Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Alumina and SiC Powders Dispersion
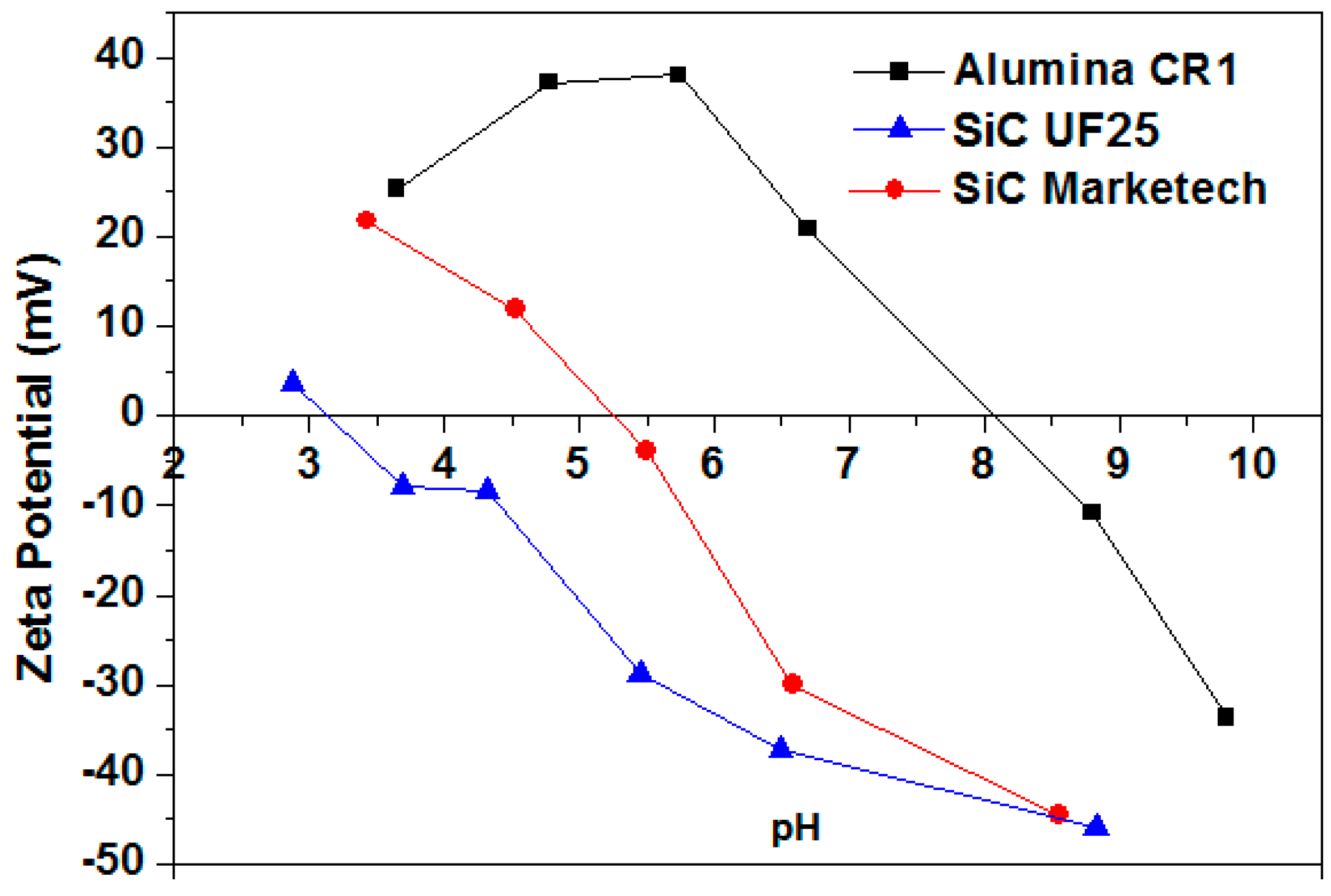
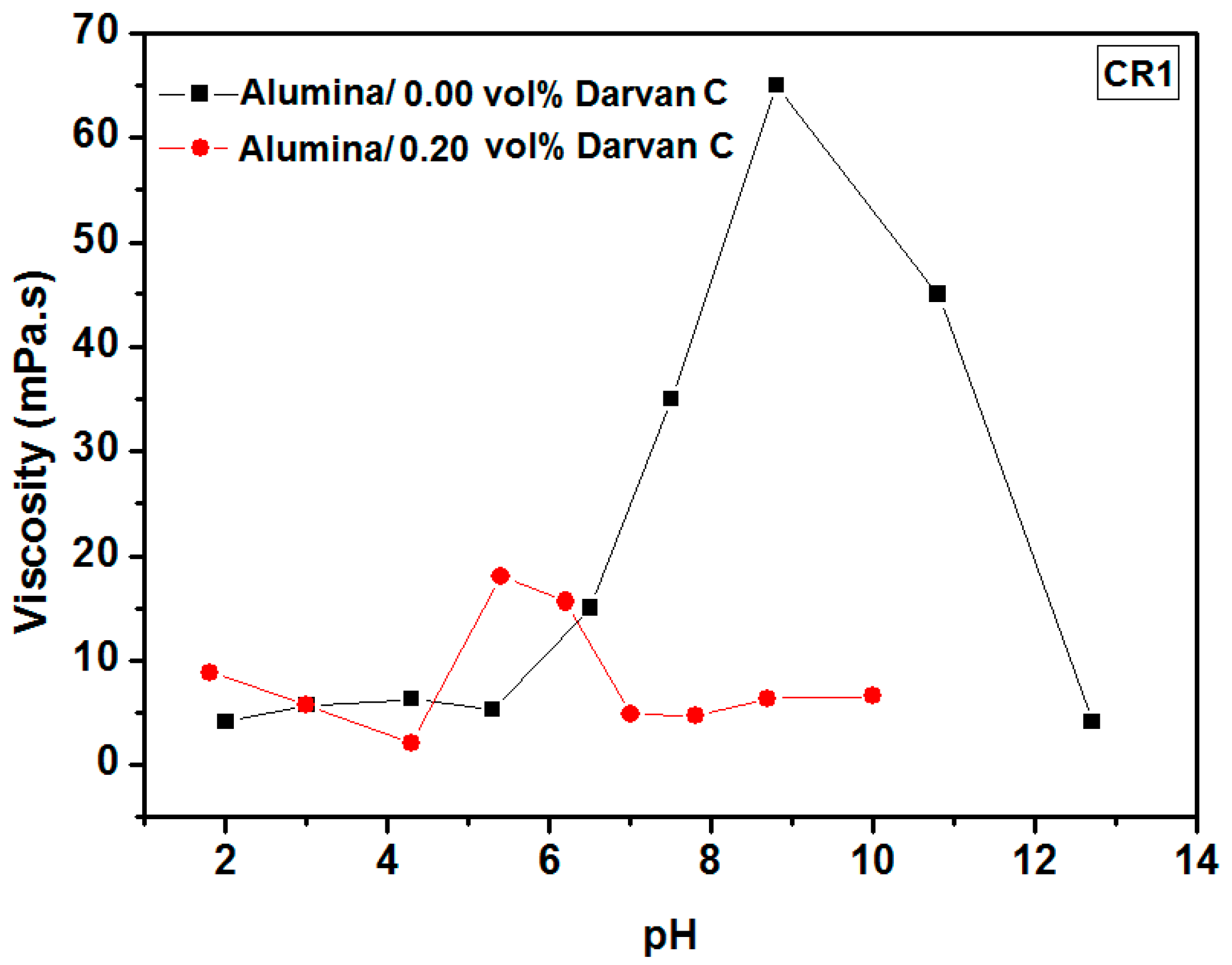
3.1.1. Determination of the Isoelectric Point (IEP) and the Effect of Darvan C
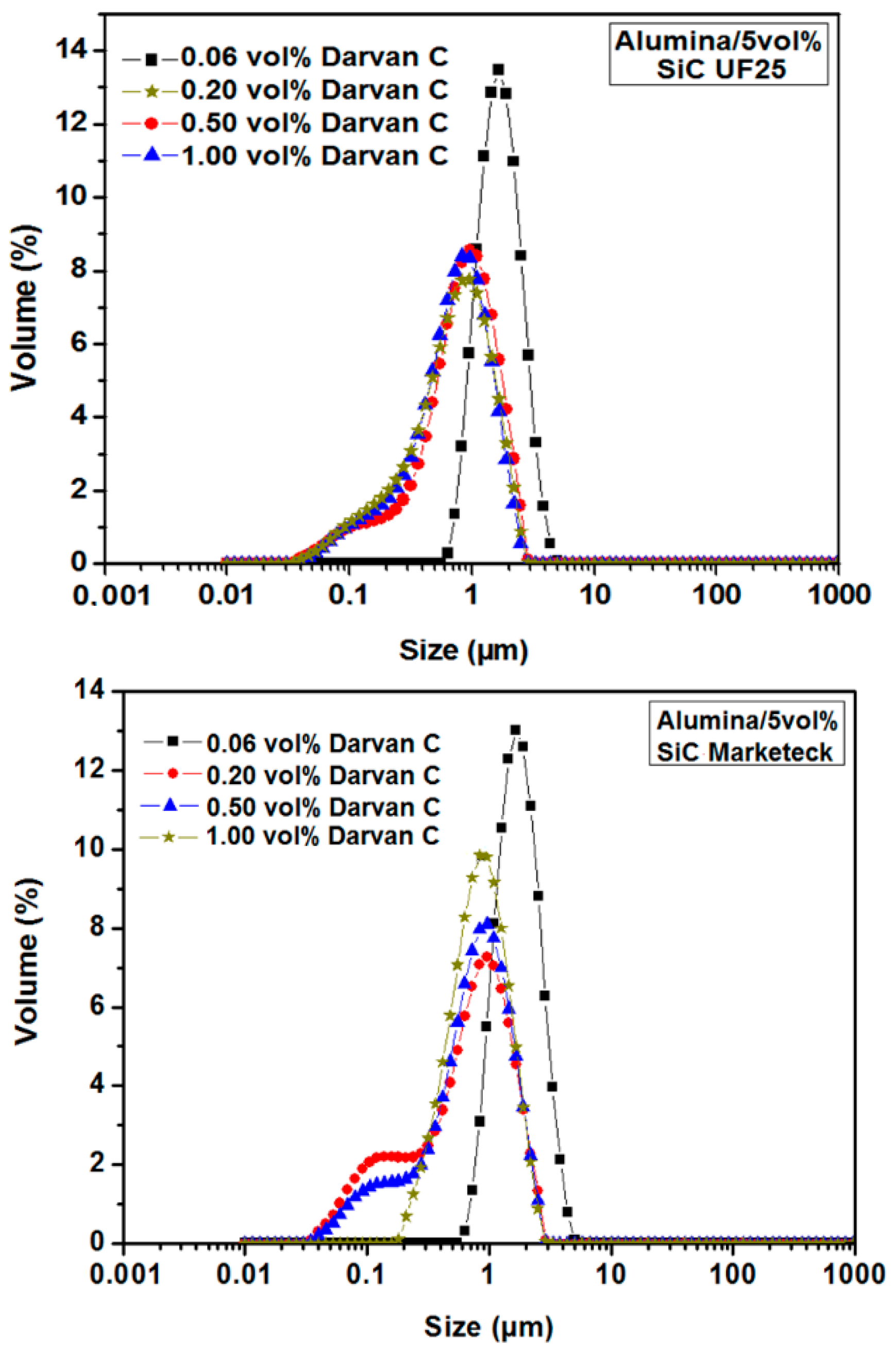
3.1.2. Optimization of the Dispersion Conditions
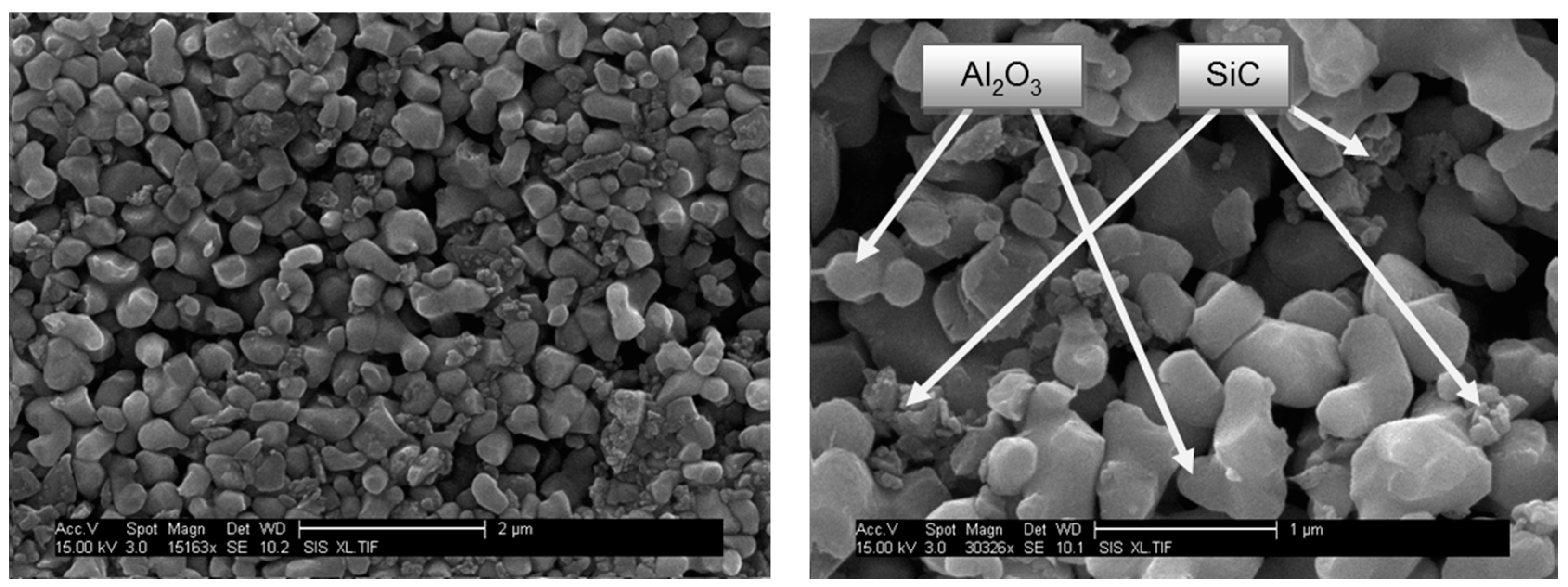
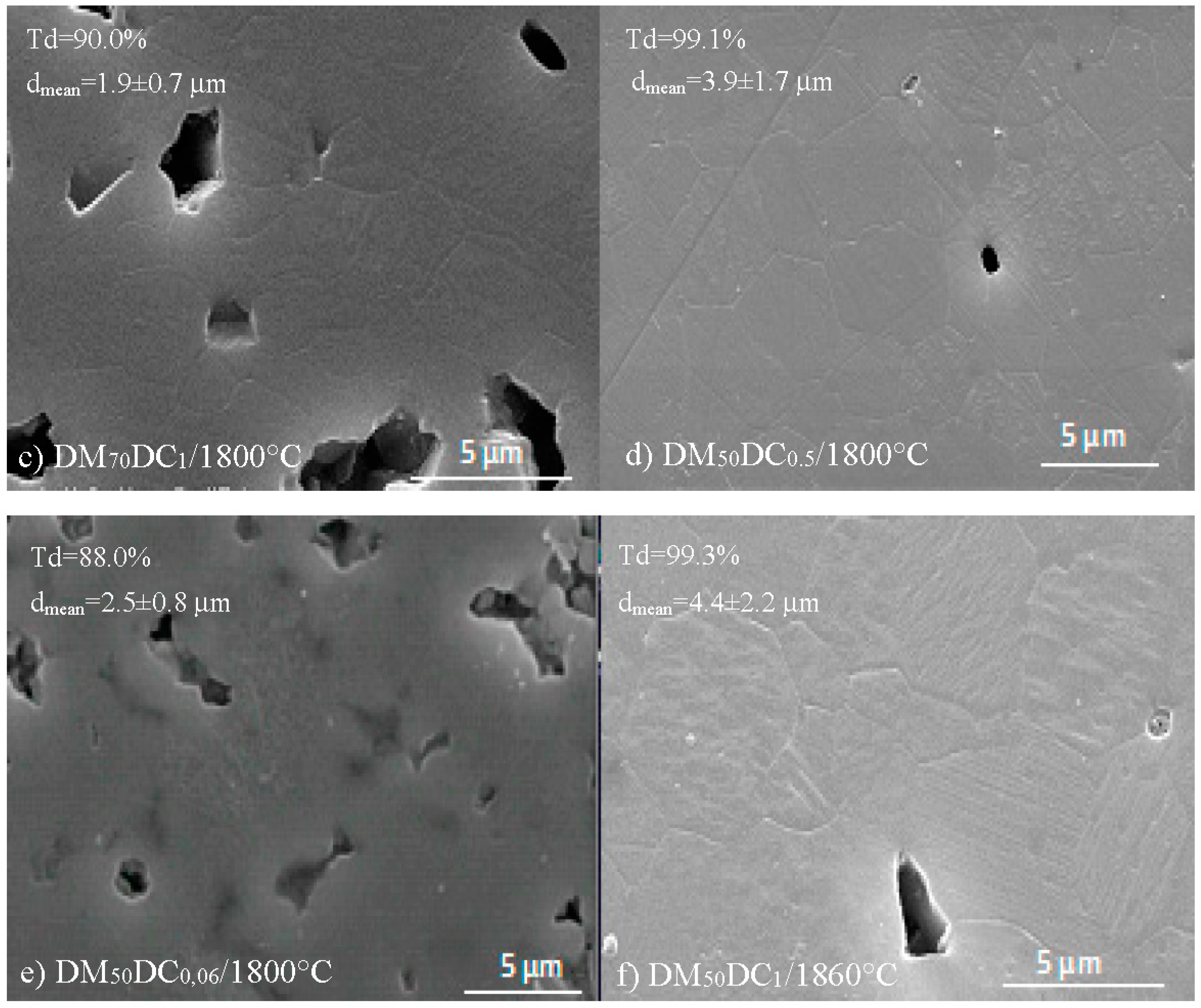
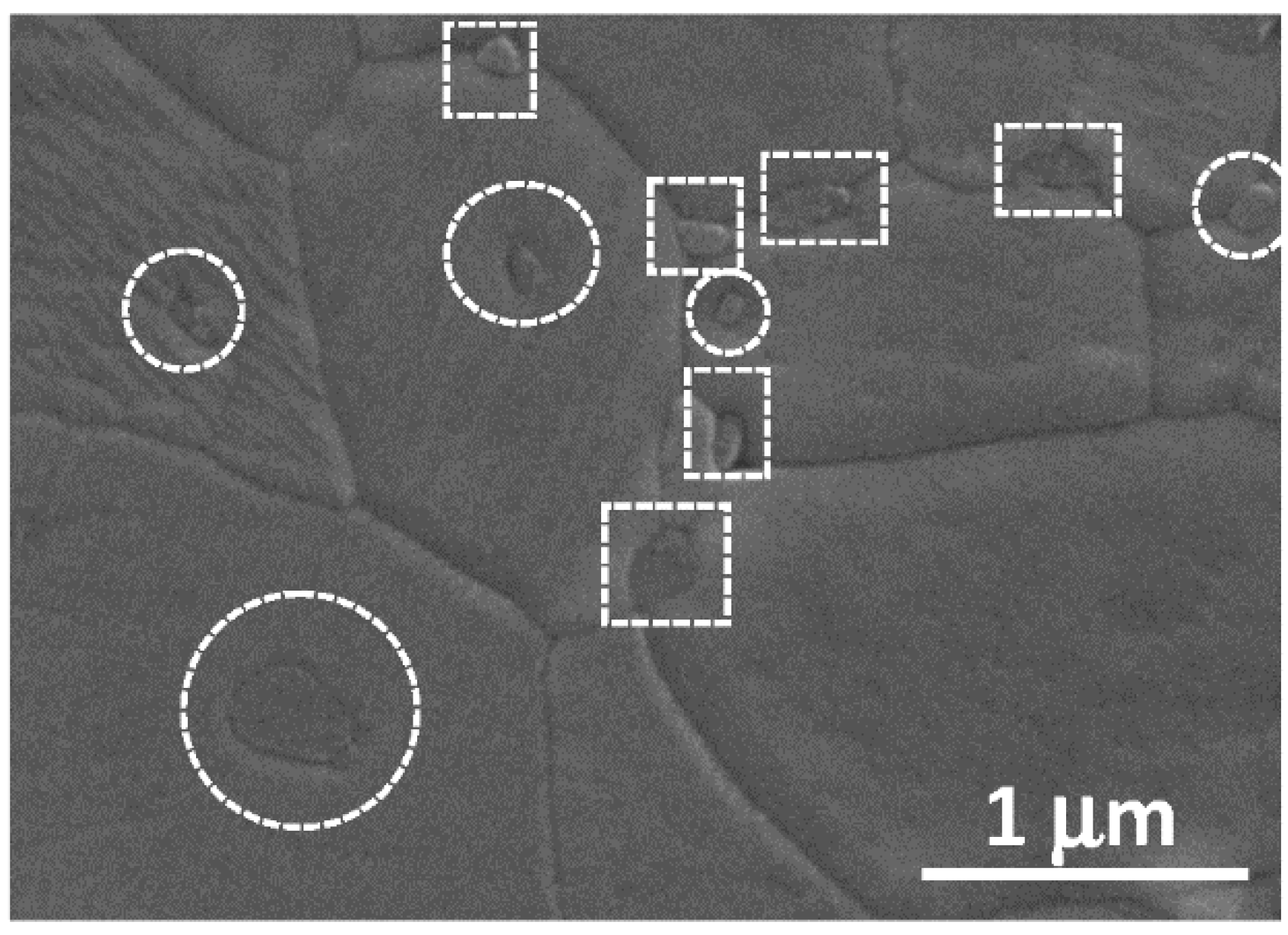
3.2. Conventional Sinstering of Alumina/SiC Composites
- Increasing the percentage of dry matter beyond 50 wt % promoted a decrease in the green density and consequently in the final density of samples.
- The amount of Darvan C had a direct influence on the densities reached before and after sintering. Samples obtained from well-dispersed suspensions (0.50 or 1.00 vol % Darvan C) exhibited higher densities than samples obtained from poorly dispersed suspensions (0.06 vol % Darvan C).
- In addition, increasing the sintering temperature resulted in higher final density and alumina grain size, especially in materials processed from 60 and 70 wt % dry matter slurries.
- DM50, 60 or 70DC0.06, 0.5 or 1 where:
- DM: Dry matter 50, 60 or 70 wt %
- DC: Darvan C 0.06, 0.50 or 1.00 vol %
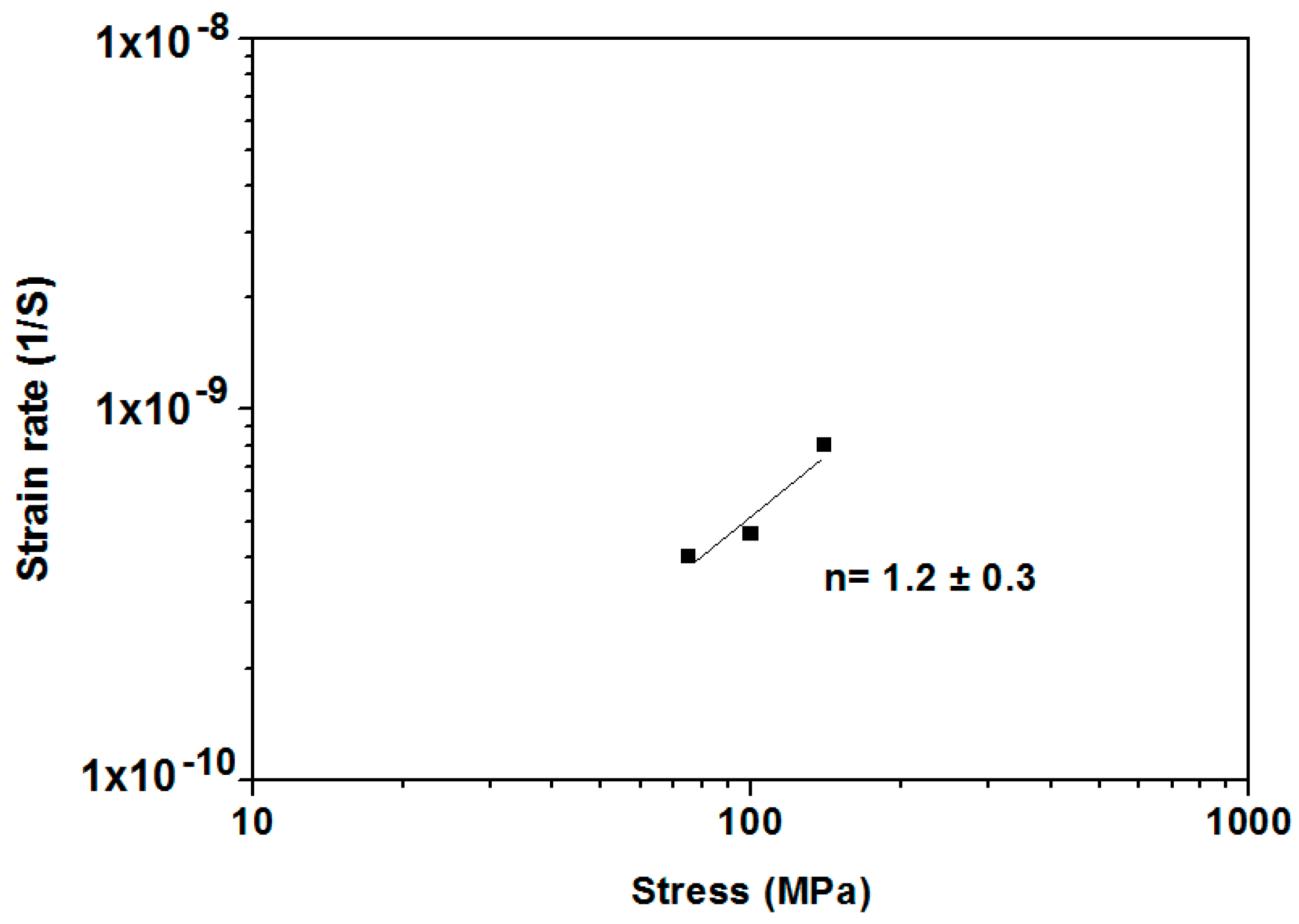
3.3. Creep Behaviour
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Niihara, K. New Design Concept of Structural Ceramics-Ceramic Nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Ohji, T.; Nakahira, A.; Hirano, T.; Niihara, K. Tensile Creep Behavior of Alumina/Silicon Carbide Nanocomposite. J. Am. Ceram. Soc. 1994, 77, 3259–3262. [Google Scholar] [CrossRef]
- Descamps, A.; O’Sullivan, D.; Poorteman, M.; Descamps, J.C.; Leriche, A.; Cambier, F. Creep behaviour of Al2O3-SiC Nanocomposites. J. Eur. Ceram. Soc. 1999, 99, 2475–2485. [Google Scholar] [CrossRef]
- Thompson, A.M.; Chan, H.M.; Harmer, M.P. Tensile Creep of Alumina-Silicon Carbide Nanocomposites. J. Am. Ceram. Soc. 1997, 80, 2221–2228. [Google Scholar] [CrossRef]
- Nakahira, A.; Niihara, K. Microstructure and High-Temperature Mechanical Properties for Al2O3/SiC Nanocomposites. In Proceedings of the 95th Annual Meeting of the American Ceramic Society, Cincinnati, OH, USA, 18–22 April 1993. [Google Scholar]
- Hackley, V.A. Colloidal processing of silicon nitride with poly(acrylic acid). I. adsorption and electrostatic interactions. J. Am. Ceram. Soc. 1997, 80, 2315–2325. [Google Scholar] [CrossRef]
- Dupont, L.; Foissy, A. Evaluation of the adsorption trends of a low molecular weight polyelectrolyte with a site-binding model. Colloid Surf. A Physicochem. Eng. Aspects 1996, 110, 235–248. [Google Scholar] [CrossRef]
- Hidber, P.C.; Graule, T.J.; Gauckler, L.J. Citric acid—A dispersant for aqueous alumina suspensions. J. Am. Ceram. Soc. 1996, 79, 1857–1867. [Google Scholar] [CrossRef]
- Adair, H.J.; Mutsuddy, B.C.; Drauglis, E.J. Stabilization of silicon carbide whisker suspensions: I, Influence of surface oxidation in aqueous suspensions. Adv. Ceram. Mater. 1988, 3, 231–234. [Google Scholar] [CrossRef]
- Whitman, P.K.; Feke, D.L. Colloidal characterization of ultrafine silicon carbide and silicon nitride powders. Adv. Ceram. Mater. 1986, 1, 366–370. [Google Scholar] [CrossRef]
- Cesarano, J., III; Aksay, I.A. Processing of highly concentrated aqueous alumina suspensions stabilized with polyelectrolytes. J. Am. Ceram. Soc. 1988, 71, 1062–1067. [Google Scholar] [CrossRef]
- Cesarano, J., III; Aksay, I.A.; Bleier, A. Stability of aqueous—Al2O3 suspension with poly(methacrylic acid) polyelectrolyte. J. Am. Ceram. Soc. 1988, 71, 250–255. [Google Scholar] [CrossRef]
- Parks, G.A. The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chem. Rev. 1965, 65, 177–198. [Google Scholar] [CrossRef]
- Tokita, M. Trends in advanced SPS systems and technology. J. Soc. Powder Tech. Jpn. 1993, 30, 790–804. [Google Scholar] [CrossRef]
- Omori, M. Sintering, consolidation, reaction and crystal growth by the spark plasma system (SPS). Mater. Sci. Eng. A 2000, 287, 183–188. [Google Scholar] [CrossRef]
- Munir, Z.A.; Amselmi-Tamburini, U.; Ohyanagi, M. The effect of electric field and pressure on the synthesis and consolidation of materials: A review of the Spark Plasma Sintering Method. J. Mater. Sci. 2006, 41, 763–777. [Google Scholar] [CrossRef]
- Kumeda, K.; Nakamura, Y.; Takata, A.; Ishizaki, K. Surface observation of pulsed electric current sintered alumina balls. J. Ceram. Soc. Jpn. 1999, 107, 187–189. [Google Scholar] [CrossRef]
- Hollenberg, G.W.; Terwilliger, G.R.; Gordon, R.S. Calculation of Stresses and Strains in Four-Point Bending Creep Tests. J. Am. Ceram. Soc. 1971, 54, 196–199. [Google Scholar] [CrossRef]
- Singh, B.M.; Jena, J.; Laxmidhar, B.; Sarama, B. Dispersion of nano-silicon carbide (SiC) powder in aqueous suspensions. J. Nanop. Res. 2007, 9, 797–806. [Google Scholar] [CrossRef]
- Duran, C.; Gozmez, H.; Yilmaz, H. Dispersion of mechanochemically activated SiC and Al2O3 powders. Mater. Sci. Eng. 2008, 475, 23–26. [Google Scholar] [CrossRef]
- Briscoe, B.; Asad, U.; Lucham, F. Optimising the dispersion on an alumina suspension using commercial polyvalent electrolyte dispersants. J. Eur. Ceram. Soc. 1998, 18, 2141–2147. [Google Scholar] [CrossRef]
- Singh, B.P.; Bhattacharjee, S.; Besra, L. Evaluation of dispersibility of aqueous alumina suspension in presence of Darvan C. Ceram. Int. 2004, 30, 939–946. [Google Scholar] [CrossRef]
- Azar, M. Mise en Forme et Frittage des Poudres de Céramique Nanostructurées: Cas d’une Alumine de Transition; Insa de Lyon: Villeurbanne, France, 2009. (In French) [Google Scholar]
- Cannon, R.; Langdon, T. Review creep of ceramics. J. Mater. Sci. 1988, 23, 1–20. [Google Scholar] [CrossRef]
- Thompson, A.M.; Fang, J.; Chan, H.M.; Harmer, M.P. High temperature behaviour of alumina/SiC nanocomposites. J. Am. Ceram. Soc. 1995, 51, 671–678. [Google Scholar]




| Material | Phase (%) | Purity (%) | Brunauer, Emmett and Teller (BET) Particle Size (nm) | Specific Surface Area (m2/g) | d50 (μm) |
|---|---|---|---|---|---|
| Alumina CR1 | 95% α-5% γ-Al2O3 | 99.9 | 457 | 3.3 | 0.60 |
| SiC UF25 | α-SiC 6H/4H polytype | 98.0 | 78 | 24.5 | 0.45 |
| SiC Marketech | α -SiC | - | - | - | 0.06 |
| Sample | Solid Loading (wt %) | Darvan C (vol %) | Sintering Temperature (°C) | Green Density (%td) | Final Density (%td) | dmean (μm) |
|---|---|---|---|---|---|---|
| 1 | 50 | 1.00 | 1800 | 60 | 99.1 | 3.7 ± 1.5 |
| 2 | 60 | 1.00 | 1800 | 58 | 94.1 | 2.5 ± 1.1 |
| 3 | 70 | 1.00 | 1800 | 57 | 90.0 | 1.9 ± 0.7 |
| 4 | 50 | 0.06 | 1800 | 45 | 88.0 | 2.5 ± 0.8 |
| 5 | 50 | 0.50 | 1800 | 60 | 99.1 | 3.9 ± 1.7 |
| 6 | 50 | 1.00 | 1860 | 60 | 99.3 | 4.4 ± 2.2 |
| 7 | 60 | 1.00 | 1860 | 58 | 99.2 | - |
| 8 | 70 | 1.00 | 1860 | 57 | 97.7 | - |
| 9 | 50 | 0.50 | 1860 | 60 | 99.3 | 4.5 ± 2.2 |
| σ (MPa) | Strain (%) | Strain Rate (1/s) |
|---|---|---|
| 70 | 0.07 | 4.0 × 10−10 |
| 100 | 0.09 | 4.3 × 10−10 |
| 140 | 0.15 | 7.5 × 10−10 |
| Sample | Grain Size (μm) | Strain Rate (1/s) |
|---|---|---|
| Alumina/5 vol % SiC UF25 (this work) | 3.7 ± 1.5 | 4.3 × 10−10 |
| Alumina/17 vol % SiC (Ohji et al. [2]) | 2.0 | 1.0 × 10−10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaafar, M.; Fantozzi, G.; Reveron, H. Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites. Ceramics 2018, 1, 13-25. https://doi.org/10.3390/ceramics1010003
Jaafar M, Fantozzi G, Reveron H. Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites. Ceramics. 2018; 1(1):13-25. https://doi.org/10.3390/ceramics1010003
Chicago/Turabian StyleJaafar, Mira, Gilbert Fantozzi, and Helen Reveron. 2018. "Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites" Ceramics 1, no. 1: 13-25. https://doi.org/10.3390/ceramics1010003
APA StyleJaafar, M., Fantozzi, G., & Reveron, H. (2018). Preparation and Characterization of Pressureless Sintered Alumina/5 vol % SiC Micro-Nanocomposites. Ceramics, 1(1), 13-25. https://doi.org/10.3390/ceramics1010003





