Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash
Abstract
1. Introduction
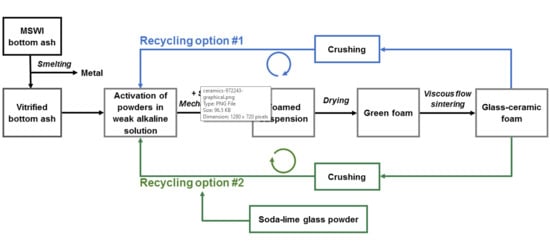
2. Materials and Methods
3. Results and Discussion
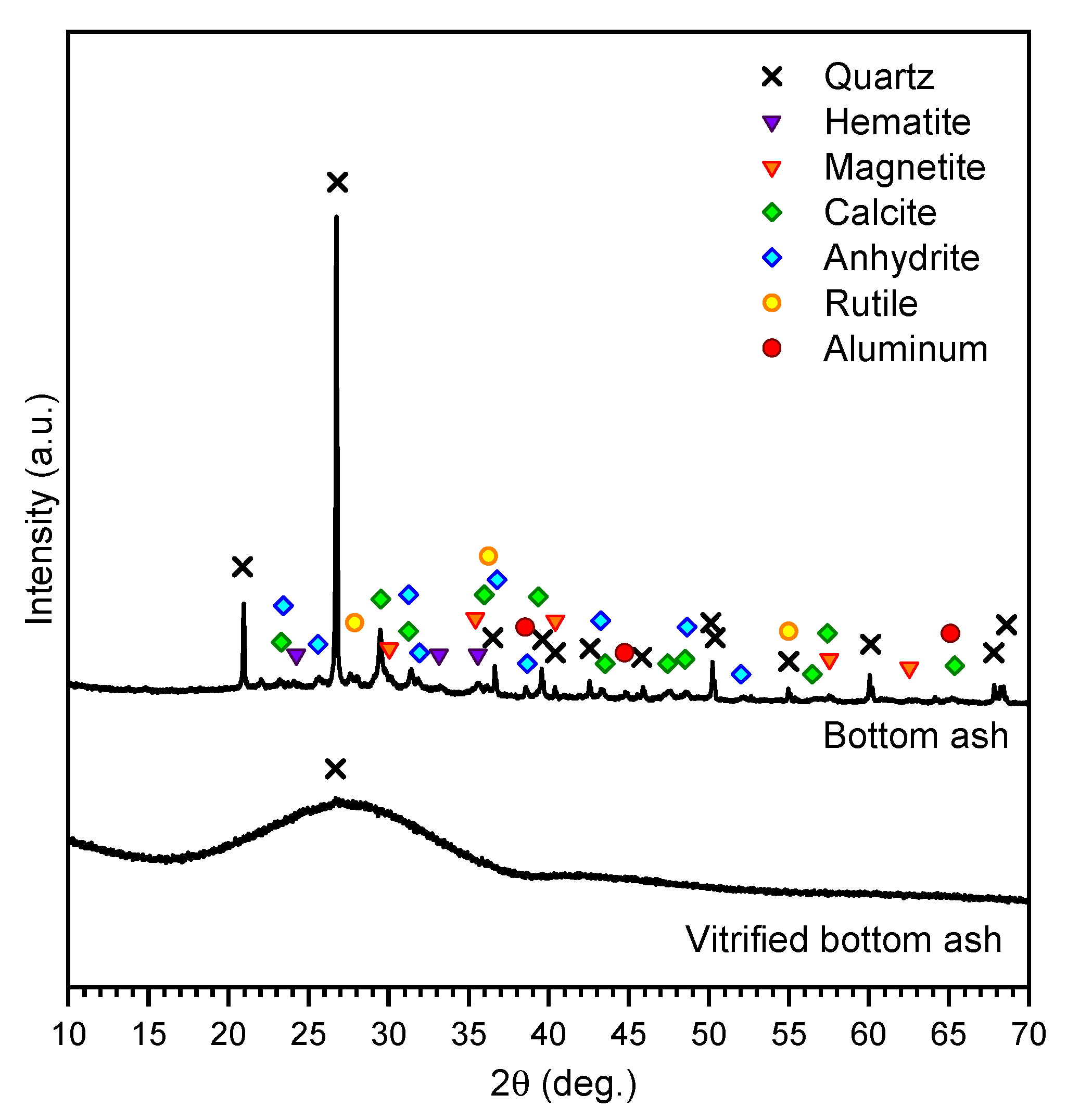
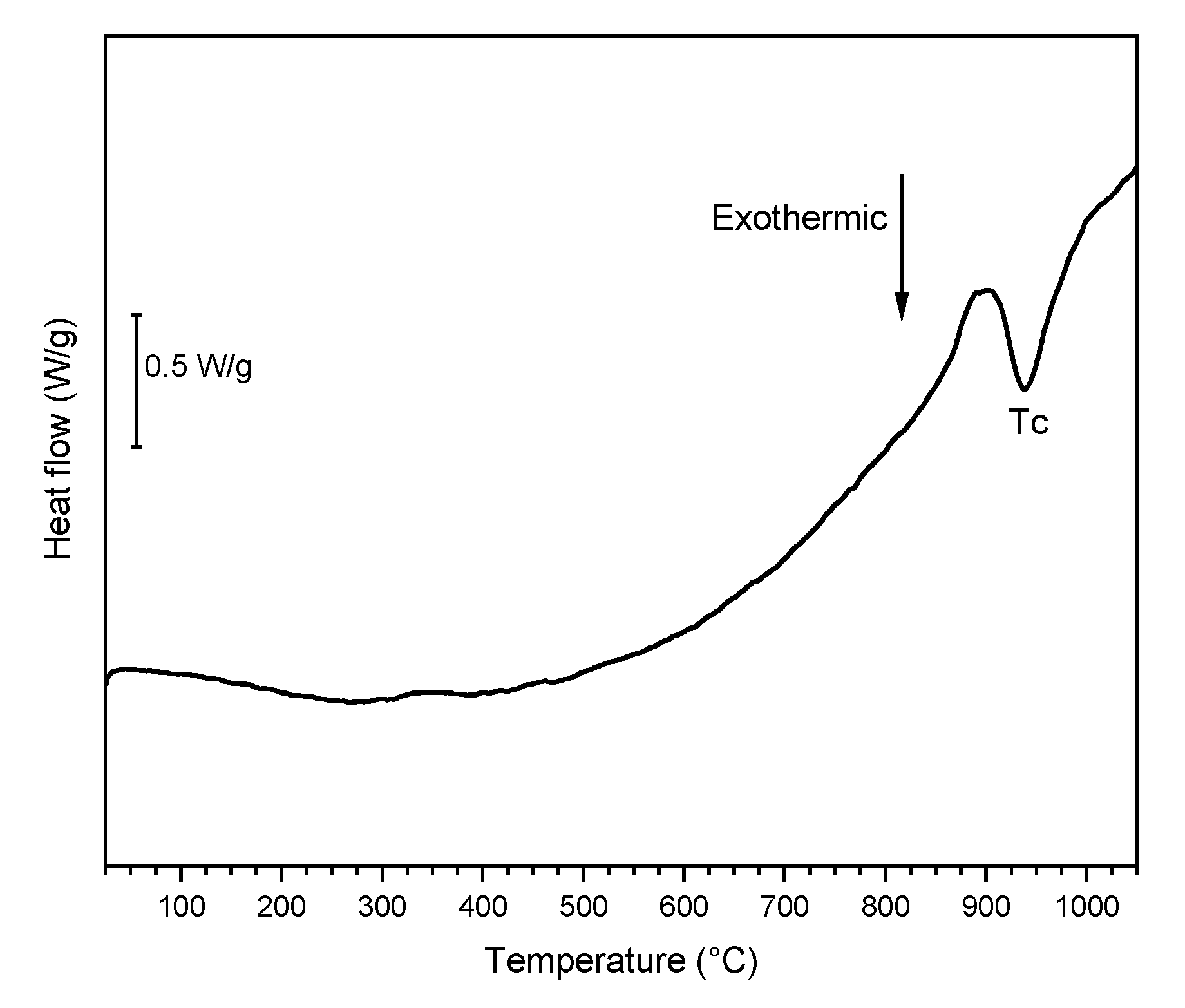
3.1. Vitrification of Bottom Ash
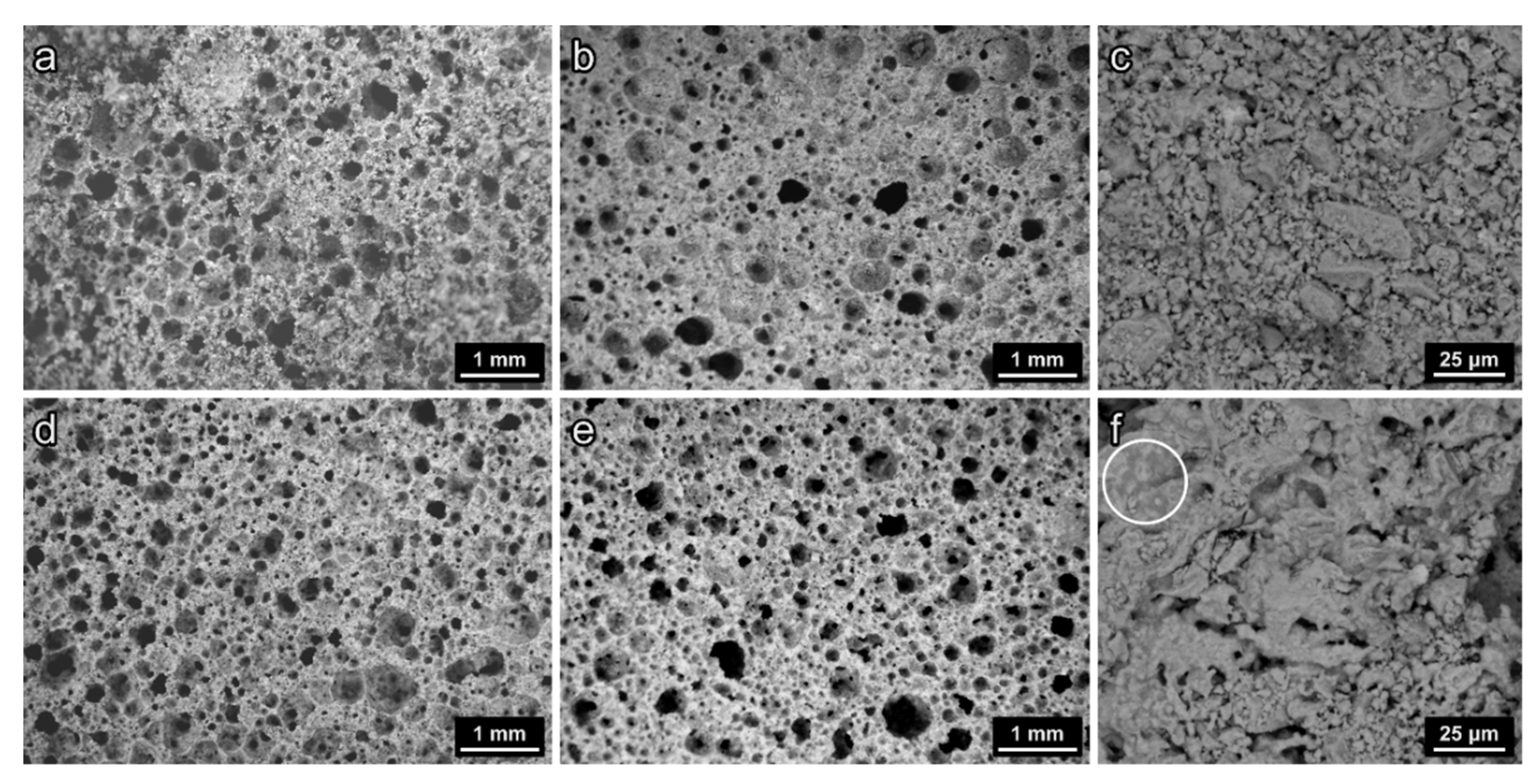
3.2. Vitrified Bottom Ash-Based Porous Glass-Ceramics
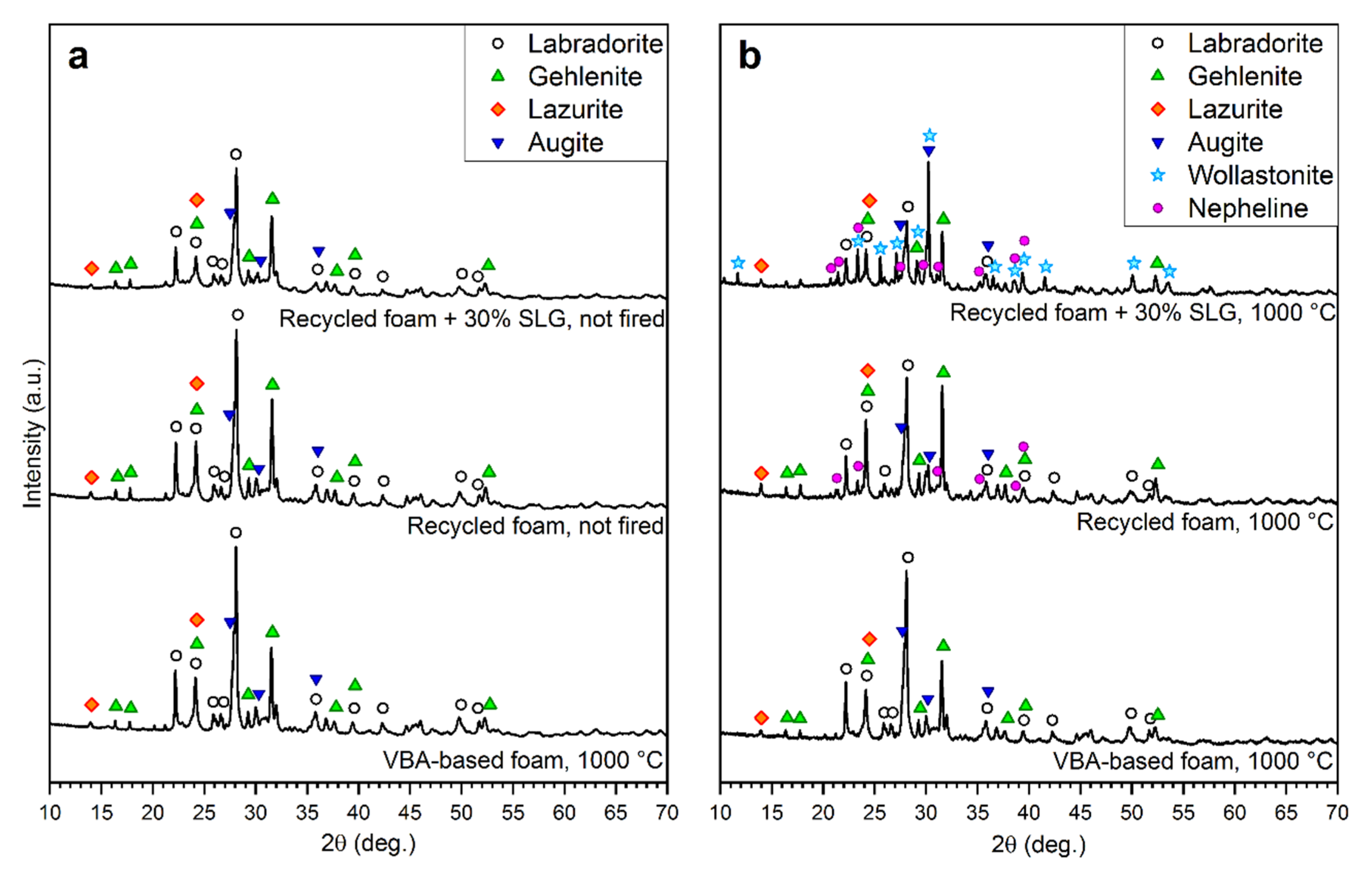
3.3. “Recycled” Porous Glass-Ceramics
4. Conclusions
- Treating MSWI BA through SAF favors the generation of an amorphous mineral precursor, which represents around 65–68 wt% of BA and a rich Fe-Cu metal phase, in an amount of 9–12 wt%. The cleaning of the starting waste and the extraction of valuable metals are the main benefits of the thermal treatment. In fact, the separation yields a vitreous slag, to be transformed into useful products, and a metal alloy, which may undergo further separation steps;
- The vitreous slag from BA smelting could be easily transformed into highly porous glass-ceramics, according to the mechanical foaming of suspensions of fine powders, undergoing gelation in alkaline aqueous solutions, followed by sintering;
- For the sake of sustainability, the gelation could be achieved with limited molarity of the alkali activator (1 M NaOH); firing, in addition, could be conducted at a moderate temperature, 1000 °C, with significant crystallization—in turn promoting the obtainment of glass-ceramic foams with a good strength-to-density ratio—coupled with an excellent stabilization of pollutants;
- The glass-ceramic foams were tested as raw material for a second manufacturing cycle. Although still successful in the stabilization of pollutants, the “recycling” approach was not acceptable for the low strength of the products;
- The stabilization of pollutants and the strength of foams from the second cycle could be optimized by the addition of 30 wt% soda-lime glass, in a condition of reactive sintering, implying an extensive transformation of the phase assemblage;
- The gel casting/sintering approach, applied to the waste-derived material, possibly combined with soda-lime glass, has a great potential for permanent waste minimization.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ragossnig, A.M.; Schneider, D.R. Circular economy, recycling and end-of-waste. Waste Manag. Res. 2019, 37, 109–111. [Google Scholar] [CrossRef] [PubMed]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. Strategic municipal solid waste management: A quantitative model for Italian regions. Energy Convers. Manag. 2014, 77, 709–720. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Ammar, M. The influence of catalysts in biomass steam gasification and catalytic potential of coal bottom ash in biomass steam gasification: A review. Renew. Sustain. Energy Rev. 2017, 73, 468–476. [Google Scholar] [CrossRef]
- Saveyn, H.; Eder, P.; Garbarino, E.; Muchova, L.; Hjelmar, O.; van der Sloot, H.; Comans, R.; van Zomeren, A.; Hyks, J.; Oberender, A. Study on Methodological Aspects Regarding Limit Values for Pollutants in Aggregates in the Context of the Possible Development of End-of-Waste Criteria Under the EU Waste Framework Directive; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Xiao, Y.; Oorsprong, M.; Yang, Y.; Voncken, J.H.L. Vitrification of bottom ash from a municipal solid waste incinerator. Waste Manag. 2008, 28, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Colombo, P.; Brusatin, G.; Bernardo, E.; Scarinci, G. Inertization and reuse of waste materials by vitrification and fabrication of glass-based products. Curr. Opin. Solid State Mater. Sci. 2003, 7, 225–239. [Google Scholar] [CrossRef]
- Rincón, A.; Marangoni, M.; Cetin, S.; Bernardo, E. Recycling of inorganic waste in monolithic and cellular glass-based materials for structural and functional applications. J. Chem. Technol. Biotechnol. 2016, 91, 1946–1961. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, R.D.; Wu, J.P.; Boccaccini, A.R. Glass-ceramics: Their production from wastes-A Review. J. Mater. Sci. 2006, 41, 733–761. [Google Scholar] [CrossRef]
- Scarinci, G.; Brusatin, G.; Bernardo, E. Glass Foams. In Cellular Ceramics: Structure, Manufacturing, Properties and Applications, 1st ed.; Scheffler, M., Colombo, P., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 158–176. [Google Scholar]
- Silva, R.V.; de Brito, J.; Lynn, C.J.; Dhir, R.K. Use of municipal solid waste incineration bottom ashes in alkali-activated materials, ceramics and granular applications: A review. Waste Manag. 2017, 68, 207–220. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Lancellotti, I.; Kamseu, E.; Michelazzi, M.; Barbieri, L.; Corradi, A.; Leonelli, C. Chemical stability of geopolymers containing municipal solid waste incinerator fly ash. Waste Manag. 2010, 30, 673–679. [Google Scholar] [CrossRef]
- Idir, R.; Cyr, M.; Pavoine, A. Investigations on the durability of alkali-activated recycled glass. Constr. Build. Mater. 2020, 236, 117477. [Google Scholar] [CrossRef]
- Rincón, A.; Giacomello, G.; Pasetto, M.; Bernardo, E. Novel ‘inorganic gel casting’ process for the manufacturing of glass foams. J. Eur. Ceram. Soc. 2017, 37, 2227–2234. [Google Scholar] [CrossRef]
- Rincon Romero, A.; Salvo, M.; Bernardo, E. Up-cycling of vitrified bottom ash from MSWI into glass-ceramic foams by means of ‘inorganic gel casting’ and sinter-crystallization. Constr. Build. Mater. 2018, 192, 133–140. [Google Scholar] [CrossRef]
- Rabelo Monich, P.; Dogrul, F.; Lucas, H.; Friedrich, B.; Bernardo, E. Strong porous glass-ceramics from alkali activation and sinter-crystallization of vitrified MSWI bottom ash. Detritus 2019, 08, 101–108. [Google Scholar] [CrossRef]
- BS EN 12457-4:2002. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 10 mm (without or with Size Reduction); BS EN: London, UK, 2002. [Google Scholar]
- Directive 2003/33/EC, 2003. Official Journal of the European Communities. 2003. L 11/27. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2003:011:0027:0049:EN:PDF (accessed on 29 December 2020).
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Haz. Mat. 2020, 393. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Lv, J.F.; Lai, Z.N.; Lan, Z.Y.; Wang, H. Innovative methodology for separating copper and iron from Fe-Cu alloy residues by selective oxidation smelting. J. Clean. Prod. 2019, 231, 110–120. [Google Scholar] [CrossRef]
- Pisciella, P.; Crisucci, S.; Karamanov, A.; Pelino, M. Chemical durability of glasses obtained by vitrification of industrial wastes. Waste Manag. 2001, 21, 1–9. [Google Scholar] [CrossRef]
- Nedeljković, M.; Ghiassi, B.; Melzer, S.; Kooij, C.; van der Laan, S.; Ye, G. CO2 binding capacity of alkali-activated fly ash and slag pastes. Ceram. Int. 2018, 44, 19646–19660. [Google Scholar] [CrossRef]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 2014; pp. 1–510. [Google Scholar]
- Höland, W.; Beall, G.H. Glass-Ceramic Technology, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 123–126. [Google Scholar]
- Rabelo Monich, P.; Romero, A.R.; Rambaldi, E.; Bernardo, E. Case studies of up-cycling of partially crystallized ceramic waste in highly porous glass-ceramics. Constr. Build. Mater. 2020, 261, 119971. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H. A low cost route for fabrication of wollastonite glass–ceramics directly using soda-lime waste glass by reactive crystallization–sintering. Ceram. Int. 2013, 39, 1943–1949. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Dong, C.; Yang, Y. The behaviour and reactions of sodium containing minerals in ash melting process. J. Energy Inst. 2017, 90, 167–173. [Google Scholar] [CrossRef]
- Grosso, M.; Niero, M.; Rigamonti, L. Circular economy, permanent materials and limitations to recycling: Where do we stand and what is the way forward? Waste Manag. Res. 2017, 35, 793–794. [Google Scholar] [CrossRef]


| Oxide (wt%) | Soda-Lime Glass (SLG) | Bottom Ash (BA) * | Vitrified BA (VBA) | Air Pollution Control Residues (APCR) |
|---|---|---|---|---|
| SiO2 | 71.9 | 40.1 | 48.1 | 25.0 |
| Al2O3 | 1.2 | 9.6 | 18.2 | 10.7 |
| CaO | 7.5 | 19.8 | 20.0 | 21.3 |
| Na2O | 14.3 | 4.8 | 4.7 | 8.4 |
| MgO | 4 | 2.2 | 2.5 | 1.9 |
| K2O | 0.4 | 1.0 | 0.8 | 1.4 |
| Fe2O3 | 10.4 | 1.1 | 7.9 | |
| TiO2 | 0.1 | 1.2 | 1.0 | 1.3 |
| MnO | 0.1 | 0.6 | 0.2 | |
| P2O5 | 1.3 | 0.1 | 2.0 | |
| ZnO | 0.7 | 0.0 | 8.1 | |
| PbO | 0.2 | 0.0 | 1.1 | |
| Cr2O3 | 0.2 | 0.0 | 0.2 | |
| BaO | 0.2 | 0.2 | 0.1 | |
| CuO | 0.7 | 0.3 | 1.9 | |
| SO3 | 2.0 | 1.5 | 3.8 | |
| Cl | 1.3 | 0.1 | 3.3 | |
| C | 4.0 | 0.6 | 0.1 |
| Element (wt%) | Fe | Cu | Si | P | Cr | Ni | Co | Sn |
| Average | 80.3 | 10.8 | 1.9 | 1.4 | 1.1 | 0.6 | 0.4 | 0.2 |
| Error | 3.0 | 1.9 | 0.4 | 0.6 | 0.3 | 0.2 | 0.2 | 0.03 |
| Trace Elements | Mo | Sb | Zn | Bi | Nb | Ag | Nd | Cd |
| ppm | 250–1550 | 700–1200 | 100–490 | 170–350 | 5–90 | 25–80 | 15–20 | 5–9 |
| Group of Samples | VBA-Based Foam | Recycled Foam | 70% Recycled Foam/30% SLG |
|---|---|---|---|
| Density Determinations | |||
| ρgeom (g/cm3) | 0.76 ± 0.03 | 0.68 ± 0.01 | 0.66 ± 0.00 |
| ρapparent (g/cm3) | 2.45 ± 0.00 | 2.66 ± 0.03 | 2.59 ± 0.01 |
| ρtrue (g/cm3) | 2.70 ± 0.00 | 2.72 ± 0.00 | 2.63 ± 0.00 |
| Porosity Distribution | |||
| Total porosity (vol%) | 71.9 | 75 | 74.8 |
| Open porosity (vol%) | 68.9 | 74.4 | 74.5 |
| Closed porosity (vol%) | 2.9 | 0.6 | 0.3 |
| Strength Determinations | |||
| σcomp (MPa) | 3.8 ± 0.9 | 1.0 ± 0.1 | 4.9 ± 1.2 |
| σbend (MPa) ** | 128.5 | 42.3 | 196.4 |
| Element | Limit Values for Inert Waste | BA before Smelting | VBA | VBA-Based Foam | Recycled Foam | 70% Recycled Foam/30% SLG |
|---|---|---|---|---|---|---|
| As | 0.5 | 0.03 | 0.01 | 0.06 | 0.11 | 0.15 |
| Ba | 20 | 1.42 | 0.24 | 0.16 | 0.95 | 1.09 |
| Cd | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 |
| Cr | 0.5 | 0.02 | 0.07 | <0.01 | 0.51 | 0.46 |
| Cu | 2 | 3.64 | 0.08 | 0.14 | 0.76 | 0.13 |
| Mo | 0.5 | 1.14 | 0.4 | 0.05 | 0.03 | 0.03 |
| Ni | 0.4 | 0.16 | <0.01 | <0.01 | <0.01 | <0.01 |
| Pb | 0.5 | <0.01 | 0.14 | 0.05 | 0.05 | <0.01 |
| Zn | 4 | <0.01 | 0.05 | 0.20 | 0.20 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabelo Monich, P.; Lucas, H.; Friedrich, B.; Bernardo, E. Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash. Ceramics 2021, 4, 1-11. https://doi.org/10.3390/ceramics4010001
Rabelo Monich P, Lucas H, Friedrich B, Bernardo E. Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash. Ceramics. 2021; 4(1):1-11. https://doi.org/10.3390/ceramics4010001
Chicago/Turabian StyleRabelo Monich, Patricia, Hugo Lucas, Bernd Friedrich, and Enrico Bernardo. 2021. "Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash" Ceramics 4, no. 1: 1-11. https://doi.org/10.3390/ceramics4010001
APA StyleRabelo Monich, P., Lucas, H., Friedrich, B., & Bernardo, E. (2021). Recyclable Porous Glass-Ceramics from the Smelting of MSWI Bottom Ash. Ceramics, 4(1), 1-11. https://doi.org/10.3390/ceramics4010001