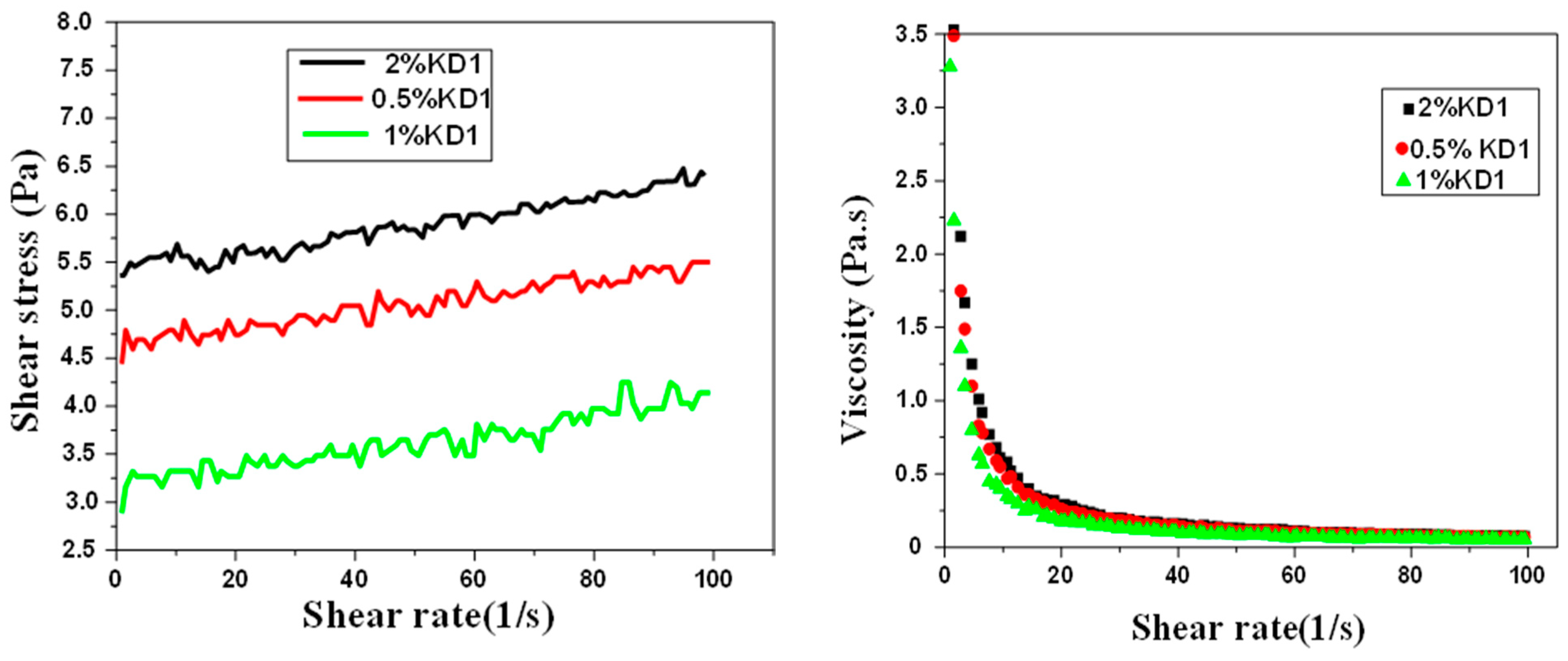
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fourati, N.; Léger, D.; Fakhfakh, Z. Etude de la défloculation de suspensions concentrées de kaolin en milieu aqueux. Cas des polyacrylates et des silicates de sodium. Les Cah. Rhéologie 1998, 15, 475–487. [Google Scholar]
- Teh, E.J.; Leong, Y.K.; Liu, Y.; Fourie, A.B.; Fahey, M. Differences in the Rheology and Surface Chemistry of Kaolin Clay Slurries: The Source of the Variations. Chem. Eng. Sci. 2009, 64, 3817–3825. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry, 2nd ed.; National Academy of Sciences: Washington, DC, USA, 1977. [Google Scholar]
- Mpofu, P.; Addai-Mensah, J.; Ralston, J. Investigation of the Effect of Polymer Structure Type on Flocculation, Rheology and Dewatering Behaviour of Kaolinite Dispersions. Int. J. Miner. Process. 2003, 71, 247–268. [Google Scholar] [CrossRef]
- Peng, F.F.; Di, P. Efecto de Las Sales Multivalentes de Calcio y Aluminio Sobre La Floculación de La Suspensión de Caolín Con Poliacrilamida Aniónica. J. Colloid Interface Sci. 1994, 164, 229. [Google Scholar] [CrossRef]
- Avadiar, L.; Leong, Y.; Fourie, A.; Nugraha, T. rheological response to ca (ii) concentration—The source of kaolin slurry rheological variation. In Proceedings of the Australasian Chemical Engineering Conference, CHEMECA, Wellington, New Zealand, 23–26 September 2012. [Google Scholar]
- Au, P.; Leong, Y. Colloids and Surfaces A: Physicochemical and Engineering Aspects Rheological and Zeta Potential Behaviour of Kaolin and Bentonite Composite Slurries. Colloids Surf. A Eng. Asp. 2013, 436, 530–541. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces, 2nd ed.; Academic Press Inc.: London, UK, 1992; pp. 139–307. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science, 3rd ed.; Academic Press Inc.: San Diego, CA, USA, 1988; pp. 230–240. [Google Scholar]
- Ersoy, B.; Evcin, A.; Uygunoglu, T.; Akdemir, Z.B.; Brostow, W.; Wahrmund, J. Zeta Potential-Viscosity Relationship in Kaolinite Slurry in the Presence of Dispersants. Arab. J. Sci. Eng. 2014, 39, 5451–5457. [Google Scholar] [CrossRef]
- Johnson, S.B.; Franks, G.V.; Scales, P.J.; Boger, D.V.; Healy, T.W. Surface Chemistry-Rheology Relationships in Concentrated Mineral Suspensions. Int. J. Miner. Process. 2000, 58, 267–304. [Google Scholar] [CrossRef]
- Yao, X.; Tan, S.; Huang, Z.; Jiang, D. Dispersion of Talc Particles in a Silica Sol. Mater. Lett. 2005, 59, 100–104. [Google Scholar] [CrossRef]
- Penner, D.; Lagaly, G. Influence of anions on the rheological properties of clay mineral dispersions. Appl. Clay Sci. 2001, 19, 131–142. [Google Scholar] [CrossRef]
- Sposito, G. Surface-reactions in natural aqueous colloidal systems. Chimia 1989, 43, 169–176. [Google Scholar] [CrossRef]
- Singh, B.P.; Menchavez, R.; Takai, C.; Fuji, M.; Takahashi, M. Stability of Dispersions of Colloidal Alumina Particles in Aqueous Suspensions. J. Colloid Interface Sci. 2005, 291, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Knowles, J.C.; Callcut, S.; Georgiou, G. Characterisation of the Rheological Properties and Zeta Potential of a Range of Hydroxyapatite Powders. Biomaterials 2000, 21, 1387–1392. [Google Scholar] [CrossRef]
- Shakeel, A.; Ali, W.; Chassagne, C.; Kirichek, A. Tuning the Rheological Properties of Kaolin Suspensions Using Biopolymers. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130120. [Google Scholar] [CrossRef]
- Liu, D. Adsorption, Rheology, Packing, and Sintering of Nanosize Ceramic Powders. Ceram. Int. 1999, 25, 107–113. [Google Scholar] [CrossRef]
- Kolli, M.; Hamidouche, M.; Bouaouadja, N.; Fantozzi, G. Physicochemical Characterization of Algerian Kaolin to Be Used in the Production of Refractories. J. Optoelectron. Adv. Mater.-Symp. 2011, 3, 134–136. [Google Scholar]
- Adeosun, S.O.; Sekunowo, O.I.; Taiwo, O.O.; Ayoola, W.A.; Machado, A. Evaluation of Secondary Aluminium Dross in Calcium Aluminate Cement. Adv. Mater. 2014, 3, 541–546. [Google Scholar] [CrossRef]
- Gil, A.; Korili, S.A. Management and Valorization of Aluminum Saline Slags: Current Status and Future Trends. Chem. Eng. J. 2016, 289, 74–84. [Google Scholar] [CrossRef]
- Meshram, A.; Singh, K.K. Recovery of Valuable Products from Hazardous Aluminum Dross. Resour. Conserv. Recycl. 2018, 130, 95–108. [Google Scholar] [CrossRef]
- Satish Reddy, M.; Neeraja, D. Aluminum Residue Waste for Possible Utilisation as a Material: A Review. Sādhanā 2018, 43, 124. [Google Scholar] [CrossRef]
- Hiraki, T.; Nosaka, A.; Okinaka, N.; Akiyama, T. Synthesis of Zeolite-X from Waste Materials. ISIJ Int. 2009, 49, 1644–1648. [Google Scholar] [CrossRef][Green Version]
- Jraba, N.; Tounsi, H.; Makhlouf, T. Valorization of Aluminum Chips into γ-Al2O3 and η-Al2O3 with High Surface Areas via the Precipitation Route. Int. J. Miner. Metall. Mater. 2018, 9, 1003–1014. [Google Scholar] [CrossRef]
- Chargui, F.; Hamidouche, M.; Belhouchet, H.; Jorand, Y.; Doufnoune, R.; Fantozzi, G. Mullite Fabrication from Natural Kaolin. Boletín Soc. Española Cerámica Vidr. 2018, 57, 169–177. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía de Gutiérrez, R.; Puertas, F. Effect of Metakaolin on Natural Volcanic Pozzolan-Based Geopolymer Cement. Appl. Clay Sci. 2016, 132–133, 491–497. [Google Scholar] [CrossRef]
- Tchakoute Kouamo, H.; Mbey, J.A.; Elimbi, A.; Kenne Diffo, B.B.; Njopwouo, D. Synthesis of Volcanic Ash-Based Geopolymer Mortars by Fusion Method: Effects of Adding Metakaolin to Fused Volcanic Ash. Ceram. Int. 2013, 39, 1613–1621. [Google Scholar] [CrossRef]
- Izak, P.; Ogłaza, L.; Mozgawa, W.; Mastalska-Popławska, J.; Stempkowska, A. Influence of the Type of Aqueous Sodium Silicate on the Stabilization and Rheology of Kaolin Clay Suspensions. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 196, 155–159. [Google Scholar] [CrossRef]
- Kouamo, H.T.; Elimbi, A.; Mbey, J.A.; Sabouang, C.J.N.; Njopwouo, D. The Effect of Adding Alumina-Oxide to Metakaolin and Volcanic Ash on Geopolymer Products: A Comparative Study. Constr. Build. Mater. 2012, 35, 960–969. [Google Scholar] [CrossRef]
- Sainz, M.A.; Serrano, F.J.; Amigo, J.M.; Bastida, J.; Caballero, A. XRD Microstructural Analysis of Mullites Obtained from Kaolinite—Alumina Mixtures. J. Eur. Ceram. Soc. 2000, 20, 403–412. [Google Scholar] [CrossRef]
- Robles, P.; Piceros, E.; Leiva, W.H.; Valenzuela, J.; Toro, N.; Jeldres, R.I. Analysis of Sodium Polyacrylate as a Rheological Modifier for Kaolin Suspensions in Seawater. Appl. Clay Sci. 2019, 183, 105328. [Google Scholar] [CrossRef]
- Rubio-Hernández, F.J.; Páez-Flor, N.M.; Gómez-Merino, A.I.; Sánchez-Luque, F.J.; Delgado-García, R.; Goyos-Pérez, L. The Influence of High-Concentration Na Hexametaphosphate Dispersant on the Rheological Behavior of Aqueous Kaolin Dis-persions. Clays Clay Miner. 2016, 64, 210–219. [Google Scholar] [CrossRef]
- Rand, B.; Melton, I.E. Isoelectric point of the edge surface of kaolinite. Nature 1975, 257, 214–216. [Google Scholar] [CrossRef]
- Zaman, A.A.; Mathur, S. Influence of Dispersing Agents and Solution Conditions on the Solubility of Crude Kaolin. J. Colloid Interface Sci. 2004, 271, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Franks, G.V.; Meagher, L. The Isoelectric Points of Sapphire Crystals and Alpha-Alumina Powder. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Durgut, E.; Cinar, M.; Terzi, M.; Kursun Unver, I.; Yildirim, Y.; Ozdemir, O. Evaluation of Different Dispersants on the Dis-persion/Sedimentation Behavior of Halloysite, Kaolinite, and Quartz Suspensions in the Enrichment of Halloysite Ore by Me-chanical Dispersion. Minerals 2022, 12, 1426. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, J.; He, B. Effect of Electrical Characteristics Modification on Viscosity of Kaolin Suspension. Adv. Mater. Res. 2011, 335–336, 1262–1266. [Google Scholar] [CrossRef]
- Management, L.; Osmond, G. Dispersion and Zeta Potential of Pure Clays as Related to Net Particle Charge under Varying PH, Electrolyte Concentration and Cation Type. Eur. J. Soil Sci. 1995, 46, 657–665. [Google Scholar]
- Yukselen-Aksoy, Y.; Kaya, A. Specific Surface Area Effect on Compressibility Behaviour of Clayey Soils. Proc. Inst. Civ. Eng. Geotech. Eng. 2013, 166, 76–87. [Google Scholar] [CrossRef]
- Singh, B.P.; Bhattacharjee, S.; Besra, L.; Sengupta, D.K. Evaluation of Dispersibility of Aqueous Alumina Suspension in Presence of Darvan C. Ceram. Int. 2004, 30, 939–946. [Google Scholar] [CrossRef]
- Lee, L.T.; Rahbari, R.; Lecourtier, J.; Chauveteau, G. Adsorption of Polyacrylamides on the Different Faces of Kaolinites. J. Colloid Interface Sci. 1991, 147, 351–357. [Google Scholar] [CrossRef]
- Lee, S.K.; Ryu, S.S.; Yoon, D.H. Synthesis of Fine Ca-Doped BaTiO3 Powders by Solid-State Reaction Method-Part II: Rheological Study on Milling. J. Electroceram. 2007, 18, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, X.; Su, P. Dispersion of Kaolin Powders in Silica Sols. Appl. Clay Sci. 2010, 49, 51–54. [Google Scholar] [CrossRef]
- Nabzar, L.; Pefferkorn, E.; Varoqui, R. Stability of Polymer-Clay Suspensions. The Polyacrylamide-Sodium Kaolinite System. Colloid Surf. A 1988, 30, 345–353. [Google Scholar] [CrossRef]
- Önen, V.; Göçer, M.; Taner, H.A. Kaolen Süspansiyonlarinin Susuzlaştirilmasinda Koagülant Ve Flokülantlari Etkisi. Ömer Halisdemir Üniversitesi Mühendislik Bilim. Derg. 2018, 7, 297–305. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Oliveira, M.; Ferreira, J.M.F. Effect of Solvent Composition on Dispersing Ability of Reaction Sialon Suspensions. J. Colloid Interface Sci. 2003, 259, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Loginov, M.; Larue, O.; Lebovka, N.; Vorobiev, E. Fluidity of Highly Concentrated Kaolin Suspensions: Influence of Particle Concentration and Presence of Dispersant. Colloids Surf. A Physicochem. Eng. Asp. 2008, 325, 64–71. [Google Scholar] [CrossRef]
- Bell, N.S.; Rodriguez, M.A. Dispersion Properties of an Alumina Nanopowder Using Molecular, Polyelectrolyte, and Steric Stabilization. J. Nanosci. 2004, 4, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wu, H.; Liu, Z.; Hu, H.; Guo, S. Study on the Adsorption Behavior of Polymeric Dispersants to S-ZnF Particles during Grinding Process. Materials 2023, 16, 1287. [Google Scholar] [CrossRef]
- Fripiat, J.J.; Gatineau, L. Interaction Eau-Argile. Sci. Geol. Bull. 1984, 37, 283–296. [Google Scholar] [CrossRef]
- Tseng, W.J.; Wu, C.H. Aggregation, Rheology and Electrophoretic Packing Structure of Aqueous Al2O3 Nanoparticle Suspensions. Acta Mater. 2002, 50, 3757–3766. [Google Scholar] [CrossRef]
- Qiang, Z.; Xiao, X.; Li, D.; Fu, F.; Ning, Y. Performance Evaluation of Laponite as a Mud—Making Material for Drilling Fluids. Pet. Sci. 2019, 16, 890–900. [Google Scholar] [CrossRef]
- Ramsay, J.D.F.; Lindner, P. Small-angle neutron scattering investigations of the structure of thixotropic dispersions of smectite clay colloids. J. Chem. Soc. Faraday Trans. 1993, 89, 4207–4214. [Google Scholar] [CrossRef]
- Rubbi, F.; Das, L.; Habib, K.; Aslfattahi, N.; Saidur, R. State-of-the-Art Review on Water-Based Nanofluids for Low Temperature Solar Thermal Collector Application. Sol. Energy Mater. Sol. Cells 2021, 230, 111220. [Google Scholar] [CrossRef]
- Rahman, M.T.; Negash, B.M.; Danso, D.K.; Idris, A.; Elryes, A.A.; Umar, I.A. Effects of Imidazolium- and Ammonium-Based Ionic Liquids on Clay Swelling: Experimental and Simulation Approach. J. Pet. Explor. Prod. Technol. 2022, 12, 1841–1853. [Google Scholar] [CrossRef]
- Tsetsekou, A.; Agrafiotis, C.; Leon, I.; Milias, A. Optimization of the Rheological Properties of Alumina Slurries for Ceramic Processing Applications Part II: Spray-Drying. J. Eur. Ceram. Soc. 2001, 21, 493–506. [Google Scholar] [CrossRef]
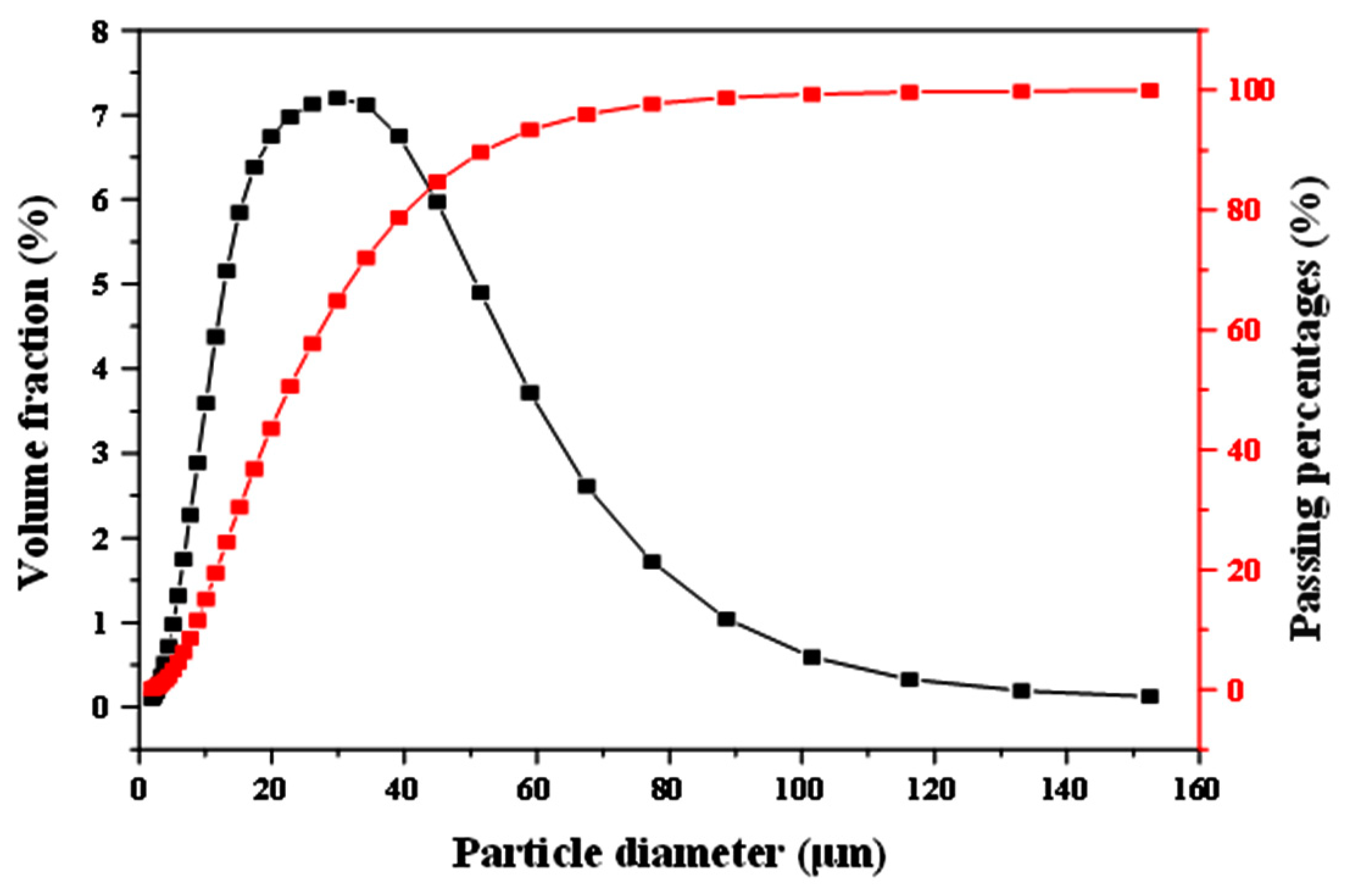

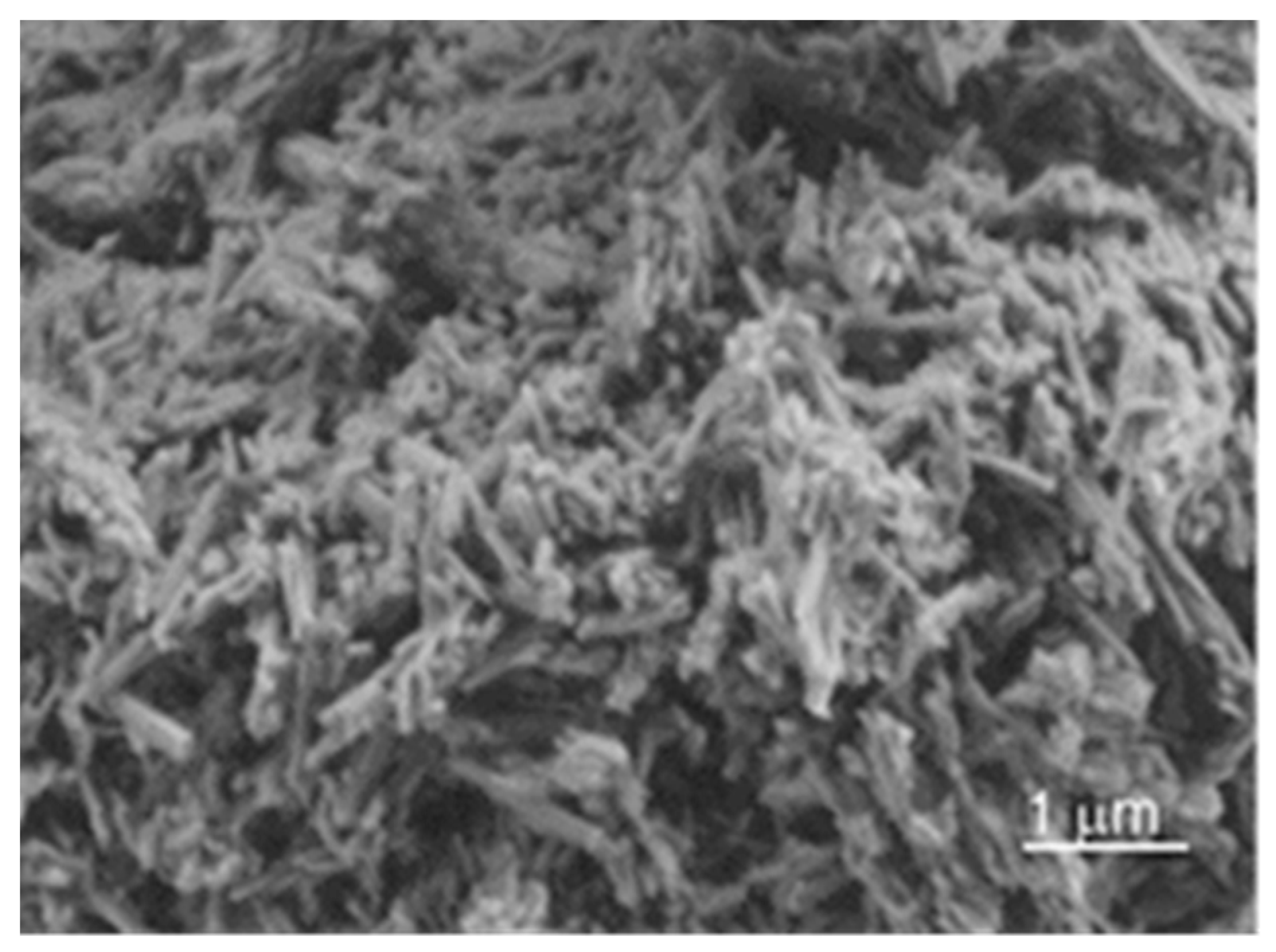
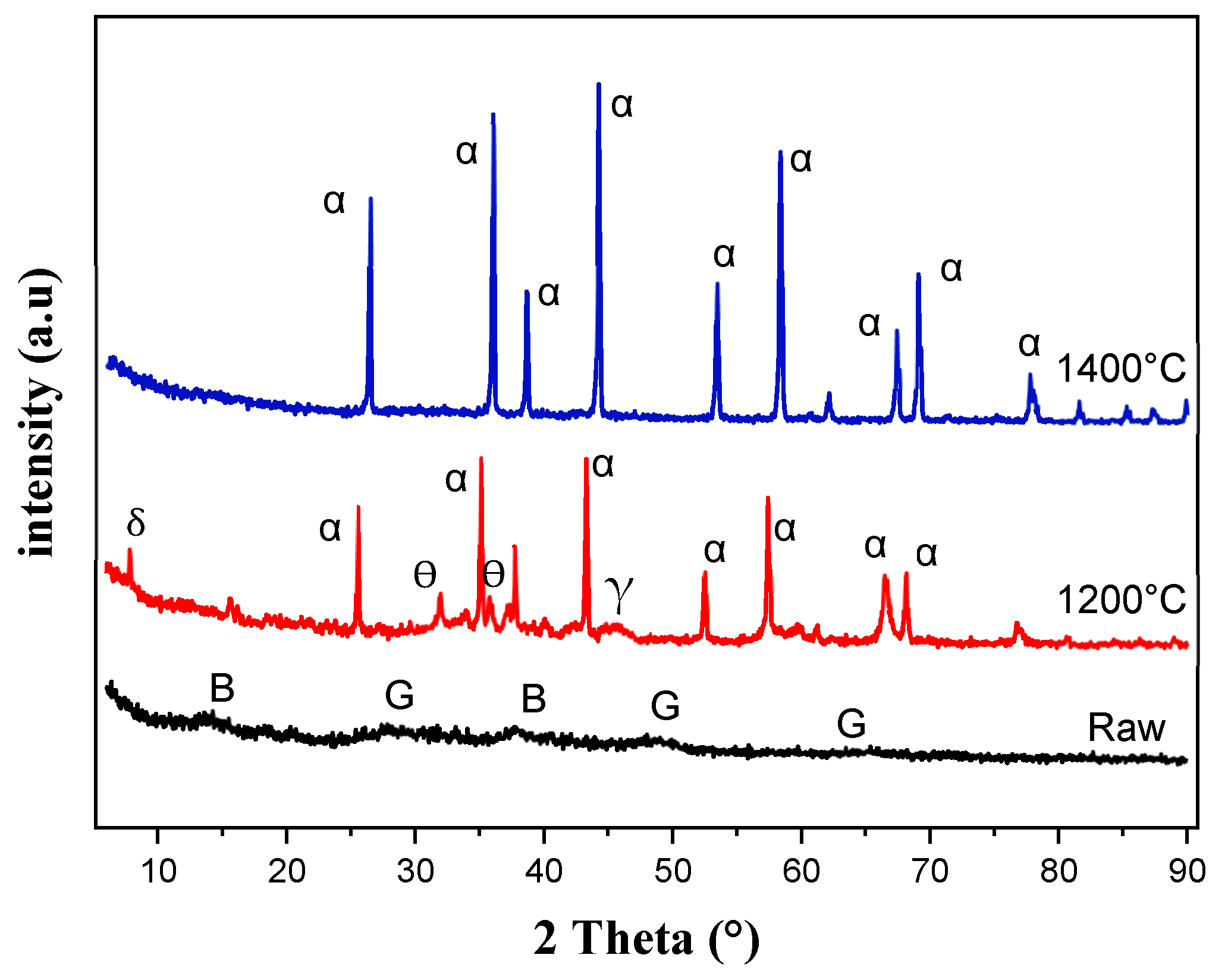
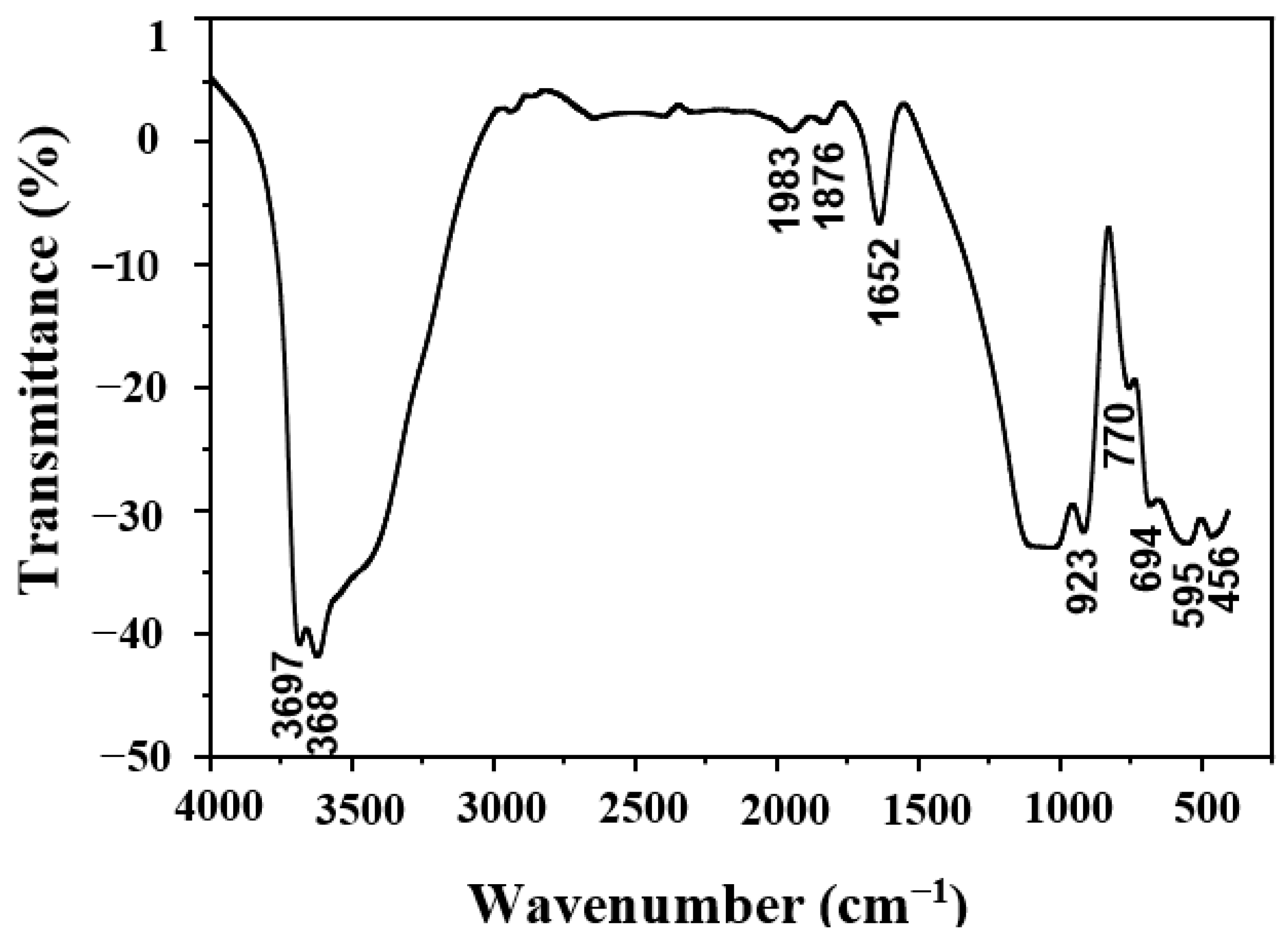

| Oxides | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Kaolin wt.% | 44.9 | 37.49 | 0.12 | 0.26 | 0.13 | 0.1 | 0.19 | 0.01 |
| Aluminum slag wt.% | 2.5 | 87 | 0.15 | 0.12 | 0.21 | 0.2 | 0.2 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chargui, F.; Hamidouche, M.; Louahdi, R.; Fantozzi, G. Rheological Behavior of an Algerian Natural Kaolin: Effect of Dispersant. Ceramics 2024, 7, 1159-1171. https://doi.org/10.3390/ceramics7030076
Chargui F, Hamidouche M, Louahdi R, Fantozzi G. Rheological Behavior of an Algerian Natural Kaolin: Effect of Dispersant. Ceramics. 2024; 7(3):1159-1171. https://doi.org/10.3390/ceramics7030076
Chicago/Turabian StyleChargui, Fouzia, Mohamed Hamidouche, Rachid Louahdi, and Gilbert Fantozzi. 2024. "Rheological Behavior of an Algerian Natural Kaolin: Effect of Dispersant" Ceramics 7, no. 3: 1159-1171. https://doi.org/10.3390/ceramics7030076
APA StyleChargui, F., Hamidouche, M., Louahdi, R., & Fantozzi, G. (2024). Rheological Behavior of an Algerian Natural Kaolin: Effect of Dispersant. Ceramics, 7(3), 1159-1171. https://doi.org/10.3390/ceramics7030076







