Enhancement of the Ionic Conductivity in Electric Field-Assisted Pressureless Sintered BITIVOX Solid Electrolytes
Abstract
1. Introduction
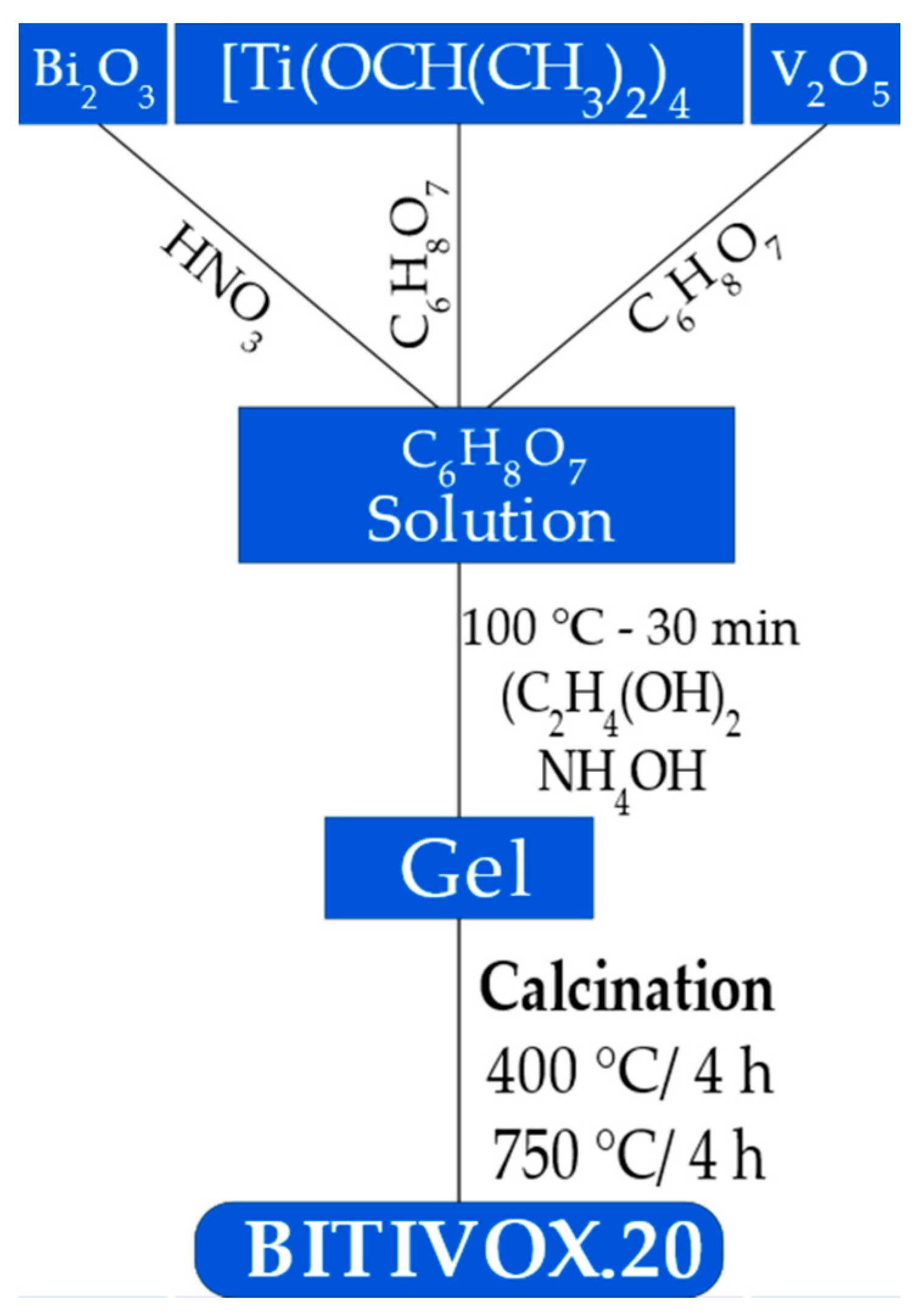
2. Materials and Methods
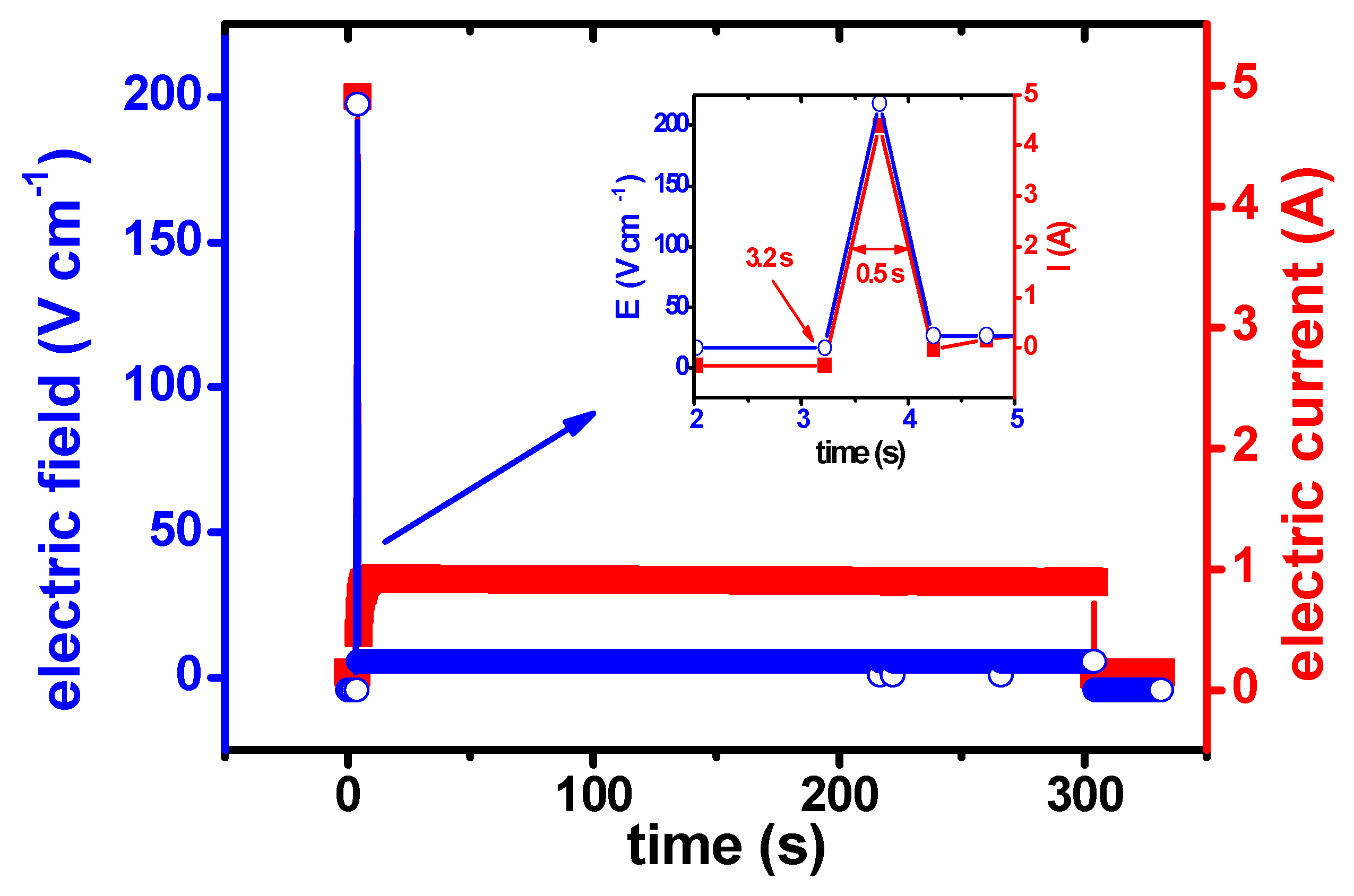
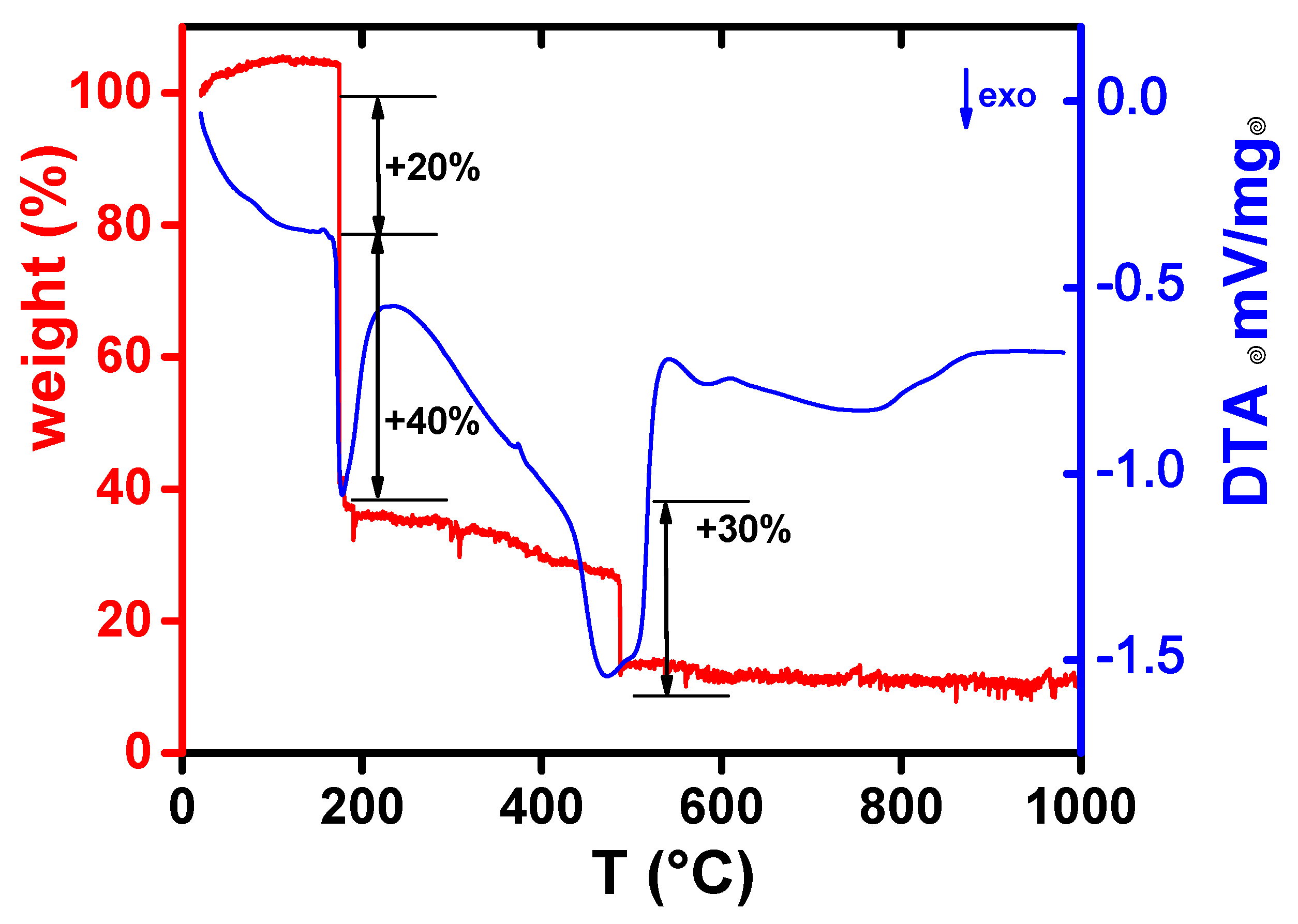
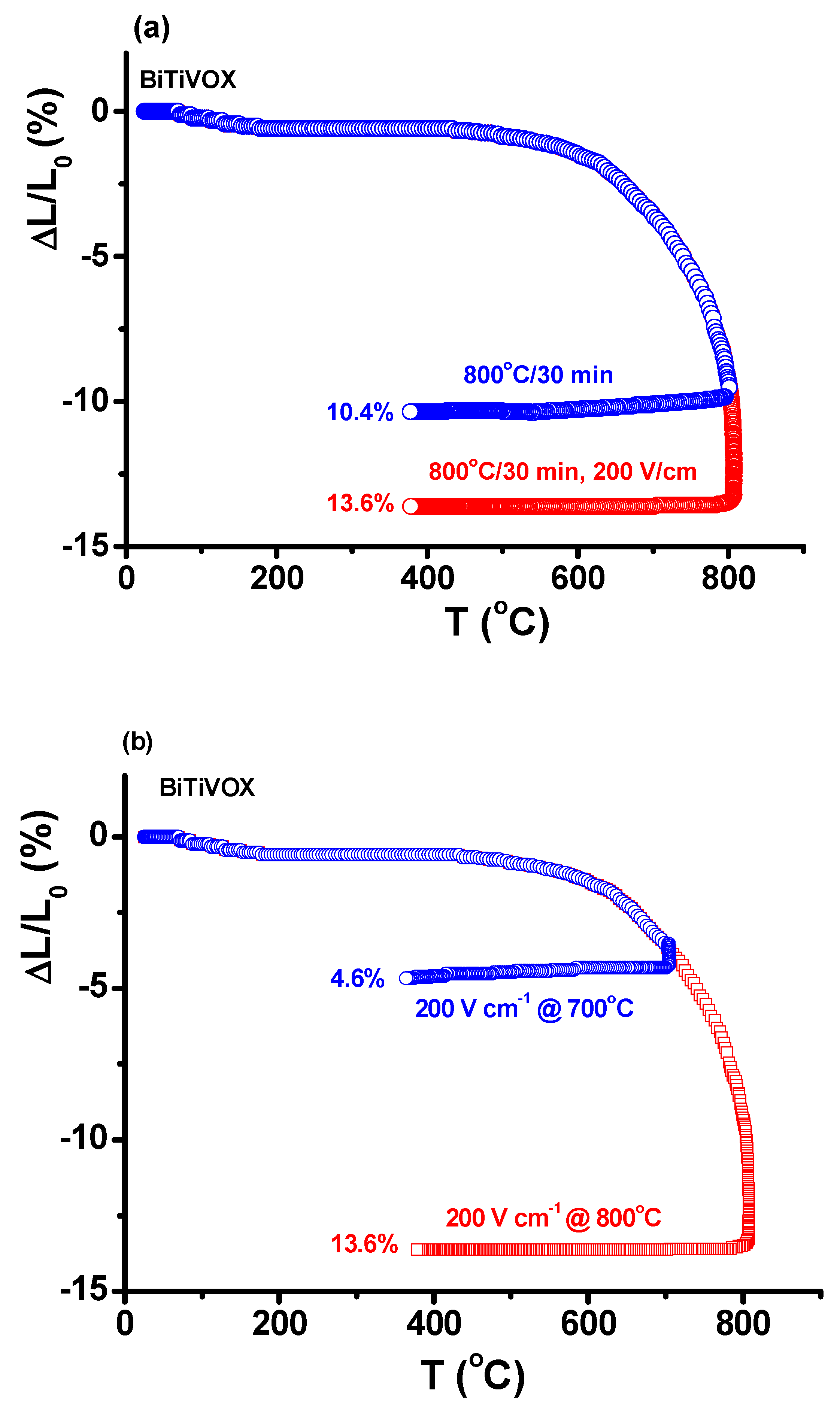
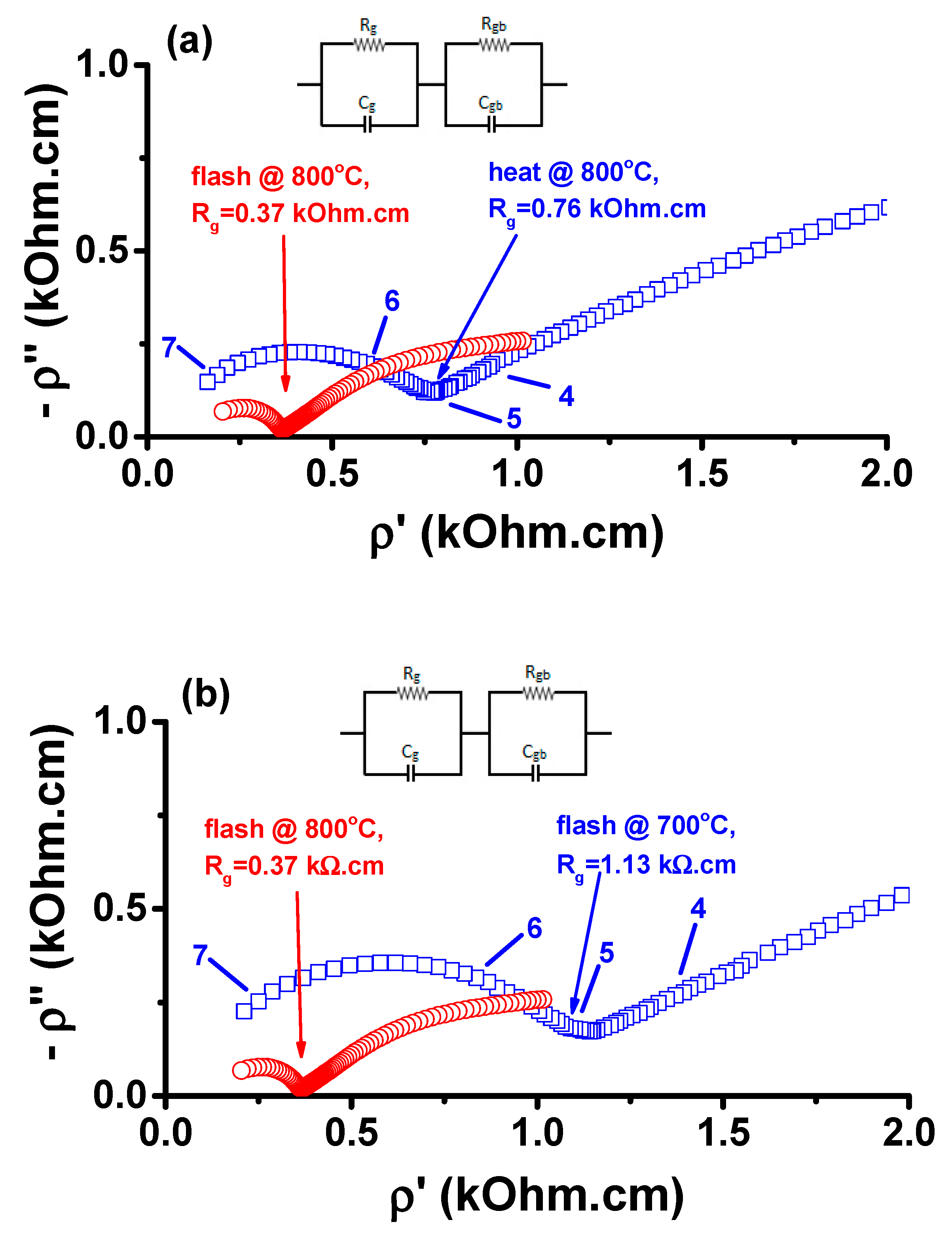
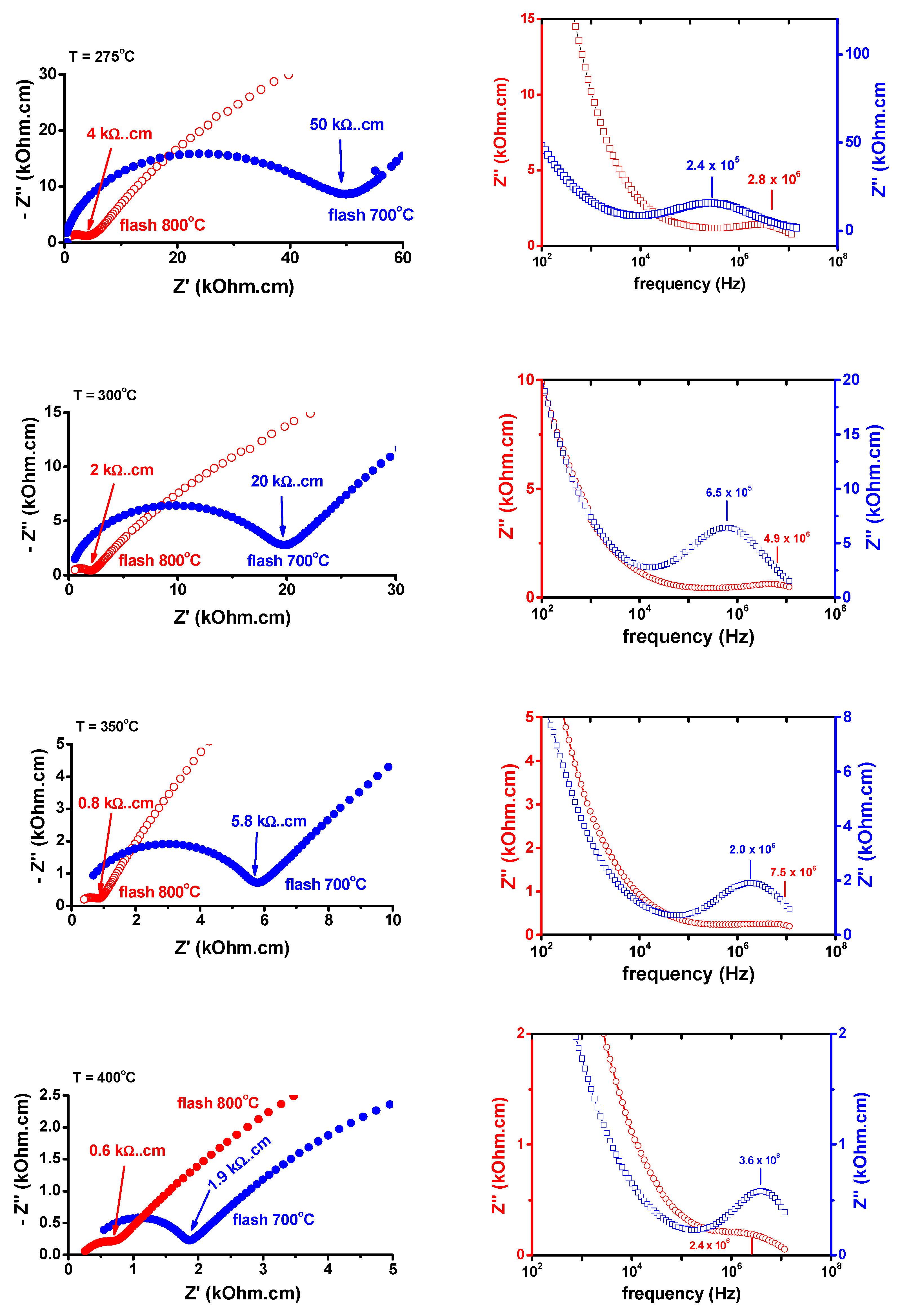
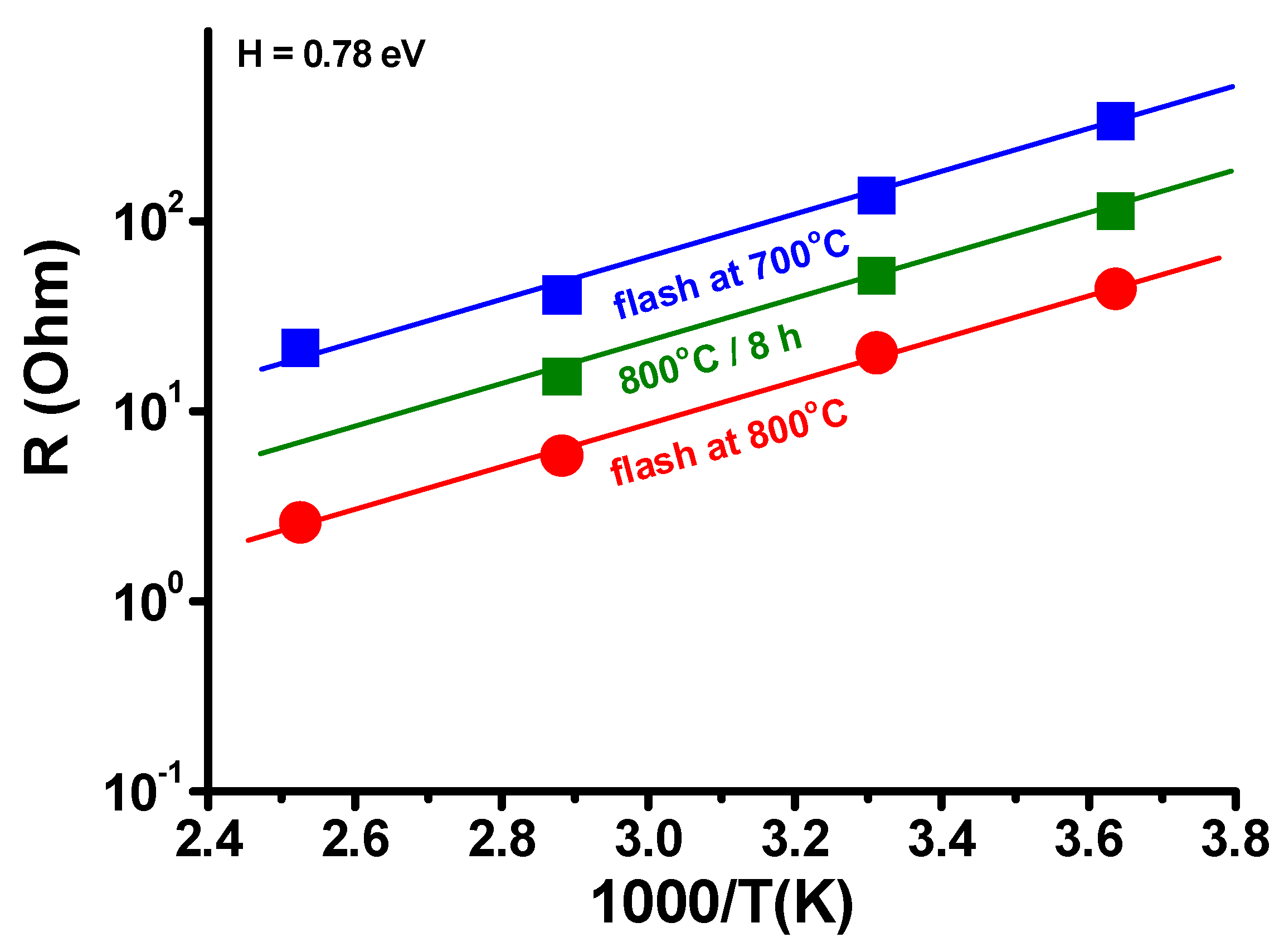
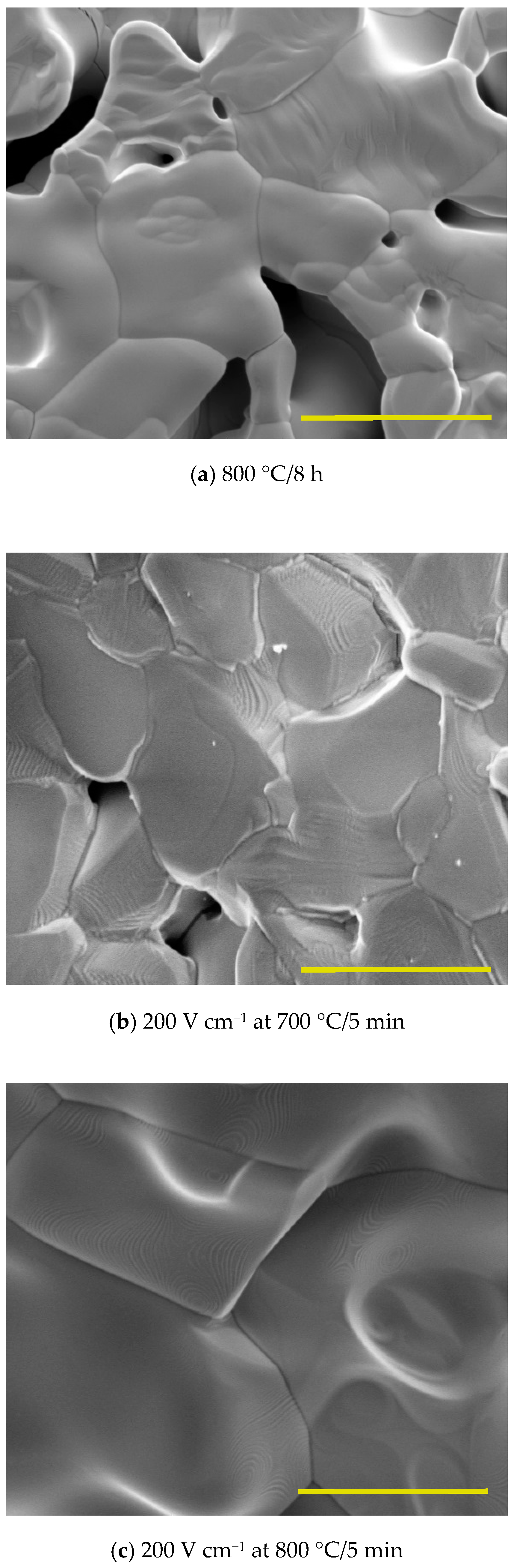
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-ion electrolytes. Annu. Rev. Mater. Res. 2003, 33, 91–128. [Google Scholar] [CrossRef]
- Fergus, J.W. Electrolytes for solid oxide fuel cells. J. Power Sources 2006, 162, 30–40. [Google Scholar] [CrossRef]
- Amado, R.S.; Malta, L.G.B.; Garrido, F.M.S.; Medeiros, M.E. Solid oxide fuel cells: Materials, components and configurations. Quim. Nova 2006, 30, 189–197. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Jagannathan, K.P.; Tiku, S.K.; Ray, H.S.; Ghosh, A.; Subbarao, E.C. Technological applications of solid electrolytes. In Solid Electrolytes and their Application; Subbarao, E.C., Ed.; Plenum Press: New York, NY, USA, 2012; pp. 201–260. [Google Scholar]
- Steele, B.C.H.; Heinzel, A. Materials for fuel cells technology. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Overs, A.; Riess, I. Properties of the solid electrolyte gadolinia-doped ceria prepared by thermal decomposition of mixed cerium-gadolinium oxalate. J. Am. Ceram. Soc. 1982, 65, 606–609. [Google Scholar] [CrossRef]
- Abraham, F.; Boivin, J.C.; Mairesse, G.; Nowogrocki, G. The BIMEVOX series a new family of high performances oxide ion conductors. Solid State Ion. 1990, 40, 934–937. [Google Scholar] [CrossRef]
- Eguchi, K.; Setogushi, T.; Inoue, T.; Arai, H. Electrical properties of ceria-based oxides and their application to solid oxide fuel cells. Solid State Ion. 1992, 52, 165–172. [Google Scholar] [CrossRef]
- Huang, P.N.; Petric, A. Superior oxygen ion conductivity of lanthanum gallate doped with strontium and magnesium. J. Electrochem. Soc. 1996, 143, 1644–1648. [Google Scholar] [CrossRef]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Krok, F.; Abrahams, I.; Bangobango, D.G.; Bogusz, W.; Nelstrop, J.A.G. Electrical and structural study of BICOVOX. Solid State Ion. 1996, 86, 261–266. [Google Scholar] [CrossRef]
- Anne, M.; Bachmann, M.; Pernot, M.; Abraaham, F.; Mairesse, G.; Strobel, P. Structure of new anionic conductors Bi4V2(1-x)M2xO11-3x, M=Cu, Ni. Physica B 1992, 180, 621–623. [Google Scholar] [CrossRef]
- Sharma, V.; Shukla, A.K.; Gopalakrishnan, J. Effect of aliovalent-cation substitution on the oxygen-ion conductivity of Bi4V2O11. Solid State Ion. 1992, 58, 359–362. [Google Scholar] [CrossRef]
- Pernot, E.; Anne, M.; Bachmann, M.; Strobel, P.; Fouletier, J.; Vannier, R.N.; Mairesse, G.; Abraham, F.; Nowogrocki, G. Structure and conductivity of Cu- and Ni-substituted Bi4V2O5 compounds. Solid State Ion. 1994, 70, 259–263. [Google Scholar] [CrossRef]
- Krok, F.; Abrahams, I.; Malys, M.; Bogusz, W.; Nelstrop, J.A.G. Structural and electrical characterisation of BICOCUVOX. Ionics 1997, 3, 235–238. [Google Scholar] [CrossRef]
- Sammers, N.M.; Tompsett, G.A.; Nafe, H.; Aldinger, F. Bismuth based oxide electrolytes structure and ionic conductivity. J. Eur. Ceram. Soc. 1999, 19, 1801–1826. [Google Scholar] [CrossRef]
- Abrahams, I.; Krok, F.; Malys, M.; Bush, A.J. Defect structure and ionic conductivity as a function of thermal history in BIMGVOX solid electrolytes. J. Mater. Sci. 2001, 36, 1099–1104. [Google Scholar] [CrossRef]
- Guillodo, M.; Fouletier, J.; Dessemond, L.; Gallo, P.D. Electrical properties of dense Me-doped bismuth vanadate (Me = Cu, Co) pO2-dependent conductivity determined by impedance spectroscopy. J. Eur. Ceram. Soc. 2001, 21, 2331–2344. [Google Scholar] [CrossRef]
- Godinho, M.J.; Bueno, P.R.; Orlandi, M.O.; Leite, E.R.; Longo, E. Ionic conductivity of Bi4Ti0.2V1.8O10.7 polycrystalline ceramics obtained by the polymeric precursor route. Mater. Lett. 2003, 57, 2540–2544. [Google Scholar] [CrossRef]
- Abrahams, I.; Krok, F. A model for the mechanism of low temperature ionic conduction in divalent-substituted γ-BIMEVOXes. Solid State Ion. 2003, 157, 139–145. [Google Scholar] [CrossRef]
- Paydar, M.H.; Hadian, A.M.; Fafilek, G. Ionic conductivity and crystal structure relationships in Ti/Cu substituted BI4V2O11. J. Mater. Sci. 2004, 39, 1357–1361. [Google Scholar] [CrossRef]
- Cho, H.S.; Sakai, G.; Shimanoe, K.; Yamazoe, N. Preparation of BiMeVOx (Me = Cu, Ti, Zr, Nb, Ta) compounds as solid electrolyte and behavior of their oxygen concentration cells. Sens. Actuators B 2005, 109, 307–314. [Google Scholar] [CrossRef]
- Chmielowiec, J.; Pasciak, G.; Bujlo, P. Ionic conductivity and thermodynamic stability of La-doped BIMEVOX. J. Alloys Compd. 2008, 451, 676–678. [Google Scholar] [CrossRef]
- Beg, S.; Salami, N.S. Study on the electrical properties of Co-Ti double substituted Bi4V2O11. J. Alloys Compd. 2014, 586, 302–307. [Google Scholar] [CrossRef]
- Beg, S.; Al-Areqi, N.A.S.; Al-Alas, A.; Hafeez, S. Co(III)-NI (II) double substituted bismuth vanadate: Synthesis, phase stabilization, and structural and electrical characterization. Ionics 2014, 20, 269–274. [Google Scholar] [CrossRef]
- Fuierer, P.; Maier, M.; Exner, J.; Moos, R. Anisotropy and thermal stability of hot-forged BICUTIVOX oxygen ion conducting ceramics. J. Eur. Ceram. Soc. 2014, 34, 943–951. [Google Scholar] [CrossRef]
- Roy, V.; Sahu, S.; Avasthi, A.; Bharadwaj, S. Synthesis, electrical and thermal properties of Bi4V2-xMexO11 (Me = Nb, Zr. Y and Cu with x = 0.0 and 0.06) ceramics. J. Therm. Anal. Calorim. 2014, 115, 1265–1271. [Google Scholar] [CrossRef]
- Beg, S.; Haneef, S. Study on phase stability and ionic conductivity in TiIV-substituted bismuth vanadate. Phase Transit. 2014, 87, 821–831. [Google Scholar] [CrossRef]
- Piva, R.H.; Biz, H.; Piva, D.H.; Morelli, M.R. Facile preparation of BIMEVOX powders via melting process: From synthesis to sintering optimization. Ceram. Int. 2016, 42, 7088–7098. [Google Scholar] [CrossRef]
- Singh, V.; Gosh, S.; Aich, S.; Roy, B. Low temperature solid oxide electrolytes (LT-SOE): A review. J. Power Sources 2017, 339, 103–135. [Google Scholar] [CrossRef]
- Yang, D.; Conrad, H. Enhanced sintering rate of zirconia (3Y-TZP) by application of a small AC electric field. Scr. Mater. 2010, 63, 328–331. [Google Scholar] [CrossRef]
- Muccillo, R.; Kleitz, M.; Muccillo, E.N.S. Flash grain welding in yttria stabilized zirconia. J. Eur. Ceram. Soc. 2011, 31, 1517–1521. [Google Scholar] [CrossRef]
- Grasso, S.; Sakka, Y.; Rendtorff, N.; Hu, C.; Maizza, G.; Borodianska, H.; Vasylkiv, O. Modeling of the temperature distribution of flash sintered zirconia. J. Ceram. Soc. Japan 2011, 119, 144–146. [Google Scholar] [CrossRef]
- Raj, R. Joule heating during flash sintering. J. Eur. Ceram. Soc. 2012, 32, 2293–2301. [Google Scholar] [CrossRef]
- Dancer, C.E.J. Flash sintering of ceramic materials. Res. Express 2016, 3, 102001. [Google Scholar] [CrossRef]
- Yu, M.; Grasso, S.; McKinnon, R.; Saunders, T.; Reece, M.J. Review of flash sintering: Materials, mechanisms and modelling. Adv. Appl. Ceram. 2016, 116, 24–60. [Google Scholar] [CrossRef]
- Muccillo, R.; Muccillo, E.N.S. An experimental setup for shrinkage evaluation during electric field-assisted flash sintering: Application to yttria-stabilized zirconia. J. Eur. Ceram. Soc. 2013, 33, 515–520. [Google Scholar] [CrossRef]
- Kleitz, M.; Kennedy, J.H. Resolution of multicomponent impedance diagrams. In Fast Ion Transport in Solids; Mundy, J.N., Shenoy, G.K., Vashishta, P., Eds.; Elsevier North Holland, Inc.: New York, NY, USA, 1979; pp. 185–188. ISBN 0444003533. [Google Scholar]
- Muccillo, E.N.S.; Muccillo, R. Electric field-assisted sintering of tin dioxide with manganese dioxide addition. J. Eur. Ceram. Soc. 2014, 34, 3699–3706. [Google Scholar] [CrossRef]
- Fletcher, J.G.; West, A.R.; Irvine, J.T.S. The AC-impedance response of the physical interface between yttria-stabilized zirconia and YBa2Cu3O7-x. J. Electrochem. Soc. 1995, 142, 2650–2654. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Impedance Spectroscopy: Theory, Experiment, and Applications, 2nd ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 978-0471647492. [Google Scholar]
- Dygas, J.R.; Krok, F.; Bogusz, P.; Kurek, P.; Reiselhuber, K.; Breiter, M.W. Impedance study of BICUVOX ceramics. Solid State Ion. 1994, 70–71, 239–247. [Google Scholar] [CrossRef]
- Kurek, P.; Breiter, M.W. Thermal stability and ionic conductivity of the BIMEVOX.10 ceramics (ME = Zn, Ni). Solid State Ion. 1996, 86–88, 131–135. [Google Scholar] [CrossRef]
- Tripathy, D.; Pandey, A. Structural and impedance studies of TiIV and NbV co-doped bismuth vanadate system. J. Alloys Compd. 2018, 737, 136–143. [Google Scholar] [CrossRef]

| Composition | T (°C) | σ (S cm−1) | E (eV) | Reference |
|---|---|---|---|---|
| Bi2V0.9Cu0.1O9 | 227 | 2.9 × 10−4 | 0.66 | [44] |
| Bi2V0.9Ni0.1O9 | 227 | 3.05 × 10−4 | 0.71 | [45] |
| Bi2V0.9Zn0.1O9 | 227 | 1.27 × 10−4 | 0.73 | [45] |
| Bi2V0.8Ti0.2O9 | 320 | 1.1 × 10−4 | 0.61 | [32] |
| Bi2V0.8Ti0.2O9 | 400 | 3.7 × 10−2 | 0.78 | this work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S. Medina, M.; G. M. Carvalho, S.; N. S. Muccillo, E.; Muccillo, R. Enhancement of the Ionic Conductivity in Electric Field-Assisted Pressureless Sintered BITIVOX Solid Electrolytes. Ceramics 2019, 2, 502-513. https://doi.org/10.3390/ceramics2030038
S. Medina M, G. M. Carvalho S, N. S. Muccillo E, Muccillo R. Enhancement of the Ionic Conductivity in Electric Field-Assisted Pressureless Sintered BITIVOX Solid Electrolytes. Ceramics. 2019; 2(3):502-513. https://doi.org/10.3390/ceramics2030038
Chicago/Turabian StyleS. Medina, Midilane, Sabrina G. M. Carvalho, Eliana N. S. Muccillo, and Reginaldo Muccillo. 2019. "Enhancement of the Ionic Conductivity in Electric Field-Assisted Pressureless Sintered BITIVOX Solid Electrolytes" Ceramics 2, no. 3: 502-513. https://doi.org/10.3390/ceramics2030038
APA StyleS. Medina, M., G. M. Carvalho, S., N. S. Muccillo, E., & Muccillo, R. (2019). Enhancement of the Ionic Conductivity in Electric Field-Assisted Pressureless Sintered BITIVOX Solid Electrolytes. Ceramics, 2(3), 502-513. https://doi.org/10.3390/ceramics2030038






