Processing and Tribological Properties of PEO Coatings on AlZn5.5MgCu Aluminium Alloy with Incorporated Al-Cu-Fe Quasicrystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Coatings Deposition
2.2. Friction and Wear Testing and Analysis
2.3. Microscopic Study
3. Results
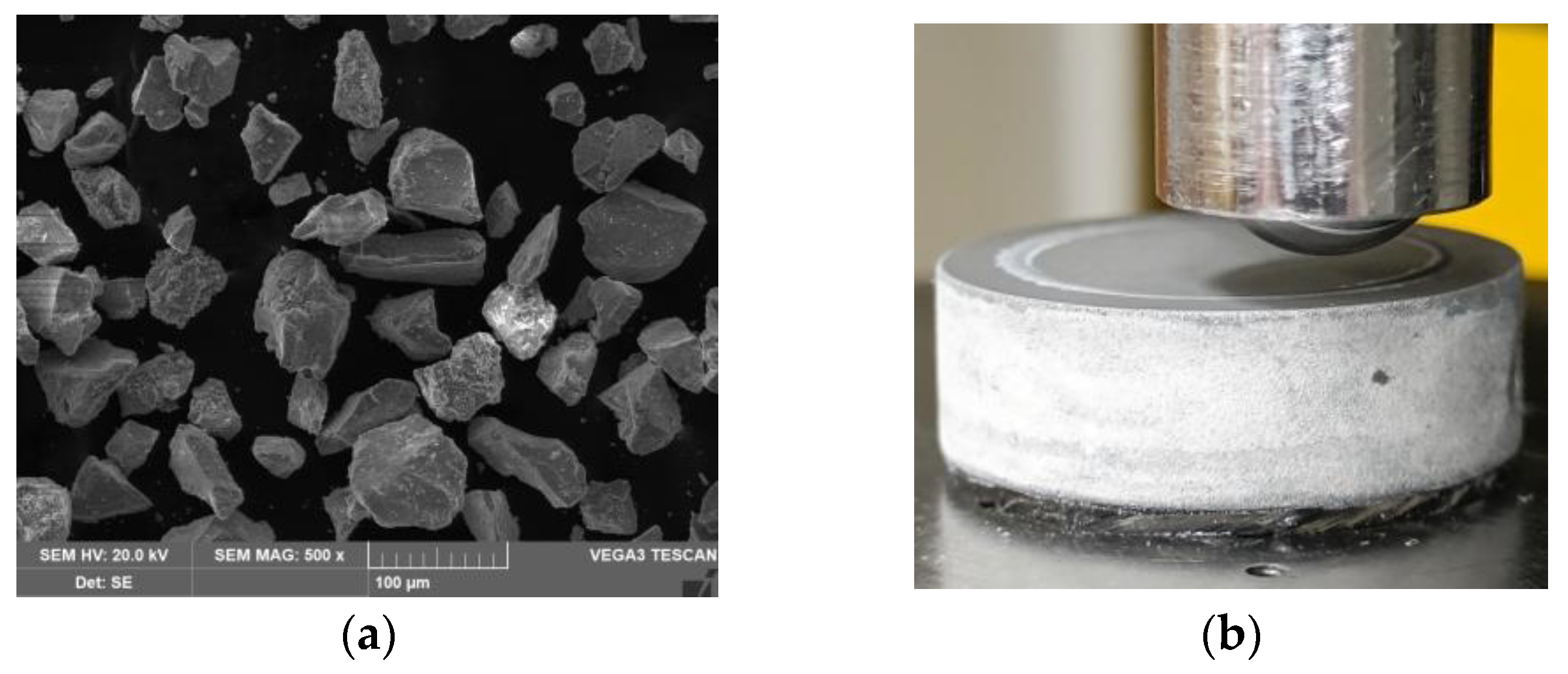
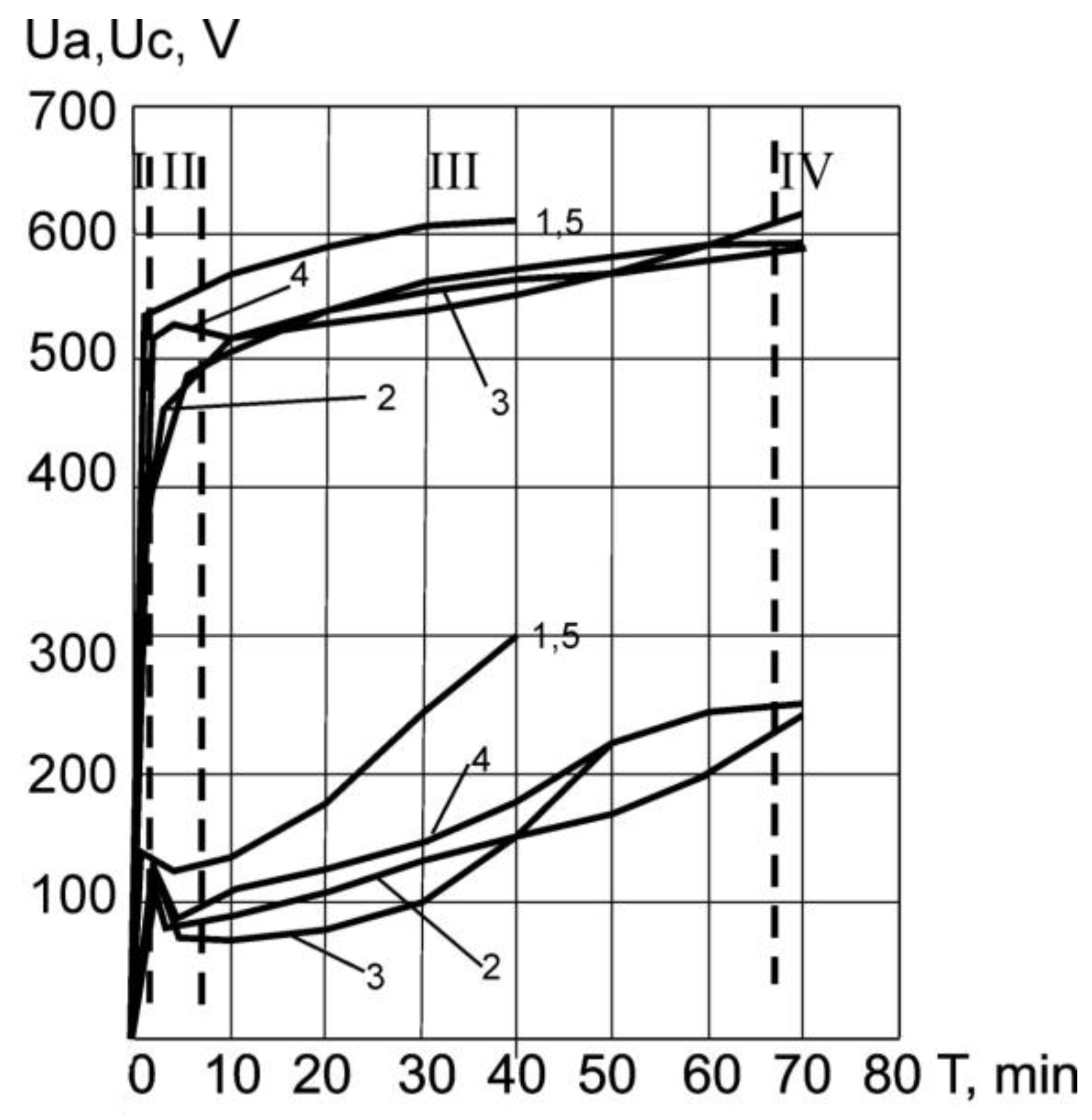
3.1. Plasma Electrolytic Oxidation (PEO)
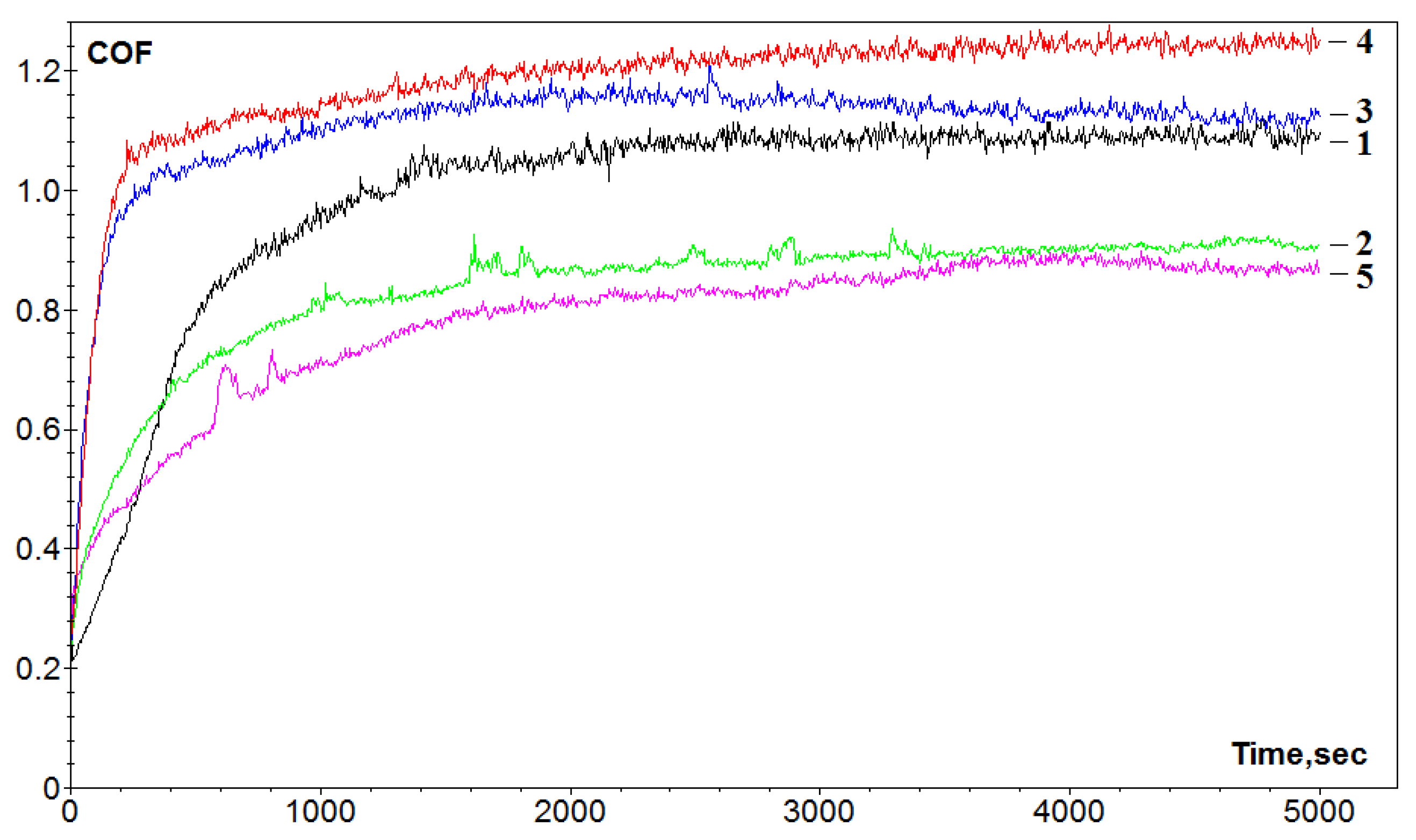
3.2. Friction and Wear Test
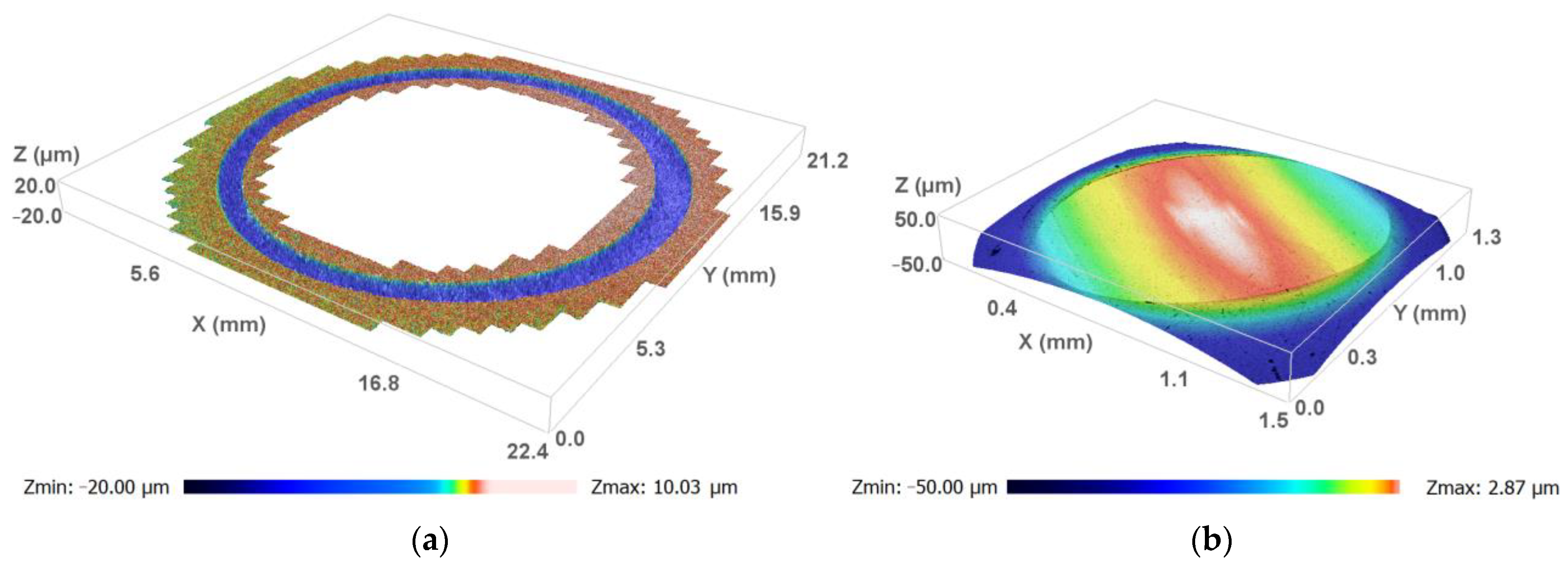
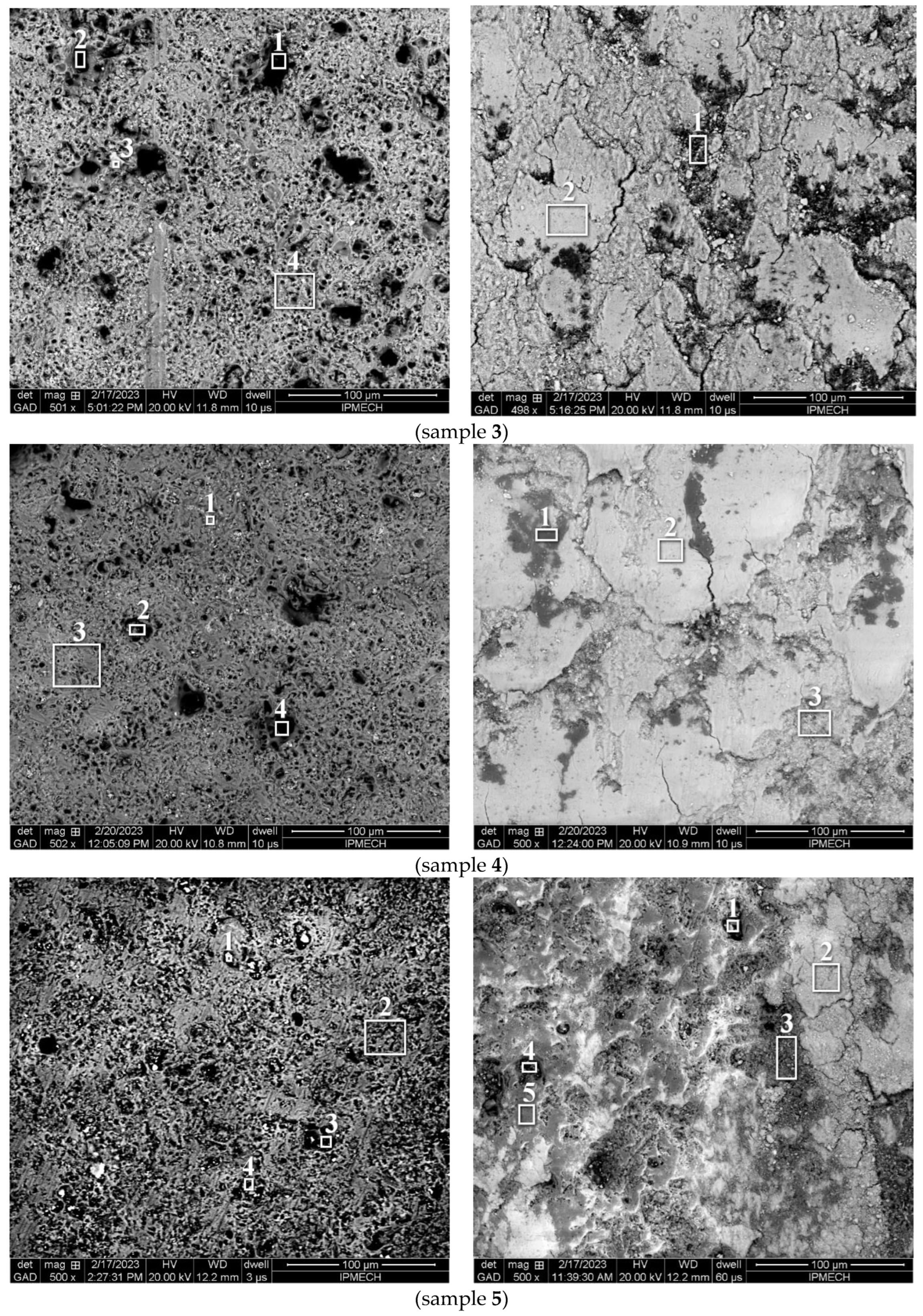
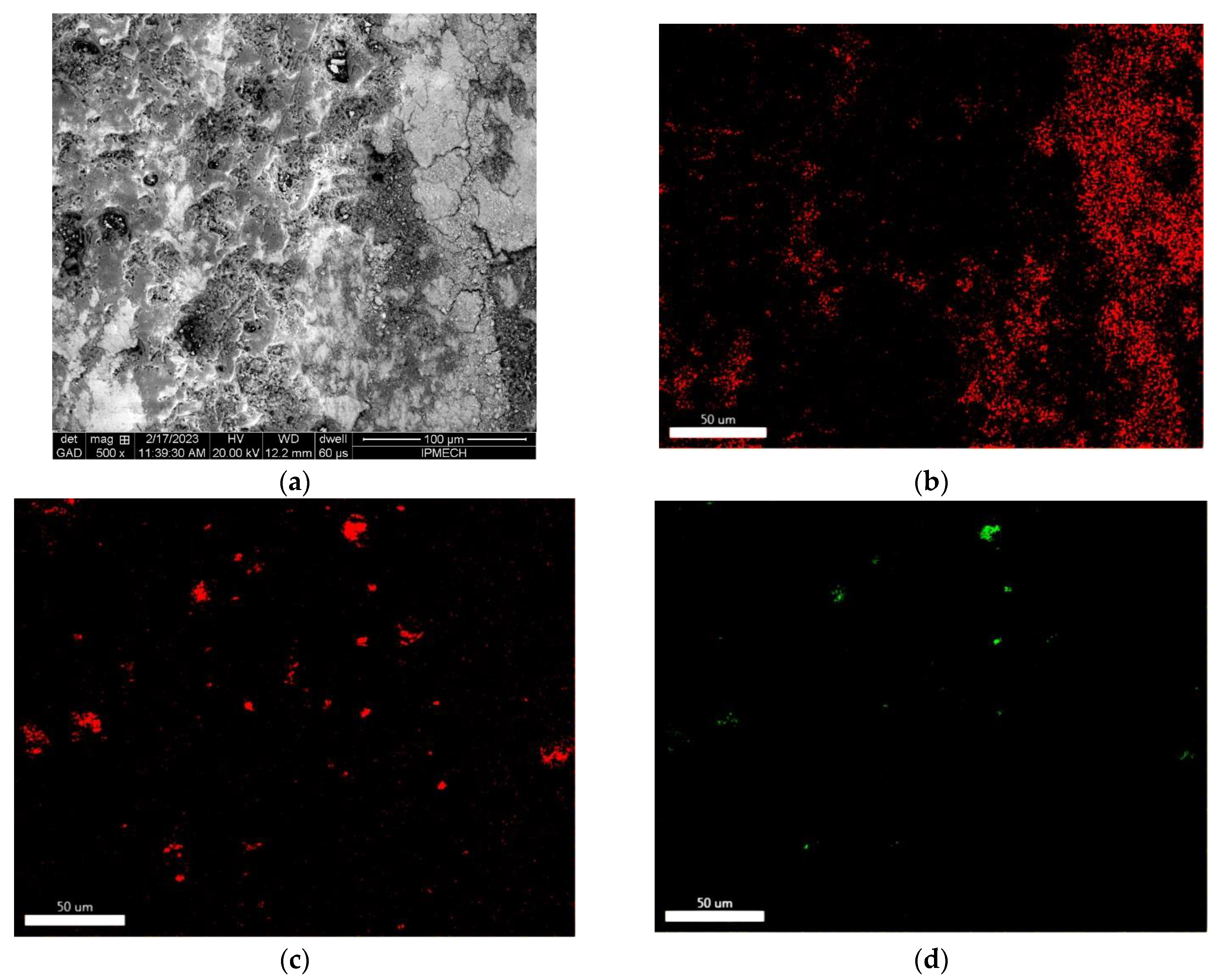
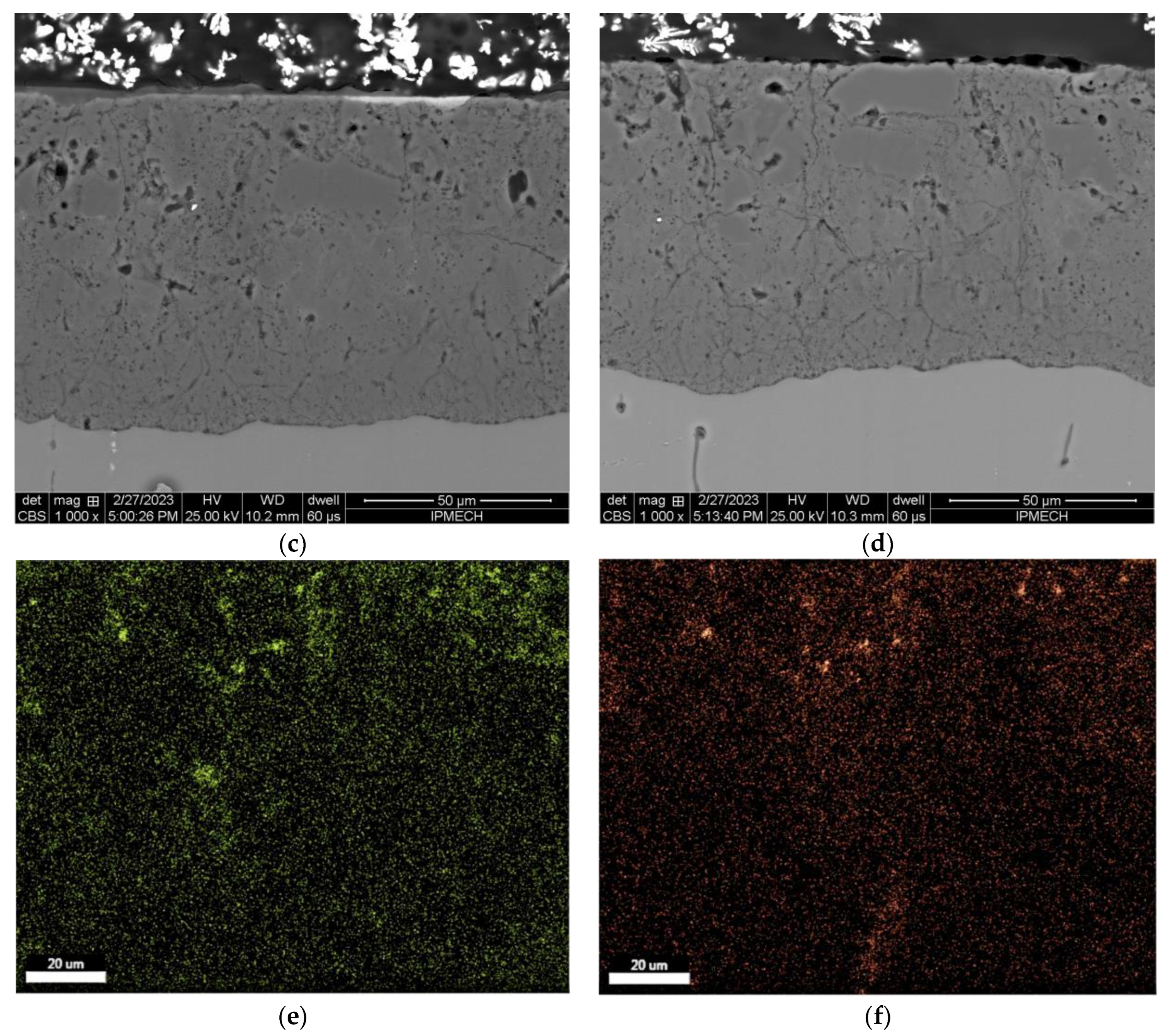
3.3. Microscopic Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huttunen-Saarivirta, E. Microstructure, fabrication and properties of quasicrystalline Al–Cu–Fe alloys: A review. J. Alloys Compd. 2004, 363, 154–178. [Google Scholar] [CrossRef]
- Janot, C. The Properties and Applications of Quasicrystals. Europhys. News 1996, 27, 60–64. [Google Scholar] [CrossRef]
- Wolf, W.; Koga, G.Y.; Schulz, R.; Savoie, S.; Kiminami, C.S.; Bolfarini, C.; Botta, W.J. Wear and Corrosion Performance of Al-Cu-Fe-(Cr) Quasicrystalline Coatings Produced by HVOF. J. Therm. Spray Technol. 2020, 29, 1195–1207. [Google Scholar] [CrossRef]
- Feitosa, F.; Gomes, R.; Silva, M.; De Lima, S.; Dubois, J. Effect of oxygen/fuel ratio on the microstructure and properties of HVOF-sprayed Al59Cu25.5Fe12.5B3 quasicrystalline coatings. Surf. Coat. Technol. 2018, 353, 171–178. [Google Scholar] [CrossRef]
- Sales, M.; Merstallinger, A.; Ustinov, A.; Polishchuk, S.; Melnichenko, T. Effect of the addition of crystalline β-phase in Al–Cu–Fe quasicrystalline coatings on their tribological properties. Surf. Coat. Technol. 2007, 201, 6206–6211. [Google Scholar] [CrossRef]
- Milman, Y.; Lotsko, D.; Dub, S.; Ustinov, A.; Polishchuk, S.; Ulshin, S. Mechanical properties of quasicrystalline Al–Cu–Fe coatings with submicron-sized grains. Surf. Coat. Technol. 2007, 201, 5937–5943. [Google Scholar] [CrossRef]
- Bigoni, D.; Capuani, D. Temperature dependence of the tribological properties of laser-melted Al-Cu-Fe quasicrystalline plasma sprayed coatings. J. Non-Cryst. Solids 2005, 351, 280–287. [Google Scholar] [CrossRef]
- Zhou, C.; Cai, F.; Kong, J.; Gong, S.; Xu, H. A study on the tribological properties of low-pressure plasma-sprayed Al–Cu–Fe–Cr quasicrystalline coating on titanium alloy. Surf. Coat. Technol. 2004, 187, 225–229. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Aleksandrova, Y.P. Reduction of Shear Stresses in Friction Units with an Electrical Insulation Coating of the ITER Blanket Modules. J. Mach. Manuf. Reliab. 2020, 49, 763–769. [Google Scholar] [CrossRef]
- Zaytzev, A.N. Plasma-Sprayed Oxide Coatings with Advanced Tribological Properties Under Dry Sliding Conditions in Air. In Proceedings of the 6th International Conference on Industrial Engineering; Radionov, A.A., Gasiyarov, V.R., Eds.; Lecture Notes in Mechanical Engineering. Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Yuan, W.; Shao, T.; Fleury, E.; Se, D.; Chen, D. Microstructure and tribological properties of plasma sprayed Al–Cu–Fe quasicrystalline coatings after laser post-treatment processing. Surf. Coat. Technol. 2004, 185, 99–105. [Google Scholar] [CrossRef]
- Shao, T.; Cao, X.; Fleury, E.; Kim, D.-H.; Hua, M.; Se, D. Tribological behavior of plasma sprayed Al–Cu–Fe+Sn quasicrystalline composite coatings. J. Non-Cryst. Solids 2004, 334–335, 466–470. [Google Scholar] [CrossRef]
- Yadav, T.; Mukhopadhyay, N. Quasicrystal: A low-frictional novel material. Curr. Opin. Chem. Eng. 2018, 19, 163–169. [Google Scholar] [CrossRef]
- Wolf, W.; Bolfarini, C.; Kiminami, C.S.; Botta, W.J. Recent developments on fabrication of Al-matrix composites reinforced with quasicrystals: From metastable to conventional processing. J. Mater. Res. 2020, 36, 281–297. [Google Scholar] [CrossRef]
- Lee, S.M.; Jung, J.H.; Fleury, E.; Kim, W.T.; Kim, D.H. Metal matrix composites reinforced by gas-atomized Al-Cu-Fe powders. Mater. Sci. Eng. A 2000, 294–296, 99–103. [Google Scholar] [CrossRef]
- Tang, F.; Anderson, I.; Biner, S. Microstructures and mechanical properties of pure Al matrix composites reinforced by Al-Cu-Fe alloy particles. Mater. Sci. Eng. A 2003, 363, 20–29. [Google Scholar] [CrossRef]
- Markov, G.A.; Slonova, A.I.; Terleeva, O.P. Chemical Composition, Structure, and Morphology of Microplasma Coatings. Prot. Met. 1997, 33, 257–262. [Google Scholar]
- Malyshev, V.N. Effective electrochemical transformation method of valve metals surface layer into high-tensile ceramic coating. In Chemical Reactions on Surfaces; Duncan, J.I., Klein, A.B., Eds.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2008; Chapter 7; pp. 211–262. [Google Scholar]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Belevantsev, V.I.; Terleeva, O.P.; Markov, G.A.; Shulepko, E.K.; Slonova, A.I.; Utkin, V.V. Microplasma Electrochemical Processes. Prot. Met. 1998, 34, 416–430. [Google Scholar]
- Simchen, F.; Sieber, M.; Kopp, A.; Lampke, T. Introduction to Plasma Electrolytic Oxidation—An Overview of the Process and Applications. Coatings 2020, 10, 628. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Zinigrad, M. Plasma Electrolytic Oxidation of Valve Metals. In Materials Science—Advanced Topics; Mastai, Y., Ed.; IntechOpen: London, UK, 2013; Chapter 4; pp. 85–102. [Google Scholar] [CrossRef]
- Rajendra, A.; Parmar, B.; Sharma, A.K.; Bhojraj, H.; Nayak, M.M.; Rajanna, K. Hard anodisation of aluminium and its application to sensorics. Surf. Eng. 2005, 21, 193–197. [Google Scholar] [CrossRef]
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mater. Rev. 2018, 64, 127–162. [Google Scholar] [CrossRef]
- Malyshev, V.N.; Markov, G.A.; Fedorov, V.A.; Petrosyants, A.A.; Terleeva, O.P. Features of the structure and properties of coatings applied by the method of microarc oxidation. Chem. Pet. Eng. 1984, 20, 41–43. [Google Scholar] [CrossRef]
- Malyshev, V.N. Friction and outwearing of ceramic coatings formed by the microarc oxidation method. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 871–879. [Google Scholar] [CrossRef]
- Curran, J.; Kalkancı, H.; Magurova, Y.; Clyne, T. Mullite-rich plasma electrolytic oxide coatings for thermal barrier applications. Surf. Coat. Technol. 2007, 201, 8683–8687. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.; Liang, J.; Chen, J. Thermal control coatings on magnesium alloys prepared by plasma electrolytic oxidation. Appl. Surf. Sci. 2013, 280, 151–155. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Sergienko, V. Composite PEO-Coatings as Defence Against Corrosion and Wear: A Review. Corros. Sci. Tech. 2019, 18, 212–219. [Google Scholar] [CrossRef]
- Malyshev, V.N.; Zorin, K.M. Formation of Wear-Resistant Compositional MAO-Coatings Containing a Dispersed Phase; Open Science Publishing: Raleigh, NC, USA, 2019; 217p. (In Russian) [Google Scholar]
- Lu, X.; Mohedano, M.; Blawert, C.; Matykina, E.; Arrabal, R.; Kainer, K.U.; Zheludkevich, M.L. Plasma electrolytic oxidation coatings with particle additions—A review. Surf. Coat. Technol. 2016, 307, 1165–1182. [Google Scholar] [CrossRef]
- Malyshev, V.; Zorin, K. Features of microarc oxidation coatings formation technology in slurry electrolytes. Appl. Surf. Sci. 2007, 254, 1511–1516. [Google Scholar] [CrossRef]
- Malyshev, V.N. Mikrolichtbogen-Oxidation—Ein neuartiges Verfahren zur Verfestigung von Aluminiumoberflaechen. Metalloberflaeche 1995, 8, 606–608. [Google Scholar]
- Available online: https://quasicrystal.eu/ (accessed on 1 March 2022).
- Malyshev, V.N. Neue Anwendungs-moeglichkeiten fuer Aluminium. Metalloberflaeche 2006, 1–2, 28–29. [Google Scholar]
- Yerokhin, A.L.; O Snizhko, L.; Gurevina, N.L.; Leyland, A.; Pilkington, A.; Matthews, A. Discharge characterization in plasma electrolytic oxidation of aluminium. J. Phys. D Appl. Phys. 2003, 36, 2110–2120. [Google Scholar] [CrossRef]
- ASTMG99-05; Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus. American Society for Testing and Materials: Conshohocken, PA, USA, 2004.
- Sobolev, A.; Bograchev, D.; Borodianskiy, K.; Zinigrad, M. Kinetics and mechanism of corrosion of oxide coating fabricated on aluminum alloy by the plasma electrolytic oxidation in molten salt. Corros. Sci. 2022, 208, 110604. [Google Scholar] [CrossRef]
- Jiang, B.L.; Wang, Y.M. Plasma electrolytic oxidation treatment of aluminium and titanium alloys. In Surface Engineering of Light Alloys; Elsevier: Amsterdam, The Netherlands, 2010; pp. 110–154. [Google Scholar] [CrossRef]
- Arrabal, R.; Moheadano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M.C. Characterization and wear behavior of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]


| Sample Codes | Quasicrystal Modifier, g·L−1 | Roughness, mkm | Average Thickness, µm | Base Electrolyte | |
|---|---|---|---|---|---|
| Ra | Rz | ||||
| 1 | 0 | 1.4 ± 0.4 | 16.6 ± 5.0 | 95 | KOH + Na2SiO39H2O + Na6P6O18 |
| 2 | 5 | 1.0 ± 0.2 | 16.1 ± 5.0 | 100 | |
| 3 | 10 | 1.2 ± 0.2 | 16.5 ± 3.0 | 115 | |
| 4 | 15 | 1.1 ± 0.1 | 16.5 ± 3.0 | 120 | |
| 5 | rubbing-in | 0.6 ± 0.1 | 7.8 ± 2.0 | 100 | |
| Sample Codes | Coefficient of Friction | Disc Volume Losses, mm3 | Disc Specific Wear Rate, mm3/Nm | Ball Volume Losses, mm3 | Hardness, HV |
|---|---|---|---|---|---|
| 1 | 1.10 ± 0.03 | 0.434 ± 0.020 | 8.7 ± 0.04 × 10−6 | 0.0048 ± 0.0008 | 365 ± 50 |
| 2 | 1.03 ± 0.08 | 0.131 ± 0.024 | 2.6 ± 0.05 × 10−6 | 0.0066 ± 0.0010 | 300 ± 30 |
| 3 | 1.13 ± 0.04 | 0.481 ± 0.089 | 9.6 ± 1.80 × 10−6 | 0.0134 ± 0.0012 | 240 ± 30 |
| 4 | 1.17 ± 0.06 | 0.310 ± 0.007 | 6.2 ± 0.01 × 10−6 | 0.0137 ± 0.0009 | 250 ± 50 |
| 5 | 0.92 ± 0.03 | 0.024 ± 0.001 | 0.5 ± 0.002 × 10−6 | 0.0048 ± 0.0009 | 350 ± 50 |
| Sample | Zone | C | O | Na | Mg | Al | Si | P | K | Mn | Fe | Cu | W | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 36.41 | 36.40 | 1.51 | 0.66 | 13.18 | 3.87 | 1.97 | 1.48 | 0.36 | - | - | 0.68 | 3.43 |
| 2 | 12.26 | 39.61 | 1.82 | 0.81 | 35.52 | 2.82 | 2.63 | 1.55 | 0.41 | - | - | 0.38 | 2.19 | |
| 3 | 13.28 | 48.91 | 3.13 | 0.58 | 4.93 | 22.46 | 3.20 | 3.12 | 0.04 | - | - | 0.14 | 0.21 | |
| 4 | 46.91 | 33.06 | 3.84 | 0.48 | 6.89 | 2.35 | 2.41 | 3.03 | 0.13 | - | - | 0.28 | 0.62 | |
| total | 18.16 | 42.99 | 3.72 | 0.71 | 16.51 | 9.96 | 3.86 | 3.14 | 0.12 | - | - | 0.16 | 0.67 | |
| 2 | 1 | 19.60 | 17.08 | 0.96 | 0.26 | 22.92 | 2.08 | 0.71 | 1.62 | 3.03 | 12.70 | 5.62 | 5.01 | 8.41 |
| 2 | 22.33 | 9.70 | 1.70 | 0.29 | 10.90 | 2.42 | 0.46 | 3.76 | 2.66 | 33.09 | 5.00 | 2.78 | 4.91 | |
| total | 16.52 | 44.45 | 2.93 | 0.95 | 20.44 | 7.79 | 3.08 | 2.20 | 0.10 | 0.35 | 0.49 | 0.18 | 0.52 | |
| 3 | 1 | 34.86 | 28.43 | 0.13 | 0.00 | 13.75 | 2.17 | 0.04 | 1.68 | 2.61 | 3.84 | 4.21 | 3.71 | 4.57 |
| 2 | 8.22 | 9.42 | 1.47 | 0.26 | 8.87 | 1.47 | 1.02 | 1.51 | 4.30 | 35.75 | 8.67 | 5.69 | 13.35 | |
| 3 | 6.26 | 35.22 | 2.60 | 0.33 | 21.63 | 9.25 | 3.83 | 3.20 | 0.33 | 1.69 | 2.32 | 11.23 | 2.11 | |
| 4 | 10.76 | 47.77 | 1.77 | 1.05 | 22.05 | 8.58 | 4.51 | 2.02 | 0.08 | 0.53 | 0.42 | 0.07 | 0.39 | |
| total | 11.04 | 43.79 | 2.79 | 0.89 | 21.90 | 10.16 | 3.79 | 2.59 | 0.18 | 0.87 | 0.89 | 0.31 | 0.80 | |
| 4 | 1 | 3.89 | 32.23 | 1.37 | 0.53 | 24.00 | 9.65 | 4.26 | 2.11 | 0.05 | 7.90 | 12.68 | 0.92 | 0.41 |
| 2 | 47.28 | 35.01 | 0.99 | 0.68 | 9.29 | 1.82 | 3.56 | 0.55 | 0.04 | 0.35 | 0.16 | 0.04 | 0.23 | |
| 3 | 6.59 | 46.79 | 0.91 | 1.01 | 29.33 | 10.25 | 1.86 | 1.67 | 0.12 | 0.75 | 0.46 | 0.08 | 0.18 | |
| 4 | 11.53 | 9.56 | 1.22 | 0.52 | 14.75 | 2.43 | 0.68 | 0.78 | 0.85 | 34.20 | 6.38 | 5.53 | 11.57 | |
| total | 9.95 | 42.28 | 3.39 | 0.76 | 21.97 | 10.90 | 3.97 | 2.60 | 0.10 | 1.96 | 1.10 | 0.19 | 0.83 | |
| 5 | 1 | 23.54 | 23.68 | 1.40 | 0.57 | 22.34 | 1.02 | 0.41 | 0.33 | 0.27 | 9.21 | 15.72 | 0.92 | 0.59 |
| 2 | 10.39 | 52.06 | 0.00 | 1.41 | 30.43 | 3.31 | 1.73 | 0.43 | 0.02 | 0.05 | 0.04 | 0.05 | 0.08 | |
| 3 | 28.78 | 45.28 | 0.84 | 1.42 | 16.13 | 2.49 | 1.77 | 1.05 | 0.08 | 0.94 | 0.72 | 0.32 | 0.18 | |
| 4 | 43.47 | 27.89 | 0.04 | 2.59 | 16.08 | 0.69 | 0.54 | 0.25 | 0.06 | 3.94 | 3.88 | 0.34 | 0.23 | |
| total | 13.59 | 47.28 | 1.09 | 1.42 | 27.41 | 5.00 | 1.86 | 1.39 | 0.06 | 0.19 | 0.27 | 0.08 | 0.36 |
| Sample | Zone | C | O | Na | Mg | Al | Si | P | K | Mn | Fe | Cu | W | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 5.70 | 48.98 | 0.83 | 0.64 | 18.51 | 6.23 | 2.72 | 1.17 | 0.15 | 0.25 | 0.23 | 13.96 | 0.63 |
| 2 | 7.17 | 53.41 | 0.38 | 0.96 | 17.92 | 5.68 | 2.58 | 1.00 | 0.11 | 0.22 | 0.21 | 9.85 | 0.51 | |
| total | 3.27 | 43.12 | 1.93 | 0.51 | 21.77 | 6.86 | 3.33 | 1.81 | 0.10 | 0.27 | 0.28 | 15.67 | 1.08 | |
| 2 | 1 | 6.73 | 57.69 | 1.18 | 1.16 | 19.23 | 6.43 | 2.42 | 1.24 | 0.11 | 0.21 | 0.18 | 3.20 | 0.22 |
| 2 | 7.30 | 46.97 | 4.76 | 1.05 | 22.14 | 8.62 | 4.06 | 3.06 | 0.10 | 0.19 | 0.33 | 0.96 | 0.46 | |
| 3 | 4.37 | 42.94 | 2.49 | 0.67 | 21.16 | 7.11 | 3.07 | 2.07 | 0.21 | 0.39 | 0.58 | 13.79 | 1.15 | |
| total | 5.91 | 47.99 | 2.00 | 0.85 | 22.48 | 7.76 | 2.87 | 1.66 | 0.08 | 0.31 | 0.40 | 6.99 | 0.70 | |
| 3 | 1 | 1.01 | 43.21 | 0.28 | 1.17 | 44.86 | 3.62 | 1.85 | 0.40 | 0.16 | 0.21 | 0.32 | 1.56 | 1.35 |
| 2 | 4.08 | 47.17 | 1.73 | 0.69 | 20.56 | 6.99 | 3.07 | 1.47 | 0.12 | 0.44 | 0.54 | 12.18 | 0.96 | |
| total | 3.04 | 42.40 | 1.97 | 0.54 | 21.59 | 7.45 | 3.27 | 1.75 | 0.12 | 0.73 | 0.79 | 15.19 | 1.16 | |
| 4 | 1 | 2.14 | 47.91 | 2.15 | 1.66 | 32.29 | 5.35 | 4.85 | 1.15 | 0.12 | 0.25 | 0.28 | 0.86 | 0.99 |
| 2 | 4.86 | 56.01 | 0.65 | 1.10 | 18.53 | 7.76 | 2.92 | 1.19 | 0.06 | 0.44 | 0.34 | 5.80 | 0.34 | |
| 3 | 2.17 | 38.55 | 2.66 | 0.64 | 23.07 | 7.50 | 3.76 | 2.29 | 0.17 | 0.81 | 1.05 | 15.70 | 1.63 | |
| total | 2.57 | 44.16 | 2.24 | 0.60 | 21.37 | 8.27 | 3.30 | 1.72 | 0.13 | 0.76 | 0.83 | 13.03 | 1.02 | |
| 5 | 1 | 26.22 | 37.98 | 0.00 | 0.84 | 24.34 | 1.94 | 1.03 | 0.23 | 0.05 | 3.18 | 3.70 | 0.26 | 0.23 |
| 2 | 6.17 | 41.04 | 1.11 | 0.86 | 28.06 | 4.96 | 1.58 | 1.44 | 0.13 | 0.34 | 0.61 | 12.35 | 1.35 | |
| 3 | 9.91 | 43.75 | 0.59 | 2.06 | 33.94 | 3.42 | 1.61 | 1.16 | 0.24 | 0.31 | 0.56 | 1.49 | 0.96 | |
| 4 | 45.91 | 21.01 | 0.00 | 0.37 | 17.54 | 0.90 | 0.29 | 0.31 | 0.17 | 5.70 | 6.56 | 0.92 | 0.32 | |
| 5 | 5.12 | 47.08 | 0.59 | 1.15 | 36.92 | 4.98 | 1.95 | 1.14 | 0.06 | 0.08 | 0.08 | 0.31 | 0.54 | |
| total | 5.01 | 46.66 | 0.72 | 0.98 | 32.06 | 5.03 | 1.67 | 1.01 | 0.11 | 0.20 | 0.29 | 5.29 | 0.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torskaya, E.V.; Morozov, A.V.; Malyshev, V.N.; Shcherbakova, O.O. Processing and Tribological Properties of PEO Coatings on AlZn5.5MgCu Aluminium Alloy with Incorporated Al-Cu-Fe Quasicrystals. Ceramics 2023, 6, 858-871. https://doi.org/10.3390/ceramics6020049
Torskaya EV, Morozov AV, Malyshev VN, Shcherbakova OO. Processing and Tribological Properties of PEO Coatings on AlZn5.5MgCu Aluminium Alloy with Incorporated Al-Cu-Fe Quasicrystals. Ceramics. 2023; 6(2):858-871. https://doi.org/10.3390/ceramics6020049
Chicago/Turabian StyleTorskaya, Elena V., Alexei V. Morozov, Vladimir N. Malyshev, and Olga O. Shcherbakova. 2023. "Processing and Tribological Properties of PEO Coatings on AlZn5.5MgCu Aluminium Alloy with Incorporated Al-Cu-Fe Quasicrystals" Ceramics 6, no. 2: 858-871. https://doi.org/10.3390/ceramics6020049
APA StyleTorskaya, E. V., Morozov, A. V., Malyshev, V. N., & Shcherbakova, O. O. (2023). Processing and Tribological Properties of PEO Coatings on AlZn5.5MgCu Aluminium Alloy with Incorporated Al-Cu-Fe Quasicrystals. Ceramics, 6(2), 858-871. https://doi.org/10.3390/ceramics6020049






