Hydroxyapatite for Biomedical Applications: A Short Overview
Abstract
1. Introduction
- Dissolution of CaPs;
- Precipitation from the solution on the surface of CaPs;
- Ion transfer and structural adjustment at the tissue/CaP interface;
- Dispersion from the boundary surface layer in the CaPs;
- Effects mediated by the solution on cell activity;
- Organic and mineral phase deposition without integration into the CaP surface;
- Deposition with integration of CaPs into the surface;
- Chemotaxis to the surface of CaPs;
- Cells attachment and proliferation;
- Differentiation of cells;
- ECM formation.
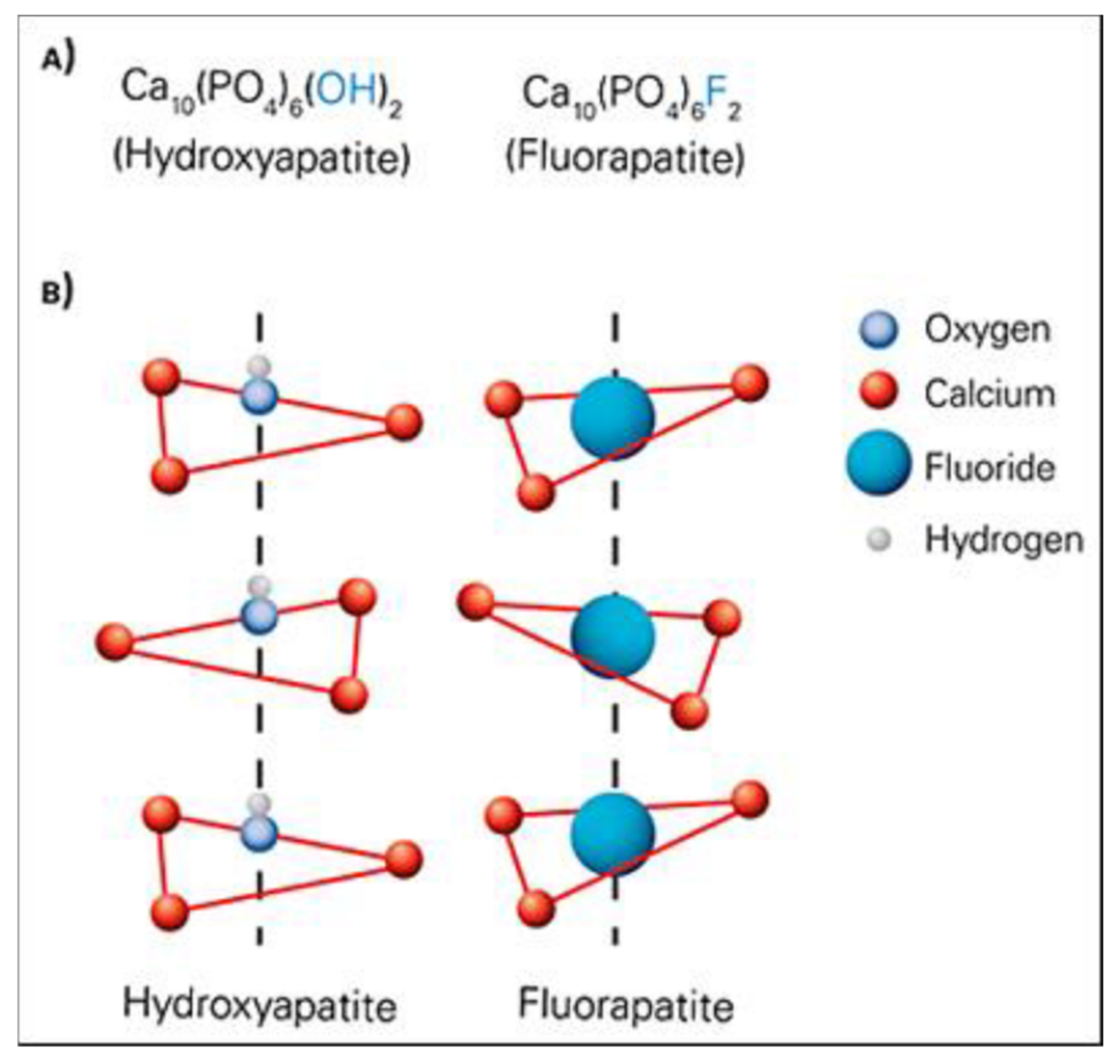
2. Chemical Structure of HA and Its Properties
- 14 Ca2+ ions: 6 of them inside the cell, and 8 shared with adjacent cells; total = 10 Ca2+ ions/unit cell
- 10 PO43− ions: 2 of them located inside the cell, and 8 peripheral ions shared with as many adjacent cells; total = 6 PO43− ions/unit cell
- 8 OH− ions: being along the edges, they all belong to the cell by ¼; total = 2 OH− ions/unit cell (see Figure 2).
3. HA Synthesis Techniques
3.1. Dry Methods
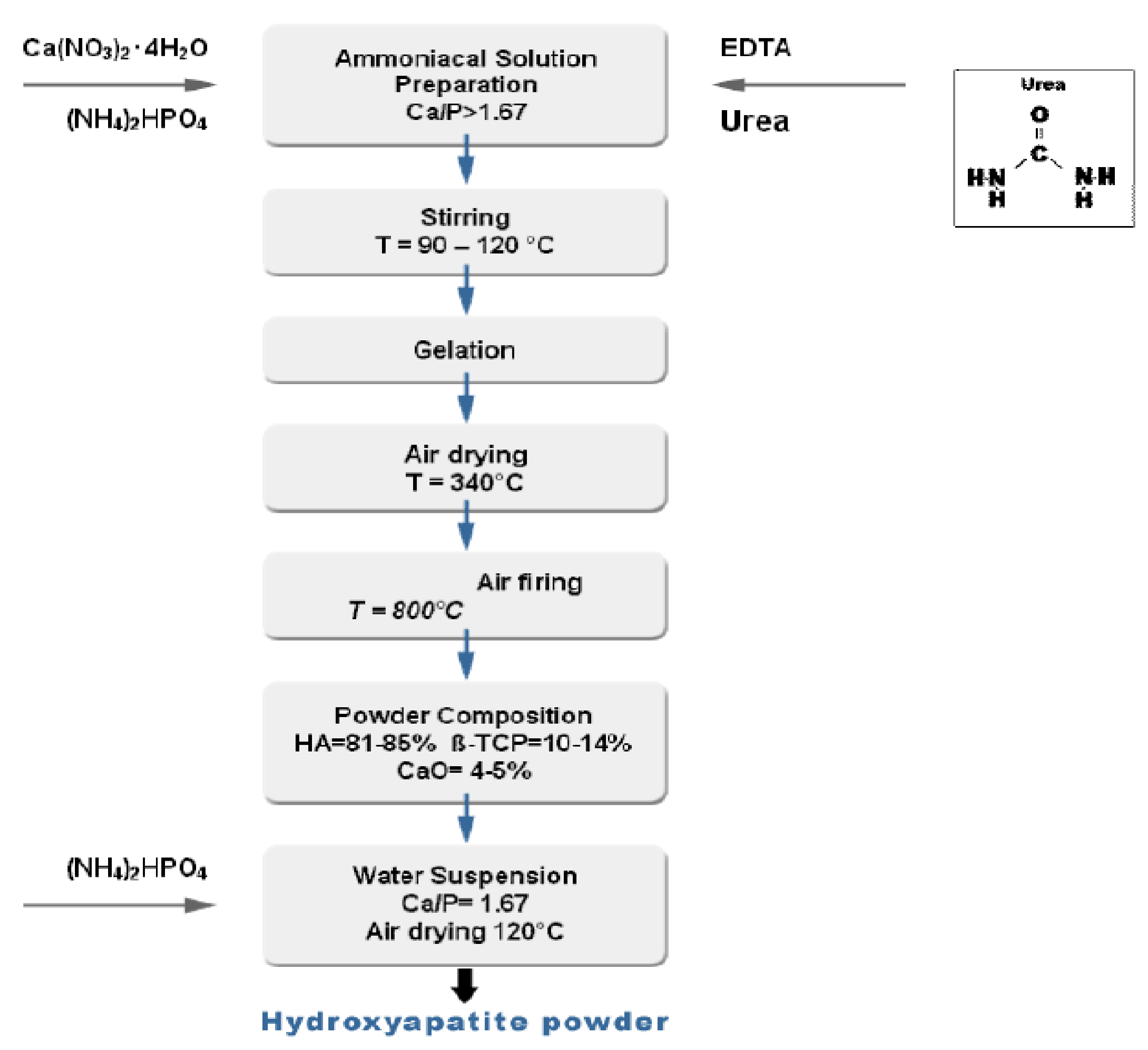
3.2. Wet Methods
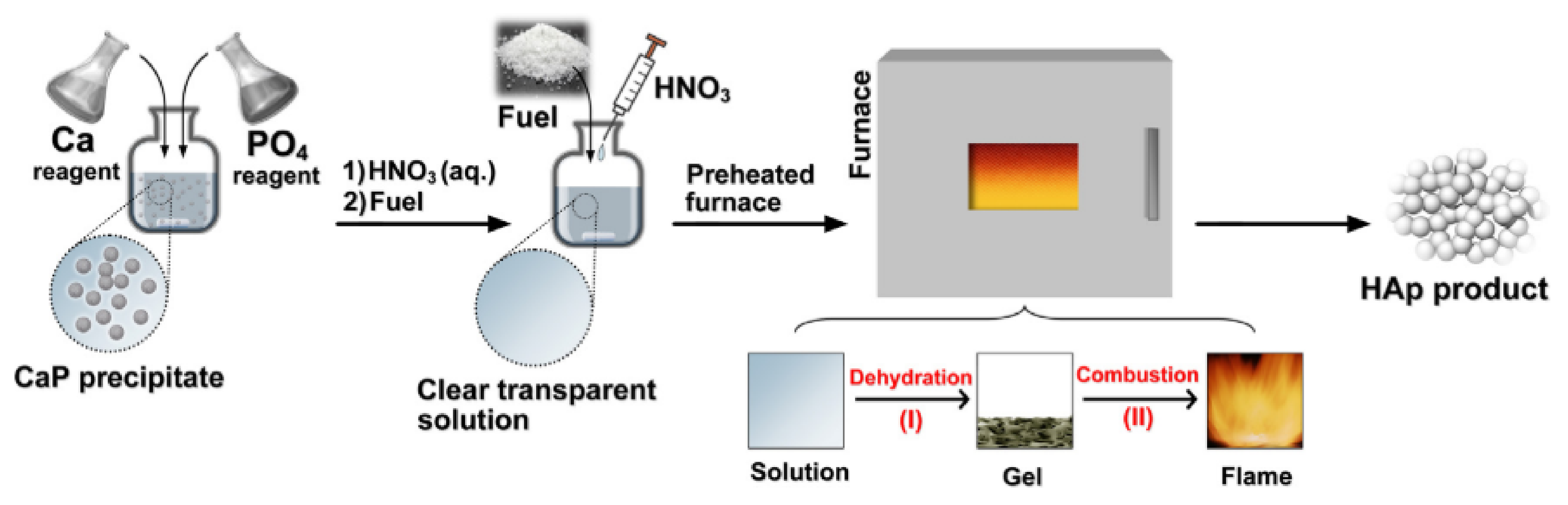
3.3. High-Temperature Processes
3.4. Synthesis Method Based on Biogenic Sources or Bioinspired Approaches
4. Dense and Porous Hydroxyapatite
- Sintering in the absence of pressure: a pressure of 60–80 MPa is used to compact the powders that are then pressurelessly sintered in air at 950–1300 °C for a few hours, with a temperature gradient of 100 °C/h. Different degree of HA density can be obtained depending on the set temperature.
- Uniaxial hot pressing (HP): dense HA is obtained without reaching too high temperatures. This prevents the formation of other phosphates, such as tricalcium phosphate, resulting in a purer product.
- Hot isostatic pressing (HIP): cold-pressed powders are then hot-pressed by means of the isostatic action of a gas. Very dense materials with excellent mechanical properties are obtained, which can also be further processed later.
5. Mechanical Properties
6. Applications of HA in Tissue Engineering
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albee, F.H.; Morrison, H.F. Studies in bone growth triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–39. [Google Scholar]
- Jazayeri, H.E.; Rodriguez-Romero, M.; Razavi, M.; Tahriri, M.; Ganjawalla, K.; Rasoulianboroujeni, M.; Malekoshoaraie, M.H.; Khoshroo, K.; Tayebi, L. The cross-disciplinary emergence of 3D printed bioceramic scaffolds in orthopedic bioengineering. Ceram. Int. 2018, 44, 1–9. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate bioceramics. Ceram. Int. 2015, 41, 13913–13966. [Google Scholar] [CrossRef]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Multiphasic calcium orthophosphate (CaPO4) bioceramics and their biomedical applications. Ceram. Int. 2016, 42, 6529–6554. [Google Scholar] [CrossRef]
- Carvalho, B.; De Rompen, E.; Lecloux, G.; Schupbach, P.; Dory, E. Effect of sintering on in vivo biological performance of bovine hydroxyapatite. Materials 2019, 12, 3946. [Google Scholar] [CrossRef] [PubMed]
- Mohamad Yunos, D.; Bretcanu, O.; Boccaccini, A.R. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 2008, 43, 4433–4442. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.G.; Dard, M.; Larjava, H. Hydoxyapatite/beta-tricalcium phosphate biphasic ceramics as regenerative material for the repair of complex bone defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2493–2512. [Google Scholar] [CrossRef]
- Søballe, K. Hydroxyapatite ceramic coating for bone implant fixation: Mechanical and histological studies in dogs. Acta Orthop. Scand. 1993, 64, 1–58. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tis-sue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Horowitz, R.A.; Mazor, Z.; Foitzik, C.; Prasad, H.; Rohrer, M.; Palti, A. β-Tricalcium Phosphate As Bone Substitute Material. J. Osseointegr. 2010, 1, 60–68. [Google Scholar]
- Islam, M.T.; Felfel, R.M.; Abou Neel, E.A.; Grant, D.M.; Ahmed, I.; Hossain, K.M.Z. Bioactive calcium phosphate–based glasses and ceramics and their biomedical applications: A review. J. Tissue Eng. 2017, 8, 2041731417719170. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Botelho, M.G.; Dorozhkin, S.V. Biphasic calcium phosphates bioceramics (HA/TCP): Concept, physico-chemical properties and the impact of standardization of study protocols in biomaterials research. Mater. Sci. Eng. C 2017, 71, 1293–1312. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef] [PubMed]
- Kumta, P.N.; Sfeir, C.; Lee, D.H.; Olton, D.; Choi, D. Nanostructured calcium phosphates for biomedical applications: Novel synthesis and characterization. Acta Biomater. 2005, 1, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Lawson, A.C.; Czernuszka, J.T. Collagen-calcium phosphate composites. Proc. Inst. Mech. Eng. H 1998, 212, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, R.; Popa, A.-C.; Enciu, A.-M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.-R.; et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Den-tistry Applications. Materials 2019, 10, 3704. [Google Scholar] [CrossRef] [PubMed]
- Szcześ, A.; Hołysz, L.; Chibowski, E. Synthesis of hydroxyapatite for biomedical applications. Adv. Colloid Interface Sci. 2017, 249, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Wopenka, B.; Pasteris, J.D. A mineralogical perspective on the apatite in bone. Mater. Sci. Eng. C 2005, 25, 131–143. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First principles investigation of mineral component of bone: CO3 substitutions in hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Best, S.M.; Porter, A.E.; Thian, E.S.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.M. Hydroxyapatite-Based Materials: Synthesis and Characterization. In Biomedical Engineering: Frontiers and Challenges; Fazel-Rezai, R., Ed.; Intech Open: Rijeka, Croatia, 2011. [Google Scholar]
- Doi, Y.; Shibutani, T.; Moriwaki, Y.; Kajimoto, T.; Iwayama, Y. Sintered carbonate apatites as bioresorbable bone substitutes. J. Biomed. Mater. Res. 1998, 39, 603–610. [Google Scholar] [CrossRef]
- Rahyussalim, A.J.; Supriadi, S.; Marsetio, A.F.; Pribadi, P.M.; Suharno, B. The potential of carbonate apatite as an alternative bone substitute material. Med. J. Indones. 2019, 28, 92–97. [Google Scholar] [CrossRef]
- Wang, A.N.; Wu, L.G.; Li, X.L.; Sun, Y.D.; Wang, J.; Wang, S.W.; Jia, A.X.; Wang, C.; Zhang, Y.Y.; Fu, Q.Q.; et al. Study on the Blend Film Prepared by Chitosan and Gelatin. Adv. Mater. Res. 2011, 201–203, 2866–2869. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Fang, Y.; Hooper, T.J.N.; Kelly, N.L.; Gupta, D.; Balani, K.; Manna, I.; Baikie, T.; Bishop, P.T.; White, T.J.; et al. Crystal chemistry and antibacterial properties of cupriferous hydroxyapatite. Materials 2019, 12, 1814. [Google Scholar] [CrossRef]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Baino, F.; Fiume, E.; Miola, M.; Leone, F.; Onida, B.; Verné, E. Fe-doped bioactive glass-derived scaffolds produced by sol-gel foaming. Mater. Lett. 2019, 235, 207–211. [Google Scholar] [CrossRef]
- Fiume, E.; Serino, G.; Bignardi, C.; Verné, E.; Baino, F. Bread-derived bioactive porous scaffolds: An innovative and sus-tainable approach to bone tissue engineering. Molecules 2019, 24, 2954. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Pinheiro, A.; De Oliveira, R.; Goes, J.C.; Aranha, N.; De Oliveira, L.; Sombra, A. Properties and in vivo investigation of nanocrystalline hydroxyapatite obtained by mechanical alloying. Mater. Sci. Eng. C 2004, 24, 549–554. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Chapter 6: Ceramics in Bone Grafts and Coated Implants. In Materials for Bone Disorders; Bose, S., Bandyopadhyay, A., Eds.; Academic Press (Elsevier): London, UK, 2017; pp. 265–314. [Google Scholar]
- Li, B.; Webster, T. Orthopedic Biomaterials: Advances and Applications; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Nasiri-Tabrizi, B.; Honarmandi, P.; Ebrahimi-Kahrizsangi, R. Synthesis of nanosize single-crystal hydroxyapatite via mechanochemical method. Mater. Lett. 2009, 63, 543–546. [Google Scholar] [CrossRef]
- Zhan, J.; Tseng, Y.H.; Chan, J.C.C.; Mou, C.Y. Biomimetic formation of hydroxyapatite nanorods by a single-crystal-to-single-crystal transformation. Adv. Funct. Mater. 2005, 15, 2005–2010. [Google Scholar] [CrossRef]
- Tenhuisen, K.S.; Brown, P.W. Formation of calcium-deficient hydroxyapatite from α-tricalcium phosphate. Biomaterials 1998, 19, 2209–2217. [Google Scholar] [CrossRef]
- Park, H.C.; Baek, D.J.; Park, Y.M.; Yoon, S.Y.; Stevens, R. Thermal stability of hydroxyapatite whiskers derived from the hydrolysis of α-TCP. J. Mater. Sci. 2004, 39, 2531–2534. [Google Scholar] [CrossRef]
- Fathi, M.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Sopyan, I.; Singh, R.; Hamdi, M. Synthesis of nano sized hydroxyapatite powder using sol-gel technique and its conver-sion to dense and porous bodies. Indian J. Chem. A 2008, 47, 1626–1631. [Google Scholar]
- Zhu, R.; Yu, R.; Yao, J.; Wang, D.; Ke, J. Morphology control of hydroxyapatite through hydrothermal process. J. Alloys Compd. 2008, 457, 555–559. [Google Scholar] [CrossRef]
- Chun, H.J.; Park, K.; Kim, C.-H.; Khang, G. Novel Biomaterials for Regenerative Medicine, 1st ed.; Springer: Singapore, 2018. [Google Scholar]
- Guo, G.; Sun, Y.; Wang, Z.; Guo, H. Preparation of hydroxyapatite nanoparticles by reverse microemulsion. Ceram. Int. 2005, 31, 869–872. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, G.; Wang, Z.; Guo, H. Synthesis of single-crystal HAP nanorods. Ceram. Int. 2006, 32, 951–954. [Google Scholar] [CrossRef]
- Li, H.; Zhu, M.Y.; Li, L.H.; Zhou, C.R. Processing of nanocrystalline hydroxyapatite particles via reverse microemul-sions. J. Mater. Sci. 2008, 43, 384–389. [Google Scholar] [CrossRef]
- Hazar Yoruç, A.B.; Ipek, Y. Sonochemical synthesis of hydroxyapatite nanoparticles with different precursor reagents. Acta Phys. Pol. A 2012, 121, 230–232. [Google Scholar] [CrossRef]
- Ruksudjarit, A.; Pengpat, K.; Rujijanagul, G.; Tunkasiri, T. Synthesis and characterization of nanocrystalline hydroxyapatite from natural bovine bone. Curr. Appl. Phys. 2008, 8, 270–272. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- White, R.A.; Weber, J.N.; White, E.W. Replamineform: A New Process for Preparing Porous Ceramic, Metal, and Poly-mer Prosthetic Materials. Science 1972, 176, 922–924. [Google Scholar] [CrossRef]
- White, E.W.; Weber, J.N.; Roy, D.M.; Owen, E.L.; Chiroff, R.T.; White, R.A. Replamineform Porous Biomaterials for Hard Tissue Implant Applications. J. Biomed. Mater. Res. 1975, 6, 23–27. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, D.; Yang, L.; Tang, H. Hydrothermal Conversion of Coral into Hydroxyapatite. Mater. Charact. 2001, 47, 83–87. [Google Scholar] [CrossRef]
- Liu, W.; Wang, T.; Shen, Y.; Pan, H.; Peng, S.; Lu, W.W. Strontium Incorporated Coralline Hydroxyapatite for Engineering Bone. ISRN Biomater. 2013, 2013, 649163. [Google Scholar] [CrossRef]
- Damien, E.; Revell, P.A. Coralline Hydroxyapatite Bone Graft Substitute: A Review of Experimental Studies and Biomedical Applications. J. Appl. Biomater. Biomech. 2004, 2, 65–73. [Google Scholar] [PubMed]
- Tampieri, A.; Sprio, S.; Ruffini, A.; Celotti, G.; Lesci, I.G.; Roveri, N. From Wood to Bone: Multi-Step Process to Convert Wood Hierarchical Structures into Biomimetic Hydroxyapatite Scaffolds for Bone Tissue Engineering. J. Mater. Chem. 2009, 19, 4973–4980. [Google Scholar] [CrossRef]
- Ruffini, A.; Sprio, S.; Tampieri, A. Wood Structures with Organized Morphology for Bone Substitutes. J. Appl. Biomater. Biomech. 2007, 5, 207. [Google Scholar]
- Sprio, S.; Ruffini, A.; Valentini, F.; D’Alessandro, T.; Sandri, M.; Panseri, S.; Tampieri, A. Biomimesis and Biomorphic Transformations: New Concepts Applied to Bone Regeneration. J. Biotechnol. 2011, 156, 347–355. [Google Scholar] [CrossRef]
- Ruffini, A.; Sprio, S.; Tampieri, A. Study of the Hydrothermal Transformation of Wood-Derived Calcium Carbonate into 3D Hierarchically Organized Hydroxyapatite. Chem. Eng. J. 2013, 217, 150–158. [Google Scholar] [CrossRef]
- Ruffini, A.; Sprio, S.; Tampieri, A. Towards Hierarchically Organized Scaffolds for Bone Substitutes from Wood Structures. Key Eng. Mater. 2008, 361–363, 959–962. [Google Scholar]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue re-placement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Al-Naib, U.M.B. Introductory chapter: A Brief Introduction to Porous Ceramic. In Recent Advances in Porous Ceramics; Al-Naib, U.M.B., Ed.; Intech Open: Rijeka, Croatia, 2018. [Google Scholar]
- Kumar, A.; Kargozar, S.; Baino, F.; Han, S.S. Additive Manufacturing Methods for Producing Hydroxyapatite and Hydroxyapatite-Based Composite Scaffolds: A Review. Front. Mater. 2019, 6, 313. [Google Scholar] [CrossRef]
- Potestio, I. Lithoz: How lithography-based ceramic AM is expanding the opportunities for technical ceramics. Powder Inject. Mould. Int. 2019, 13, 2–5. [Google Scholar]
- Schwentenwein, M.; Homa, J. Additive manufacturing of dense alumina ceramics. Int. J. Appl. Ceram. Technol. 2015, 12, 1–7. [Google Scholar] [CrossRef]
- Schlacher, J.; Hofer, A.-K.; Geier, S.; Kraleva, I.; Papšík, R.; Schwentenwein, M.; Bermejo, R. Additive manufacturing of high-strength alumina through a multi-material approach. Open Ceram. 2021, 5, 100082. [Google Scholar] [CrossRef]
- Baino, F.; Magnaterra, G.; Fiume, E.; Schiavi, A.; Tofan, L.; Schwentenwein, M.; Verné, E. Digital light processing stereolithography of hydroxyapatite scaffolds with bone-like architecture, permeability, and mechanical properties. J. Am. Ceram. Soc. 2021, 1–10. [Google Scholar] [CrossRef]
- Tanner, K.E. Bioactive ceramic-reinforced composites for bone augmentation. J. R. Soc. Interface 2012, 7, S541–S557. [Google Scholar] [CrossRef]
- Feng, C.; Zang, K.; He, R.; Ding, G.; Xia, M. Additive manufacturing of hydroxyapatite bioceramic scaffolds: Dispersion, digital light processing, sintering, mechanical properties, and biocompatibility. J. Adv. Ceram. 2020, 9, 360–373. [Google Scholar] [CrossRef]
- Gervaso, F.; Scalera, F.; Kunjalukkal Padmanabhan, S.; Sannino, A.; Licciulli, A. High performace hydroxyapatite scaffolds for bone tissue engineering applications. Int. J. Appl. Ceram. Technol. 2012, 9, 507–516. [Google Scholar] [CrossRef]
- Levitt, G.E.; Crayton, P.H.; Monroe, E.A.; Condrate, R.A. Forming methods for apatite prosthesis. J. Biomed. Mater. Res. 1969, 3, 683–685. [Google Scholar] [CrossRef]
- Dorozhkin, S. Medical Application of Calcium Orthophosphate Bioceramics. BIO 2011, 1, 1–51. [Google Scholar] [CrossRef]
- Furlong, R.J.; Osborn, J.F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Jt. Surg. 1991, 73, 741–745. [Google Scholar] [CrossRef]
- Ferreira-Ermita, D.A.; Valente, F.L.; Carlo-Reis, E.C.; Araújo, F.R.; Ribeiro, I.M.; Cintra, C.C.; Borges, A.P. Characterization and in vivo biocompatibility analysis of synthetic hydroxyapatite compounds associated with magnetite nanoparticles for a drug delivery system in osteomyelitis treatment. Results Mater. 2020, 5, 100063. [Google Scholar] [CrossRef]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Vacanti, C.A.; Bonassar, L.J.; Vacanti, M.P.; Shufflebarger, J. Replacement of an Avulsed Phalanx with Tissue-Engineered Bone. N. Engl. J. Med. 2001, 344, 1511–1514. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Honoki, K.; Ohgushi, H.; Kotobuki, N.; Matsushima, A.; Takakura, Y. Tissue engineering approach to the treatment of bone tumors: Three cases of cultured bone grafts de-rived from patients’ mesenchymal stem cells. Artif. Organs 2006, 30, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.K. Recent advances in the synthesis, functionalization and biomedical applica-tions of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Ignjatovic, N.; Savic, V.; Najman, S.; Plavsic, M.; Uskokovic, D. A study of HAp/PLLA composite as a substitute for bone powder. Biomaterials 2001, 22, 571–575. [Google Scholar] [CrossRef]
- Wang, M.; Joseph, R.; Bonfield, W. Hydroxyapatite-polyethylene composites for bone substitution: Effects of ceramic particle size and morphology. Biomaterials 1998, 19, 2357–2366. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7, 9–19. [Google Scholar] [CrossRef]
- Baino, F. Ceramics for bone replacement: Commercial products and clinical use. In Advances in Ceramic Biomaterials; Palmero, P., Cambier, F., De Barra Editors, E., Eds.; Woodhead Publishing (Elsevier): Cambridge, UK, 2017; pp. 249–278. [Google Scholar]
- Cuzmar, E.; Perez, R.A.; Manzanares, M.-C.; Ginebra, M.-P.; Franch, J. In Vivo Osteogenic Potential of Biomimetic Hydroxyapatite/Collagen Microspheres: Comparison with Injectable Cement Pastes. PLoS ONE 2015, 10, e0131188. [Google Scholar]
- Kargozar, S.; Singh, R.K.; Kim, H.W.; Baino, F. “Hard” ceramics for “Soft” tissue engineering: Paradox or opportunity? Acta Biomater. 2020, 115, 1–28. [Google Scholar] [CrossRef]
- Baino, F.; Vitale-Brovarone, C. Ceramics for oculo-orbital surgery. Ceram. Int. 2015, 41, 5213–5231. [Google Scholar] [CrossRef][Green Version]




| Material | Chemical Formula | Ca/P Molar Ratio | Solubility at 25 °C, g/L | pH Stability Range in Aqueous Solutions (25 °C) |
|---|---|---|---|---|
| Monocalcium phosphate monohydrate (MCPM) | Ca(H2PO4)2·H2O | 0.5 | ~18 | 0.0–2.0 |
| Dicalcium phosphate dehydrate (DCPD), mineral brushite | CaHPO4·2H2O | 1.0 | ~0.088 | 2.0–6.0 |
| Octacalcium phosphate (OCP) | Ca8(HPO4)2(PO4)4·5H2O | 1.33 | ~0.0081 | 5.5–7.0 |
| α–Tricalcium phosphate (α-TCP) | α-Ca3(PO4)2 | 1.5 | ~0.0025 | a |
| β–Tricalcium phosphate (β-TCP) | β-Ca3(PO4)2 | 1.5 | ~0.0005 | a |
| Amorphous calcium phosphate (ACP) | CaxHy(PO4)z·nH2O, n = 3–4.5, 15–20% H2O | 1.0–2.2 c | b | 5.0–12.0 |
| Hydroxyapatite (HA) | Ca10(PO4)6(OH)2 | 1.67 | ~0.0003 | 9.5–12.0 |
| Fluorapatite (FA) | Ca10(PO4)6F2 | 1.67 | ~0.0002 | 7.0–12.0 |
| Oxyapatite (OA) | Ca10(PO4)6O | 1.67 | ~0.087 | a |
| Tetracalcium phosphate (TTC) | Ca10(PO4)2O | 2.0 | ~0.0007 | a |
| Property | Value | Property | Value |
|---|---|---|---|
| Density | 3.16 g/cm3 | Poisson’s ratio | 0.27 |
| Decomposition temperature | >1000 °C | Fracture Energy | 2.3–20 J/m2 |
| Dielectric costant | 7.40–10.47 | Fracture toughness | 0.7–1.2 MPa·m½ (decrease with porosity) |
| Thermal conductivity | 0.013 W/cm·K | Fracture hardness | 3–7 GPa (dense HA) |
| Melting point | 1614 °C | Biocompatibility | High |
| Tensile strength | 38–300 MPa (dense HA) ~3 MPa (porous HA) | Biodegradation | Low |
| Bending strength | 38–250 MPa (dense HA) 2–11 MPa (porous HA) | Bioactivity | High |
| Compressive strength | 120–900 MPa (dense HA) 2–100 MPa (porous HA) | Osteoconduction | High |
| Young’s elastic modulus | 35–120 GPa | Osteoinduction | Nil |
| Processing Technique | Typical Procedure | Powder Property |
|---|---|---|
| Solid-state synthesis | Calcium and phosphate containing compounds. Sintering ~ 1250 °C | HA particles with heterogeneous size (from nano- to micro-scale) and shape. |
| Mechanochemical method | Slow mixing of Ca(OH) + H3PO4/(CH3COO)2Ca + KH2PO4/Ca(NO3) + (NH4)2HPO4 solutions using vigorous stirring, followed by aging | HA nanoparticles of 50–100 nm length. HA nanorods of 50 nm diameter. HA nanospheres of 200 nm size |
| Hydrothermal method | Hydrothermal treatment of an aqueous mixture of pH 4.5 comprising Ca(NO3)2, NaH2PO4, HNO3 and urea at 160 °C for about 3 h | HA whiskers of 10 μm width and 150 μm length |
| Sol-gel method | Aging an ethanol solution of pH 10 comprising Ca(NO3)2, (NaH)2PO4, NH4OH, and PEG at 85 °C for 4 h, followed by drying | Sintered HA nanocrystals of 50–70 nm size |
| Sonochemical method | Ultrasonic irradiation (28–34 kHz, 100 W) of a pseudo-body solution containing NaCl, KCl, NaH2PO4, KH2PO4, CaCl2 and MgCl2 | Spherical HA nanoparticles of 18 nm size with a specific surface area up to |
| Synthesis method based on biogenic sources | Thermal treatment of deproteinized bovine bone, then crushing and ball milling, followed by vibro-milling using ethanol | Needle-like HA nanopowder of ~100 nm size |
| Material | Compressive Strength (MPa) | Tensile Strength (MPa) | Elastic Modulus (GPa) | Fracture Toughness (MPa) |
|---|---|---|---|---|
| Dense HA | ~400 | ~40 | ~100 | ~1.0 |
| Porous HA (82–86 vol.%) | 0.2–0.4 | - | 0.8–1.6 × 10−3 | - |
| Cortical bone | 130–180 | 50–151 | 12–18 | 6–8 |
| Cancellous bone | 1–20 | - | 0.1–0.5 | - |
| Tradename | Producer | Country |
|---|---|---|
| Actifuse | ApaTech | UK |
| ApaPore | ApaTech | UK |
| Apaceram | Pentax | Japan |
| Bonefil | Pentax | Japan |
| Bonetite | Pentax | Japan |
| Bonoceram | Sumitomo Osaka Cement | Japan |
| Bioroc | Depuy—Bioland | France |
| Cerapatite | Ceraver | France |
| BoneSource | Stryker Orthopaedics | NJ, USA |
| Calcitite | Zimmer | IN, USA |
| Osteograf | Ceramed | CO, USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiume, E.; Magnaterra, G.; Rahdar, A.; Verné, E.; Baino, F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics 2021, 4, 542-563. https://doi.org/10.3390/ceramics4040039
Fiume E, Magnaterra G, Rahdar A, Verné E, Baino F. Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics. 2021; 4(4):542-563. https://doi.org/10.3390/ceramics4040039
Chicago/Turabian StyleFiume, Elisa, Giulia Magnaterra, Abbas Rahdar, Enrica Verné, and Francesco Baino. 2021. "Hydroxyapatite for Biomedical Applications: A Short Overview" Ceramics 4, no. 4: 542-563. https://doi.org/10.3390/ceramics4040039
APA StyleFiume, E., Magnaterra, G., Rahdar, A., Verné, E., & Baino, F. (2021). Hydroxyapatite for Biomedical Applications: A Short Overview. Ceramics, 4(4), 542-563. https://doi.org/10.3390/ceramics4040039









