Different Techniques of Creating Bone Digital 3D Models from Natural Specimens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photogrammetry Protocol|Reality Capture
- Launch the RealityCapture program.
- Import images into the program.
- Adjust alignment settings:
- Max feature per mpx.: 20,000;
- Max features per image: 80,000;
- Preselector features: 20,000;
- Image overlap: low;
- Force component rematch: yes;
- Detector sensitivity: high.
- Launch the alignment process.
- Define the reconstruction region.
- Use reconstruction with High detail option to initialize the meshing process.
- Use Clean Model tool to remove topology defects (non-manifold vertices, non-manifold edges, holes, isolated vertices).
- Use the Texture instrument with the following setting to create a texture for the model:
- Imported-model default texture resolution: 16,384 × 16,384;
- Correct colors: Yes.
- Export the 3D model along with the texture as an OBJ (file format) object.
2.2. Segmentation Protocol|3D Slicer
- Launch the 3D Slicer program.
- Import CT data into the program:
- Set the image contrast to ensure better visibility.
- Add a new segment using the tool:
- Segment Editor:
- ○
- Set up the Threshold tool;
- ○
- Using Scissors, Draw, Islands, manually segment the required structure.
- When segmentizing one structure is complete, proceed to the second one by adding a new segment and repeat the segmentation procedure if needed.
- Export the completed segment or segments as a 3D model in the OBJ (file format) file format.
2.3. Simplification and Optimization Protocol|MeshLab
- Launch the MeshLab program.
- Import a 3D model into the program.
- Remove artifacts, simplify, and optimize the model using tools (all the default values with modifications indicated below):
- Remove isolated pieces (wrt diameter);
- Remove duplicated faces;
- Remove duplicated vertex;
- Remove zero area faces;
- Repair non-manifold edges by removing faces;
- Repair non-manifold vertices by splitting;
- Remove unreferenced vertices;
- Simplification: quadric edge collapse decimation:
- ○
- Preserve boundary of the mesh: on;
- ○
- Preserve normal: on;
- ○
- Preserve topology: on;
- ○
- Planar simplification: on.
- Remeshing: isotropic explicit remeshing:
- ○
- Adaptive remeshing: on;
- ○
- Collapse step: off.
- Export the completed segment or segments as a 3D model in the binary PLY file format.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, Z.; Dun, A.; Jiang, H.; Nie, C.; Zhao, S.; Wang, T.; Zhai, J. The role of 3D printed models in the teaching of human anatomy: A systematic review and meta-analysis. BMC Med. Educ. 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Schrot, J.; Pietila, T.; Sahu, A. State of the art: 3D printing for creating compliant patient-specific congenital heart defect models. J. Cardiovasc. Magn. Reason. 2014, 16 (Suppl. 1), W19. [Google Scholar] [CrossRef] [Green Version]
- Kantaros, A.; Piromalis, D. Fabricating lattice structures via 3d printing: The case of porous bio-engineered scaffolds. Appl. Mech. 2021, 2, 289–302. [Google Scholar] [CrossRef]
- Kantaros, A.; Diegel, O.; Piromalis, D.; Tsaramirsis, G.; Khadidos, A.O.; Khadidos, A.O.; Khan, F.Q.; Jan, S. 3D printing: Making an innovative technology widely accessible through makerspaces and outsourced services. Mater. Today Proc. 2022, 49, 2712–2723. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A. Industry 4.0 applications in medical field: A brief review. Curr. Med. Res. Pract. 2019, 9, 102–109. [Google Scholar] [CrossRef]
- Tsaramirsis, G.; Kantaros, A.; Al-Darraji, I.; Piromalis, D.; Apostolopoulos, C.; Pavlopoulou, A.; Alrammal, M.; Ismail, Z.; Buhari, S.M.; Stojmenovic, M.; et al. A modern approach towards an industry 4.0 model: From driving technologies to management. J. Sens. 2022, 2022, 5023011. [Google Scholar] [CrossRef]
- Hammerton, C.; Yip, S.W.L.; Manobharath, N.; Myers, G.; Sturrock, A. Are 3D printed models acceptable in assessment? Clin. Teach. 2022, 19, 221–228. [Google Scholar] [CrossRef]
- Yuen, J. What is the role of 3d printing in undergraduate anatomy education? A scoping review of current literature and recommendations. Med. Sci. Edu. 2020, 30, 1321–1329. [Google Scholar] [CrossRef]
- Garas, M.; Vaccarezza, M.; Newland, G.; McVay-Doornbusch, K.; Hasani, J. 3D-Printed specimens as a valuable tool in anatomy education: A pilot study. Ann. Anat. 2018, 219, 57–64. [Google Scholar] [CrossRef]
- Zou, Y.; Han, Q.; Weng, X.; Zou, Y.; Yang, Y.; Zhang, K.; Yang, K.; Xu, X.; Wang, C.; Qin, Y.; et al. The precision and reliability evaluation of 3-dimensional printed damaged bone and prosthesis models by stereo lithography appearance. Medicine 2018, 97, e9797. [Google Scholar] [CrossRef]
- Day, K.M.; Kelley, P.K.; Harshbarger, R.J.; Dorafshar, A.H.; Kumar, A.R.; Steinbacher, D.M.; Patel, P.; Combs, P.D.; Levine, J.P. Advanced three-dimensional technologies in craniofacial reconstruction. Plast. Reconstr. Surg. 2021, 148, 94e–108e. [Google Scholar] [CrossRef] [PubMed]
- Bastawrous, S.; Wu, L.; Liacouras, P.C.; Levin, D.B.; Ahmed, M.T.; Strzelecki, B.; Amendola, M.F.; Lee, J.T.; Coburn, J.; Ripley, B. Establishing 3d printing at the point of care: Basic principles and tools for success. Radiographics 2022, 42, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.T.O.; Evans, D.J.R. Art, anatomy, and medicine: Is there a place for art in medical education? Art, Anatomy, and Medical Education. Anat. Sci. Edu. 2014, 7, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Govsa, F.; Yagdi, T.; Ozer, M.A.; Eraslan, C.; Alagoz, A.K. Building 3D anatomical model of coiling of the internal carotid artery derived from CT angiographic data. Eur. Arch. Otorhinolaryngol. 2017, 274, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Pugalendhi, A.; Arumugam, S.; Ranganathan, R.; Ganesan, S. 3D printed patient-specific bone models for anatomy education from medical imaging. J. Eng. Res. 2021, 1–12. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, G.; Liang, Y.; Fan, G. Three-dimensional physical model in urologic cancer. Front. Surg. 2022, 9, 757337. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Jones, J.F.X. Dual-extrusion 3D printing of anatomical models for education: Two Materials 3D Printing in Anatomy. Anat. Sci. Edu. 2018, 11, 65–72. [Google Scholar] [CrossRef]
- Smith, C.F.; Tollemache, N.; Covill, D.; Johnston, M. Take away body parts! An investigation into the use of 3D-printed anatomical models in undergraduate anatomy education. Anat. Sci. Edu. 2018, 11, 44–53. [Google Scholar] [CrossRef]
- Diaz, C.M.; Linden, K.; Solyali, V. Novel and innovative approaches to teaching human anatomy classes in an online environment during a pandemic. Med. Sci. Edu. 2021, 31, 1703–1713. [Google Scholar] [CrossRef]
- Ugidos Lozano, M.T.; Blaya Haro, F.; Ruggiero, A.; Manzoor, S.; Nuere Menendez-Pidal, S.; Juanes Méndez, J.A. Different digitalization techniques for 3d printing of anatomical pieces. J. Med. Syst. 2018, 42, 46. [Google Scholar] [CrossRef]
- Wickramasinghe, N.; Thompson, B.R.; Xiao, J. The opportunities and challenges of digital anatomy for medical sciences: Narrative review. JMIR Med. Educ. 2022, 8, e34687. [Google Scholar] [CrossRef] [PubMed]
- Al-Mosawe, A.; Agha, H.; Al-Hadeethi, L.; Al-Mahaidi, R. Efficiency of image correlation photogrammetry technique in measuring strain. Aust. J. Struct. Eng. 2018, 19, 207–213. [Google Scholar] [CrossRef]
- Jones, D.G. Three-dimensional printing in anatomy education: Assessing potential ethical dimensions. Anat. Sci. Educ. 2019, 12, 435–443. [Google Scholar] [CrossRef]
- Flaxman, T.E.; Cooke, C.M.; Miguel, O.X.; Sheikh, A.M.; Singh, S.S. A review and guide to creating patient specific 3D printed anatomical models from MRI for benign gynecologic surgery. 3D Print Med. 2021, 7, 17. [Google Scholar] [CrossRef]
- Lima, L.F.; Barros, A.J.; Martini, A.D.; Stocco, M.B.; Kuczmarski, A.H.; Souza, R.L. Photogrammetry and 3D prototyping: A low-cost resource for training in veterinary orthopedics. Cienc. Rural 2019, 49, e20180929. [Google Scholar] [CrossRef]
- Sikes, R.W.; Sniezek, C.M.; Clancey, B.T.; Johnson, C.J. Virtual 3d brain slices: Improving learning of cross-sectional neuroanatomy by expanding access to human brain cross-sections through photogrammetric 3d scanning. FASEB J. 2018, 32 (Suppl. 1), 635-17. [Google Scholar] [CrossRef]
- Tashiro, M.; Minohara, S.; Yusa, K.; Sakurai, H.; Kanai, T.; Baba, M.; Miyamoto, T.; Nakano, T. 242 Quantitative evaluation of 3d lung motion with anatomical feature tracking technique for precise particle radiotherapy. Radiother. Oncol. 2006, 78, S85–S86. [Google Scholar] [CrossRef]
- Bartikian, M.; Ferreira, A.; Gonçalves-Ferreira, A.; Neto, L.L. 3D printing anatomical models of head bones. Surg. Radiol. Anat. 2019, 41, 1205–1209. [Google Scholar] [CrossRef]
- Ratinam, R.; Quayle, M.; Crock, J.; Lazarus, M.; Fogg, Q.; McMenamin, P. Challenges in creating dissectible anatomical 3D prints for surgical teaching. J. Anat. 2019, 234, 419–437. [Google Scholar] [CrossRef] [Green Version]
- Aimar, A.; Palermo, A.; Innocenti, B. The role of 3d printing in medical applications: A state of the art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Gartrell, R.; Yeung, J.M. A pipeline for generating interactive, schematic 3d surgical anatomy models. J. Surg. Educ. 2021, 78, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3d printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Betancourt, M.C.; Araújo, C.; Marín, S.; Buriticá, W. The quantitative impact of using 3d printed anatomical models for surgical planning optimization: Literature review. 3D Print Addit. Manuf. 2022, 3dp.2021.0188. [Google Scholar] [CrossRef]
- Formisano, M.; Iuppariello, L.; Mirone, G.; Cinalli, G.; Casaburi, A.; Guida, P.; Clemente, F. 3d printed anatomical model for surgical planning: A pediatric hospital experience. In Proceedings of the International Conference on E-Health and Bioengineering (EHB), Iasi, Romania, 18–19 November 2021. [Google Scholar] [CrossRef]
- Cornejo, J.; Cornejo-Aguilar, J.A.; Vargas, M.; Helguero, C.G.; Milanezi de Andrade, R.; Torres-Montoya, S.; Asensio-Salazar, J.; Rivero Calle, A.; Martínez Santos, J.; Damon, A.; et al. Anatomical engineering and 3d printing for surgery and medical devices: International review and future exponential innovations. Biomed. Res. Int. 2022, 2022, 6797745. [Google Scholar] [CrossRef] [PubMed]
- Fasel, J.H.D.; Malis, D.D.; Wiederer, C.; Hagenbuch, N. 3D printing of anatomical models for surgeons: An investigation on repeatability. IJIDeM 2018, 12, 621–627. [Google Scholar] [CrossRef]
- Osti, F.; Santi, G.; Neri, M.; Liverani, A.; Frizziero, L.; Stilli, S.; Maredi, E.; Zarantonello, P.; Gallone, G.; Stallone, S.; et al. Ct conversion workflow for intraoperative usage of bony models: From dicom data to 3d printed models. Appl. Sci. 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Narayan, Y.S.; Prakash, K.J.; Rajashekhar, S.; Narendra, P. 3D printed human humerus bone with proximal implant prototype for arthroplasty. Int. J. Health Sci. 2022, 6 (Suppl. 4). [Google Scholar] [CrossRef]
- Salazar, D.A.; Cramer, J.; Markin, N.W.; Hunt, N.H.; Linke, G.; Siebler, J.; Zuniga, J. Comparison of 3D printed anatomical model qualities in acetabular fracture representation. Ann. Transl. Med. 2022, 10, 391. [Google Scholar] [CrossRef]
- Saleh, Y.; Piper, R.; Richard, M.; Jeyaretna, S.; Cosker, T. Designing a 3d printed model of the skull-base: A collaboration between clinicians and industry. J. Med. Educ. Curric. Dev. 2022, 9, 238212052210807. [Google Scholar] [CrossRef]
- Martinez-Marquez, D.; Mirnajafizadeh, A.; Carty, C.P.; Stewart, R.A. Application of quality by design for 3D printed bone prostheses and scaffolds. PLoS ONE 2018, 13, e0195291. [Google Scholar] [CrossRef]
- Rungrojwittayakul, O.; Kan, J.Y.; Shiozaki, K.; Swamidass, R.S.; Goodacre, B.J.; Goodacre, C.J.; Lozada, J.L. Accuracy of 3d printed models created by two technologies of printers with different designs of model base. J. Prosthodont. 2020, 29, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Carew, R.M.; Iacoviello, F.; Rando, C.; Moss, R.M.; Speller, R.; French, J.; Morgan, R.M. A multi-method assessment of 3D printed micromorphological osteological features. Int. J. Leg. Med. 2022, 136, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Ammanuel, S.; Brown, I.; Uribe, J.; Rehani, B. Creating 3d models from radiologic images for virtual reality medical education modules. J. Med. Syst. 2019, 43, 166. [Google Scholar] [CrossRef] [PubMed]
- Silén, C.; Karlgren, K.; Hjelmqvist, H.; Meister, B.; Zeberg, H.; Pettersson, A. Three-dimensional visualisation of authentic cases in anatomy learning–An educational design study. BMC Med. Educ. 2022, 22, 477. [Google Scholar] [CrossRef]
- Edelmers, E.; Kazoka, D.; Pilmane, M. Creation of Anatomically Correct and Optimized for 3D Printing Human Bones Models. Appl. Syst. Innov. 2021, 4, 67. [Google Scholar] [CrossRef]
- Hochman, J.B.; Rhodes, C.; Wong, D.; Kraut, J.; Pisa, J.; Unger, B. Comparison of cadaveric and isomorphic three-dimensional printed models in temporal bone education. Laryngoscope 2015, 125, 2353–2357. [Google Scholar] [CrossRef]
- McMenamin, P.G.; Quayle, M.R.; McHenry, C.R.; Adams, J.W. The production of anatomical teaching resources using three-dimensional (3d) printing technology: 3D Printing in Anatomy Education. Anat. Sci. Educ. 2014, 7, 479–486. [Google Scholar] [CrossRef]
- Blahuta, R.I.; Blikhar, V.S.; Dufeniuk, O.M. Transfer of 3d scanning technologies into the field of criminal proceedings. Sci. Innov. 2020, 16, 84–91. [Google Scholar] [CrossRef]
- Higueras, M.; Calero, A.I.; Collado-Montero, F.J. Digital 3D modeling using photogrammetry and 3D printing applied to the restoration of a Hispano-Roman architectural ornament. DAACH 2021, 20, e00179. [Google Scholar] [CrossRef]
- Baltsavias, E.P. A comparison between photogrammetry and laser scanning. ISPRS J. Photogramm. Remote Sens. 1999, 54, 83–94. [Google Scholar] [CrossRef]
- Bridger, C.A.; Reich, P.D.; Caraça Santos, A.M.; Douglass, M.J.J. A dosimetric comparison of CT- and photogrammetry- generated 3D printed HDR brachytherapy surface applicators. Phys. Eng. Sci. Med. 2022, 45, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.; Ford, A.L.J.; Smith, M.J. Standard methods for creating digital skeletal models using structure-from-motion photogrammetry. Am. J. Phys. Anthropol. 2019, 169, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Wesencraft, K.M.; Clancy, J.A. Using Photogrammetry to Create a Realistic 3D Anatomy Learning Aid with Unity Game Engine. Adv. Exp. Med. Biol. 2019, 1205, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Carew, R.M.; Morgan, R.M.; Rando, C. Experimental assessment of the surface quality of 3D printed bones. Aust. J. Forensic Sci. 2021, 53, 592–609. [Google Scholar] [CrossRef]
- Petriceks, A.H.; Peterson, A.S.; Angeles, M.; Brown, W.P.; Srivastava, S. Photogrammetry of human specimens: An innovation in anatomy education. J. Med. Educ. Curric. Dev. 2018, 5, 238212051879935. [Google Scholar] [CrossRef] [Green Version]
- Ripley, B.; Levin, D.; Kelil, T.; Hermsen, J.L.; Kim, S.; Maki, J.H.; Wilson, G.J. 3d printing from mri data: Harnessing strengths and minimizing weaknesses: 3d printing from mri data. J. Magn. Reason. Imaging 2017, 45, 635–645. [Google Scholar] [CrossRef]
- Bois, M.C.; Morris, J.M.; Boland, J.M.; Larson, N.L.; Scharrer, E.F.; Aubry, M.-C.; Maleszewski, J.J. Three-dimensional surface imaging and printing in anatomic pathology. J. Pathol. Inform. 2021, 12, 22. [Google Scholar] [CrossRef]
- Bücking, T.M.; Hill, E.R.; Robertson, J.L.; Maneas, E.; Plumb, A.A.; Nikitichev, D.I. From medical imaging data to 3D printed anatomical models. PLoS ONE 2017, 12, e0178540. [Google Scholar] [CrossRef] [Green Version]
- Andreß, S.; Achilles, F.; Bischoff, J.; Kußmaul, A.C.; Böcker, W.; Weidert, S. A method for finding high accuracy surface zones on 3D printed bone models. Comput. Biol. Med. 2021, 135, 104590. [Google Scholar] [CrossRef]
- van Eijnatten, M.; van Dijk, R.; Dobbe, J.; Streekstra, G.; Koivisto, J.; Wolff, J. CT image segmentation methods for bone used in medical additive manufacturing. Med. Eng. Phys. 2018, 51, 6–16. [Google Scholar] [CrossRef]
- Labranche, L.; Wilson, T.D.; Terrell, M.; Kulesza, R.J. Learning in stereo: The relationship between spatial ability and 3d digital anatomy models. Anat. Sci. Educ. 2022, 15, 291–303. [Google Scholar] [CrossRef]
- Lau, I.; Sun, Z. The role of 3D printed heart models in immediate and long-term knowledge acquisition in medical education. Rev. Cardiovasc. Med. 2022, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, P.G.; Hussey, D.; Chin, D.; Alam, W.; Quayle, M.R.; Coupland, S.E.; Adams, J.W. The reproduction of human pathology specimens using three-dimensional (3d) printing technology for teaching purposes. Med. Teach. 2021, 43, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Douglass, M.J.J. Can optical scanning technologies replace CT for 3D printed medical devices in radiation oncology? J. Med. Radiat. Sci. 2022, 69, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Crowe, S.; Luscombe, J.; Maxwell, S.; Simpson-Page, E.; Poroa, T.; Wilks, R.; Li, W.; Cleland, S.; Chan, P.; Lin, C.; et al. Evaluation of optical 3D scanning system for radiotherapy use. J. Med. Radiat. Sci. 2022, 69, 218–226. [Google Scholar] [CrossRef]
- Fogarasi, M.; Coburn, J.C.; Ripley, B. Algorithms used in medical image segmentation for 3D printing and how to understand and quantify their performance. 3D Print Med. 2022, 8, 18. [Google Scholar] [CrossRef]
- Brouwers, L.; Teutelink, A.; van Tilborg, F.A.J.B.; de Jongh, M.A.C.; Lansink, K.W.W.; Bemelman, M. Validation study of 3D-printed anatomical models using 2 PLA printers for preoperative planning in trauma surgery, a human cadaver study. Eur. J. Trauma Emerg. Surg. 2019, 45, 1013–1020. [Google Scholar] [CrossRef]
- Paramasivam, V.; Sindhu; Singh, G.; Santhanakrishnan, S. 3d printing of human anatomical models for preoperative surgical planning. Procedia Manuf. 2020, 48, 684–690. [Google Scholar] [CrossRef]
- Odeh, M.; Levin, D.; Inziello, J.; Lobo Fenoglietto, F.; Mathur, M.; Hermsen, J.; Stubbs, J.; Ripley, B. Methods for verification of 3D printed anatomic model accuracy using cardiac models as an example. 3D Print Med. 2019, 5, 6. [Google Scholar] [CrossRef]
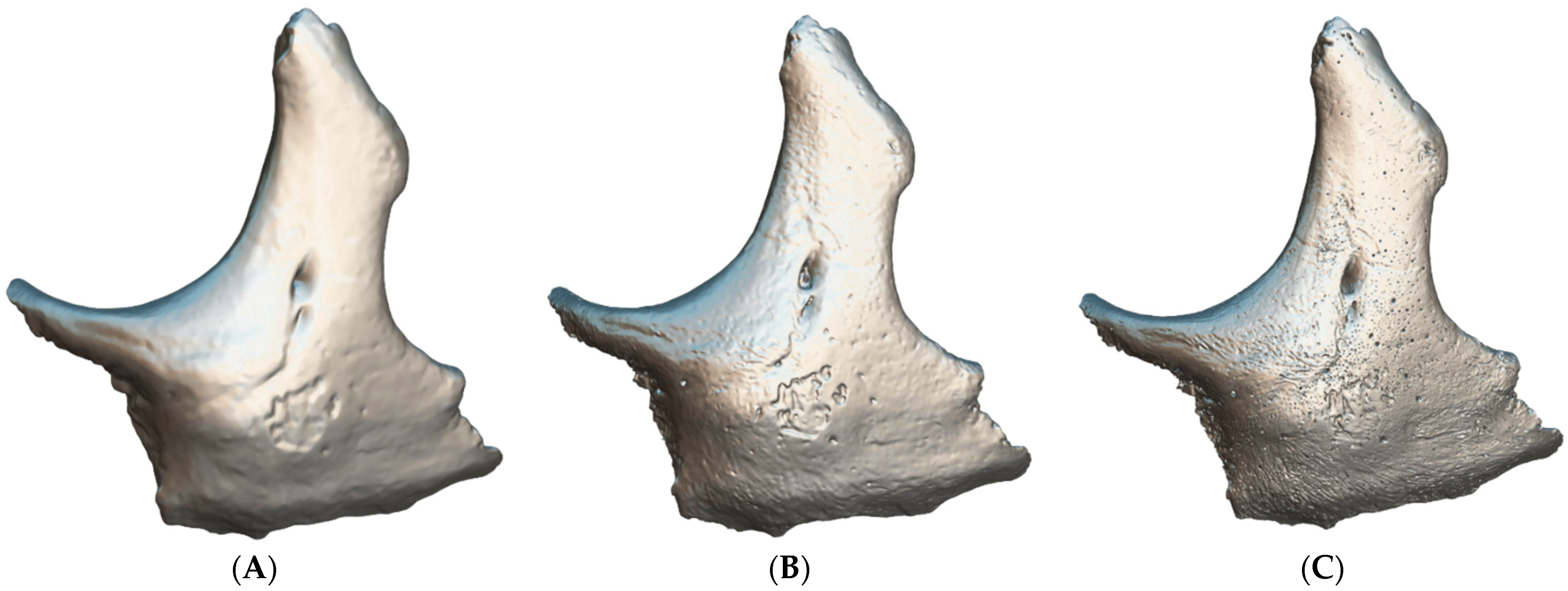
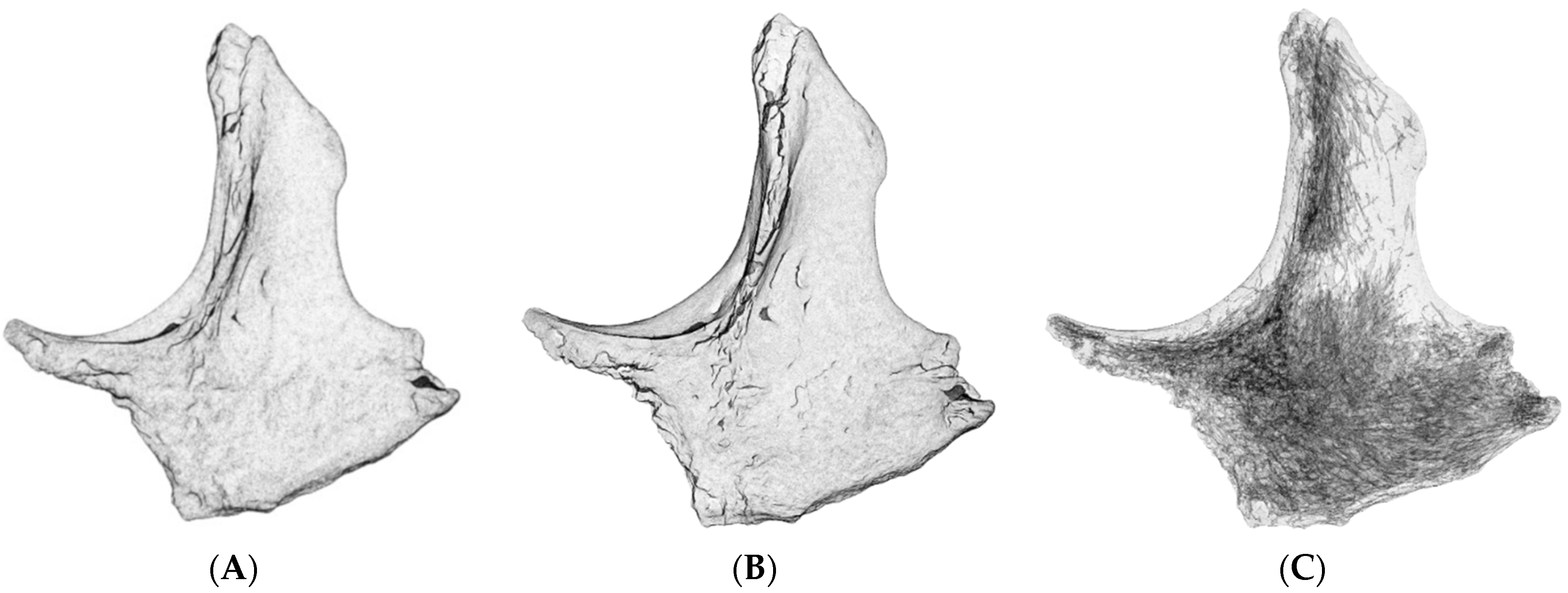
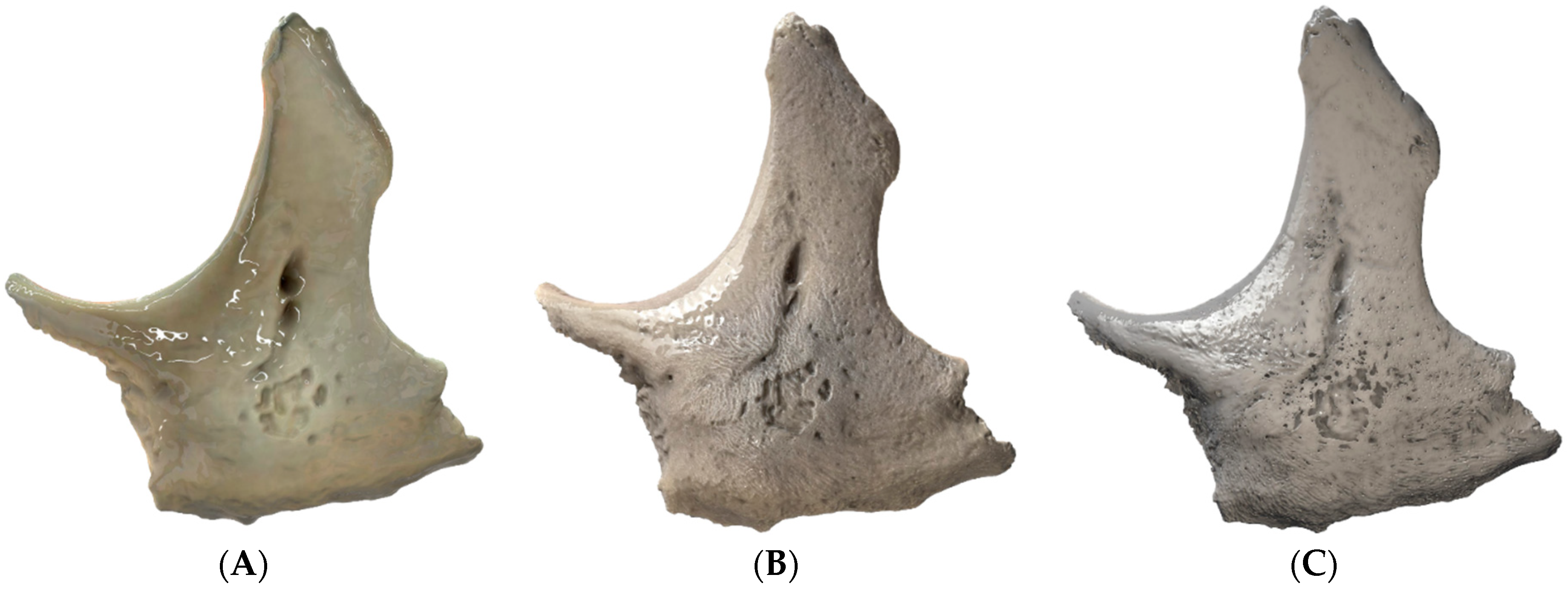
| Equipment | Manufacturer | Model | Specification |
|---|---|---|---|
| Camera | Sony | ILCE-7RM2 | electronics.sony.com/imaging/interchangeable-lens-cameras/full-frame/p/ilce7rm2-b (accessed on 17 August 2022) |
| Lens | Sigma | 70 mm F2.8 DG MACRO Art | sigma-global.com/en/lenses/a018_70_28 (accessed on 17 August 2022) |
| X-ray micro-computed tomography | SCANCO Medical | µCT50 | scanco.ch/microct50.html (accessed on 17 August 2022) |
| Computer | Lenovo | Legion 7 | Windows 11 Pro, AMD Ryzen 7 5800H, NVIDIA GeForce RTX 3080 16 GB, 64 GB DDR4 3200 MHz, 1000 GB solid-state drive. |
| 3D Scanner | Shining3D | EinScan-S | einscan.com/desktop-3d-scanners/einscan-se/einscan-se-specs (accessed on 17 August 2022) |
| Software|Platform | Version | Information |
|---|---|---|
| EinScan-S | 2.5.0.7 | einscan.com/support/download/software/?scan_model=einscan-s (accessed on 17 August 2022). |
| Micro-CT | -//- | Was shipped along with the µCT50 micro-CT machine. |
| 3D Slicer | 5.02 | slicer.org (accessed on 17 August 2022) |
| MeshLab | 2022.02 | meshlab.net (accessed on 17 August 2022) |
| RealityCapture | 1.2.0.17385 | capturingreality.com/realitycapture (accessed on 17 August 2022) |
| Adobe Photoshop | 23.1.1 | For textures’ color correction. |
| Capture One 22 Pro | 15.0.1.4 | For cameras’ RAW images procession. |
| Sketchfab | -//- | sketchfab.com (accessed on 17 August 2022) |
| Techniques | Before Simplification (Faces|Vertices) | After Simplification (Faces|Vertices) |
|---|---|---|
| 3D scanning | 700,002 350,003 | Has not been simplified |
| Micro Computed Tomography | 70,195,566 35,073,613 | 7,019,556 3 485,608 |
| Photogrammetry | 13,716,318 6,882,203 | 700,842 350,423 |
| Techniques | Size of the Texture Map (Pixels; Width × Height) |
|---|---|
| 3D scanning | 766 × 998 |
| Micro Computed Tomography | No visual data havebeen captured |
| Photogrammetry | 16,384 × 16,384 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmers, E.; Kazoka, D.; Bolocko, K.; Pilmane, M. Different Techniques of Creating Bone Digital 3D Models from Natural Specimens. Appl. Syst. Innov. 2022, 5, 85. https://doi.org/10.3390/asi5040085
Edelmers E, Kazoka D, Bolocko K, Pilmane M. Different Techniques of Creating Bone Digital 3D Models from Natural Specimens. Applied System Innovation. 2022; 5(4):85. https://doi.org/10.3390/asi5040085
Chicago/Turabian StyleEdelmers, Edgars, Dzintra Kazoka, Katrina Bolocko, and Mara Pilmane. 2022. "Different Techniques of Creating Bone Digital 3D Models from Natural Specimens" Applied System Innovation 5, no. 4: 85. https://doi.org/10.3390/asi5040085
APA StyleEdelmers, E., Kazoka, D., Bolocko, K., & Pilmane, M. (2022). Different Techniques of Creating Bone Digital 3D Models from Natural Specimens. Applied System Innovation, 5(4), 85. https://doi.org/10.3390/asi5040085








