Preparation and Properties of Co-Doped Magnesium Lanthanum Hexaluminat Blue Ceramics
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Microstructural Characterization
2.3. Optical Performance Characterization
3. Result and Discussion
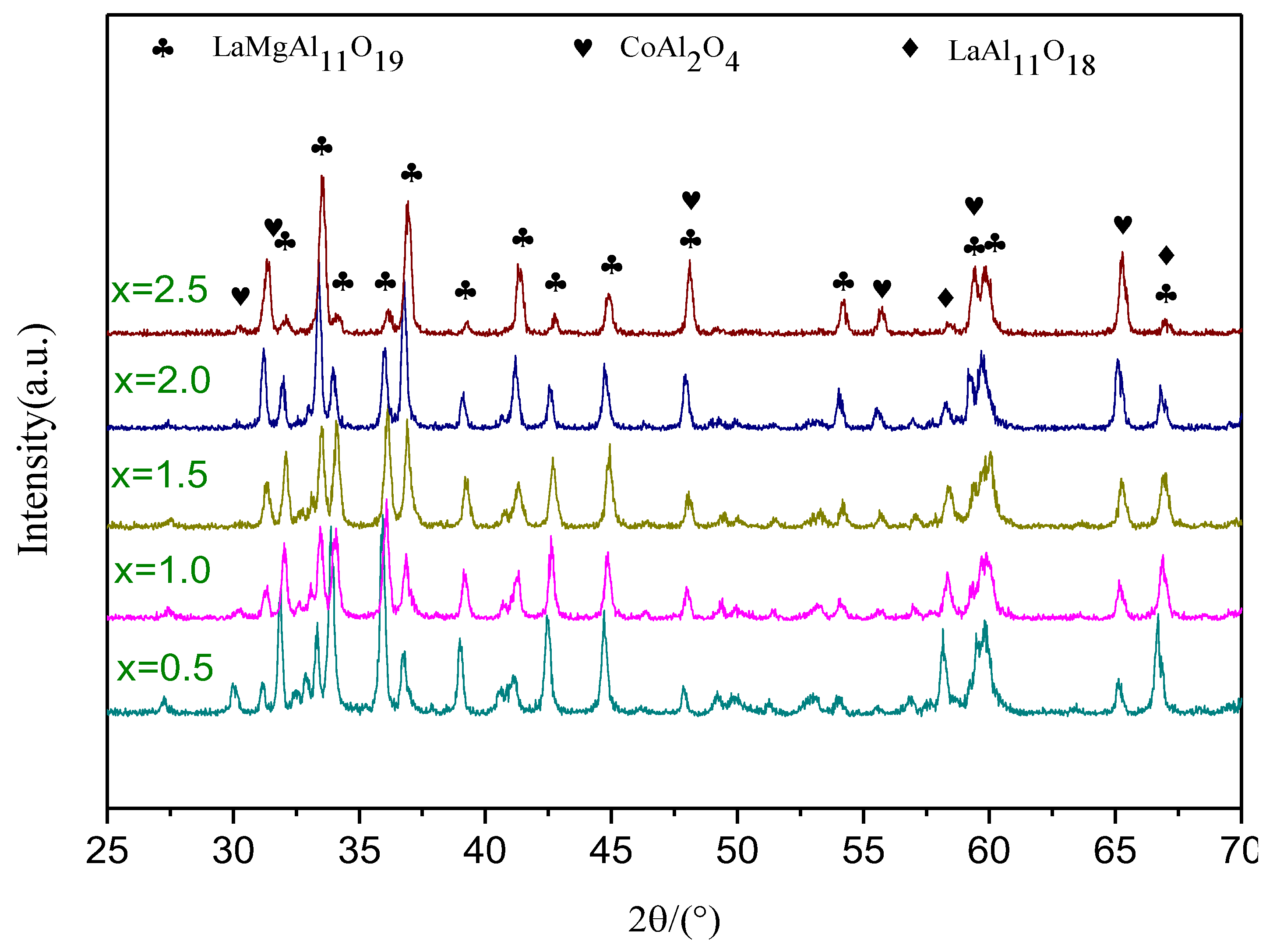
3.1. Phase Analysis
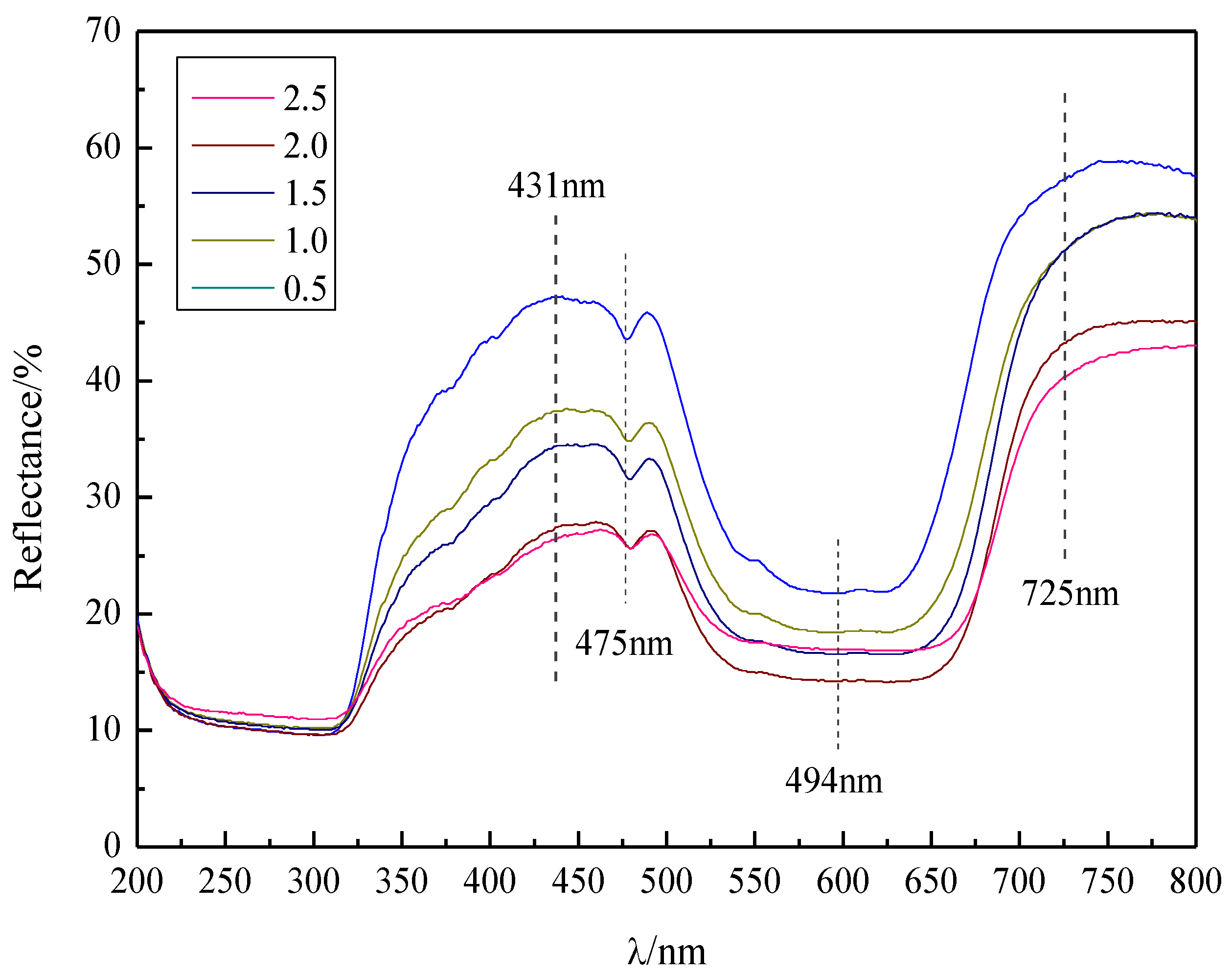
3.2. The Diffuse Reflectance and the Chromatic Properties
3.3. Influence of Other Factors on the Optimal Doping Amount
4. Conclusions
- (1)
- The doping content of Co is the key factor for the color development of the sample, and x = 1.0 is the optimal doping amount. At this time, the blue tone of the sample −b* reached the maximum value (−b* = 35.36).
- (2)
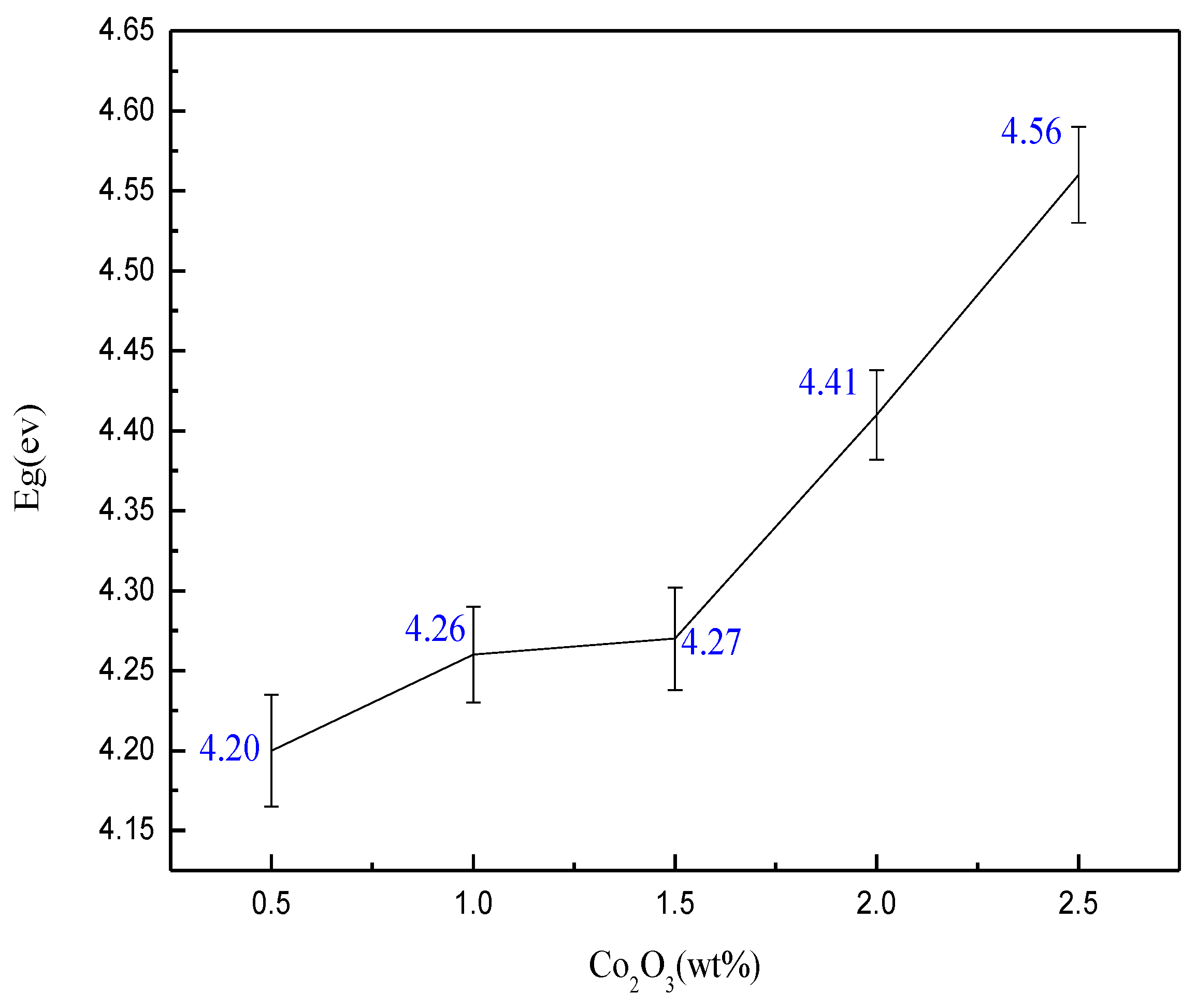
- The forbidden band width also plays a certain role in the color development of the sample. When Eg = 4.26 ev in this system, the sample color is the bluest.
- (3)
- Increasing the temperature can promote the synthesis of the phase, thereby increasing the color depth of the sample. In this system, the color of the sample reaches the deepest at 1450 °C. Continuing to increase the temperature will only promote the synthesis of the phase with little effect on the color of the sample.
- (4)
- This material can maintain color stability when exposed to air, and can be used to prepare various jewelry and decorative items.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lv, H.D.; Bao, J.X.; Ruan, F.; Zhou, F. Preparation and properties of black Ti-doped zirconia ceramics. J. Mater. Res. Technol. 2020, 1, 19–23. [Google Scholar] [CrossRef]
- Toriumi, K.; Ozima, M.; Akaogi, M. Electron-density distribution in crystals of CoAl2O4. Acta Crystallogr. Sect. B Struct. Sci. 1978, 34, 1093–1096. [Google Scholar] [CrossRef]
- Walsh, A.; Yan, Y.; Al-Jassim, M.M. Electronic, Energetic, and Chemical Effects of Intrinsic Defects and Fe-Doping of CoAl2O4: A DFT+U Study. J. Phys. Chem. C 2008, 112, 12044–12050. [Google Scholar] [CrossRef]
- Fu, W.; Yuan, Z.; Chen, K.Y. High-temperature solid-phase synthesis of spinel-type CoAl2O4 blue pigment. J. Ceram. 2019, 4, 11–16. [Google Scholar]
- Lv, H.D.; Bao, J.X.; Qi, S.Y.; Jin, Q.; Guo, W.R. Optical and mechanical properties of purple zirconia ceramics. Asian Ceram. Soc. 2019, 7, 306–311. [Google Scholar] [CrossRef]
- Alvarez-Docio, C.M.; Reinosa, J.J.; Campo, A. 2D particles forming a nanostructured shell: A step forward cool NIR reflectivity for CoAl2O4 pigments. Dye. Pigment. 2017, 137, 1–11. [Google Scholar] [CrossRef]
- Zhou, F.F.; Wang, Y.; Cui, Z.Y. Thermal cycling behavior of nanostructured 8YSZ, SZ/8YSZ and 8CSZ/8YSZ thermal barrier coatings fabricated by atmospheric plasma spraying. Ceram. Int. 2017, 43, 4102. [Google Scholar] [CrossRef]
- Zhu, R.X.; Liu, Z.G.; Ouyang, J.H. Preparation and characterization of LnMgAl11O19 (Ln = La, Nd, Gd) ceramic powders. Ceram. Int. 2013, 39, 884. [Google Scholar] [CrossRef]
- He, M.T.; Meng, H.M.; Wang, Y.C.; Ren, P.W. Preparation of Magnesium-based Lanthanum Hexaluminate Spraying Powder and Its Heat Treatment Process. Powder Metall. Technol. 2018, 36, 370–376. [Google Scholar]
- Jiang, B.; Fang, M.H.; Huang, Z.H. Preparation of Gd3 + doped La1-xMgAl11O19 (x = 0-1) ceramics and its thermal properties. J. Chin. Ceram. Soc. 2010, 3, 1263–1267. [Google Scholar]
- Cui, J.J.; Liu, Z.G. Hot corrosion behavior of LaMgAl11O19 ceramic coated with molten CMAS deposits at temperature of 1250 °C in air. J. Alloy. Compd. 2016, 685, 316–321. [Google Scholar] [CrossRef]
- Melo, D.; Vieira, F.T.G.; Costa, T.C.C.; Soledade, L.E.B.; Paskocimas, C.A.; Melo, D.M.A.; Longod, E.; Marinhoe, E.P.; Souzaa, A.G.; Santos, I.M.G. Lanthanum cobaltite black pigments with perovskite structure. Dye. Pigment. 2013, 98, 459–463. [Google Scholar] [CrossRef]
- Li, J.; Medina, E.A.; Stalick, J.K.; Sleight, A.W.; Subramanian, M.A. Colored oxides with hibonite structure: A potential route to non-cobalt blue pigments. Prog. Solid State Chem. 2016, 44, 107–127. [Google Scholar] [CrossRef]
- He, L.H.; Wang, H.H.; Mou, B.J. Introduction of several yellow pigments. Ceramics 2013, 5, 46–47. [Google Scholar]
- Liu, X.Y.; Lu, F.; Liu, S.F. Sintering and molten salt corrosion behavior of Mg-based lanthanum hexaaluminate ceramics. Chin. Rare Earths 2018, 39, 73–79. [Google Scholar]
- Wang, Y.Q.; Yang, W.J.; Ma, J. Ultralow Temperature Synthesis and Properties of Cobalt Blue Materials. J. Ceram. 2019, 4, 497–502. [Google Scholar]
- Horlyck, J.; Pokhrel, S.; Lovell, E. Unifying double flame spray pyrolysis with lanthanum doping to restrict cobalt-aluminate formation in Co/Al2O3 catalysts for the dry reforming of methane. Catal. Sci. Technol. 2019, 9, 4970–4980. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.L.; Sun, C. Properties of LnMgAl11O19 (Ln = La, Nd) powders with thermal barrier coating ceramic layer material. Chin. J. Mater. Res. 2019, 33, 6. [Google Scholar]
- Merino, M.C.G.; Estrella, A.L.; Rodriguez, M.E.; Acuña, L.; Lassa, M.S.; Lascalea, G.E.; Vázquez, P. Combustion syntheses of CoAl2O4 powders using different fuels. Procedia Mater. Sci. 2015, 8, 519–525. [Google Scholar] [CrossRef]
- Ioana, M.; Gabriela, M.; Dana, G. Blue CoAl2O4 spinel via complexation method. Mater. Chem. Phys. 2010, 122, 491–497. [Google Scholar]
- Han, P.W.; Xiao, L.; Wang, Y.L. Cobalt Recovery by the Chlorination-Volatilization Method. Metall. Mater. Trans. B 2019, 50, 1128–1133. [Google Scholar] [CrossRef]





| Co2O3/(wt%) | Volume Density/(g/cm3) | Relative Density/(%) |
|---|---|---|
| x = 0.5 | 3.79 | 88.88 |
| x = 1.0 | 3.71 | 86.92 |
| x = 1.5 | 3.65 | 85.55 |
| x = 2.0 | 3.53 | 82.71 |
| x = 2.5 | 3.47 | 81.30 |
| Co2O3 (wt%) | L* | a* | b* |
|---|---|---|---|
| x = 0.5 | 54.33 | −3.87 | −34.02 |
| x = 1.0 | 47.81 | −3.17 | −35.36 |
| x = 1.5 | 44.14 | −2.71 | −34.11 |
| x = 2.0 | 40.98 | −2.07 | −26.89 |
| x = 2.5 | 38.93 | −2.43 | −23.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Wang, Q.; Bao, J.; Song, X. Preparation and Properties of Co-Doped Magnesium Lanthanum Hexaluminat Blue Ceramics. Ceramics 2020, 3, 235-244. https://doi.org/10.3390/ceramics3020021
Guo R, Wang Q, Bao J, Song X. Preparation and Properties of Co-Doped Magnesium Lanthanum Hexaluminat Blue Ceramics. Ceramics. 2020; 3(2):235-244. https://doi.org/10.3390/ceramics3020021
Chicago/Turabian StyleGuo, Rui, Qingchun Wang, Jinxiao Bao, and Xiwen Song. 2020. "Preparation and Properties of Co-Doped Magnesium Lanthanum Hexaluminat Blue Ceramics" Ceramics 3, no. 2: 235-244. https://doi.org/10.3390/ceramics3020021
APA StyleGuo, R., Wang, Q., Bao, J., & Song, X. (2020). Preparation and Properties of Co-Doped Magnesium Lanthanum Hexaluminat Blue Ceramics. Ceramics, 3(2), 235-244. https://doi.org/10.3390/ceramics3020021




