Preparation of Fly Ash-Based Porous Ceramic with Alumina as the Pore-Forming Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Characterization
3. Results and Discussion
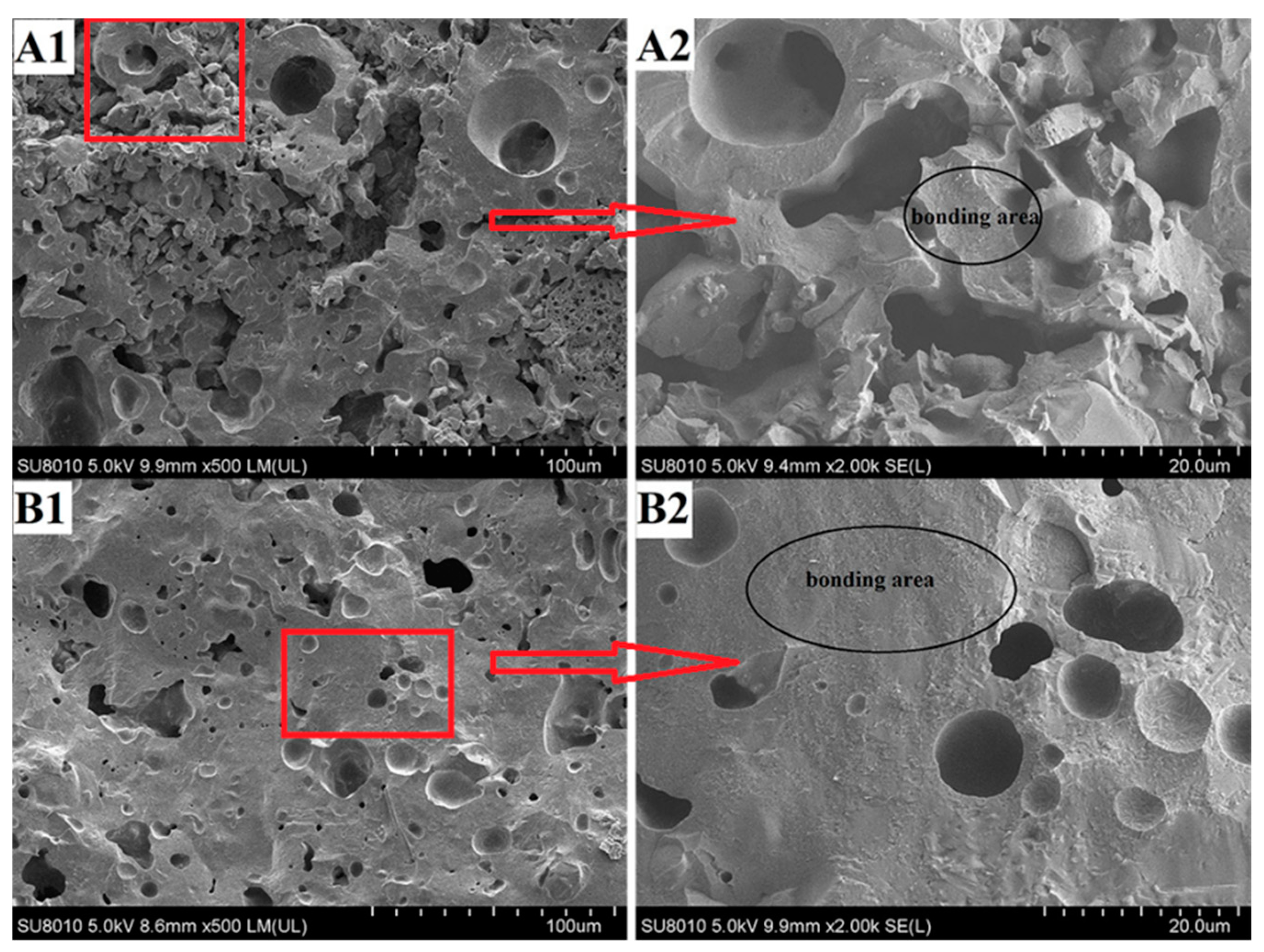
3.1. Effect of the Alumina Addition on the Bending Strength and Porosity of the Porous Ceramic
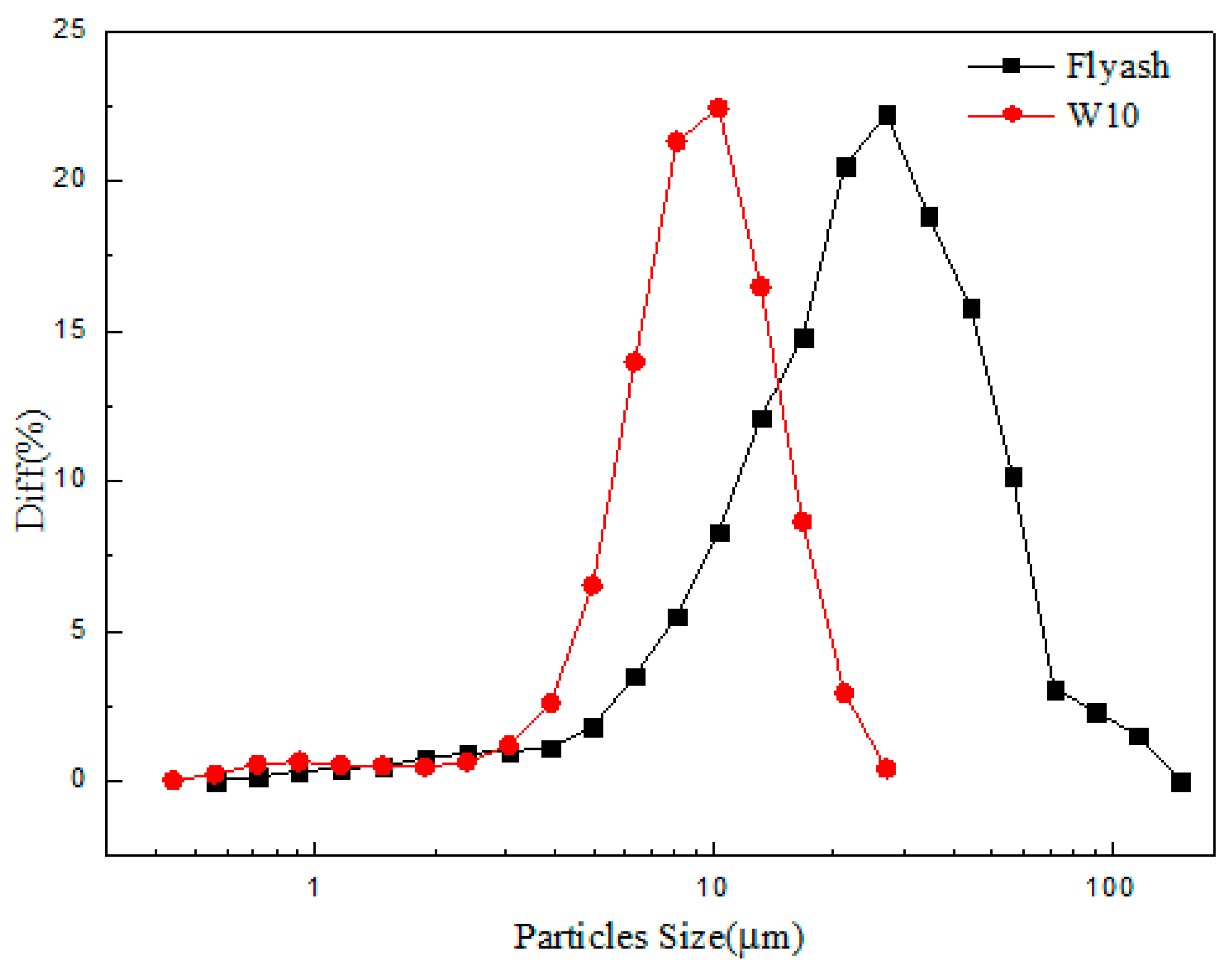
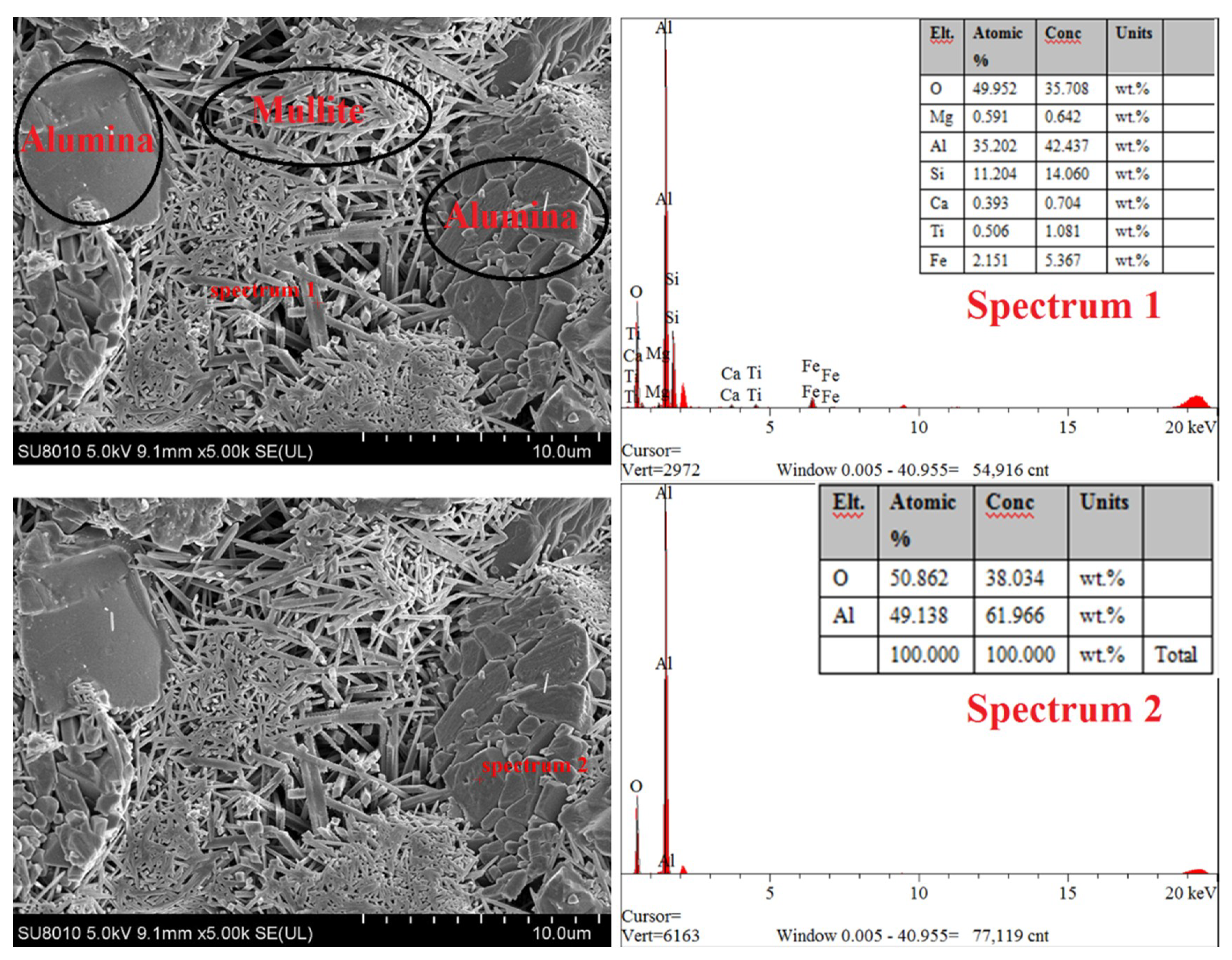
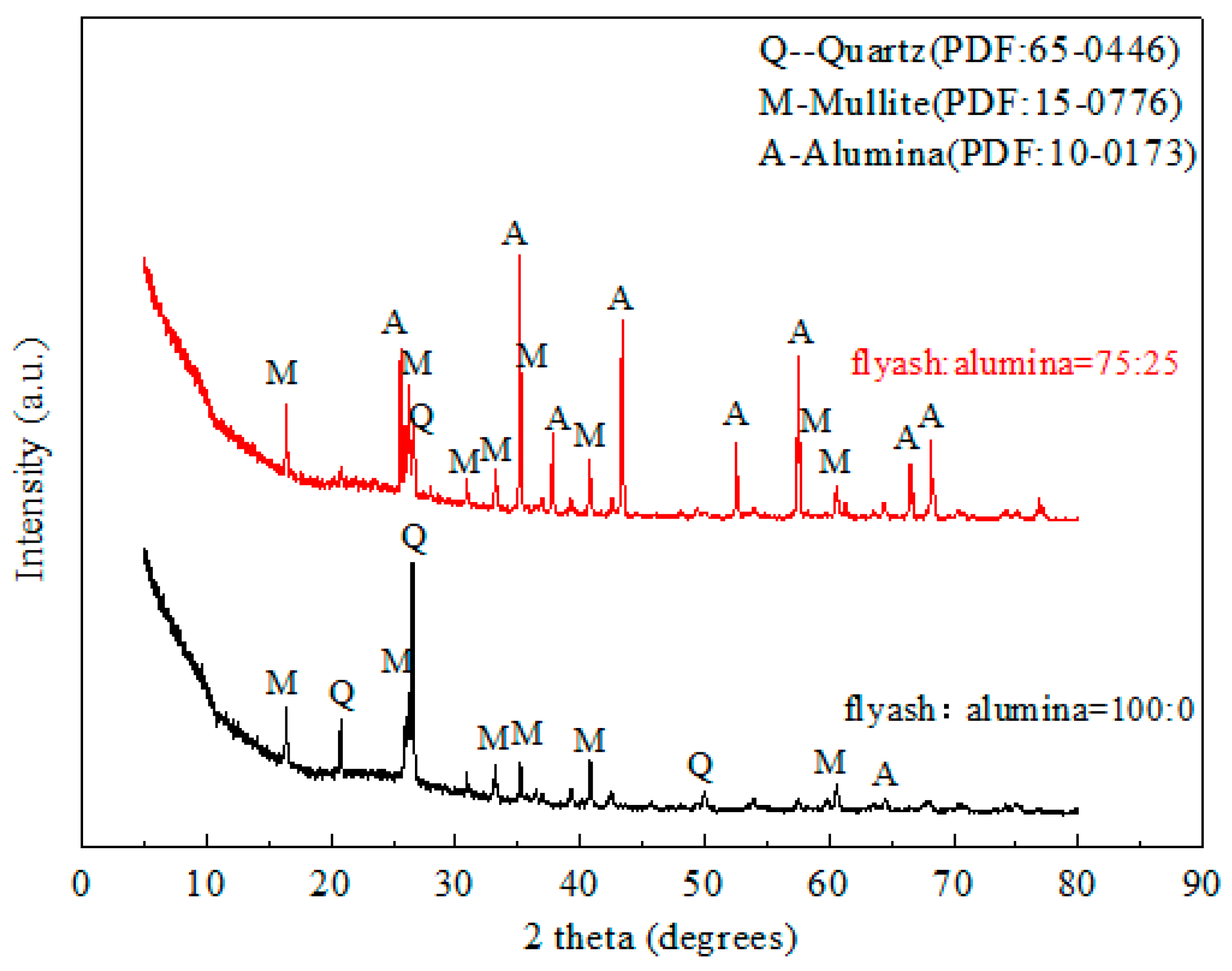
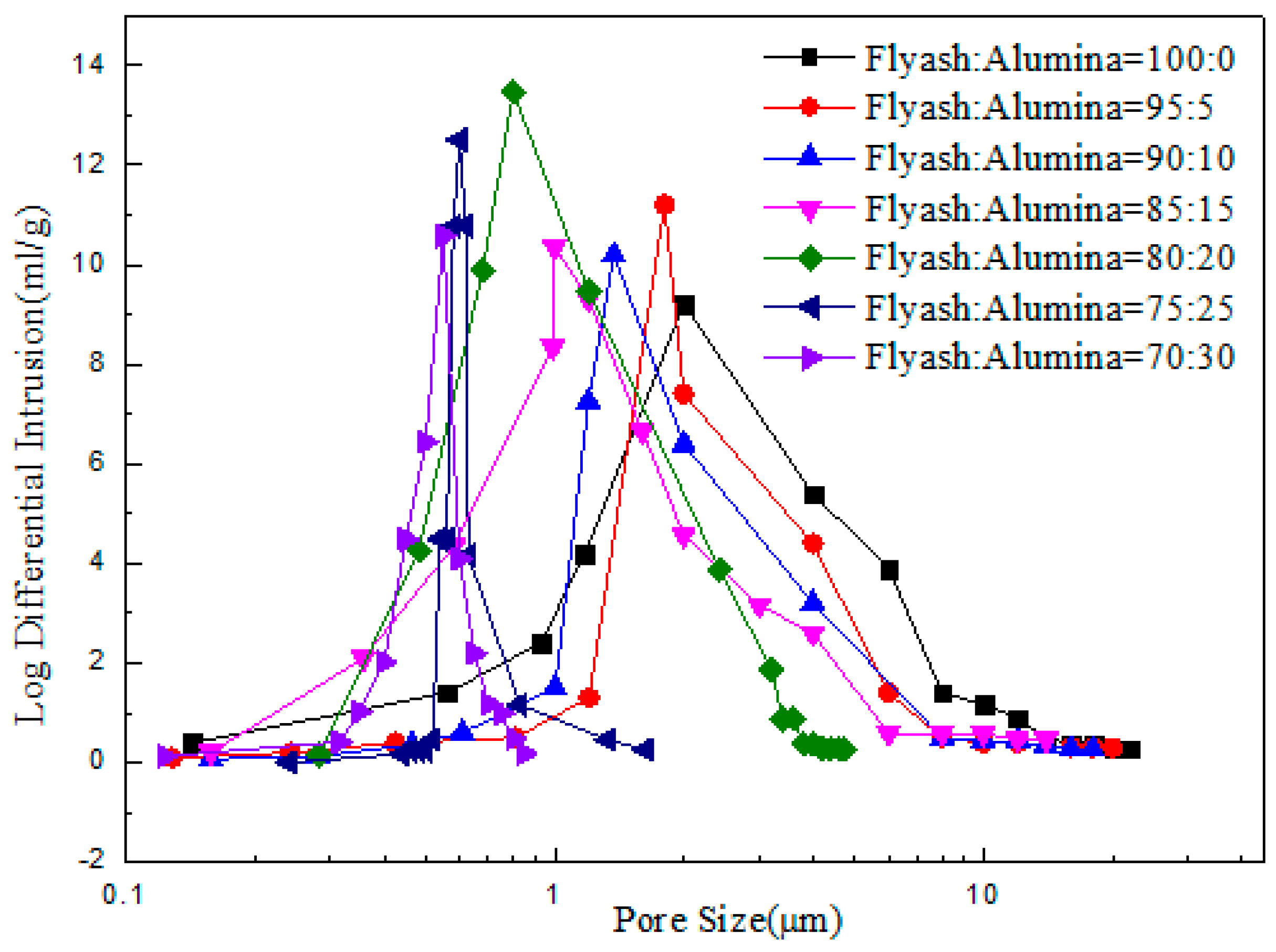
3.2. Effect of the Alumina Addition on the Pore Size Distribution of the Porous Ceramic
3.3. Effect of the Addition Content of Alumina on Permeability of the Porous Ceramic
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.; Bandyopadhyay, S.; Larbot, A.; Cerneaux, S. New clay–alumina porous capillary supports for filtration application. J. Membr. Sci. 2018, 392, 130–136. [Google Scholar] [CrossRef]
- Shkrabina, R.A.; Boneckamp, B.; Pex, P.; Veringa, H.; Ismagilov, Z. Porous structure of alumina ceramic support for gas separation membranes, II. Study of porous structure of ceramic composition. React. Kinet. Catal. Lett. 1995, 54, 193–201. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, S.; Zhang, X.; Yang, J.; Liu, X.; Meng, G. Fabrication and characterization of low cost tubular mineral-based ceramic membranes for micro-filtration from natural zeolite. J. Membr. Sci. 2006, 281, 592–599. [Google Scholar] [CrossRef]
- Yan, W.; Li, N.; Han, B.; Liu, J.; Liu, G. Preparation and Characterization of Porous Cordierite Ceramics with Well-distributed Interconnected Pores. Trans. Indian Ceram. Soc. 2011, 70, 65–69. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Shlyapin, S.D.; Val’yano, G.E.; Fedorova, L.V. Structure and Physicomechanical Properties of Porous Ceramic Based on Al2O3 Prepared Using a Filtration Combustion Method. Refract. Ind. Ceram. 2018, 58, 538–541. [Google Scholar] [CrossRef]
- Sun, Z.; Lu, C.; Fan, J.; Yuan, F. Porous silica ceramics with closed-cell structure prepared by inactive hollow spheres for heat insulation. J. Alloy. Compd. 2016, 662, 157–164. [Google Scholar] [CrossRef]
- Han, J.; Hong, C.; Zhang, X.; Du, J.; Zhang, W. Highly Porous ZrO2 Ceramics Fabricated by a Camphene-Based Freeze-Casting Route: Microstructure and Properties. J. Eur. Ceram. Soc. 2010, 30, 53–60. [Google Scholar] [CrossRef]
- Chen, J.; Liu, G.; Button, T.W. Mechanical properties of porous TiO2 ceramics fabricated by freeze casting process. Adv. Appl. Ceram. 2013, 112, 436–441. [Google Scholar] [CrossRef]
- Shin, J.H.; Kumar, B.V.M.; Kim, J.H.; Hong, S.H. Tribological Properties of Si3N4/SiC Nano–Nano Composite Ceramics. J. Am. Ceram. Soc. 2011, 94, 3683–3685. [Google Scholar] [CrossRef]
- Chang, Q.B.; Liu, X.Q.; Wang, X.; Wang, Y.Q.; Zhou, J.E. Preparation of Porous ZrO2-Al2O3 Composite Ceramic with High Strength and Good Corrosion-Resistance. Adv. Mater. Res. 2011, 197, 1545–1548. [Google Scholar] [CrossRef]
- Cao, J.; Dong, X.; Li, L.; Dong, Y.; Hampshire, S. Recycling of waste fly ash for production of porous mullite ceramic membrane supports with increased porosity. J. Eur. Ceram. Soc. 2014, 34, 3181–3194. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, L.; Mukiza, E.; Qi, Z.; Cang, D. Formation mechanism of high apparent porosity ceramics prepared from fly ash cenosphere. J. Alloy. Compd. 2018, 749, 750–757. [Google Scholar] [CrossRef]
- Jedidi, I.; Saïdi, S.; Khemakhem, S.; Larbot, A.; Elloumi-Ammar, N.; Fourati, A.; Charfi, A.; Salah, A.B.; Amar, R.B. Elaboration of new ceramic microfiltration membranes from mineral coal fly ash applied to waste water treatment. J. Hazard. Mater. 2009, 172, 152–158. [Google Scholar] [CrossRef]
- Dong, Y.C.; Hampshire, S.; Zhou, J.E.; Ji, Z.; Wang, J.; Meng, G. Sintering and characterization of flyash-based mullite with MgO addition. J. Eur. Ceram. Soc. 2011, 31, 687–695. [Google Scholar] [CrossRef]
- Jung, J.S.; Park, H.C.; Stevens, R. Mullite ceramics derived from coal fly ash. J. Mater. Sci. Lett. 2001, 20, 1089–1091. [Google Scholar] [CrossRef]
- Li, S.; Du, H.; Guo, A.; Xu, H.; Yang, D. Preparation of self-reinforcement of porous mullite ceramics through in situ synthesis of mullite whisker in flyash body. Ceram. Int. 2012, 38, 1027–1032. [Google Scholar] [CrossRef]
- Li, J.H.; Ma, H.W.; Huang, W.H. Effect of V2O5 on the properties of mullite ceramics synthesized from high-aluminum fly ash and bauxite. J. Hazard. Mater. 2009, 166, 1535–1539. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.C.; Feng, X.; Feng, X.; Ding, Y.; Liu, X.; Meng, G. Preparation of low-cost mullite ceramics from natural bauxite and industrial waste fly ash. J. Alloy. Compd. 2008, 460, 599–606. [Google Scholar] [CrossRef]
- Zhu, J.B.; Yan, H. Microstructure and properties of mullite-based porous ceramics produced from coal fly ash with added Al2O3. Int. J. Min. Met. Mater. 2017, 24, 309–315. [Google Scholar] [CrossRef]
- Liu, X.R.; Xiong, L.; Chen, A.Y. Effect of Bentonite on Properties of Fly Ash-Based Porous Ceramics. Adv. Mater. Res. 2011, 412, 195–198. [Google Scholar] [CrossRef]
- Živcová, Z.; Gregorová, E.; Pabst, W.; Smith, D.S.; Michot, A.; Poulier, C. Thermal conductivity of porous alumina ceramics prepared using starch as a pore-forming agent. J. Eur. Ceram. Soc. 2009, 29, 347–353. [Google Scholar] [CrossRef]
- Jamaludin, A.R.; Kasim, S.R.; Abdullah, M.Z.; Ahmad, Z.A. Sago starch as binder and pore-forming agent for the fabrication of porcelain foam. Ceram. Int. 2014, 40, 4777–4784. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Verweij, H.; Biesheuvel, P. Design of ceramic membrane supports: permeability, tensile strength and stress. J. Membr. Sci. 1999, 156, 141–152. [Google Scholar] [CrossRef]
- Knudsen, F.P. Dependence of Mechanical Strength of Brittle Polycrystalline Specimens on Porosity and Grain Size. J. Am. Ceram. Soc. 1959, 42, 376–387. [Google Scholar] [CrossRef]
- Chang, Q.; Yang, Y.; Zhang, X.; Wang, Y.; Zhou, J.E.; Wang, X.; Cerneaux, S.; Zhu, L.; Dong, Y. Effect of particle size distribution of raw powders on pore size distribution and bending strength of Al2O3 microfiltration membrane supports. J. Eur. Ceram. Soc. 2014, 34, 3819–3825. [Google Scholar] [CrossRef]
- Wu, X.; Huo, Z.; Ren, Q.; Li, H.; Lin, F.; Wei, T. Preparation and characterization of ceramic proppants with low density and high strength using fly ash. J. Alloy. Compd. 2017, 702, 442–448. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Xie, W.; Liu, Y.; Du, G. Preparation of porous ceramic from fly ash and alumina. China Ceram. 2008, 44, 27–29. (In Chinese) [Google Scholar]
- Lee, J.H.; Kim, W.Y.; Yang, T.Y.; Yoon, S.Y.; Kim, B.K.; Park, H.C. Fabrication of porous ceramic composites with improved compressive strength from coal fly ash. Adv. Appl. Ceram. 2011, 110, 244–250. [Google Scholar] [CrossRef]
- Zhou, J.E.; Yang, Y.L.; Chang, Q.B.; Wang, Y.Q.; Yang, K.; Bao, Q.F. Effect of particle size gradients of alumina powders on the pore size distribution and bending strength of ceramic membrane supports. J. Synth. Cryst. 2014, 43, 2125–2131. (In Chinese) [Google Scholar]



| Component | Al2O3 | SiO2 | Fe2O3 | CaO | MgO | K2O | Na2O | TiO2 | P2O3 | SO3 | L.O.I |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt%) | 37.12 | 55.39 | 3.25 | 1.78 | 1.26 | 2.83 | 0.18 | 1.1 | 0.19 | 0.19 | 6.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Liu, F.; Chang, Q.; Hu, Z.; Wang, Q.; Wang, Y. Preparation of Fly Ash-Based Porous Ceramic with Alumina as the Pore-Forming Agent. Ceramics 2019, 2, 286-295. https://doi.org/10.3390/ceramics2020023
Yang Y, Liu F, Chang Q, Hu Z, Wang Q, Wang Y. Preparation of Fly Ash-Based Porous Ceramic with Alumina as the Pore-Forming Agent. Ceramics. 2019; 2(2):286-295. https://doi.org/10.3390/ceramics2020023
Chicago/Turabian StyleYang, Yulong, Fengli Liu, Qibing Chang, Zhiwen Hu, Qikun Wang, and Yongqing Wang. 2019. "Preparation of Fly Ash-Based Porous Ceramic with Alumina as the Pore-Forming Agent" Ceramics 2, no. 2: 286-295. https://doi.org/10.3390/ceramics2020023
APA StyleYang, Y., Liu, F., Chang, Q., Hu, Z., Wang, Q., & Wang, Y. (2019). Preparation of Fly Ash-Based Porous Ceramic with Alumina as the Pore-Forming Agent. Ceramics, 2(2), 286-295. https://doi.org/10.3390/ceramics2020023





